Introduction
Ulcerative colitis (UC) is a form of chronic
inflammatory bowel disease that affects the colon and large
intestine, causing swelling and irritation (1). UC is typically diagnosed during young
adulthood and affects an individual for their entire life (2). The incidence rate of UC varies across
the world, with high incidence rates observed in North America and
Western Europe (3). With the
development of a prevention program, the prevalence of UC has been
stabilized in areas that were typically associated with high
incidence (3). However, an
increasing trend in UC incidence has been observed in Eastern
Europe and Asia in the last 10 years (3). It is well established that UC not only
reduces the quality of life of patients, but also increases the
risk of colorectal cancer (4,5).
As UC is a type of inflammatory disease (6), the inhibition of inflammation is a
promising approach for UC treatment (7). Inflammatory factors, including
interleukin (IL)-36α, IL-17 and IL-23, are critical mediators of UC
(8,9). In addition to these cytokines, long
non-coding RNAs (lncRNAs; >200 nucleotides in length) also play
pivotal roles in inflammation, and the regulation of the expression
of certain lncRNAs can contribute to the amelioration of UC
symptoms (10). In a previous
study, Padua et al (11)
reported a novel lncRNA, interferon-γ antisense RNA I (IFNG-AS1),
as an enhancer of inflammation in UC. In another study, lncRNA
Mirt2 was characterized as a lipopolysaccharide (LPS)-inducible
inflammation inhibitor (12). It is
known that LPS-induced inflammation and UC share a similar
pathogenesis (13), suggesting that
the opposite roles of IFNG-AS1 and Mirt2 indicate a possible
interaction between the two lncRNAs during UC. Therefore, the
present study aimed to investigate the interactions between
IFNG-AS1 and Mirt2 in UC.
Materials and methods
Participants and plasma samples
The present study included 60 patients with UC (34
males and 26 females; age range, 19-64 years; mean ± SD, 40.1±6.2
years) and 60 healthy controls (34 males and 26 females; age range,
20-65 years; mean ± SD, 39.8±6.5 years). All the participants were
recruited at the Department of Gastroenterology, Hainan General
Hospital between April 2016 and April 2019. The 60 healthy
volunteers were recruited from the Physical Health Center of Hainan
General Hospital to match the age and gender distributions of the
patients with UC. The present study was approved by the Ethics
Committee of Hainan General Hospital. All participants were
informed of the details of the protocols of the present study and
provided written informed consent. The inclusion criteria for
patients with UC were as follows: i) No treatment received within
100 days before admission; and ii) newly diagnosed cases. The
exclusion criteria for patients with UC were as follows: i)
Presence of other clinical disorders; and ii) patients transferred
from other hospitals.
Before the initiation of any therapies, ~5 ml blood
was extracted from the median cubital vein under fasting
conditions. To separate the plasma, blood was centrifuged in EDTA
tubes for 20 min at 1,200 x g at room temperature.
Human colonic epithelial cells
(HCnEpCs) and transfections
HCnEpCs from Cell Applications were used in the
present study. The rationale behind using HCnEpCs was that HCnEpCs
are usually affected by UC (6).
Cell culture was performed according to the manufacturer's
instructions. HCnEpCs were cultured in colonic epithelial cell
medium (ScienCell Research Laboratories, Inc.) at 37˚C with 5%
CO2 containing 5 µg/ml LPS (Sigma-Aldrich; Merck KGaA)
for 12, 24, 36 and 48 h at 37˚C with 5% CO2 prior to
further experimentation.
Mirt2 and IFNG-AS1 overexpression vectors were
constructed using the pcDNA3.1 vector (Sangon BioTech Co., Ltd.).
HCnEpCs were harvested and counted, and 4x106 cells were
transfected with 10 nM vector using Lipofectamine® 2000
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
instructions. Empty vectors were used for the negative control
group. All transfections were performed using un-transfected
HCnEpCs as control cells. Subsequent experimentation was performed
after cells were incubated at 37˚C for 24 h.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
The total RNA in 0.2 ml plasma and 4x105
HCnEpCs (collected at 24 h post-transfection) was extracted using
RiboZol (Sigma-Aldrich; Merck KGaA) according to the manufacturer's
instructions. To remove genomic DNA, all RNA samples were digested
with DNase I for 90 min at 37˚C. The digested RNA samples were
reverse transcribed into cDNA using a Tetro Reverse Transcriptase
kit (Bioline) at the following thermal conditions: 55˚C for 20 min
and 80˚C for 10 min. qPCR was performed using Power
SYBR® Green PCR Master mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. GAPDH was used as an endogenous control to measure
the expression levels of Mirt2 and IFNG-AS1. The following
thermocycling conditions were used for qPCR: 95˚C for 1 min, then
95˚C for 10 sec and 55˚C for 40 sec for a total of 40 cycles. The
following primer sequences were used: Mirt2 forward,
5'-TCAACACTTTCCATAGGT-3' and reverse, 5'-ATTGTGAGGTCCAGATAG-3';
IFNG-AS1 forward, 5'-GCTGATGATGGTGGTGGCAATCT-3' and reverse,
5'-TTAGCAGTTGGTGGGCTTCT-3'; and GAPDH forward,
5'-GTCTCCTCTGACTTCAACAGCG-3' and reverse,
5'-ACCACCCTGTTGCTGTAGCCAA-3'. All qPCR reactions were performed in
triplicate. mRNA levels were quantified using the
2-ΔΔCq method and normalized to
the loading control GAPDH (14).
Cell apoptosis analysis
HCnEpCs were harvested and counted at 24 h
post-transfection. Single-cell suspensions were prepared by mixing
4x103 HCnEpCs with 1 ml colonic epithelial cell medium.
Cells were transferred to a 6-well cell culture plate (2 ml per
well) and were supplemented with 5 µg/ml LPS per well to induce
cell apoptosis. Cells were incubated at 37˚C for 48 h. After
incubation, cells were harvested and washed with PBS. Subsequently,
a fluorescein isothiocyanate-labeled Annexin V and propidium iodide
kit (cat. no V13242; Thermo Fisher Scientific, Inc.) was used to
stain cells in the dark at 4˚C for 20 min, according to the
manufacturer's instructions). Early apoptosis was analyzed using a
flow cytometer. Data were analyzed using Invitrogen Attune NxT flow
cytometry software (version 3.1, Thermo Fisher Scientific,
Inc.).
Statistical analysis
The mean ± SD values of data derived from three
biological replicates of each experiment were calculated. All data
analysis was performed using mean values. Correlation analysis was
conducted using the Pearson's correlation test. Differences between
two groups of participants and among different cell groups were
compared by the unpaired Student's t-test and one-way ANOVA
followed by Tukey's post hoc test, respectively. Receiver operating
characteristic (ROC) curve analysis was used for diagnostic
analysis. All statistical analyses were performed using GraphPad
Prism software (version 6.0; GraphPad Software, Inc.). P<0.05
was considered to indicate a statistically significant
difference.
Results
Mirt2 and IFNG-AS1 are inversely
correlated in plasma from patients with UC
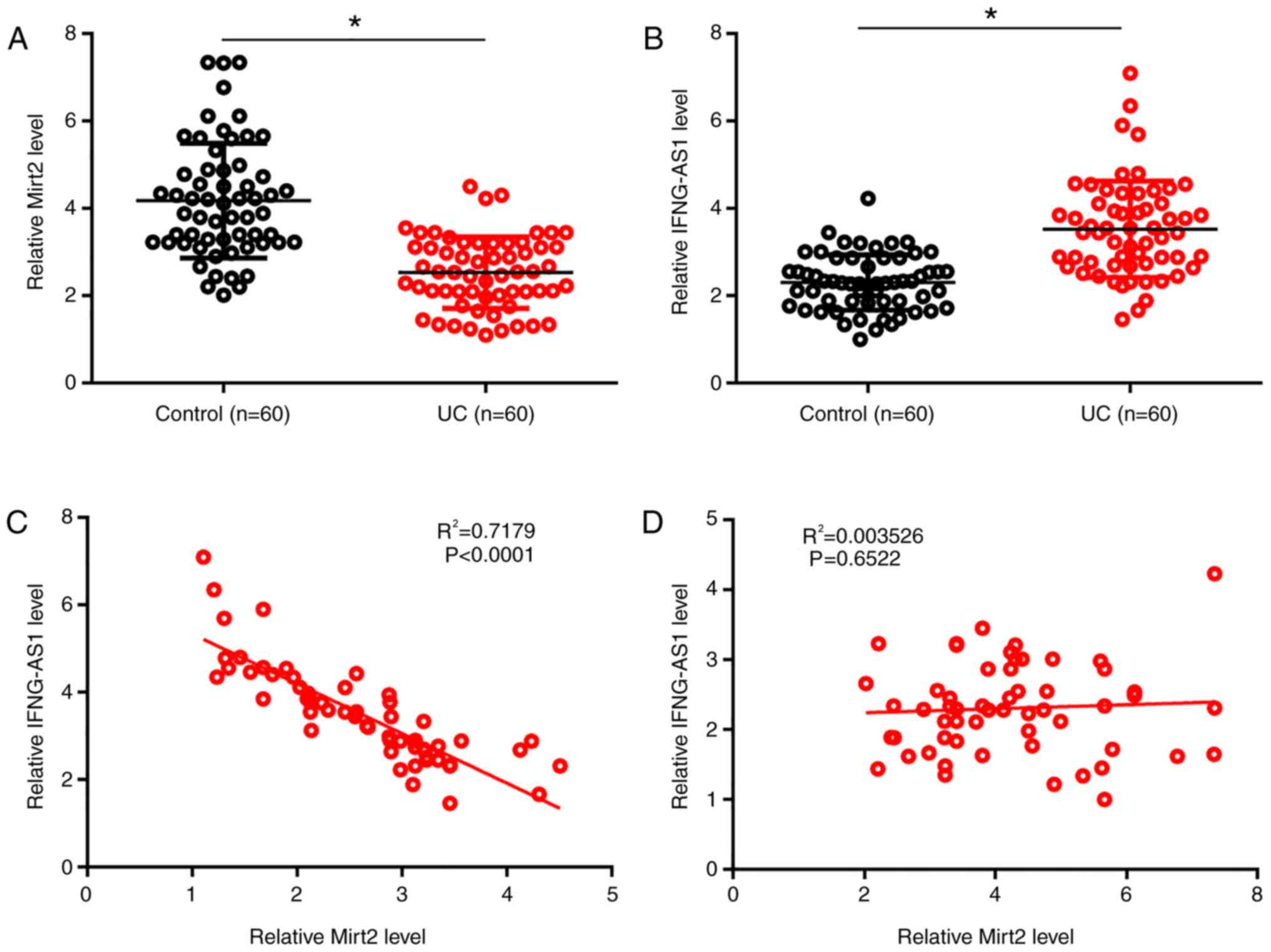
RT-qPCR and the Student's t-test were used to
measure and compare levels of Mirt2 and IFNG-AS1 in plasma derived
from patients with UC and healthy controls. Compared with the
control group, significantly lower levels of Mirt2 and
significantly higher levels of IFNG-AS1 were observed in plasma
derived from patients with UC (P<0.05; Fig. 1A and B). The relationship between Mirt2 and
IFNG-AS1 was analyzed by the Pearson's correlation test. Plasma
levels of Mirt2 were inversely and significantly correlated with
plasma levels of IFNG-AS1 in patients with UC (P<0.05;
R2=0.7179; Fig. 1C).
However, this correlation was not significant in the control group
(P>0.05; R2=0.004; Fig.
1D).
Altered plasma levels of Mirt2 and
IFNG-AS1 exhibit diagnostic values for UC
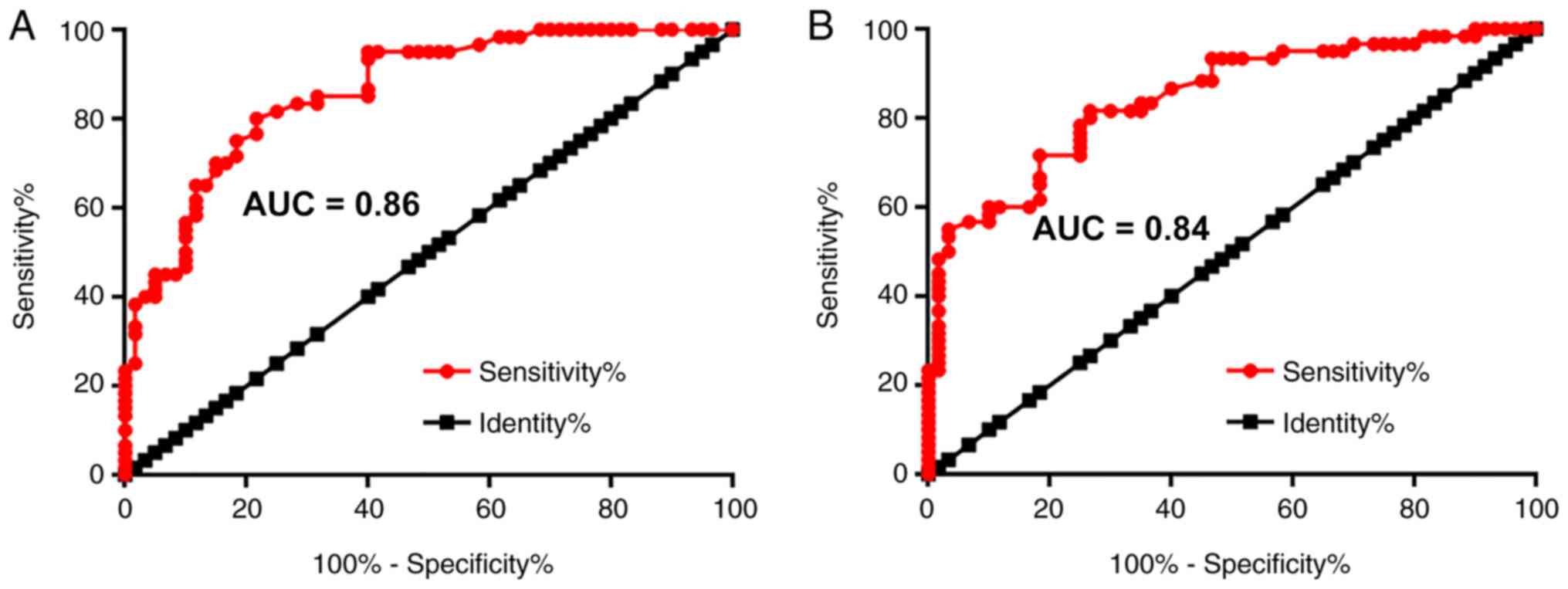
ROC curve analysis was performed to evaluate the
diagnostic potential of measuring plasma Mirt2 and IFNG-AS1 levels
in UC. Patients with UC were always the true positive cases and
healthy volunteers were always the true negative cases. An area
under the curve (AUC) >0.65 indicated diagnostic value. For
plasma Mirt2, the AUC was 0.86 (95% confidence interval, 0.80-0.93;
standard error, 0.032; Fig. 2A).
For plasma IFNG-AS1, the AUC was 0.84 (95% confidence interval,
0.78-0.91; standard error, 0.035; Fig.
2B).
LPS treatment leads to altered
expression of Mirt2 and IFNG-AS1 in HCnEpCs
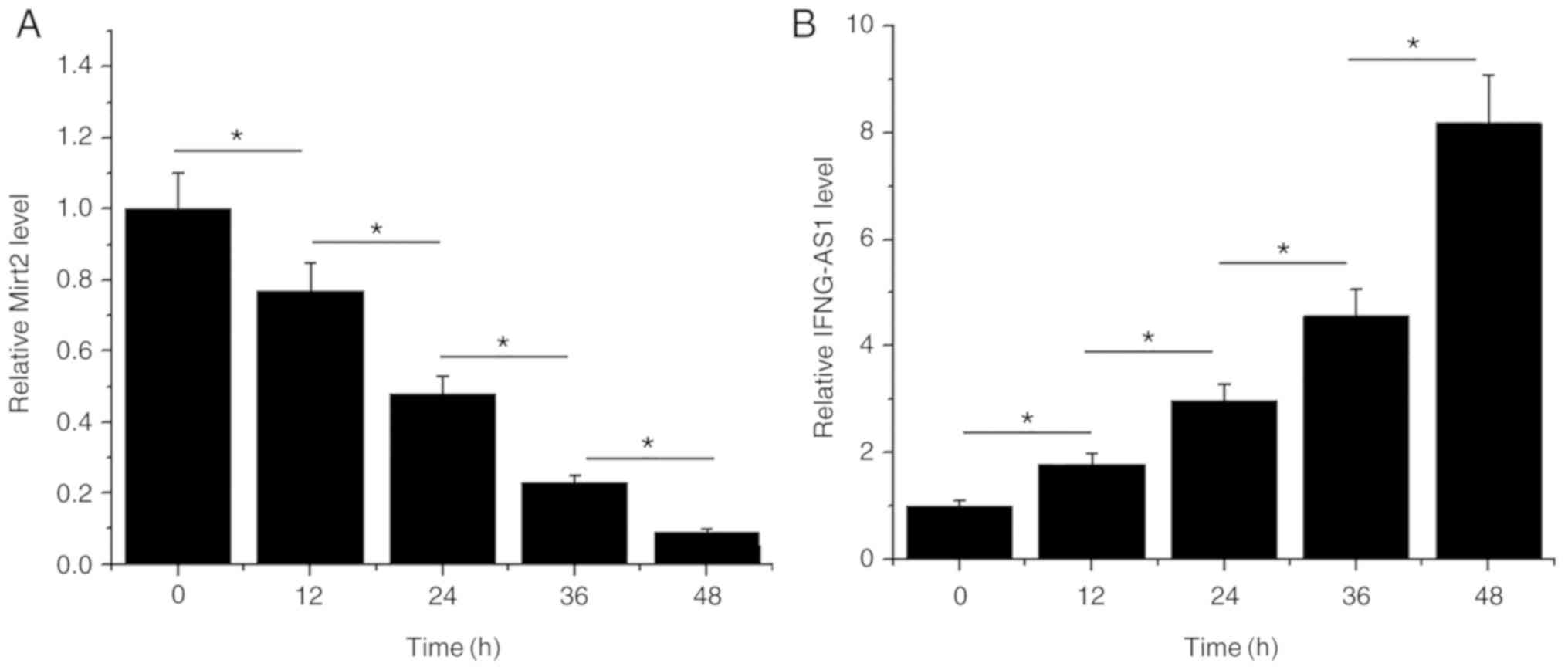
HCnEpCs were cultured in cell culture medium
containing 5 µg/ml LPS for 12, 24, 36 and 48 h, prior to the
measurement of Mirt2 and IFNG-AS1 expression levels. LPS treatment
led to the downregulation of Mirt2 in a time-dependent manner
(P<0.05; Fig. 3A). Moreover, LPS
treatment led to the upregulation of Mirt2 in a time-dependent
manner (P<0.05; Fig. 3B).
Mirt2 and IFNG-AS1 downregulate each
other in HCnEpCs
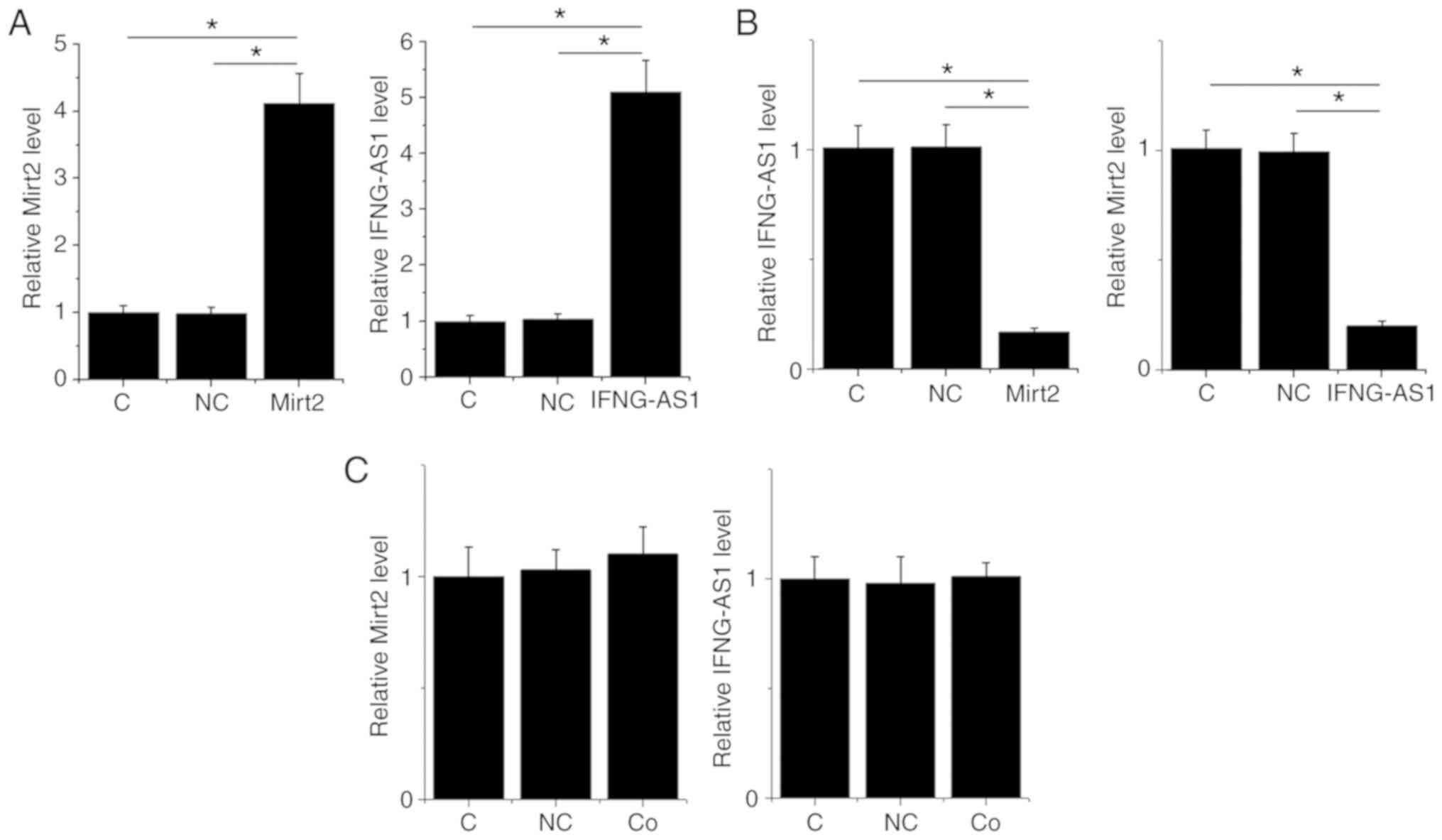
Mirt2 and IFNG-AS1 expression vectors were
transfected into HCnEpCs. At 24 h post-transfection, the expression
levels of Mirt2 and IFNG-AS1 were measured by RT-qPCR and compared
by one-way ANOVA followed by Tukey's post hoc test. Compared with
the control and negative control groups, the expression levels of
Mirt2 and IFNG-AS1 were significantly upregulated following
transfection with the overexpression vectors (P<0.05; Fig. 4A). Moreover, overexpression of Mirt2
and IFNG-AS1 resulted in downregulated expression levels of
IFNG-AS1 and Mirt2, respectively (P<0.05; Fig. 4B). However, co-transfection of the
expression vector of IFNG-AS1 and Mirt2 failed to significantly
alter the expression levels of IFNG-AS1 and Mirt2 (P>0.05;
Fig. 4C).
Interaction between Mirt2 and IFNG-AS1
affects the apoptosis of HCnEpCs
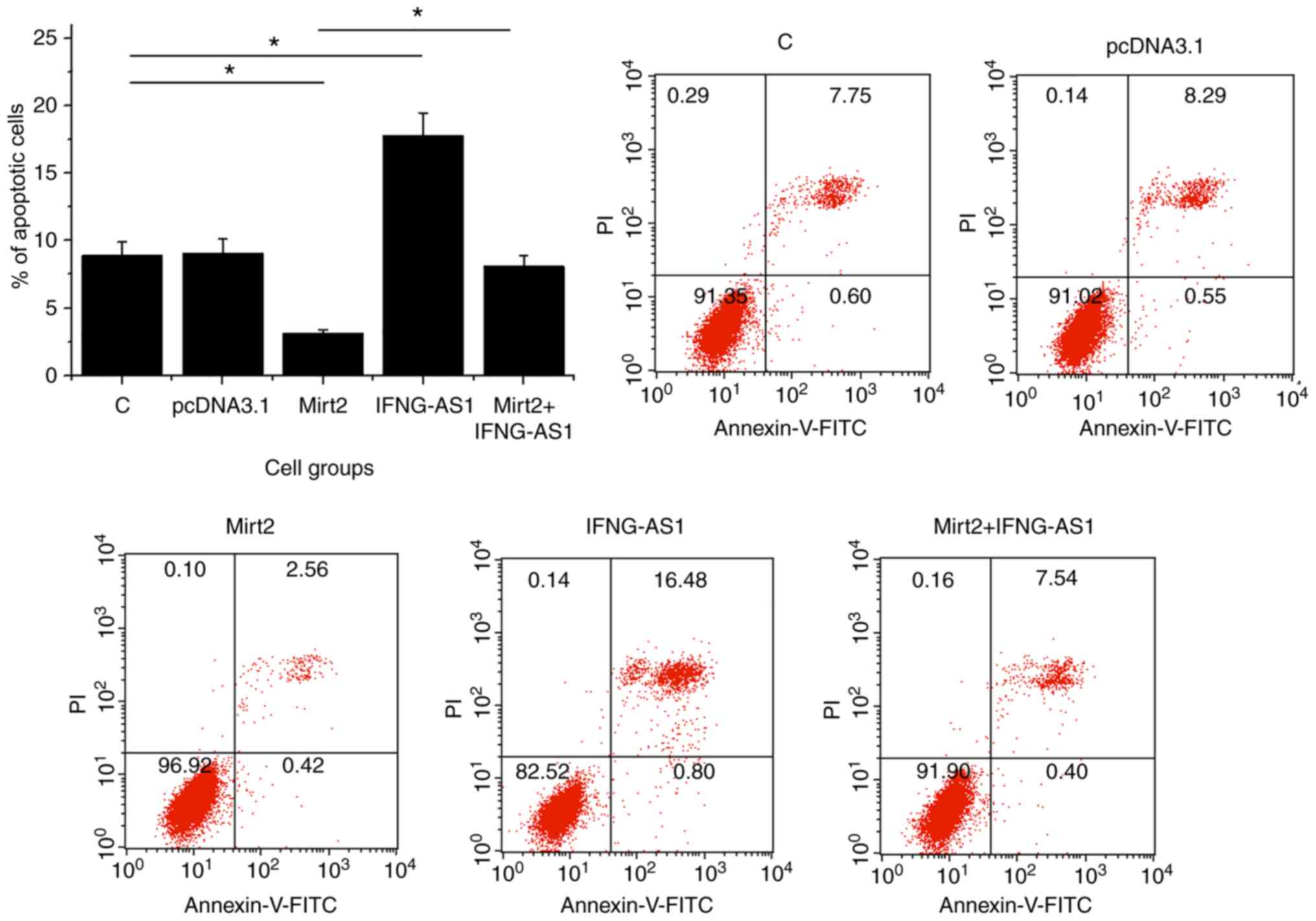
The effect of Mirt2 and IFNG-AS1 overexpression on
the late apoptosis of HCnEpCs was analyzed by a cell apoptosis
assay. Compared with the control and negative control groups,
overexpression of Mirt2 led to a decreased rate of apoptosis in the
colonic epithelial cells, while IFNG-AS1 overexpression led to an
increased rate of apoptosis (P<0.05; Fig. 5). Moreover, IFNG-AS1 overexpression
attenuated the effects of Mirt2 overexpression (P<0.05; Fig. 5).
Discussion
The present study investigated the interactions
between two inflammatory-related lncRNAs in UC (11,12).
Mirt2 and IFNG-AS1 were dysregulated in UC and were inversely
correlated. Furthermore, Mirt2 and IFNG-AS1 may form a negative
regulation feedback loop to regulate the apoptosis of HCnEpCs.
A previous study reported that the expression level
of IFNG-AS1 was increased by 5.27 times in UC (11). The study reported that in CD4 T
cells, IFNG-AS1 regulated the key inflammatory cytokine,
interferon-γ, to promote inflammation in patients with UC (11). Consistently, the present study also
observed upregulation of IFNG-AS1 in the plasma from patients with
UC and in HCnEpCs treated with LPS. In another study, Mirt2 was
reported to interact with toll-like receptor 4 to negatively
regulate inflammation (12). In the
present study, Mirt2 was downregulated in plasma from patients with
UC and in HCnEpCs treated with LPS. Collectively, these results
suggested that IFNG-AS1 and Mirt2 may be involved in UC.
Cell apoptosis, including the apoptosis of HCnEpCs,
contributes to the progression of UC (15,16).
Therefore, suppression of HCnEpC apoptosis is a potential
therapeutic approach for UC (15,16).
lncRNAs have been characterized as key players in the regulation of
cell behavior (17). In cellular
processes, lncRNAs regulate the expression of downstream genes or
sponge miRNAs (18,19). To the best of our knowledge, the
interactions between different lncRNAs have not been well
characterized in general. In the present study, the results
suggested that IFNG-AS1 and Mirt2 may negatively regulate the
expression of one another to regulate the apoptosis of HCnEpCs.
However, the mechanism that mediates the interaction between the
two lncRNAs requires further investigation. Based on the findings
obtained in the present study, it could be speculated that the
interaction between IFNG-AS1 and Mirt2 is mediated by certain
pathological mediators. This speculation is supported by the
observation that IFNG-AS1 and Mirt2 were only inversely correlated
in patients with UC, but not in healthy controls. Further
investigation is required to identify the possible mediators
between IFNG-AS1 and Mirt2.
To the best of our knowledge, the present study is
the first to report the involvement of Mirt2 in UC and to analyze
the interaction of two lncRNAs in UC. However, the present study
did not include a detailed analysis of the mechanism of the roles
of Mirt2 and IFNG-AS1 in UC, therefore further investigation is
required. Additionally, the present study did not analyze the
effects of Mirt2 on inflammatory and apoptotic factors in HCnEpCs.
However, it is known that LPS induces multiple inflammatory and
apoptotic pathways in HCnEpCs, such as the mitogen-activated
protein kinase signaling pathway (20). Mirt2 is an LPS-inducible inhibitor
of inflammation (12), therefore,
it is reasonable to speculate that Mirt2 may also interact with
inflammatory pathways, such as the mitogen-activated protein
kinase, NF-κB and JAK-STAT pathways, in HCnEpCs.
In conclusion, IFNG-AS1 was upregulated in UC, while
Mirt2 was downregulated. IFNG-AS1 and Mirt2 may negatively effect
the expression of one another and participate in the pathogenesis
of UC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81360603) and the
Natural Science Foundation of Hainan Province (grant no.
813215).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL performed the clinical studies, experimental
work, data analysis and wrote the manuscript. LC, SL and ML
performed the experiments and carried out literature research. XM
conceived and designed the study and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hainan General Hospital. Written informed consent was
provided by all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Choy MC, Seah D, Faleck DM, Shah SC, Chao
CY, An YK, Radford-Smith G, Bessissow T, Dubinsky MC, Ford AC, et
al: Systematic review and meta-analysis: Optimal salvage therapy in
acute severe ulcerative colitis. Inflamm Bowel Dis. 25:1169–1186.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cosnes J, Gower-Rousseau C, Seksik P and
Cortot A: Epidemiology and natural history of inflammatory bowel
diseases. Gastroenterology. 140:1785–1794. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Molodecky NA, Soon IS, Rabi DM, Ghali WA,
Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema
HW and Kaplan GG: Increasing incidence and prevalence of the
inflammatory bowel diseases with time, based on systematic review.
Gastroenterology. 142:46–54.e42.e30. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bernstein CN, Ng SC, Lakatos PL, Moum B
and Loftus EV Jr: Epidemiology and Natural History Task Force of
the International Organization of the Study of Inflammatory Bowel
Disease: A review of mortality and surgery in ulcerative colitis:
Milestones of the seriousness of the disease. Inflamm Bowel Dis.
19:2001–2010. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kaplan GG, Seow CH, Ghosh S, Molodecky N,
Rezaie A, Moran GW, Proulx MC, Hubbard J, MacLean A, Buie D and
Panaccione R: Decreasing colectomy rates for ulcerative colitis: A
population-based time trend study. Am J Gastroenterol.
107:1879–1887. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Head KA and Jurenka JS: Inflammatory bowel
disease Part 1: Ulcerative colitis-pathophysiology and conventional
and alternative treatment options. Altern Med Rev. 8:247–283.
2003.PubMed/NCBI
|
|
7
|
Mansfield JC, Holden H, Tarlow JK, Di
Giovine FS, McDowell TL, Wilson AG, Holdsworth CD and Duff GW:
Novel genetic association between ulcerative colitis and the
anti-inflammatory cytokine interleukin-1 receptor antagonist.
Gastroenterology. 106:637–642. 1994.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Russell SE, Horan RM, Stefanska AM, Carey
A, Leon G, Aguilera M, Statovci D, Moran T, Fallon PG, Shanahan F,
et al: IL-36α expression is elevated in ulcerative colitis and
promotes colonic inflammation. Mucosal Immunol. 9:1193–1204.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cătană CS, Berindan Neagoe I, Cozma V,
Magdaş C, Tăbăran F and Dumitraşcu DL: Contribution of the
IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease.
World J Gastroenterol. 21:5823–5830. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mirza AH, Berthelsen CH, Seemann SE, Pan
X, Frederiksen KS, Vilien M, Gorodkin J and Pociot F:
Transcriptomic landscape of lncRNAs in inflammatory bowel disease.
Genome Med. 7(39)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Padua D, Mahurkar-Joshi S, Law IK,
Polytarchou C, Vu JP, Pisegna JR, Shih D, Iliopoulos D and
Pothoulakis C: A long noncoding RNA signature for ulcerative
colitis identifies IFNG-AS1 as an enhancer of inflammation. Am J
Physiol Gastrointest Liver Physiol. 311:G446–G457. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Du M, Yuan L, Tan X, Huang D, Wang X,
Zheng Z, Mao X, Li X, Yang L, Huang K, et al: The LPS-inducible
lncRNA Mirt2 is a negative regulator of inflammation. Nat Commun.
8(2049)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Feakins RM: British Society of
Gastroenterology: Inflammatory bowel disease biopsies: Updated
British Society of Gastroenterology reporting guidelines. J Clin
Pathol. 66:1005–1026. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pott J, Kabat AM and Maloy KJ: Intestinal
epithelial cell autophagy is required to protect against
tnf-induced apoptosis during chronic colitis in mice. Cell Host
Microbe. 23:191–202.e194. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang SQ, Ni WK, Xiao MB, Jiang F, Lu CH,
Wang RH and Ni RZ: Actin related protein 3 (ARP3) promotes
apoptosis of intestinal epithelial cells in ulcerative colitis.
Pathol Res Pract. 215:235–242. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Kornienko AE, Guenzl PM, Barlow DP and
Pauler FM: Gene regulation by the act of long non-coding RNA
transcription. BMC Biol. 11(59)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859:169–176. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cario E, Rosenberg IM, Brandwein SL, Beck
PL, Reinecker HC and Podolsky DK: Lipopolysaccharide activates
distinct signaling pathways in intestinal epithelial cell lines
expressing Toll-like receptors. J Immunol. 164:966–972.
2000.PubMed/NCBI View Article : Google Scholar
|