Introduction
Acute respiratory distress syndrome (ARDS) has
always been a controversial topic in medical research due to its
high incidence and high risk/rate of mortality. Mechanical
ventilation has a key role in the treatment of patients with ARDS
(1). However, mechanical
ventilation may cause ventilator-induced lung injury (VILI) and
cause or accelerate pulmonary fibrotic changes (2). A previous study indicated that the
severity of pulmonary fibrosis is closely linked to mortality,
prognosis and long-term quality of life in patients with ARDS
(3). However, the mechanism has
remained to be completely clarified and there is still a lack of
effective prevention and treatment measures in clinical settings
(4). Studies have suggested a close
association of mechanical stimulation, transforming growth factor
β1 (TGF-β1) and collagen with lung tissue remodeling in ARDS
(5,6), but the regulatory mechanism has
remained elusive. In the present study, MRC-5 human embryonic lung
fibroblasts were treated with lipopolysaccharides (LPS) at the
cellular level to establish an ARDS cell injury model and the cells
were further cultured with different mechanical stretching
amplitudes through the Flexcell system to establish a cellular
mechanical damage model. The protein and gene expression levels of
TGF-β1 and collagen were detected by ELISA and reverse
transcription-quantitative (RT-q) PCR, respectively, and the
viscoelastic behavior of human embryonic lung fibroblasts under LPS
and mechanical stretch stimulation was further explored. The
present study supported the notion that in patients with mechanical
ventilation, mechanical stretch aggravates ARDS-associated lung
injury and promotes lung structural remodeling. It provided
underlying mechanobiological mechanisms and guidance for early
prevention and treatment of ventilator-induced lung injury and
pulmonary structural remodeling during ARDS treatment.
Materials and methods
Cell lines and reagents
The human embryonic lung fibroblast cell line, MRC-5
(Cell Bank of Type Culture Collection, Chinese Academy of
Sciences), was maintained in minimal essential medium (Invitrogen;
Thermo Fisher Scientific, Inc.) which contained 2.0 mmol/l
glutamine. The medium was supplemented with 10% fetal calf serum
(Invitrogen; Thermo Fisher Scientific, Inc.) and cells were
cultured at 37˚C in a humidified atmosphere containing 5% carbon
dioxide and 95% air. The MRC-5 cells were inoculated on a flexible
substrate culture plate (FX-5000, Flexcell International Corp.).
MRC-5 cells in the logarithmic growth phase were cultured for 48 h
with different concentrations of LPS (0, 5, 20 or 50 µg/ml).
Subsequently, different amplitudes of mechanical stretch
stimulation were applied to the cells via the Flexcell loading
system (Flexcell Corp., USA) and culture was continued for 48 h
(loading parameters: Frequency, 0.1 Hz; sine wave, stretching
amplitude of 5, 10, 15 and 20%).
Detection of cell proliferation
MRC-5 cells were collected after LPS stimulation
and/or mechanical stretch treatment. The cells were labeled with
carboxyfluorescein succinimidyl ester (CFSE) and cell proliferative
activity was assessed by flow cytometry (BD FACSCalibur; BD
Biosciences). CFSE is a non-toxic dye for cells and its chemical
properties are stable. Once CFSE enters the cell, it cannot be
released from the cell and will not be metabolized. The only way to
reduce the CFSE content in cells is through cell proliferation and
division. The CFSE contained in the cells enters the progeny cells
as the cells proliferate. The cells were labeled with CFSE before
inoculation on a flexible substrate culture plate where the initial
fluorescence intensity was measured. Subsequently, the fluorescence
intensity after proliferation was measured after LPS stimulation
and/or mechanical stretch treatment by flow cytometry. To determine
the proliferative activity of chondrocytes, the proliferation index
was calculated as follows: Proliferation index=initial fluorescence
intensity/fluorescence intensity after proliferation.
Detection of protein expression
levels
MRC-5 cells were treated by LPS stimulation and/or
mechanical stretch for 48 h. The supernatant was collected after
centrifugation (503.1 x g for 5 min at 25˚C) to remove impurities
and refrigerated at -70˚C. An ELISA kit (1R443; Rapidbio) was used
according to the manufacturer's protocol. The absorbance at 450 nm
was detected with a microplate reader (iMark; Bio-Rad Laboratories,
Inc.) and the content of TGF-β1 in the sample was extrapolated by
using a standard curve.
Detection of gene expression
levels
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from from
the control and treated MRC-5 cells and reverse transcribe RNA into
cDNA. The expression of TGF-β1 mRNA and collagen type I α1 (Col1α1)
mRNA in human embryonic lung fibroblasts were detected by RT-qPCR
(7). The PCR detection was
determined using an Exicycler™ 96 real-time system (Bioneer) with
SYBR Premix ExTaqII (Takara Bio, Inc.). The PCR conditions were as
follows: Enzyme activation at 94˚C for 5 min, amplification at 94˚C
for 10 sec, annealing at 60˚C for 20 sec and extension at 72˚C for
30 sec, 40 cycles, final extension at 72˚C for 6 min. The
expression level of the target genes, TGF-β1 and Col1α1, were
quantified using the 2-ΔΔCq method (7). β-actin was used as a reference gene.
The primers were as follows: β-actin forward,
5'-GACAGGATGCAGAAGGAGATTACT-3' and reverse,
5'-TGATCCACATCTGCTGGAAGGT-3', TGF-β1 forward,
5'-GCCCTGGACACCAACTATTGC-3' and reverse,
5'-AGGCTCCAAATGTAGGGGCAG-3'; Col1α1 forward,
5'-GCCTAGCAACATGCCAATC-3' and reverse,
5'-GCAAAGTTCCCACCGAGA-3'.
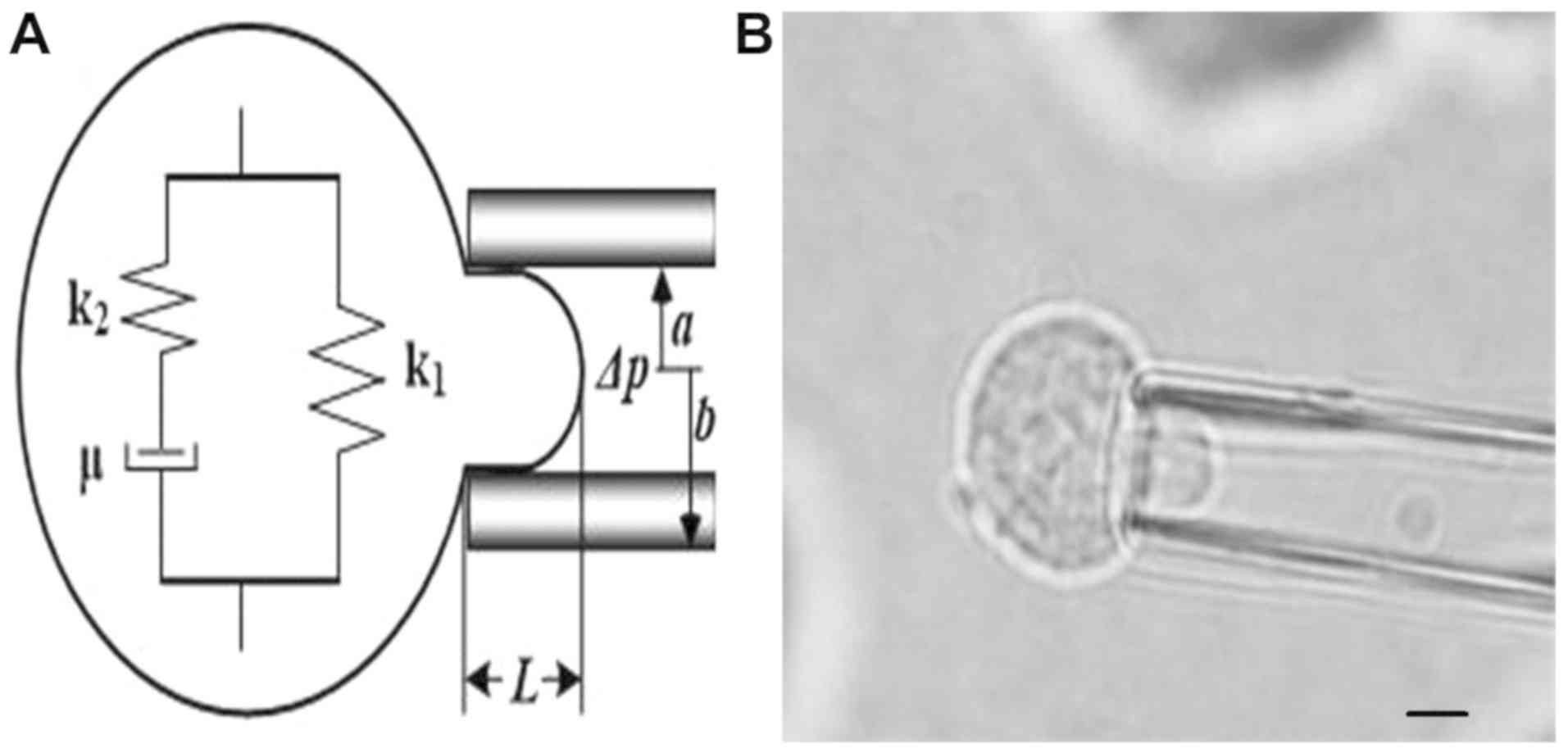
Micropipette aspiration and
viscoelasticity calculation
The deformation of control and treated MRC-5 cells
under the negative pressure (range from 0.245 to 0.392 kPa) were
examined using a micropipette aspiration system, which produced the
association between aspirated lengths and time (8-10).
The cellular viscoelastic parameters [the instantaneous modulus
(E0), the equilibrium modulus associated with
long-term equilibrium (E∞) and the apparent
viscosity (µ)] were calculated using the Kelvin standard
linear viscoelastic solid model as previously described, and the
cellular viscoelastic parameters (E0,
E∞ and µ) were calculated according to the
association between aspirated lengths and time (8-10).
The viscoelastic model and a cell sucked in by the microtubules are
shown in Fig. 1.
Statistical analysis
In the present study, all the independent
experiments were repeated three times. Data processing and mapping
were performed using SPSS 22.0 statistical software (IBM Corp.) and
GraphPad Prism 6.0 (GraphPad Software, Inc.). The measurement data
were expressed as the mean ± standard deviation. Differences
between the groups were compared by one-way analysis of variance
with the Student-Newman-Keuls post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
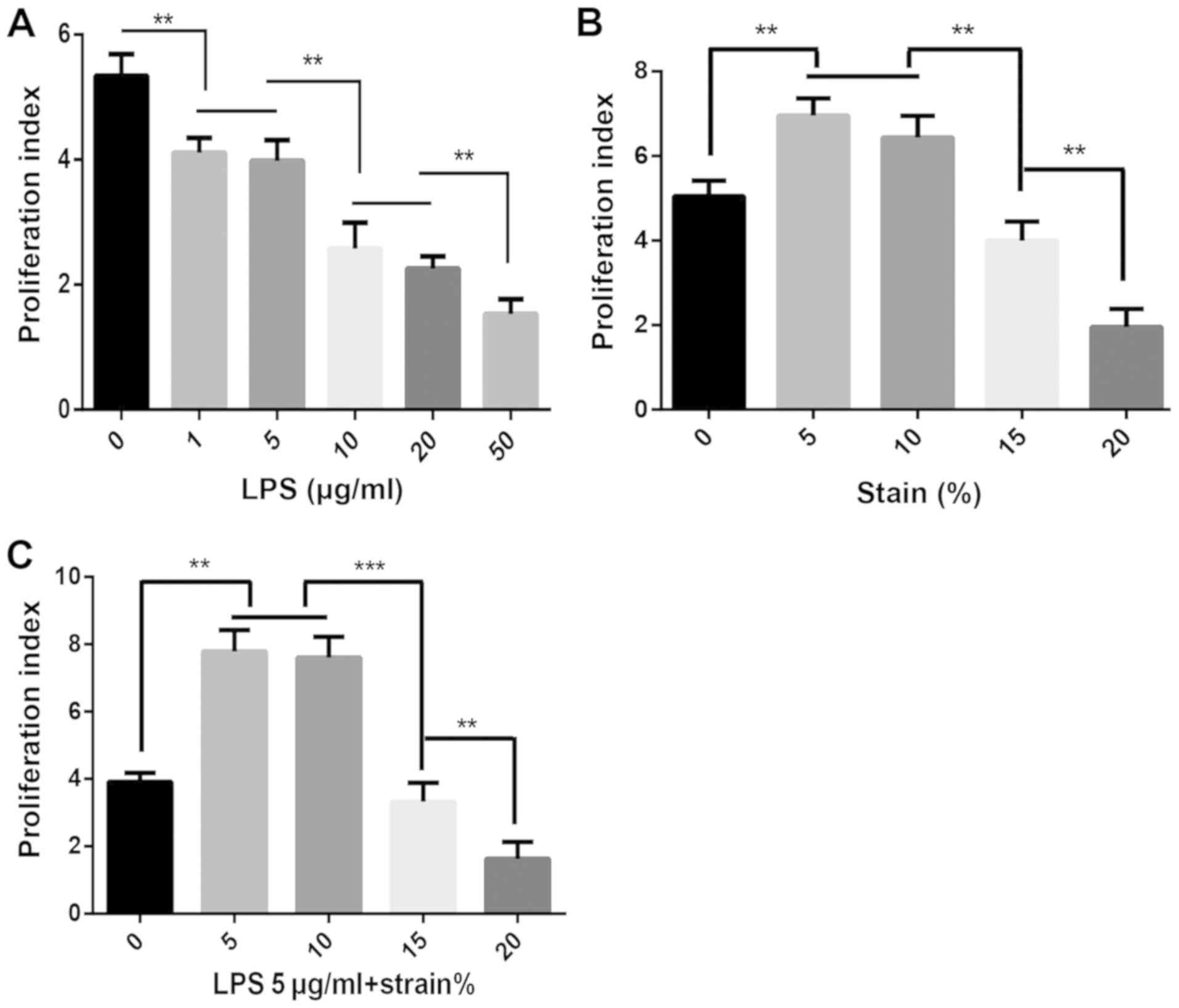
Effect on MRC-5 cell proliferation
activity
MRC-5 cells were treated with different
concentrations of LPS and the cell proliferation activity was
analyzed by flow cytometry. It was indicated that LPS induced cell
damage and decreased the proliferative activity. As the
concentration of LPS increased, the cell proliferation activity
continued to significantly decrease (P<0.01; Fig. 2A). MRC-5 cells were cultured under
different stretch amplitudes and the cell proliferation activity
was analyzed by the same method. The results suggested that the
proliferative activity of MRC-5 cells cultured under a
low-amplitude stretch (5 and 10%) was significantly increased, but
as the stretch amplitude increased (15 and 20%), the proliferative
activity of MRC-5 cells exhibited a significant decrease
(P<0.01; Fig. 2B). According to
the experimental results obtained with different concentrations of
LPS combined with clinical practice to simulate ARDS induced
injury, cells treated with LPS at low concentrations (5 µg/ml) were
selected to construct a cell injury model similar to the
pathophysiological state of ARDS, and culture was then continued at
different stretch widths to observe the effects on the
proliferative activity. The results also indicated that the
proliferation activity of MRC-5 cells stimulated by LPS cultured
with a low-amplitude stretch (5 and 10%) was improved; however,
under high traction (15 and 20%), the cell proliferation was
significantly decreased (P<0.05; Fig. 2C).
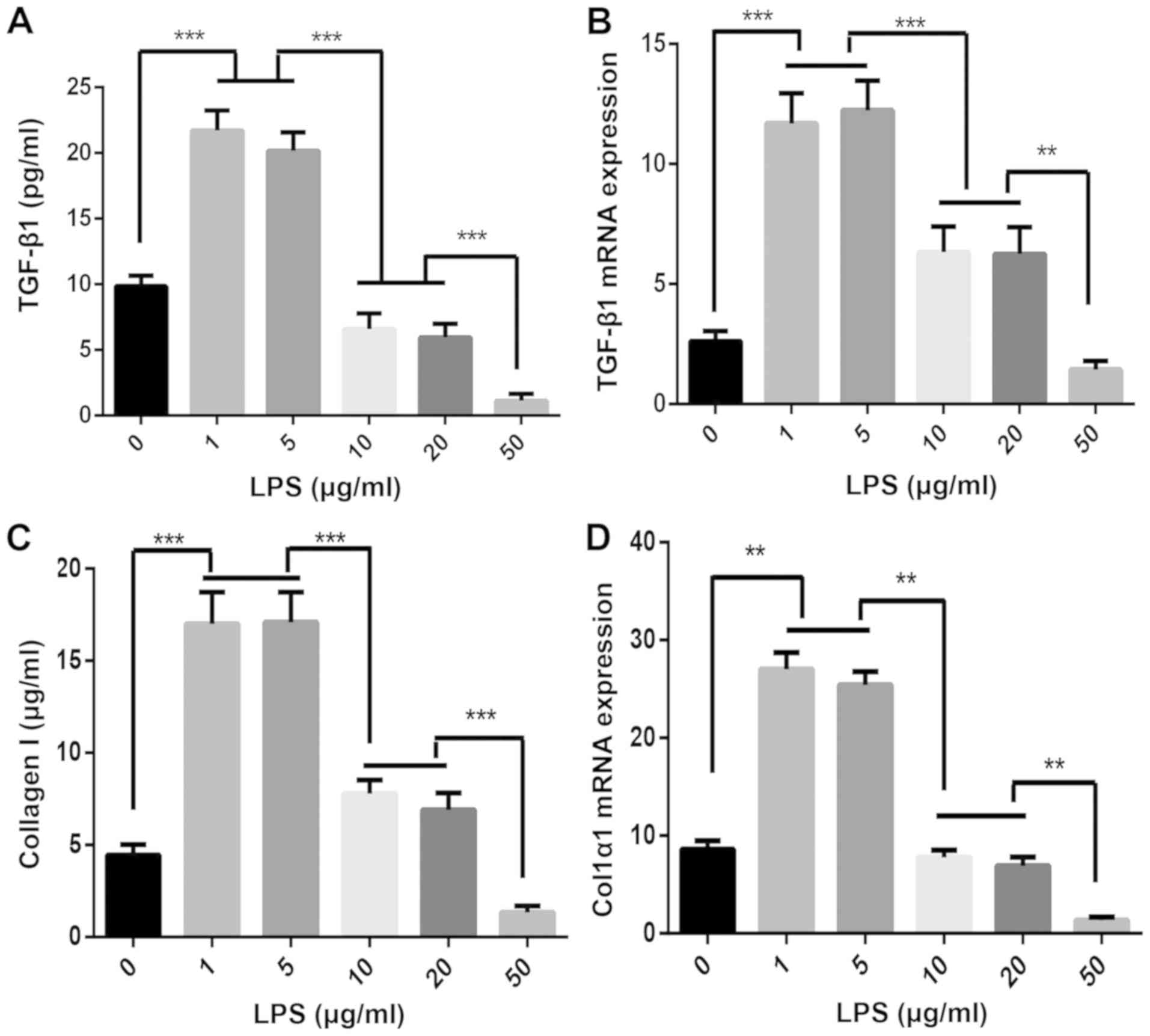
TGF-β1 and collagen I expression by
MRC-5 cells following LPS stimulation
As provided in Fig.
3, it was indicated that low concentrations of LPS upregulated
TGF-β1 and collagen I expression after cell injury, while high
concentrations of LPS affected the cell proliferation activity and
reduced the expression of TGF-β1 and collagen I in the supernatant
(Fig. 3A and C). The RT-qPCR results suggested that low
concentrations of LPS led to upregulated expression of TGF-β1 and
Col1α1 mRNA, while high concentrations of LPS decreased their
expression (Fig. 3B and D).
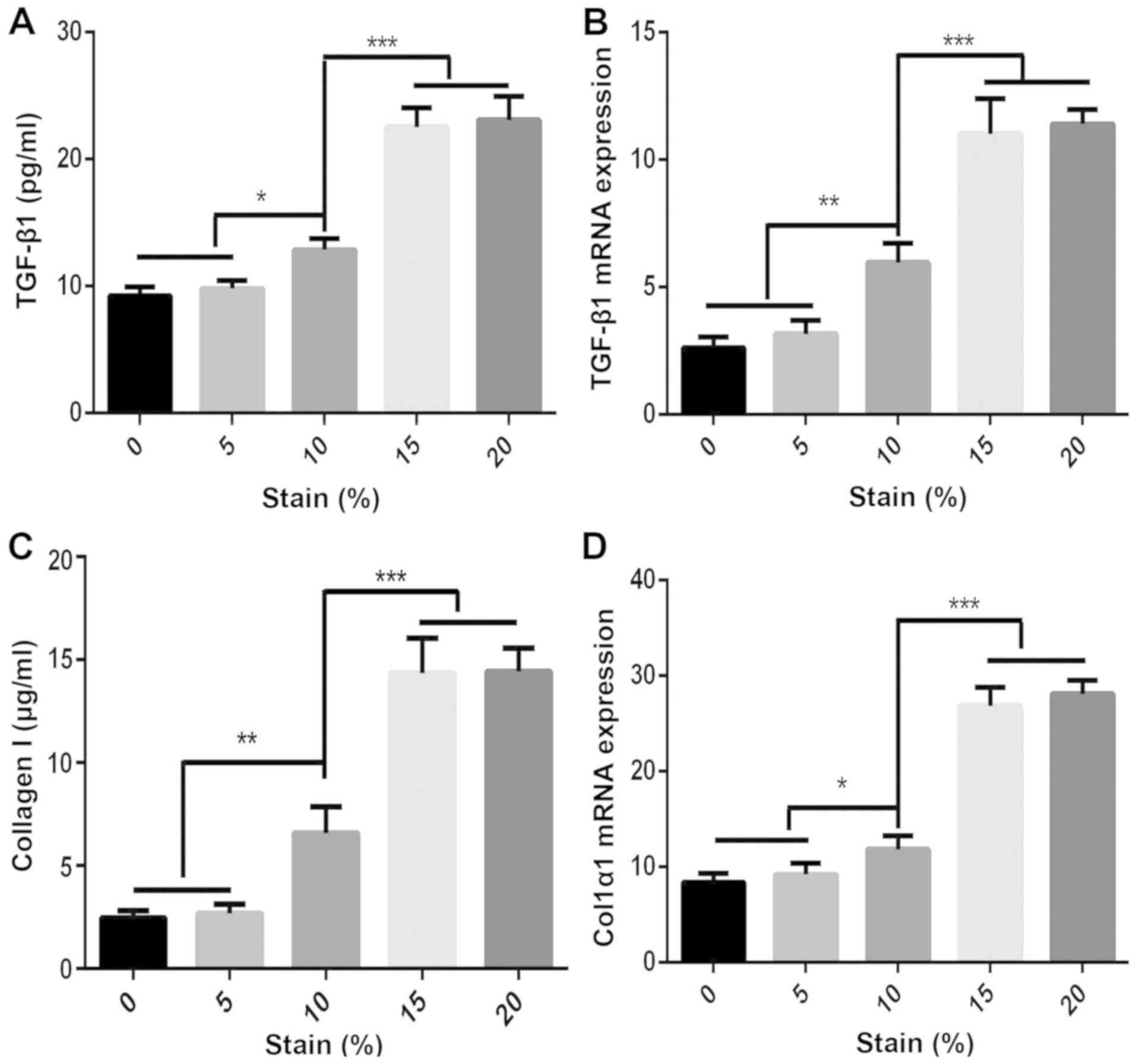
TGF-β1 and collagen I expression by
MRC-5 cells after mechanical stretch stimulation
MRC-5 cells were cultured under different mechanical
stretches and the concentration of TGF-β1 and collagen in the cells
or supernatant was detected (Fig.
4). The results indicated that mechanical stimulation with a
stretch of 5% had no effect on the protein levels of TGF-β1 and
collagen I, while a 10% stretch induced a significant increase in
TGF-β1 and collagen I levels, and a much greater mechanical
stimulation (15 and 20%) significantly increased the levels of
TGF-β1 and collagen I than the 10% stretch (Fig. 4A and C). Total RNA was extracted from the
control and mechanical stretch-treated MRC-5 cells, and the
expression of TGF-β1 and Col1α1 mRNA was detected by RT-qPCR.
Mechanical stimulation with a stretch of 10% induced upregulation
of TGF-β1 and Col1α1 mRNA expression and a much larger mechanical
stretch stimulation (15 and 20%) significantly increased the
expression of TGF-β1 mRNA and Col1α1 mRNA in the cells (Fig. 4B and D).
TGF-β1 and collagen I expression by
MRC-5 cells following combined stimulation with LPS and mechanical
stretch
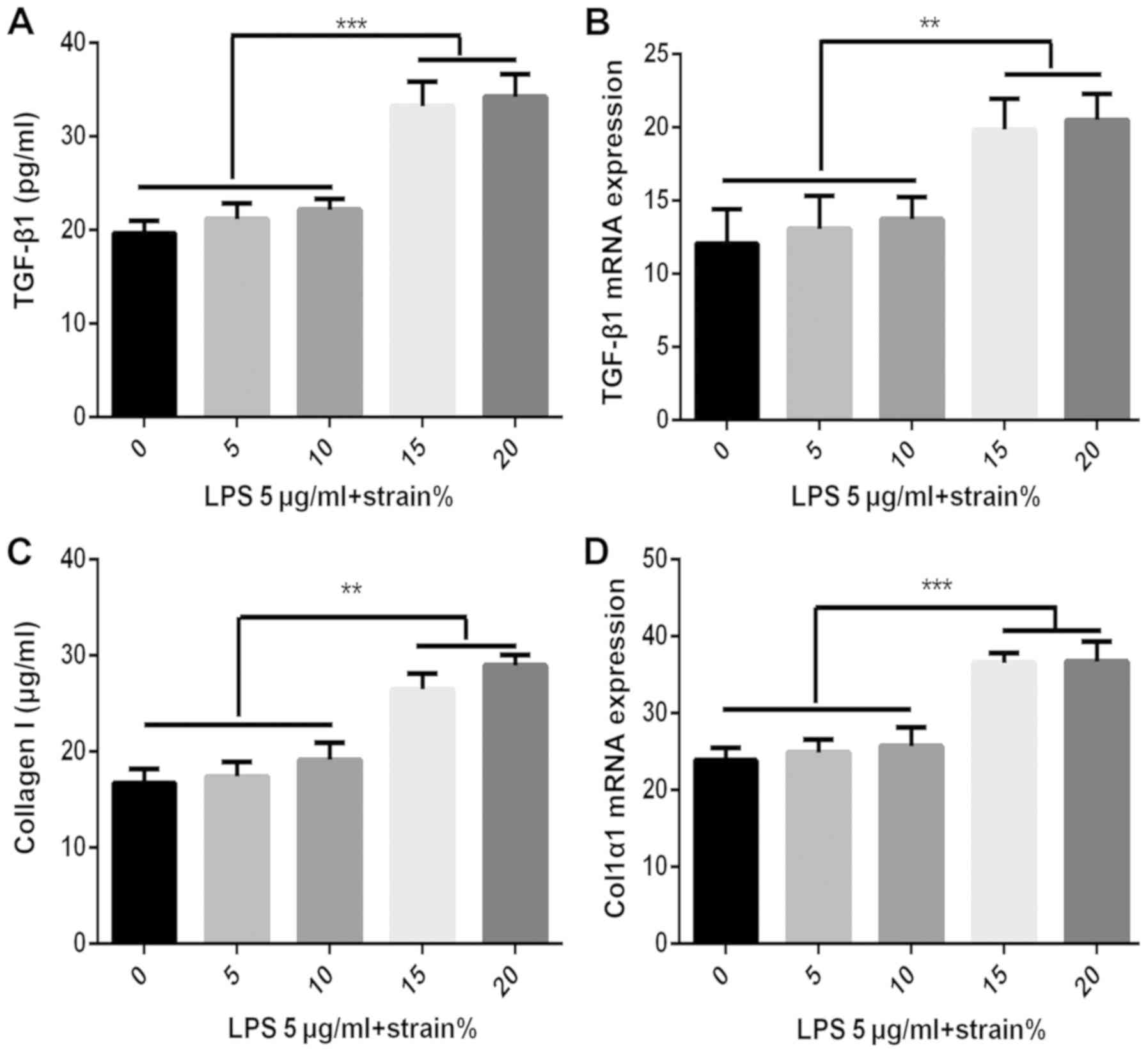
MRC-5 cells were treated with LPS at 5 µg/ml to
construct the cell injury model and those cells were cultured for a
further 48 h under mechanical stimulation with different stretch
amplitudes. The results indicated that mechanical stimulation with
a stretch amplitude of up to 10% did not result in any significant
increase in TGF-β1 or collagen I protein, or their gene expression
levels in cells following culture with 5 µg/ml LPS (P>0.05).
However, much larger mechanical stimulation (15 and 20%) caused
further damage to the LPS cell injury model. At the same time, the
levels of TGF-β1 and collagen I, and their gene expression levels
were significantly increased (P<0.05; Fig. 5).
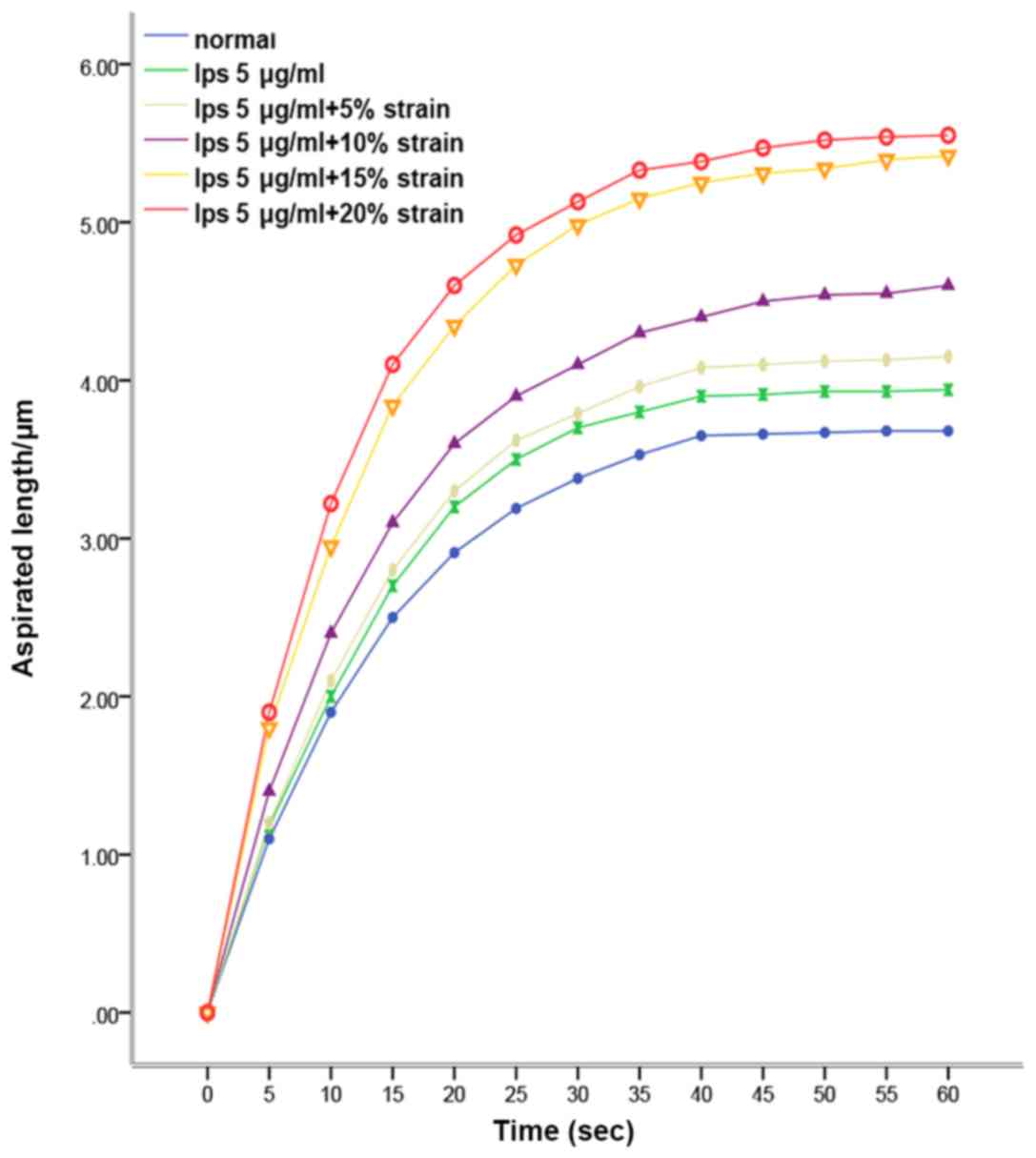
Viscoelastic changes of MRC-5 cells
under different mechanical stimulation
MRC-5 cells treated with 5 µg/ml of LPS were
cultured under different mechanical stimulation conditions. A cell
microtubule suction test indicated that MRC-5 cells exhibited
typical viscoelastic solid creep characteristics under constant
negative pressure. MRC-5 cells exhibited an instantaneous
viscoelastic deformation. The trend of cell inhalation length
changing with time at a constant negative pressure of 294 Pa in
each group suggested that LPS stimulation and mechanical stretch
treatment increased the inhalation length of cells and the length
of inhalation was positively correlated with the stretch amplitude
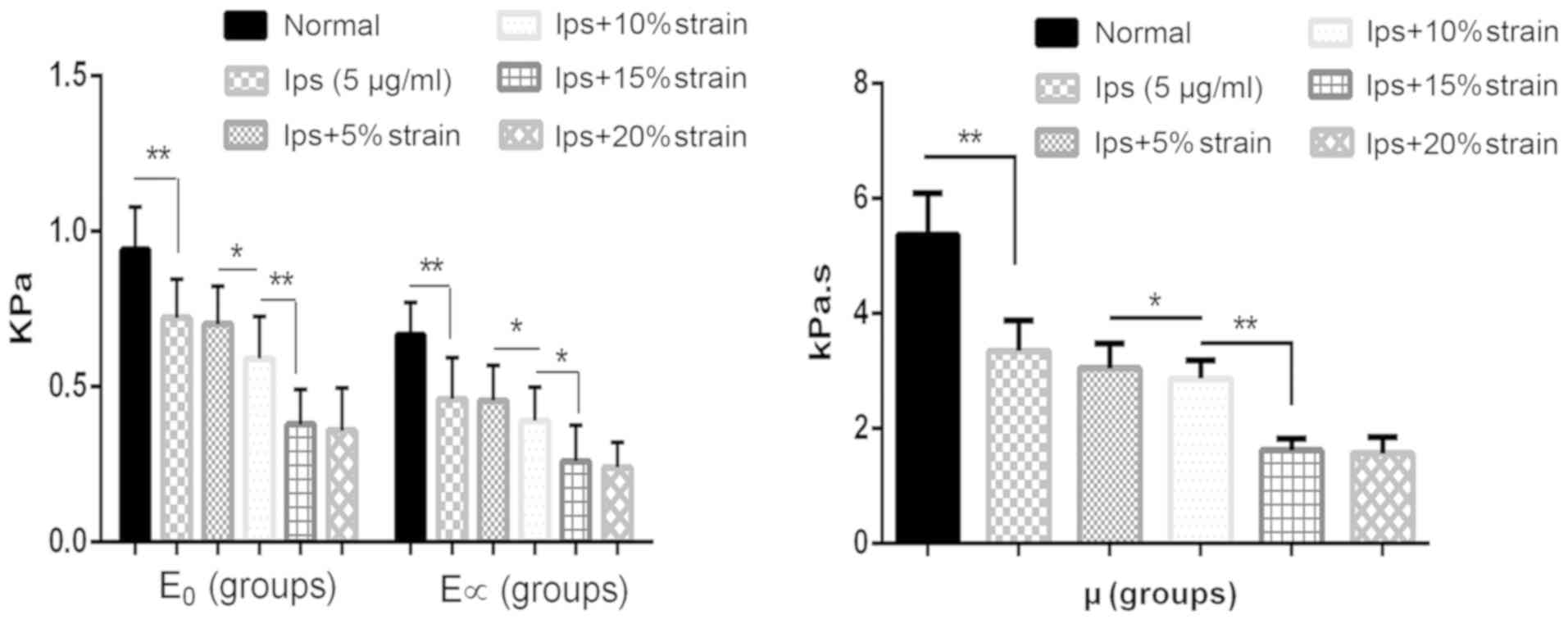
(Fig. 6). The viscoelastic
parameters (E0, E∞ and µ) of
treated and control MRC-5 cells are presented in Fig. 7. The results showed that the LPS
treatment significantly reduced all viscoelastic parameters of
MRC-5 cells (P<0.05). After LPS treatment, the biomechanical
properties of MRC-5 cells were reduced and the cells were softened.
A mechanical stretch of 5% had no effect on the biomechanical
properties of MRC-5 cells treated with only 5 µg/ml LPS. As the
mechanical stretch increased, the viscoelasticity of MRC-5 cells
gradually decreased (P<0.05), but there was no significant
difference in cell viscoelasticity between the two groups of MRC-5
cells cultured with a 15 and 20% mechanical stretch
(P>0.05).
Discussion
ARDS is serious common disease of intensive care
medicine and the mortality rate is as high as 46% (11). The use of an ARDS-associated
mechanical ventilator is a key treatment of patients with ARDS;
however, it may cause ARDS-associated ventilator-induced lung
injury and pulmonary fibrosis. A previous study indicated that have
indicated that pulmonary fibrosis is closely related to mortality,
prognosis and quality of life in patients with ARDS (12). However, to date, the cellular
mechanisms of pulmonary fibrosis in patients with ARDS have
remained to be clarified and the clinical prevention and treatment
effects are not satisfactory. In the present study, an ARDS cell
injury model was generated by LPS stimulation of MRC-5 human
embryonic lung fibroblasts. Furthermore, mechanical stretch
stimulation was performed on MRC-5 cells to study the biomechanical
changes of lung fibroblasts during the pathological progression of
ARDS-associated pulmonary fibrosis.
TGF-β is a class of cytokines with autocrine and
paracrine functions, which are considered to be the most critical
fibrotic factors. TGF-β regulates the expression of effector genes,
including collagen, by transducing signals through intracellular
signaling molecules (1). The
present study indicated that TGF-β1 is activated in the early stage
of ARDS and participates in the process of lung tissue damage
repair. As a potent profibrotic cytokine, it regulates the
expression and secretion of collagen in lung tissue (13). The present study indicated that
MRC-5 cells treated with different concentrations of LPS expressed
different levels of TGF-β1 mRNA and Col1α1 mRNA. Cell injury was
induced by low concentrations of LPS, thereby leading to enhanced
expression of TGF-β1 and collagen I and promoting the progression
of lung fibrosis. High concentrations of LPS affected the cell
proliferative activity and altered and destroyed the normal
structure of lung fibroblasts, causing serious damage to cells and
severely reducing the expression of TGF-β1 and collagen I. This may
be consistent with the pathophysiological manifestations of
clinically severe ARDS cases due to the massive endotoxin release
caused by severe infection, which results in severe lung damage. In
such cases, patients die due to rapid deterioration of lung
function, losing treatment opportunities, but the lungs do not have
time to progress to pulmonary fibrosis. This also explains the high
mortality rate of ARDS.
The lung is a mechanical organ. Mechanical
ventilation causes repeated stress on the lung tissue and exerts
mechanical stretch effects on alveolar epithelial cells, lung
fibroblasts and pulmonary macrophages (14). In the pathological state of ARDS,
different mechanical stretch modes and amplitudes have different
molecular biological and cytological effects on lung tissue cells,
affecting gene expression and metabolism of cells. Biomechanical
signal transduction has a key role in the initiation of mechanical
activation of intracellular inflammatory signaling pathways
(15). During the mechanical
ventilation treatment of ARDS, the connective tissue of the lung
continuously withstands and transmits mechanical signals. The
fibroblasts of connective tissue are cells that respond to
mechanical stretch stimulation. They are able to change their own
cellular mechanical characteristics and alter the gene and protein
expression of their own extracellular matrix under external
physical (e.g. stretch force), chemical (e.g. chemical poisons) and
biological (e.g. infectious toxins) factors, maintaining the
structure and function of organ tissues (16,17).
In the present study, mechanical stimulation at different stretch
amplitudes was performed on normal and 5 µg/ml LPS-treated human
embryonic lung fibroblasts. The results indicated that appropriate
mechanical stimulation is able to increase the proliferative
activity of cells and also slightly stimulate the expression of
TGF-β1 and collagen I in human embryonic lung fibroblasts. Larger
mechanical stimulation directly causes cell damage, reduces the
cell proliferation activity and induces the expression of TGF-β1,
which leads to a significant increase in collagen expression and
accelerates the process of pulmonary fibrosis.
The present study demonstrated that mechanical
stretch is able to regulate multiple functions of lung fibroblasts,
including cell proliferation and gene and protein expression. It
also indicated that the biomechanical viscoelastic parameters of
MRC-5 cells after LPS and mechanical stimulation with different
stretch amplitudes were significantly smaller than those of normal
cells (P<0.05). After mechanical stimulation of different
stretch amplitudes, the biomechanical properties of MRC-5 cells are
reduced and the lung fibroblasts appear to be 'softened',
indicating that lung fibroblasts are deformed in a lower order than
the linear stress-strain association. This feature is not a
reflection of the specific molecular mechanism but of certain
higher structural changes, indicating that the cytoskeleton may
have been damaged (18). LPS and
excessive mechanical stretch stimulation may re-integrate the
structure and stress distribution of cytoskeletal fibers and
molecular connections from the cell surface to the nucleus, and the
cytoskeletal structure and cell membrane structure may be changed
(19). These changes may involve
the repair and remodeling of lung tissue by regulating cell surface
receptors and extracellular matrix alone or in synergistic action
(20). The present study only
provided a preliminary assessment of the biomechanical properties
of lung fibroblasts in the short-term after LPS and mechanical
stretch stimulation. Changes in the composition and structure of
the lung fibroblast cytoskeleton during pulmonary fibrosis and the
mutual regulation of cell mechanical properties and biological
properties require further study.
A major limitation of the present study is the lack
of validation of the results in samples from patients with ARDS to
confirm the in vitro data. Furthermore, the mechanisms of
how mechanical stretch stimulation regulates the remodeling of ARDS
lung structure were not sufficiently explored and require further
study.
In conclusion, mechanical stimulation may lead to
changes in the proliferation, bioviscoelastic properties and
expression levels of TGF-β1 in lung fibroblasts, which in turn
affects the transcription and translation of collagen I genes,
resulting in changes in collagen expression in lung tissue and
leading to remodeling of lung tissue. The present study provides
further in-depth information for the early prevention and treatment
of ARDS ventilator-induced lung injury and lung structural
remodeling from a mechanobiology perspective and provides
cell-level laboratory evidence for the implementation of a
pulmonary protective ventilation strategy.
Acknowledgements
Not applicable.
Funding
This study was funded by the Lianyungang City
Science and Technology Plan Funding Project (grant no. SH1601).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL conceived and designed the present study. YX and
YQ performed the experiments. YX and YW analyzed the data. KL
provided the reagents, materials and analysis tools and interpreted
the data. YX and YQ wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-β/Smad signaling in
tissue fibrosis. Chem Biol Interact. 292:76–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nieman GF, Satalin J, Andrews P, Aiash H,
Habashi NM and Gatto LA: Personalizing mechanical ventilation
according to physiologic parameters to stabilize alveoli and
minimize ventilator induced lung injury (VILI). Intensive Care Med
Exp. 5(8)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bates J and Smith BJ: Ventilator-induced
lung injury and lung mechanics. Ann Transl Med.
6(378)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gattinoni L, Marini JJ, Collino F, Maiolo
G, Rapetti F, Tonetti T, Vasques F and Quintel M: The future of
mechanical ventilation: Lessons from the present and the past. Crit
Care. 21(183)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Souma K, Shichino S, Hashimoto S, Ueha S,
Tsukui T, Nakajima T, Suzuki HI, Shand FHW, Inagaki Y, Nagase T and
Matsushima K: Lung fibroblasts express a miR-19a-19b-20a
sub-cluster to suppress TGF-β-associated fibroblast activation in
murine pulmonary fibrosis. Sci Rep. 8(16642)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lv Z, Wang Y, Liu YJ, Mao YF, Dong WW,
Ding ZN, Meng GX, Jiang L and Zhu XY: NLRP3 inflammasome activation
contributes to mechanical stretch-induced endothelial-mesenchymal
transition and pulmonary fibrosis. Crit Care Med. 46:e49–e58.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xie Y, Wang M, Cheng M, Gao Z and Wang G:
The viscoelastic behaviors of several kinds of cancer cells and
normal cells. J Mech Behav Biomed Mater. 91:54–58. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang G and Chen W: Effects of mechanical
stimulation on viscoelasticity of rabbit scleral fibroblasts after
posterior scleral reinforcement. Exp Biol Med (Maywood).
237:1150–1154. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xie Y, Liu X, Wang S, Wang M and Wang G:
Proper mechanical stimulation improve the chondrogenic
differentiation of mesenchymal stem cells: Improve the
viscoelasticity and chondrogenic phenotype. Biomed Pharmacother.
115(108935)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Maca J, Jor O, Holub M, Sklienka P, Burša
F, Burda M, Janout V and Ševčík P: Past and present ARDS mortality
rates: A systematic review. Respir Care. 62:113–122.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pais FM, Sinha P, Liu KD and Matthay MA:
Influence of clinical factors and exclusion criteria on mortality
in ARDS observational studies and randomized controlled trials.
Respir Care. 63:1060–1069. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang X, Lai R, Su X, Chen G and Liang Z:
Edaravone attenuates lipopolysaccharide-induced acute respiratory
distress syndrome associated early pulmonary fibrosis via
amelioration of oxidative stress and transforming growth
factor-β1/Smad3 signaling. Biochem Biophys Res Commun. 495:706–712.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yen S, Preissner M, Bennett E, Dubsky S,
Carnibella R, O'Toole R, Roddam L, Jones H, Dargaville PA, Fouras A
and Zosky GR: The link between regional tidal stretch and lung
injury during mechanical ventilation. Am J Respir Cell Mol Biol,
2018.
|
|
15
|
Shimbori C, Upagupta C, Bellaye PS, Ayaub
EA, Sato S, Yanagihara T, Zhou Q, Ognjanovic A, Ask K, Gauldie J,
et al: Mechanical stress-induced mast cell degranulation activates
TGF-β1 signalling pathway in pulmonary fibrosis. Thorax.
74:455–465. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
White ES: Lung extracellular matrix and
fibroblast function. Ann Am Thorac Soc. 12 (Suppl 1):S30–S33.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Erdogan B and Webb DJ: Cancer-associated
fibroblasts modulate growth factor signaling and extracellular
matrix remodeling to regulate tumor metastasis. Biochem Soc Trans.
45:229–236. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wu Y, Zhuang J, Zhao D, Zhang F, Ma J and
Xu C: Cyclic stretch-induced the cytoskeleton rearrangement and
gene expression of cytoskeletal regulators in human periodontal
ligament cells. Acta Odontol Scand. 75:507–516. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bartolák-Suki E, Imsirovic J, Nishibori Y,
Krishnan R and Suki B: Regulation of mitochondrial structure and
dynamics by the cytoskeleton and mechanical factors. Int J Mol Sci.
18(E1812)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Manou D, Caon I, Bouris P,
Triantaphyllidou IE, Giaroni C, Passi A, Karamanos NK, Vigetti D
and Theocharis AD: The complex interplay between extracellular
matrix and cells in tissues. Methods Mol Biol. 1952:1–20.
2019.PubMed/NCBI View Article : Google Scholar
|