Introduction
Colon adenocarcinoma (COAD) is a common malignant
tumor in the digestive tract (1).
The etiology of COAD remains unclear. However, COAD is often
associated with a fatty diet (2).
The lack of significant symptoms in the early stages of COAD
increases the difficulty of diagnosis. The existing treatment
methods for advanced stages of COAD, including radiotherapy,
chemotherapy and surgical resection, have little effect on this
disease, leading to a high incidence of mortality, with >600,000
patients succumbing to COAD worldwide every year (3,4).
Recently, targeted therapy has had a significant effect on COAD
(4). However, it is crucial to
discover more effective therapeutic targets to improve patient
survival.
Histone methyltransferases are a group of epigenetic
regulators which promote histone lysine methylation (5). SET and MYND domain-containing protein
3 (SMYD3) is a type of methyltransferase that methylates histones 3
or 4 at lysine 4 or 20, respectively (H3K4 or H4K20) (6,7). A
previous study indicated that SMYD3 promoted homologous
recombination by altering H3K4-mediated gene expression (8). Chitooligosaccharides regulate
glucose-Lipid metabolism by inhibiting SMYD3 and mediating gut
microflora (9). Additionally, SMYD3
is recruited by Ebola virus nucleoprotein to facilitate viral mRNA
transcription (10).
The effects of SMYD3 on cancer progression and
metastasis have been widely investigated (11-14).
SMYD3 was found to be overexpressed in several types of tumors,
including bladder cancer, gastric cancer, lung cancer and
pancreatic cancer (15-20).
In breast cancer, SMYD3 contributes to epithelial-mesenchymal
transition progression (12).
Additionally, SMYD3 promoted cancer progression and intrahepatic
metastasis of hepatocellular carcinoma by regulating CDK2 and MMP2
expression (16,21). Although the involvement of SMYD3 in
cancer progression has been widely reported, its possible role in
COAD remains unclear.
The present study demonstrated the high expression
of SMYD3 in human COAD tissues and revealed that high expression of
SMYD3 was correlated with the prognosis and clinical pathological
features of patients with COAD. Furthermore, the results
demonstrated the effects of SMYD3 on the proliferation of COAD
in vitro and in mice. Therefore, we hypothesized that SMYD3
serves as a promising molecular target for COAD treatment.
Materials and methods
TCGA database analysis
The TCGA database analysis was performed through
GEPIA (http://gepia.cancer-pku.cn/detail.php?gene=SMYD3/) to
analyze the data with a threshold of P<0.05 and LogFC >1 or
<-1 for the differential genes. Additionally, the median value
was used as the basis for dividing the patients into two groups for
the Kaplan-Meier (KM) survival analysis, and the dotted line
indicated the 95% confidence interval.
Antibodies, primers and short hairpin
(sh)RNA plasmids
Anti-SMYD3 (for IHC assays, 1:200; for
immunoblotting assays, 1:1,000; cat. no. ab187149) and anti-β-actin
(1:3,000; cat. no. ab8226) were purchased from Abcam.
The quantitative PCR (qPCR) primer sequences used
were as follows: SMYD3 forward, 5'-CGCGTCGCCAAATACTGTAGT-3' and
reverse, 5'-CAAGAAGTCGAACGGAGTCTG-3'; and GAPDH forward,
5'-CGACCACTTTGTCAAGCTCA-3' and reverse,
5'-GGTTGAGCACAGGGTACTTTATT-3'. The shRNA plasmids of SMYD3 were
purchased from Addgene.
Human tissue samples and analysis
A total of 92 human COAD tissues and adjacent normal
tissues (3 cm from tumor tissues) were collected from patients at
Tianjin Baodi Hospital, Tianjin, China. The clinicopathological
characteristics are listed in Table
I.
 | Table ICorrelation between SMYD3 expression
and the clinical pathological features of 92 patients with colon
adenocarcinoma. |
Table I
Correlation between SMYD3 expression
and the clinical pathological features of 92 patients with colon
adenocarcinoma.
| | SMYD3
expression | |
|---|
| Feature | No. | Low n=25 | High n=67 | χ2 | P-value |
|---|
| Age (years) | | | | 1.689 | 0.194 |
|
<55 | 47 | 10 | 37 | | |
|
≥55 | 45 | 15 | 30 | | |
| Sex | | | | 0.038 | 0.846 |
|
Male | 50 | 14 | 36 | | |
|
Female | 42 | 11 | 31 | | |
| Tumor stage | | | | 4.318 | 0.038a |
|
T2 | 29 | 12 | 17 | | |
|
T3/T4 | 63 | 13 | 50 | | |
| Tumor grade | | | | 1.234 | 0.267 |
|
Low | 22 | 8 | 14 | | |
|
High | 70 | 17 | 53 | | |
| Lymph node
metastasis | | | | 2.425 | 0.119 |
|
Yes | 49 | 10 | 39 | | |
|
No | 43 | 15 | 28 | | |
| Metastasis | | | | 0.398 | 0.528 |
|
Yes | 38 | 9 | 29 | | |
|
No | 54 | 16 | 38 | | |
To explore the correlation between SMYD3 expression
and the progression of COAD, immunohistochemical (IHC) assays were
performed. Briefly, resin was used for tissue embedding tissue, and
3 µm sections were performed. Sections were subsequently fixed with
4% paraformaldehyde (PFA) at room temperature for 20 min and
blocked with 2% BSA for 20 min at room temperature. Slides were
incubated with SMYD3 antibodies (1:200; cat. no. ab187149; Abcam)
at room temperature for 2 h. Sections were then incubated with mice
or rabbit biotinylated secondary antibodies (1:500; cat. no.
ab201485; cat. no. ab99807, respectively; Abcam) at room
temperature for 1.5 h. Diaminobenzidine was used as the chromogen
substrate.
SMYD3 was mainly located in the cytoplasm of COAD
tissues. The proportion of positive stained cells was graded as
follows: 0, 0% stained cells; 1, 1-30% stained cells; 2, 31-70%
stained cells; 3, 71-100% stained cells. Staining intensity was
evaluated on a score of 0 (no or low staining), 1 (modest staining)
and 2 (high staining). Tissues were divided into high or low SMYD3
expression groups according to the staining index: Score of
staining intensity + score of staining cells %. A staining index of
<3 indicated low SMYD3 expression and ≥3 high expression.
Cell culture and transfection
Human colorectal cancer cell lines, HCT116 and
HT-29, were purchased from the American Type Culture Collection.
HCT116 and HT-29 cells were incubated in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) in a 5% CO2 incubator.
SMYD3 shRNA plasmids were transfected into HCT116 and HT-29 cells
using Lipofectamine 2000 (cat. no. 11668019; Invitrogen; Thermo
Fisher Scientific, Inc.). The shRNA target sequences were presented
as follows: Control: 5'-CCUACAUCCCGAUCGAUGAUGUUGA-3'; SMYD3:
5'-AGCCTGATTGAAGATTTGATT-3'.
The further in vitro assays were performed 24
h after the transfection. Stably SMYD3 depleted HCT116 cells were
screened by the infection of its shRNA lentivirus and used for
in vivo tumor growth assays.
qPCR assays
TRIzol (cat. no. 15596026; Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from HCT116 and
HT-29 cells. RNA was reverse transcribed using M-MLV reverse
transcriptase (cat. no. M1701; Promega Corporation). Total mRNA was
reverse transcribed to produce cDNA using a cDNA synthesis system
(cat. no. 6110A; Takara Bio, Inc.). qPCR was performed using a SYBR
Ex Taq kit (cat. no. 638319; Takara Bio, Inc.), which was used
according to the manufacturer's protocol. The reaction conditions
were as follows: Pre-denaturation, 95˚C, 3 min; Denaturation, 95˚C,
30 sec; Annealing, 58˚C, 30 sec; Extension, 72˚C, 30 sec. There are
35 cycles. The 2-ΔΔCq method was used to quantify
results (22). SMYD3 expression
levels were normalized to GAPDH.
Immunoblotting assays
HCT116 and HT-29 cells or COAD tissues were lysed
using RIPA buffer (cat. no. 9800; Cell Signaling Technology, Inc.).
BSA method was used for protein determination, and 20 µl sample was
loaded in each lane at a concentration of 1 mg/ml. Total protein
was separated using 10% SDS-PAGE and transferred onto
nitrocellulose membranes. Membranes were blocked with 5% milk in
TBST at room temperature for 2 h and incubated with primary
antibodies for the detection of SMYD3 (1:1,000; cat. no. ab187149;
Abcam) and β-actin (1:3,000; cat. no. Ab8226; Abcam) at room
temperature for 2 h at room temperature. Following this, membranes
were incubated with horseradish peroxidase-conjugated secondary
antibodies for 45 min at room temperature. Signals were detected
using an ECL kit (Novex™ ECL Chemiluminescent Substrate Reagent
kit; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol.
Colony formation assays
HCT116 and HT-29 cells were added into six-well
culture plates with the seeding density of 1,000 cell/well and
transfected with control or SMYD3 shRNA plasmids. The medium was
replaced with fresh medium every 3 days. After 2 weeks, cells were
fixed with PFA for 30 min at room temperature, stained with 0.2%
crystal violet at room temperature for 20 min and washed twice with
PBS. Colony numbers were counted manually.
MTT assays
HCT116 and HT-29 cells were plated into 96-well
plates (seeding density, 2x103/well), respectively,
transfected with control or SMYD3 shRNA plasmids using
Lipofectamine 2000 (cat. no. 11668019; Invitrogen; Thermo Fisher
Scientific, Inc.) and incubated at 37˚C for 2 days. Following this,
cells were incubated with MTT for 4 h at room temperature and
removed the culture medium. After washing twice with PBS buffer,
150 µ DMSO was added into each well to extract cells. OD values
were measured with a microplate reader at a wavelength of 570 nm
and the relative proliferation degrees were assessed.
Flow cytometry (FCM) assays
For the detection of cell apoptosis, HCT116 or HT-29
cells were fixed with 70% ethyl alcohol for h hours at -20˚C and
treated with Annexin V-FITC/propidium iodide (PI) using a kit
(Annexin V-FITC Apoptosis Detection Kit, APOAF; Sigma-Aldrich;
Merck KGaA) for 20 min at room temperature. Cells were detected
using a FACS Calibur flow cytometer (FACSAria III; BD Biosciences),
and the analysis software was the supporting software of the flow
cytometer. The apoptosis cells, including early and late apoptotic
cells were analyzed.
For cell cycle assays, cells were fixed with 70%
ethyl alcohol for 24 h at -20˚C and incubated with 50 µg/ml PI at
37˚C for 30 min. Samples were detected using a flow cytometer
(FACSAria III; BD Biosciences). The percentage of cells at
different phases (Including the G1, S and G2/M phases) were
analyzed.
Tumor growth assays
All animal experiments were approved by the
Institutional Animal Care and Use Committee of Tianjin Baodi
Hospital, Tianjin, China. Female BALB/c nude mice (10-week-old)
were supplied by Beijing Weitong Lihua Experimental Animal
Technology Co., Ltd. Mice weight was approximately 22-24 g. Mice
were fed with food and water ad libitum, and were fed at
Specific Pathogen Free (SPF) level at 20˚C and a humidity of 60%,
alternating between light and dark for 12 h. In vivo assays
were performed to according to the following criteria. A total of
16 athymic nude mice were included in control (n=8) and shRNA
treatment (n=8) groups. None of the mice died during the study.
HCT116 cells were stably infected with control or
SMYD3 shRNA lentiviruses, as previously described. Subsequently,
~2x106 HCT116 cells infected with the indicated
lentiviruses were subcutaneously implanted into the mice. Tumors
formation began at 14 days post-implantation. All mice were
maintained until the assays were finished. Tumor volumes were
measured every 3 days from the third week through a vernier caliper
after 14 days.
After 29 days, all mice were sacrificed via cervical
dislocation and tumor tissues were subsequently excised A lack of a
heartbeat was established to confirm death. Adequate humanitarian
care was given.
Tumors were isolated and photographed, and tumor
growth curves were calculated and compared. Tumor volume was
calculated as follows: Tumor volume (mm3)=tumor length
(mm) x tumor width (mm)2/2. In terms of weight, mice
gained no more than 5% and lost no more than 8% of their body
weight. The weight of different groups of mice were not
significantly different between groups during the whole feeding
period. The maximum diameter of tumors was ~12 mm and maximum tumor
weight was ~4% of total body weight.
Statistical analysis, GraphPad
software (version 5.0; GraphPad Software, Inc.)
was used for statistical analysis. Data are
presented as mean ± SD. The correlation between clinical
pathological features and SMYD3 expression was analyzed by
Pearson's χ2 analysis. Student's t-test was used for
statistical comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
SMYD3 expression is enhanced in human
COAD tissues and correlates with the prognosis and clinical
features of patients with COAD
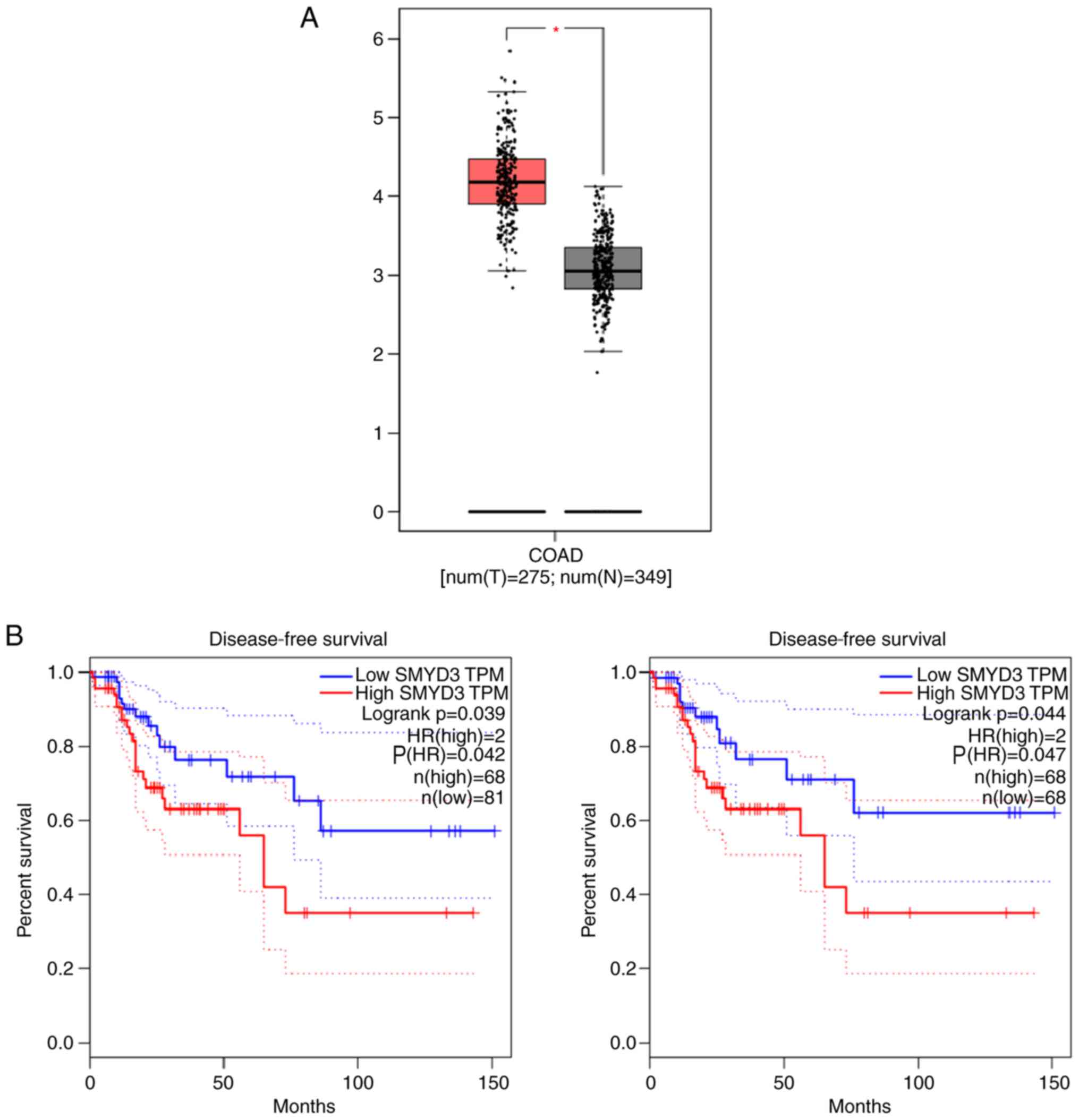
To assess the potential effects of SMYD3 in the
progression of COAD, the mRNA levels of SMYD3 in tumor tissues and
normal tissues were assessed using The Cancer Genome Atlas (TCGA)
database. A total of 275 COAD tissues and 349 adjacent tissues were
included. The results demonstrated significantly high SMYD3 mRNA
levels in human COAD tissues compared with adjacent normal tissues
(Fig. 1A). Furthermore, the
correlation between SMYD3 expression and prognosis of patients with
COAD was evaluated using the TCGA database. K-M survival analysis
was performed to assess whether SMYD3 expression impaired the
disease-free survival rates of patients with COAD based on the TCGA
database. Also according to the TCGA database, two sets of patients
were included (n=149 and n=136). Notably, SMYD3 expression was
correlated with the disease-free survival rates of patients with
COAD (P=0.042 and P=0.047, respectively; Fig. 1B). Therefore, the results indicated
high SMYD3 expression in human COAD tissues and that this
expression was correlated with the prognosis of COAD.
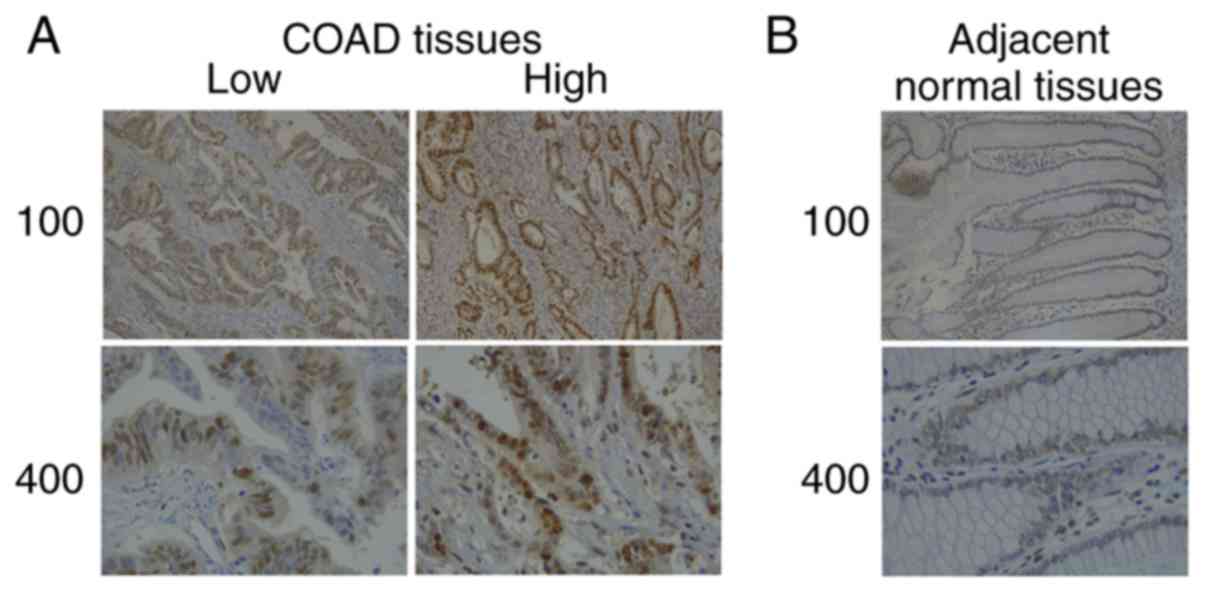
Subsequently, the expression of SMYD3 in COAD
tissues and adjacent normal tissues collected at the hospital was
evaluated. The results of IHC assays revealed that SMYD3 was mainly
located in the cytoplasm of tumor tissues (Fig. 2). Furthermore, high expression of
SMYD3 in COAD tissues compared to the adjacent tissues was
observed, which was consistent with the data presented in Fig. 1.
The correlation analysis between SMYD3
expression and clinical features of patients with COAD
Following this, the clinical pathological features
of 92 patients with COAD were analyzed (Table I). Patients were divided into two
groups according to a scoring method: Low or high SMYD3 expression.
A total of 25 patients exhibited low SMYD3 expression and 67
patients exhibited high SMYD3 expression. Clinical features,
including patient age, sex, tumor stage, tumor grade, lymph node
metastasis and metastasis, of COAD patients were analyzed. The
results demonstrated no significant correlation between SMYD3
expression and patient age (P=0.194), sex (P=0.846), tumor grade
(P=0.267), lymph node metastasis (P=0.119) or metastasis (P=0.528)
in patients with COAD. However, the data demonstrated that SMYD3
expression was correlated with tumor stage (P=0.038) in patients
with COAD.
SMYD3 affects the proliferation and
apoptosis of COAD cells in vitro
Since the results reported a potential association
between SMYD3 expression and COAD, the effects of SMYD3 on COAD
cells were examined. shRNA plasmids targeting SMYD3 were used to
deplete the expression of SMYD3 in two types of colorectal cancer
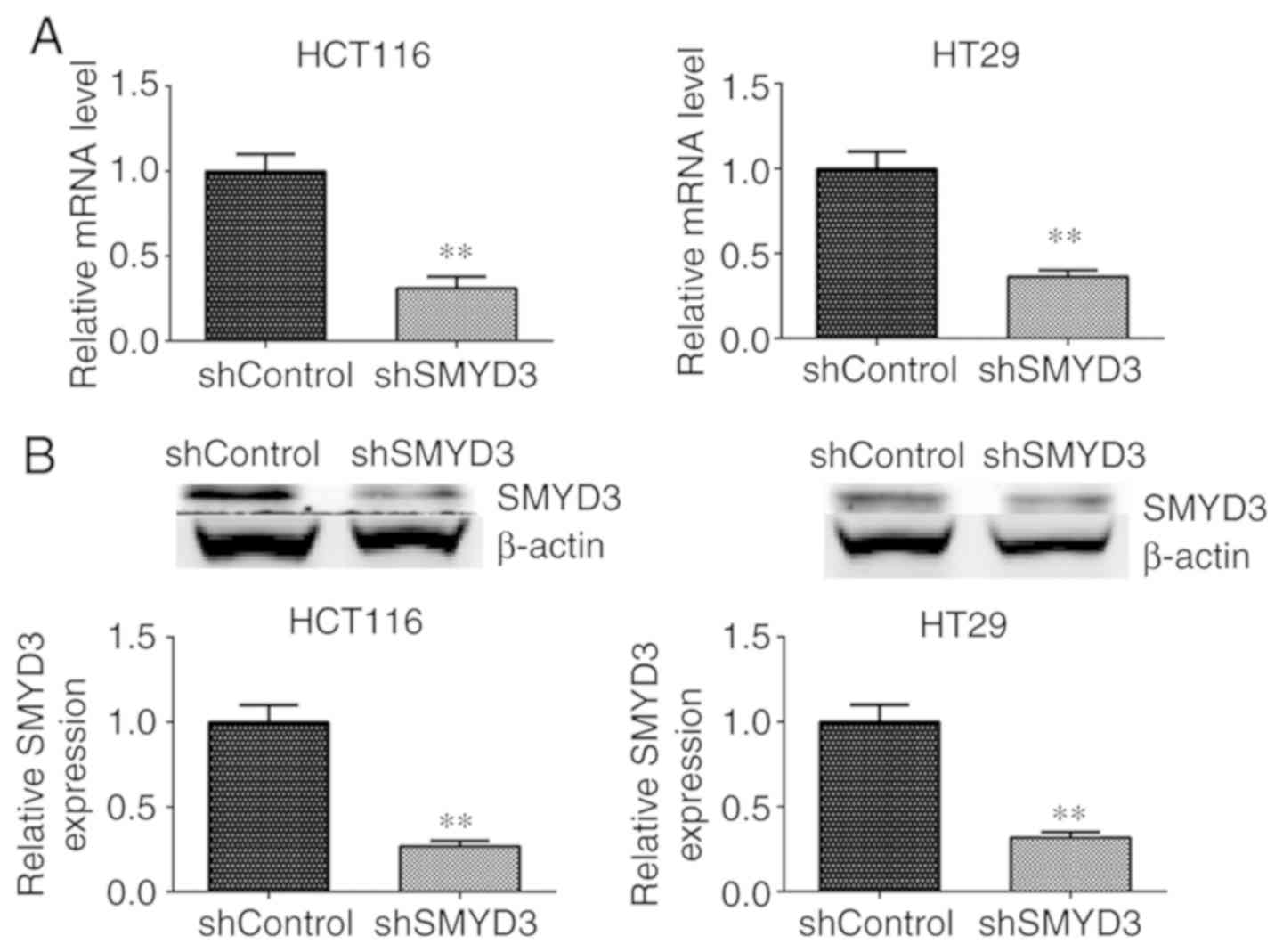
cells, HCT116 and HT-29. qPCR and immunoblotting assays were
performed to detect SMYD3 expression in controls or SMYD3
shRNA-transfected HCT116 and HT-29 cells. According to the results,
the mRNA and protein levels of SMYD3 were significantly decreased
following the transfection shRNA plasmids in HCT116 and HT-29 cells
compared with controls (Fig. 3A and
B).
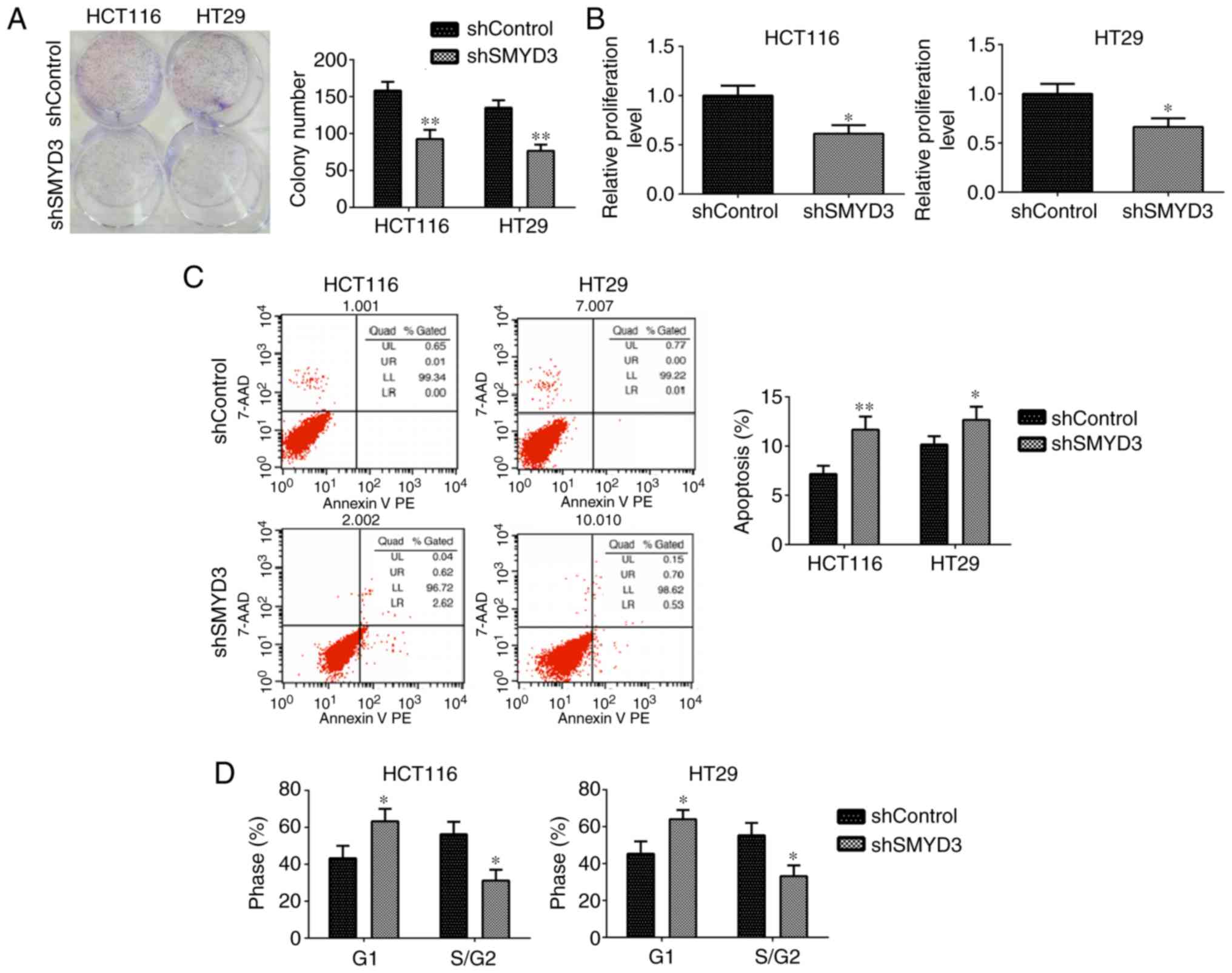
Subsequently, a series of in vitro assays
were performed to evaluate the effects of SMYD3 on the progression
of COAD. Colony formation assays demonstrated that SMYD3 ablation
led to a significant decrease in the colony numbers of HCT116 and
HT-29 cells compared with controls (Fig. 4A). Additionally, SMYD3 depletion
resulted in a significantly decreased OD value at 570 nm wavelength
in HCT116 and HT-29 cells compared with controls, as reported by
MTT assays (Fig. 4B). These data
confirmed the effects of SMYD3 on COAD cell proliferation.
Furthermore, FCM assays were performed to detect the effects of
SMYD3 on cell apoptosis and the cell cycle. The results
demonstrated that SMYD3 knockdown significantly stimulated
apoptosis in HCT116 and HT-29 cells (Fig. 4C). FCM results further demonstrated
that SMYD3 depletion resulted in the arrest of cell cycle in these
cells (Fig. 4D). In summary, these
results confirmed the effects of SMYD3 on proliferation, apoptosis
and the cell cycle in HCT116 and HT-29 cells in vitro.
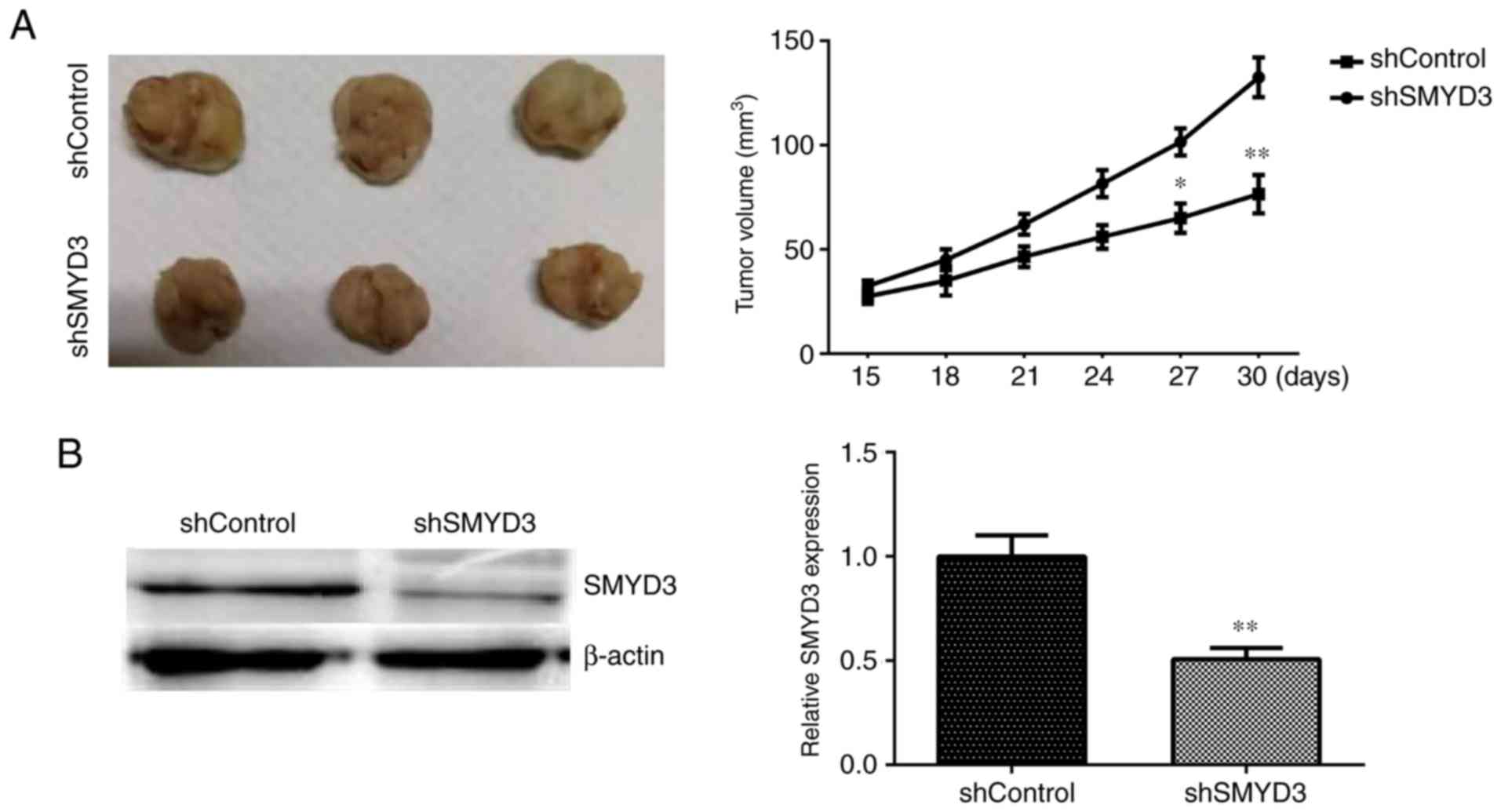
SMYD3 promotes tumor growth of HCT116
and HT-29 cells in mice in vivo
To further confirm the previous in vitro
data, whether SMYD3 contributed to tumor growth of COAD cells in an
animal model was investigated. Briefly, HCT116 cells were infected
with control or SMYD3 shRNA lentiviruses and were subcutaneously
injected into nude mice. Tumors began forming after 14 days and
tumor volumes were measured every 3 days. After 29 days, the mice
were sacrificed and all tumors were isolated and photographed. The
representative images of the tumors and the tumor growth curves are
presented in Fig. 5A. Tumor volumes
in the SMYD3-depleted groups were significantly decreased compared
with controls (Fig. 5A).
Furthermore, immunoblot assays were performed to detect SMYD3
expression in tumor tissues from mice in control and SMYD3-deplted
depletion groups. The results demonstrated that SMYD3 expression
levels were decreased in SMYD3-depleted tumor tissues compared with
controls (Fig. 5B). Therefore, we
hypothesized that SMYD3 promoted tumor growth of COAD cells in
vivo.
Discussion
During early COAD, surgical resection is generally
curative and patients with advanced COAD are often treated with
surgery combined with chemoradiotherapy (23). Due to the high metastasis of cancer,
advances in targeted therapy are required (24,25).
Targeted therapy is the most effective method to treat COAD
(26). Numerous molecular targets
of COAD have been discovered, including EGFR and sirtuin 6
(27,28). Targeted therapy drugs, including
Gefitinib and Erlotinib, are on the market or are currently in
clinical trials (29). However,
more therapeutic targets need to be developed to improve prognosis.
The current study reported that a methyltransferase, SMYD3, was
exhibited significantly high expression in human COAD tissues
compared with controls. Furthermore, the results that confirmed
SMYD3 was correlated with prognosis and clinical feature of
patients with COAD. Therefore, we hypothesized that SMYD3 may serve
as a novel and promising molecular target for COAD treatment.
Through the investigation of SMYD3 on COAD
progression in vitro, colony formation, MTT and FCM assays
were performed and the results demonstrated that the depletion of
SMYD3 impaired proliferation, stimulated apoptosis and induced cell
cycle arrest in COAD cells. Furthermore, in vivo assays
reported that SMYD3 contributed to tumor growth in mice, which was
consistent with the in vitro data. Therefore, the results
confirmed that SMYD3 regulated COAD progression by mediating cell
proliferation, apoptosis and the cell cycle. Similarly, several
previous studies have demonstrated the effects of SMYD3 on cancer
progression (30-33).
SMYD3 stimulated epithelial ovarian cancer metastasis by
suppressing p53 protein stability and promoting p53 ubiquitination
(30). Additionally, SMYD3 was
associated with the proliferation and apoptosis of ovarian cancer
by regulating methylation (18).
SMYD3 inhibition also suppressed cell proliferation, migration and
invasion of pancreatic cancer cells (34). These previous studies, together with
the results of the current study, indicated the critical role of
SMYD3 in tumor growth and development. Therefore, developing novel
inhibitors for SMYD3 may be an effective target for cancer
treatment.
Post-translational modifications mediated various
cellular processes, including mitosis and cell migration, by
regulating protein function (13,35).
As a methyltransferase, SMYD3 methylated H3K4 and H4K20, altering
the expression of downstream genes (36). Additionally, SMYD3 methylated
non-histone proteins, regulating body development and tumor
progression (37). Furthermore,
SMYD3 promoted myogenesis by activating the myogenin regulatory
network (38). The current study
demonstrated that SMYD3 affected proliferation and apoptosis of
COAD cells in vitro and tumor growth in mice in vivo.
We hypothesized that SMYD3 likely influenced the development of
COAD by regulating the methylation of key tumor-regulated proteins.
However, further experiments are required to confirm this
hypothesis. Additionally, whether SMYD3 affects the migration and
invasion of COAD cells deserves further investigation.
The SMYD methyltransferase family is known to affect
the progression of several types of tumors, such as breast cancer
and lung cancer (39). SMYD1 has
been reported to be associated with several solid tumors, including
breast cancer (40). Additionally,
SMYD2 exhibited high expression in multiple types of tumors,
including gastric cancer and colorectal cancer, and affected the
migration and invasion of tumor cells (41,42).
Combined with the results of the current study, we hypothesized
that the development of inhibitors targeting SMYD family proteins
has potential advantages for cancer targeted therapy.
In summary, the results of the current study
demonstrated that SMYD3 was highly expressed in human COAD tissues
and was correlated with the prognosis and a clinical feature, tumor
stage, in patients with COAD. SMYD3 affected cell growth by
mediating cell proliferation, apoptosis and the cell cycle in
vitro, and tumor growth in mice in vivo. Therefore, we
hypothesize that SMYD3 has potential to serve as a promising COAD
therapeutic target.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FRY, ZBW and RZY performed the molecular biology
experiments and drafted the manuscript. QHG and BL participated in
the design of the study and performed statistical analysis. FRY,
JHZ and ZL conceived the current study, participated in design and
coordination, and assisted in drafting the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in the current study were
approved by the Ethics Committee of Tianjin Baodi Hospital,
Tianjin, China. Written informed consent was obtained from all
patients or their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chang HK, Gen Y, Yeo SG, Jung JH and Park
DC: Primary adenocarcinoma with choriocarcinomatous differentiation
of the sigmoid colon misdiagnosed choriocarcinoma of the uterus:
Case report and review of the literature. Eur J Gynaecol Oncol.
40:468–470. 2019.
|
|
2
|
Lian J, Xia L, Chen Y, Zheng J, Ma K, Luo
L and Ye F: Aldolase B impairs DNA mismatch repair and induces
apoptosis in colon adenocarcinoma. Pathol Res Pract.
215(152597)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kai K, Hidaka H, Nakamura T, Ueda Y,
Marutsuka K, Ikeda T and Nanashima A: A case of poorly
differentiated adenocarcinoma with lymphoid stroma originated in
the ascending colon diagnosed as lymphoepithelioma-like carcinoma.
Clin J Gastroenterol. 13:538–544. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li Z, Tan H, Yu H, Deng Z, Zhou X and Wang
M: DNA methylation and gene expression profiles characterize
epigenetic regulation of lncRNAs in colon adenocarcinoma. J Cell
Biochem. 121:2406–2415. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bottino C, Peserico A, Simone C and
Caretti G: SMYD3: An oncogenic driver targeting epigenetic
regulation and signaling pathways. Cancers (Basel).
12(142)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dong S and Zhang P: Advances of histone
methyltransferase SMYD3 in tumors. Zhongguo Fei Ai Za Zhi.
17:689–694. 2014.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
7
|
Eberle CA, Zayas M, Stukalov A, Pichlmair
A, Alvisi G, Muller AC, Bennett KL, Bartenschlager R and
Superti-Furga G: The lysine methyltransferase SMYD3 interacts with
hepatitis C virus NS5A and is a negative regulator of viral
particle production. Virology. 462-463:34–41. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen YJ, Tsai CH, Wang PY and Teng SC:
SMYD3 promotes homologous recombination via regulation of
H3K4-mediated gene expression. Sci Rep. 7(3842)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang Q, Jiang Y, Luo X, Wang C, Wang N, He
H, Zhang T and Chen L: Chitooligosaccharides modulate glucose-lipid
metabolism by suppressing SMYD3 pathways and regulating gut
microflora. Mar Drugs. 18(69)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen J, He Z, Yuan Y, Huang F, Luo B and
Zhang J, Pan T, Zhang H and Zhang J: Host factor SMYD3 is recruited
by Ebola virus nucleoprotein to facilitate viral mRNA
transcription. Emerg Microbes Infect. 8:1347–1360. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Al-Eitan LN and Rababa'h DM: Correlation
between a variable number tandem repeat (VNTR) polymorphism in
SMYD3 gene and breast cancer: A genotype-phenotype study. Gene.
728(144281)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen D, Liu L, Luo X, Mu A, Yan L, Chen X,
Wang L, Wang N, He H, Zhou H and Zhang T: Effect of SMYD3 on the
microRNA expression profile of MCF-7 breast cancer cells. Oncol
Lett. 14:1831–1840. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cock-Rada AM, Medjkane S, Janski N, Yousfi
N, Perichon M, Chaussepied M, Chluba J, Langsley G and Weitzman JB:
SMYD3 promotes cancer invasion by epigenetic upregulation of the
metalloproteinase MMP-9. Cancer Res. 72:810–820. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Colon-Bolea P and Crespo P: Lysine
methylation in cancer: SMYD3-MAP3K2 teaches us new lessons in the
Ras-ERK pathway. Bioessays. 36:1162–1169. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dong QQ, Wang QT, Wang L, Jiang YX, Liu
ML, Hu HJ, Liu Y, Zhou H, He HP, Zhang TC and Luo XG:
SMYD3-associated pathway is involved in the anti-tumor effects of
sulforaphane on gastric carcinoma cells. Food Sci Biotechnol.
27:1165–1173. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fei X, Ma Y, Liu X and Meng Z:
Overexpression of SMYD3 is predictive of unfavorable prognosis in
hepatocellular carcinoma. Tohoku J Exp Med. 243:219–226.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fenizia C, Bottino C, Corbetta S,
Fittipaldi R, Floris P, Gaudenzi G, Carra S, Cotelli F, Vitale G
and Caretti G: SMYD3 promotes the epithelial-mesenchymal transition
in breast cancer. Nucleic Acids Res. 47:1278–1293. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jiang Y, Lyu T, Che X, Jia N, Li Q and
Feng W: Overexpression of SMYD3 in ovarian cancer is associated
with ovarian cancer proliferation and apoptosis via methylating
H3K4 and H4K20. J Cancer. 10:4072–4084. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li B, Pan R, Zhou C, Dai J, Mao Y, Chen M,
Huang T, Ying X, Hu H, Zhao J, et al: SMYD3 promoter
hypomethylation is associated with the risk of colorectal cancer.
Future Oncol. 14:1825–1834. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu C, Wang C, Wang K, Liu L, Shen Q, Yan
K, Sun X, Chen J, Liu J, Ren H, et al: SMYD3 as an oncogenic driver
in prostate cancer by stimulation of androgen receptor
transcription. J Natl Cancer Inst. 105:1719–1728. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang Y, Xie BH, Lin WH, Huang YH, Ni JY,
Hu J, Cui W, Zhou J, Shen L, Xu LF, et al: Amplification of SMYD3
promotes tumorigenicity and intrahepatic metastasis of
hepatocellular carcinoma via upregulation of CDK2 and MMP2.
Oncogene. 38:4948–4961. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nowak A, Zaklos-Szyda M, Zyzelewicz D,
Koszucka A and Motyl I: Acrylamide decreases cell viability, and
provides oxidative stress, DNA damage, and apoptosis in human colon
adenocarcinoma cell line caco-2. Molecules. 25(368)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang Y, Zhao Z, Xie C and Zhao Y:
Dual-targeting liposome modified by glutamic hexapeptide and folic
acid for bone metastatic breast cancer. Chem Phys Lipids.
228(104882)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao Z, Zhao Y, Xie C, Chen C, Lin D, Wang
S, Lin D, Cui X, Guo Z and Zhou J: Dual-active targeting liposomes
drug delivery system for bone metastatic breast cancer: Synthesis
and biological evaluation. Chem Phys Lipids.
223(104785)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ruan GT, Zhu LC, Gong YZ, Liao XW, Wang
XK, Liao C, Wang S, Yan L, Xie HL, Zhou X, et al: The diagnosis and
prognosis values of WNT mRNA expression in colon adenocarcinoma. J
Cell Biochem. 121:3145–3161. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ni X, Ding Y, Yuan H, Shao J, Yan Y, Guo
R, Luan W and Xu M: Long non-coding RNA ZEB1-AS1 promotes colon
adenocarcinoma malignant progression via miR-455-3p/PAK2 axis. Cell
Prolif. 53(e12723)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chang CL, Yuan KS, Wu ATH and Wu SY:
Adjuvant therapy for high-risk stage II or III colon
adenocarcinoma: A propensity score-matched, nationwide,
population-based cohort study. Cancers (Basel).
11(2003)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chan KWK, Chung HY and Ho WS: Anti-tumor
activity of atractylenolide I in human colon adenocarcinoma in
vitro. Molecules. 25(212)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang L, Jin Y, Yang H, Li Y, Wang C, Shi
Y and Wang Y: SMYD3 promotes epithelial ovarian cancer metastasis
by downregulating p53 protein stability and promoting p53
ubiquitination. Carcinogenesis. 40:1492–1503. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu N, Sun S and Yang X: Prognostic
significance of stromal SMYD3 expression in colorectal cancer of
TNM stage I-III. Int J Clin Exp Pathol. 10:8901–8907.
2017.PubMed/NCBI
|
|
32
|
Liu TT, Xu H, Gao WP, Zhang SX, Zhou XH,
Tang J and Liu QN: SET and MYND domain-containing protein 3 (SMYD3)
polymorphism as a risk factor for susceptibility and poor prognosis
in ovarian cancer. Med Sci Monit. 22:5131–5140. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lyu T, Jiang Y, Jia N, Che X, Li Q, Yu Y,
Hua K, Bast RC Jr and Feng W: SMYD3 promotes implant metastasis of
ovarian cancer via H3K4 trimethylation of integrin promoters. Int J
Cancer. 146:1553–1567. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhu CL and Huang Q: Overexpression of the
SMYD3 promotes proliferation, migration, and invasion of pancreatic
cancer. Dig Dis Sci. 65:489–499. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bai H, Li Y, Gao H, Dong Y, Han P and Yu
H: Histone methyltransferase SMYD3 regulates the expression of
transcriptional factors during bovine oocyte maturation and early
embryonic development. Cytotechnology. 68:849–859. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sun J, Shi F and Yang N: Exploration of
the substrate preference of lysine methyltransferase SMYD3 by
molecular dynamics simulations. ACS Omega. 4:19573–19581.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Van Aller GS, Reynoird N, Barbash O,
Huddleston M, Liu S, Zmoos AF, McDevitt P, Sinnamon R, Le B, Mas G,
et al: Smyd3 regulates cancer cell phenotypes and catalyzes histone
H4 lysine 5 methylation. Epigenetics. 7:340–343. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Codato R, Perichon M, Divol A, Fung E,
Sotiropoulos A, Bigot A, Weitzman JB and Medjkane S: The SMYD3
methyltransferase promotes myogenesis by activating the myogenin
regulatory network. Sci Rep. 9(17298)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Song J, Liu Y, Chen Q, Yang J, Jiang Z,
Zhang H, Liu Z and Jin B: Expression patterns and the prognostic
value of the SMYD family members in human breast carcinoma using
integrative bioinformatics analysis. Oncol Lett. 17:3851–3861.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tan X, Rotllant J, Li H, De Deyne P and Du
SJ: SmyD1, a histone methyltransferase, is required for myofibril
organization and muscle contraction in zebrafish embryos. Proc Natl
Acad Sci USA. 103:2713–2718. 2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yan L, Ding B, Liu H, Zhang Y, Zeng J, Hu
J, Yao W, Yu G, An R, Chen Z, et al: Inhibition of SMYD2 suppresses
tumor progression by down-regulating microRNA-125b and attenuates
multi-drug resistance in renal cell carcinoma. Theranostics.
9:8377–8391. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ohtomo-Oda R, Komatsu S, Mori T, Sekine S,
Hirajima S, Yoshimoto S, Kanai Y, Otsuji E, Ikeda E and Tsuda H:
SMYD2 overexpression is associated with tumor cell proliferation
and a worse outcome in human papillomavirus-unrelated nonmultiple
head and neck carcinomas. Hum Pathol. 49:145–155. 2016.PubMed/NCBI View Article : Google Scholar
|