Introduction
Prolonged isoflurane exposure has been implicated in
postoperative cognitive dysfunction, particularly in the elderly
(1,2). Several previous studies have
demonstrated the effect of isoflurane exposure on neurotoxicity and
cognitive impairment due to a rise in neuronal inflammation and
apoptosis, especially in the hippocampus region of the brain
(2-5).
However, the molecular mechanisms that lead to isoflurane-induced
cognitive decline in aged rats remain elusive.
Epigenetic modification regulates the transcription
of genes that are essential for the maintenance of memory processes
(6). Accumulating evidence supports
the crucial role of chromatin-mediated epigenetic regulation of
behavior and memory formation (7,8). The
most common mechanism of epigenetic modification involves histone
acetylation, which is altered dynamically during the course of
learning and memory formation (9).
Histone acetylation is endogenously achieved by maintaining a
balance between the activity of histone acetyltransferases and
histone deacetylases (HDACs). An increase in histone acetylation
results in a more relaxed chromatin structure and enhances the
transcription of genes vital for long-term memory, whereas histone
deacetylation induces a negative effect (10). It has also been found that
epigenetic variations inside the brain are crucial for short- and
long-term behavioral adaptation to a wide range of environmental
stimuli (11). These external
environmental stimuli can stimulate intracellular pathways that
directly remodel the epigenome and lead to differences in gene
expression and neuronal function in the early development as well
as the adult stages of life (12,13).
Isoflurane exposure is one such external stimulus, and thus,
targeting histone acetylation using HDAC inhibitors (HDACis) before
isoflurane exposure might reverse neurotoxicity and cognitive
decline. One such HDACi is MS-275, which is specific to HDACs 1-3.
In contrast to other HDACi, MS-275 can enter the brain even at low
doses, and raise the levels of acetylated histone 3 (Ac-H3). In
addition, MS-275 has a long half-life (14).
Epigenetic programming is largely reliant on the
activation of transcription factors binding to promoter regions of
downstream target genes, and on intracellular signaling cascades,
such as the MAPK pathway. The MAPK family of serine-threonine
protein kinases comprises ERK, p38 and JNK as its main members
(15). The dynamic expression of
MAPK signaling proteins in the mature nervous system, mainly in the
hippocampus, suggests a role for the MAPK cascade in synaptic
plasticity, learning and memory formation (16-18).
Previous studies have also provided insight into the function of
MAPK pathways in reducing sevoflurane-induced neurotoxicity
(19,20). However, to the best of the authors'
knowledge, no study to date has investigated how epigenetic
modification of MAPK signaling pathways might lead to functional
changes in hippocampal neurons following isoflurane exposure.
Therefore, in the present study, it was hypothesized
that pre-treatment with an HDACi could alleviate isoflurane-induced
neuronal apoptosis and cognitive decline in aged rats. The present
study identified a direct association between epigenetic
programming and MAPK signaling following isoflurane exposure.
Materials and methods
Animals
Male, 22-month-old Sprague-Dawley rats (280-300 g;
n=96) were purchased from The Shanghai SLAC Laboratory Animal Co.,
Ltd. All the experiments in the present study were approved by the
local ethical committee of Renmin Hospital of Wuhan University and
performed according to the guidelines presented in the Declaration
of the National Institutes of Health Guide for Care and Use of
Laboratory Animals. All animals were kept under a 12 h light/dark
cycle with a relative humidity of 40-70% in a temperature-regulated
room at 22-24˚C with unrestricted access to food and water.
Isoflurane exposure
The animals were exposed to isoflurane as previously
described (21). Briefly, the
animals were placed in a chamber pre-filled with 3% isoflurane in
30% O2 for the first 10 min followed by1.5% isoflurane
exposure to maintain constant anesthesia for 2 h on 7 consecutive
days. The concentrations of gases inhaled and exhaled were
regularly monitored using the Datex Capnomac Ultima stand-alone
multi gas analyzer (GE Healthcare).
MS-275 administration and experimental
design
The animals were divided into three groups: Control
(Cont), isoflurane (ISO) and MS-275-treated (n=32 in each group).
For hippocampal-dependent spatial memory evaluation, rats (n=8 in
each group) were randomly chosen for Morris water maze (MWM)
training before test exposures. Prior to ISO exposure, MS-275 (2
mg/kg; Sigma-Aldrich; Merck KGaA) and PBS were administered
intraperitoneally to the animals in the MS-275 and ISO groups,
respectively. The rats in the Cont group were not exposed to
isoflurane but were kept in the chamber filled with 30%
O2 for 2 h on 7 consecutive days. All rats then
recovered in a chamber with 30% O2 at 37˚C for 20 min.
The rats were sacrificed following isoflurane exposure and
cognitive testing by CO2 inhalation based on the AVMA
Guidelines on Euthanasia (22). The
hippocampus of each animal was harvested, rapidly frozen in liquid
nitrogen and stored at -80˚C for further analysis.
Reverse transcription-quantitative PCR
(RT-qPCR)
For RT-qPCR analysis, total RNA was extracted from
the hippocampus using the RNA-spin™ Total RNA Extraction kit
(Intron Biotechnology, Inc.). RNA concentrations were measured
using the NanoDrop 1000 system (Thermo Fisher Scientific, Inc.).
cDNA synthesis was carried out using the First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.) with the following
cycling conditions: Digestion at 37˚C for 2 min, annealing at 65˚C
for 5 min, RT at 50˚C for 10 min, and enzyme inactivation 85˚C for
5 min. Subsequently, RT-qPCR was performed on the ABI 7500
Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using SYBR Green PCR Master Mix (Thermo Fisher Scientific,
Inc.). The thermocycling conditions for RT-qPCR were: 95˚C initial
template denaturation, 40 cycles of 95˚C denaturation and 60˚C
annealing/extension. GAPDH was used as the endogenous control to
normalize mean Cq values using the
2-ΔΔCq method (23). The sequences of the primers used are
presented in Table SI.
TUNEL assay
Hippocampi from each rat were fixed with 4%
paraformaldehyde for 48 h at 4˚C, paraffin-embedded, then sectioned
at a thickness of 5 µm. The Dead End™ fluorometric TUNEL system kit
(Promega Corporation) was used to perform the assay according to
the manufacturer's guidelines. Hoechst dye (Hoechst 33342; Thermo
Fisher Scientific, Inc.) was used as a nuclear stain and diluted to
1 µg/ml in PBS from 10 mg/ml stock prepared in deionized water.
Samples were stained for 10 min at room temperature, with
protection from light. SlowFade™ Glass Soft-set Antifade Mountant
(Thermo Fisher Scientific, Inc.) was used for mounting.
TUNEL-positive cells were examined using NIS-Elements BR imaging
processing and analysis software version 4.00 (Nikon Corporation),
and 10 microscopic fields were analyzed per group (magnification,
x200). TUNEL-positive cell density in the CA1 region of the brain
was evaluated by dividing the number of TUNEL-positive cells by the
area of that region of the brain.
Western blotting
Protein for western blot analysis was isolated from
the hippocampal tissue samples homogenized in ice-cold RIPA Pierce
™ buffer (Thermo Fisher Scientific, Inc.) supplemented with
protease inhibitor at final 1X concentration (Halt™ Phosphatase
Inhibitor Cocktail, Thermo Fisher Scientific, Inc.). Protein
concentrations were measured using a BCA protein assay kit (Thermo
Fisher Scientific, Inc.). Proteins were then separated by SDS-PAGE
on 10% gels and 20 µg total protein/lane was transferred to a
nitrocellulose membrane. After the transfer, the blot was kept in
5% w/v non-fat dry milk blocking solution for 1 h at room
temperature, then incubated with primary antibodies overnight at
4˚C. The primary antibodies used were: Anti-HDAC1 (1:400; Abcam;
cat. no. ab53091), anti-HDAC2 (1:400; Abcam; cat. no. ab32117),
anti-HDAC3 (1:400; Abcam; cat. no. ab32369), anti-cleaved caspase-3
(1:1,000; Abcam; cat. no. ab2302), anti-Bcl-2 (1:1,000; Abcam; cat.
no. ab32124) anti-Bax (1:1,000; Abcam; cat. no. ab32503),
anti-phospho-ERK1/2 (p-ERK1/2; 1:1,000; Abcam, cat. no. ab223500),
anti-ERK1/2 (1:1,000; Abcam; cat. no. ab17942), anti-phospho-JNK
(p-JNK;1:1,000; Abcam; cat. no. ab47337), anti-JNK (1:1,000; Abcam;
cat. no. ab199380), anti-phospho-p38 (p-p38;1:1,000; Abcam; cat.
no. ab4822) and anti-p38 (1:1,000; Abcam; cat. no. ab31828).
Anti-β-actin (1:500; Abcam; cat. no. ab1801) was used as the
internal control for each sample. After incubation with primary
antibodies, the blot was washed three times with PBS + 0.1%
Tween-20 and incubated with horseradish peroxidase-conjugated goat
anti-mouse IgG secondary antibody (1:500; Abcam; cat. no. ab97023)
at room temperature for 2 h. Protein bands were detected using an
enhanced chemiluminescent kit (cat. no. EK1001; CliniSciences,
Nanterre) and the band density was visualized and quantified using
ImageJ software version 1.48 (National Institutes of Health). The
quantification is presented as the ratios between protein band
densities of the protein of interest with respect to the internal
control protein band density. The protein expression of p-ERK1/2,
p-JNK or p-p38 was normalized to total ERK1/2, JNK or p38,
respectively. The signal intensity of bands of other proteins of
interest were normalized to β-actin.
MWM experiments
The MWM test was performed to evaluate spatial
learning and memory in rodents (24). A circular pool with a diameter of
180- and 50-cm deep was filled with opaque water at a temperature
of 26˚C. A round platform with a diameter of 10 cm was immersed 2
cm beneath the water surface in one quadrant. The position of the
platform was unaltered for each rat. In total, 8 rats from each
group were randomly chosen for MWM training. Rats were trained on 4
trials/day for 5 consecutive days before receiving anesthesia. The
minimum interval between every trial was 15 min. If the rats failed
to find the hidden platform within 90 sec, they were manually
guided for 30 sec towards the hidden platform. After training, the
rats were subjected to the experimental protocol as described. On
the 7th day of isoflurane exposure, the platform was raised 2 cm
above the surface of water so that the platform was visible to
rats. This also helped to evaluate the effect of isoflurane
exposure and MS-275 pre-treatment on non-spatial factors like
visual acuity, sensorimotor performance and impetus on cognitive
function. Each trial was considered complete either when the rat
successfully climbed to the platform or when the trail duration
reached 90 sec. After spending 30 sec on the platform in between
trials, mice were put in a cage warmed with electric heating pad
for 5 min to allow recovery. The time taken for each trial in a day
was averaged and statistically analyzed.
Statistical analysis
The data are presented as the mean ± SEM. One-way
ANOVA was used for comparisons between variables, followed by
Bonferroni post hoc test using GraphPad Prism 5.0 (GraphPad
Software, Inc.). P<0.01 and P<0.001 were used to indicate a
statistically significant difference. Each experiment was performed
in triplicate and repeated thrice.
Results
HDAC expression following isoflurane
exposure
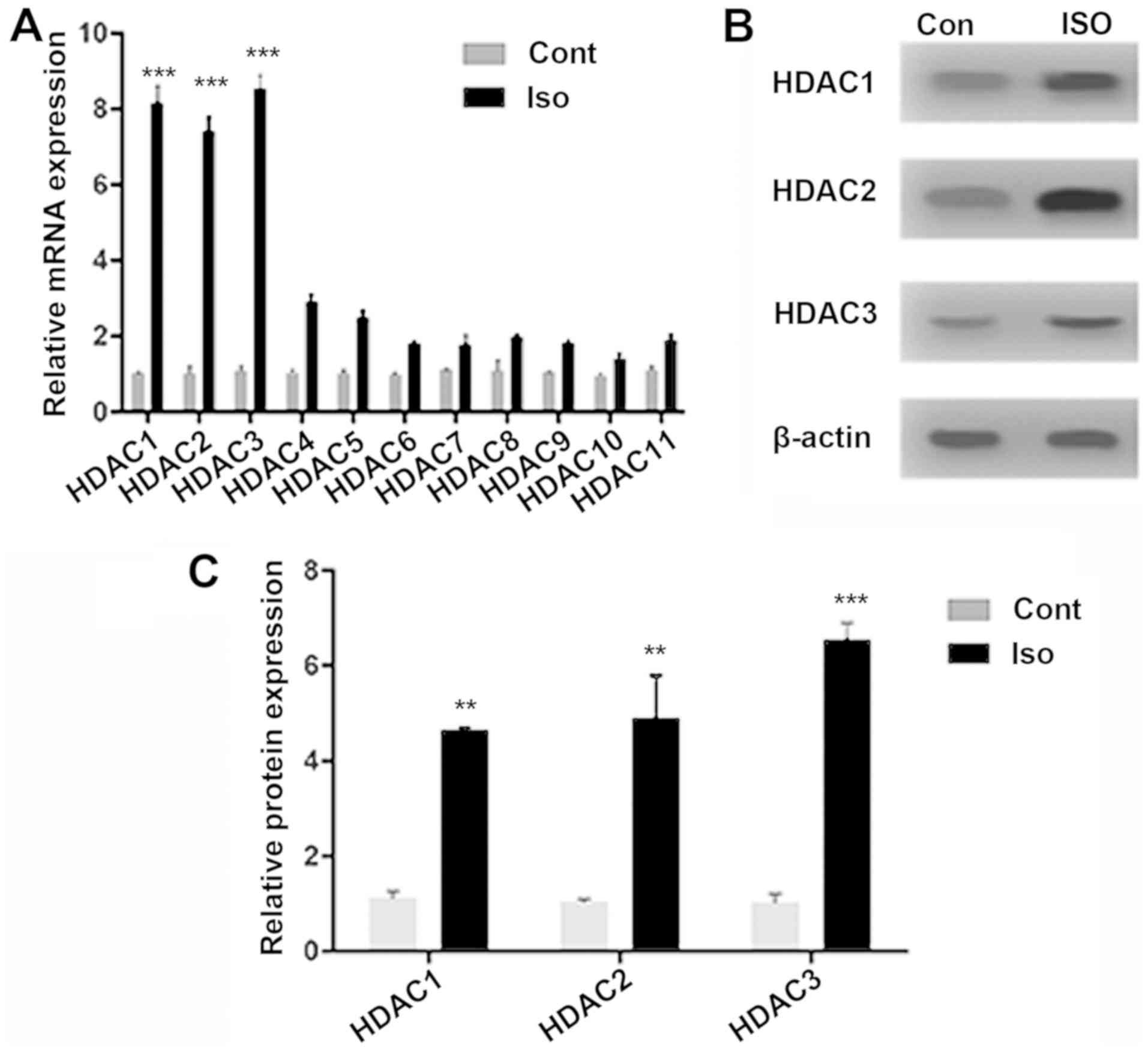
The expressions of different HDAC isoforms were
quantified following isoflurane exposure. RT-qPCR was performed for
all HDAC isoforms (HDAC1-11), identifying strong signals for HDAC1
(8.15-fold; P<0.001), HDAC2 (7.4-fold; P<0.001) and HDAC3
(8.55-fold; P<0.001) genes, compared with Cont. The increase in
mRNA expression was notably lower forHDAC4 (1.87-fold; P>0.01)
and for HDACs 5-11 (Fig. 1A).
HDAC1-3 protein expression significantly increased, compared with
Cont (Figs. 1B and C; P<0.001 and P<0.01), suggesting
isoflurane-induced epigenetic dysregulation. Notably, HDAC8, which
is also a class-I HDAC, did not exhibit a significant change in
mRNA expression after isoflurane exposure when compared with the
Cont group. This could be attributed to the fact that HDAC8 is not
recruited to chromatin through large multi-protein complexes like
HDAC1-3(25). The localization of
class-I HDACs in the nucleus (26)
has allowed for the elucidation of how HDAC inhibition modulates
MAPK signaling proteins in isoflurane-induced aged rats.
MS-275 pre-treatment decreases
isoflurane-induced neuronal apoptosis in the hippocampus
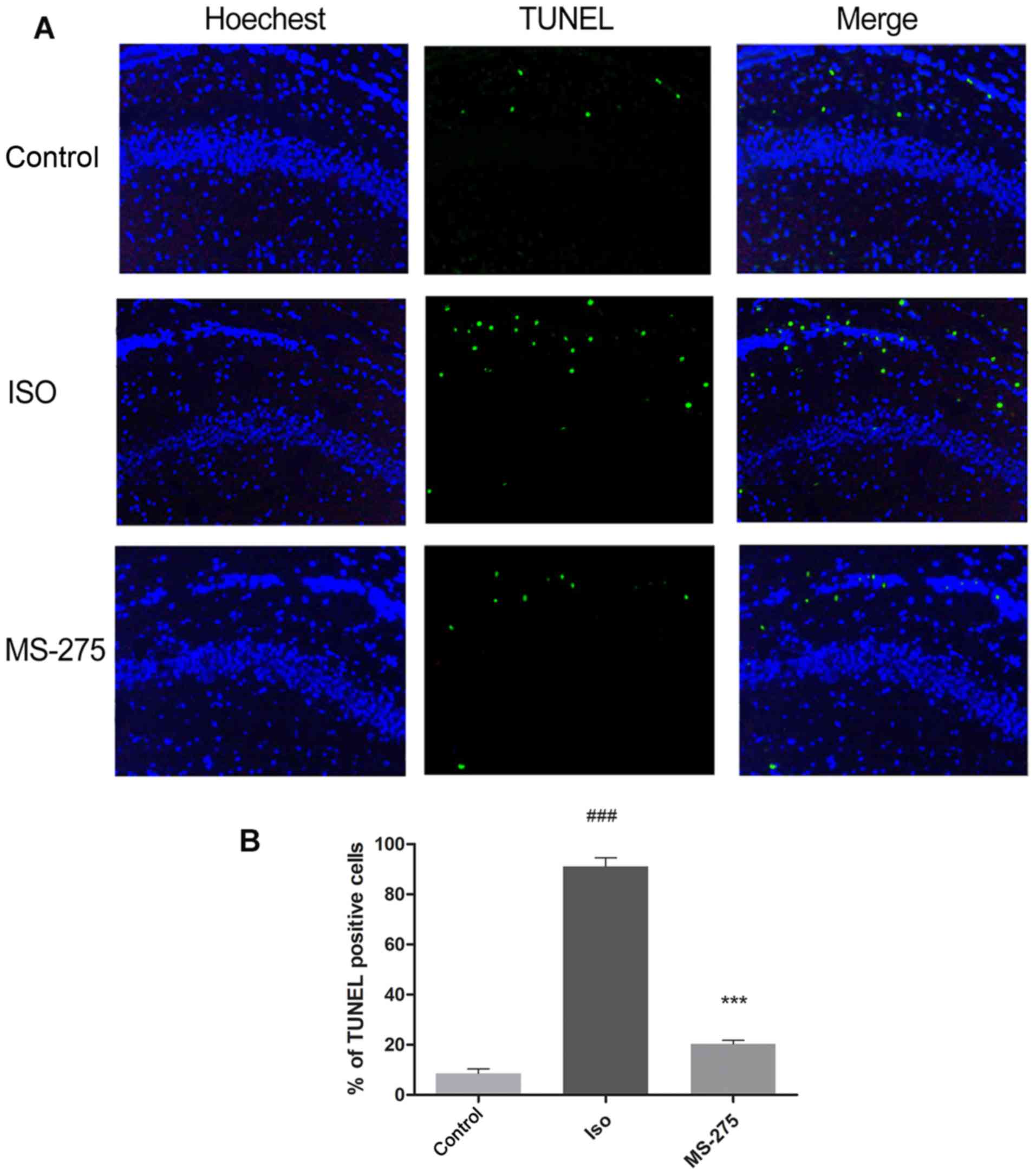
To determine the effect of MS-275 on
isoflurane-induced neuronal apoptosis, TUNEL staining was performed
on the CA1 region of the hippocampus. Following isoflurane
exposure, the number of TUNEL-positive cells increased by 82.47% in
the hippocampal CA1 region compared with Cont (Fig. 2; P<0.001). Pre-treatment with
MS-275 significantly inhibited the increase in TUNEL-positive cells
after isoflurane exposure. A70.72% decrease in TUNEL-positive cells
was observed in rats pre-treated with MS-275, compared with the ISO
group (Fig. 2; P<0.001). These
results demonstrated that MS-275 could alleviate isoflurane-induced
neuronal apoptosis.
MS-275 pre-treatment alters the
expression of apoptotic regulatory proteins
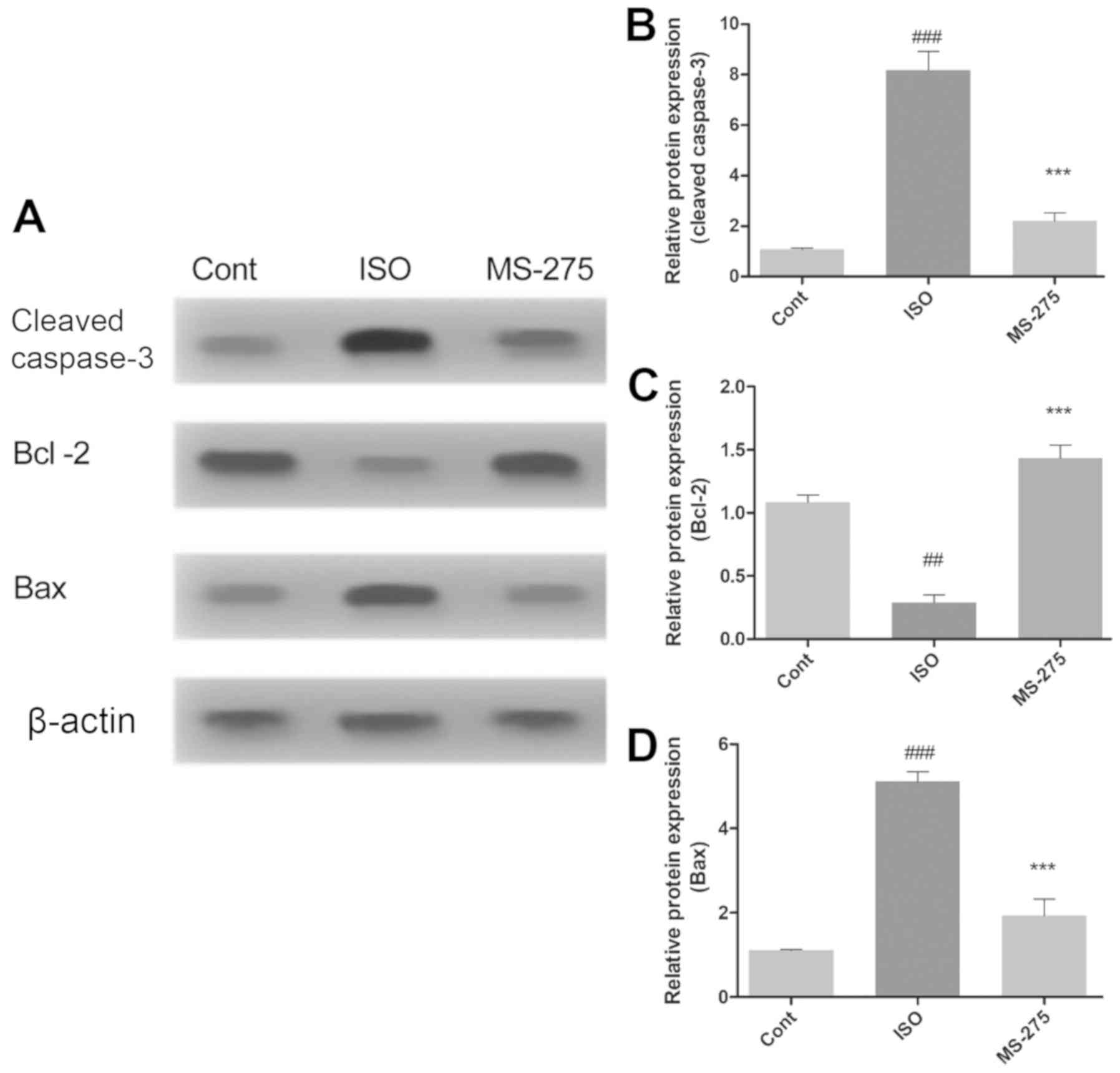
The effect of isoflurane exposure and MS-275
pre-treatment on neuronal apoptosis was further assessed by
evaluating the expression levels of proteins directly involved in
apoptosis. Protein expression of cleaved caspase-3, Bcl-2 and Bax
in the whole hippocampus was quantified using western blot analysis
(Fig. 3A). Isoflurane significantly
increased the levels of cleaved caspase-3 (7.1-fold; P<0.001;
Fig. 3B) and Bax (3.98-fold
increase; P<0.001; Fig. 3D),
compared with Cont. In addition, isoflurane also reduced Bcl-2
protein levels, relative to Cont (0.795-fold, P<0.01; Fig. 3C). However, pre-treatment with
MS-275 significantly decreased caspase-3 (5.975-fold; P<0.001;
Fig. 3B) and Bax protein levels
(3.18-fold; P<0.001; Fig. 3C),
compared with ISO. Bcl-2 protein expression was also increased
following MS-275 treatment, compared with ISO (1.145-fold;
P<0.001; Fig. 3C).
MS-275 pre-treatment reverses
isoflurane-induced variations in MAPK signaling protein levels
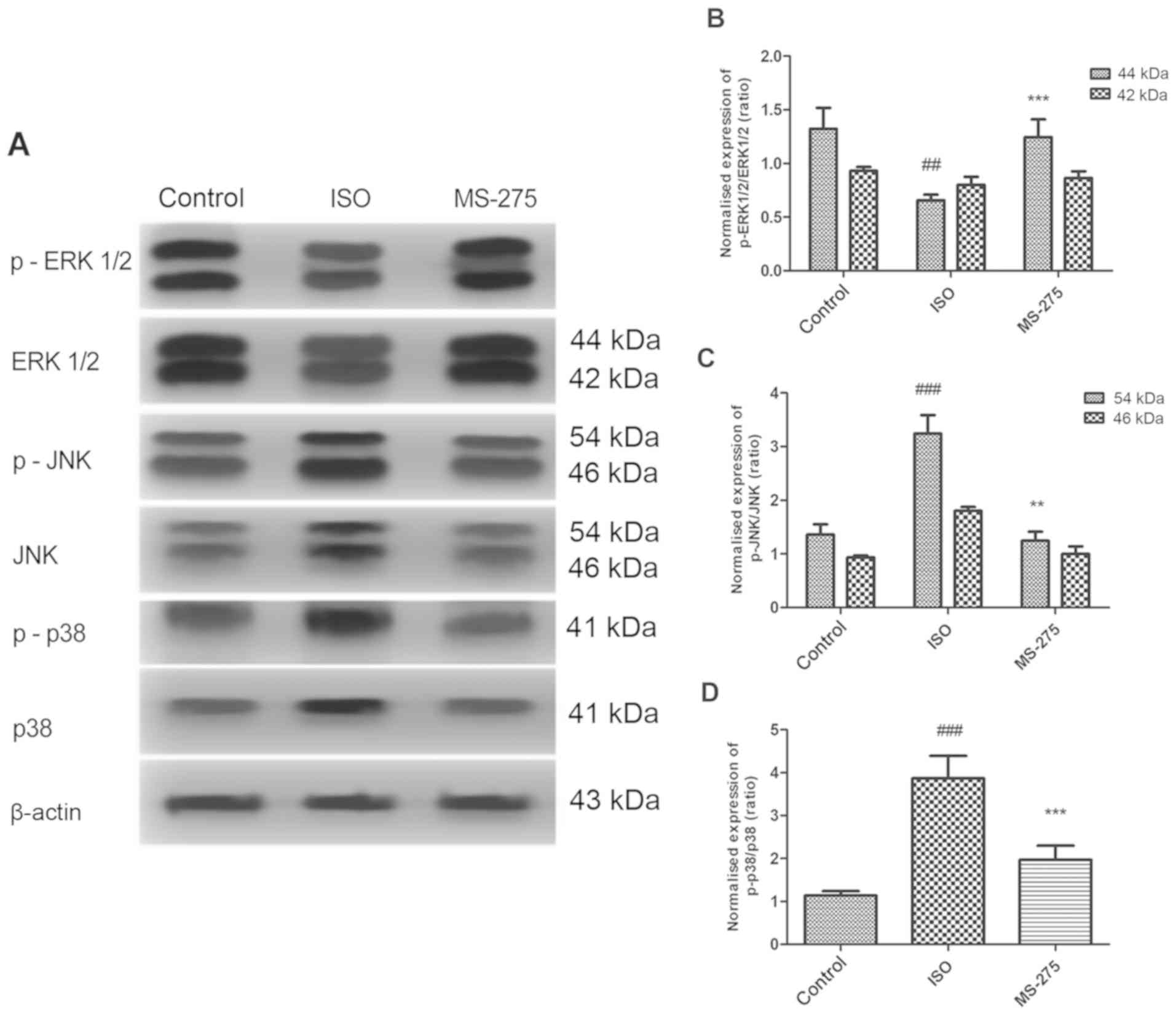
Isoflurane significantly reduced protein expression
of p-ERK1/2 (P<0.01), while increasing the expression of p-JNK
(P<0.001) and p-p38 (P<0.001), compared with Cont (Fig. 4). However, pre-treatment with HDACi
MS-275 reversed the isoflurane-induced changes in MAPK signaling
proteins. Indeed, p-ERK1/2 expression was increased, compared with
the ISO group (Fig. 4B).
Furthermore, MS-275 reduced the isoflurane-induced increase of
p-JNK proteins (Fig. 4C) and p-p38
proteins (Fig. 4D). These results
suggested that MS-275 directly influenced the expression of MAPK
signaling proteins following isoflurane exposure.
MS-275 pre-treatment improves
isoflurane-induced spatial memory impairment
The MWM test is a highly sensitive test which
evaluates cognitive function, especially hippocampus-dependent
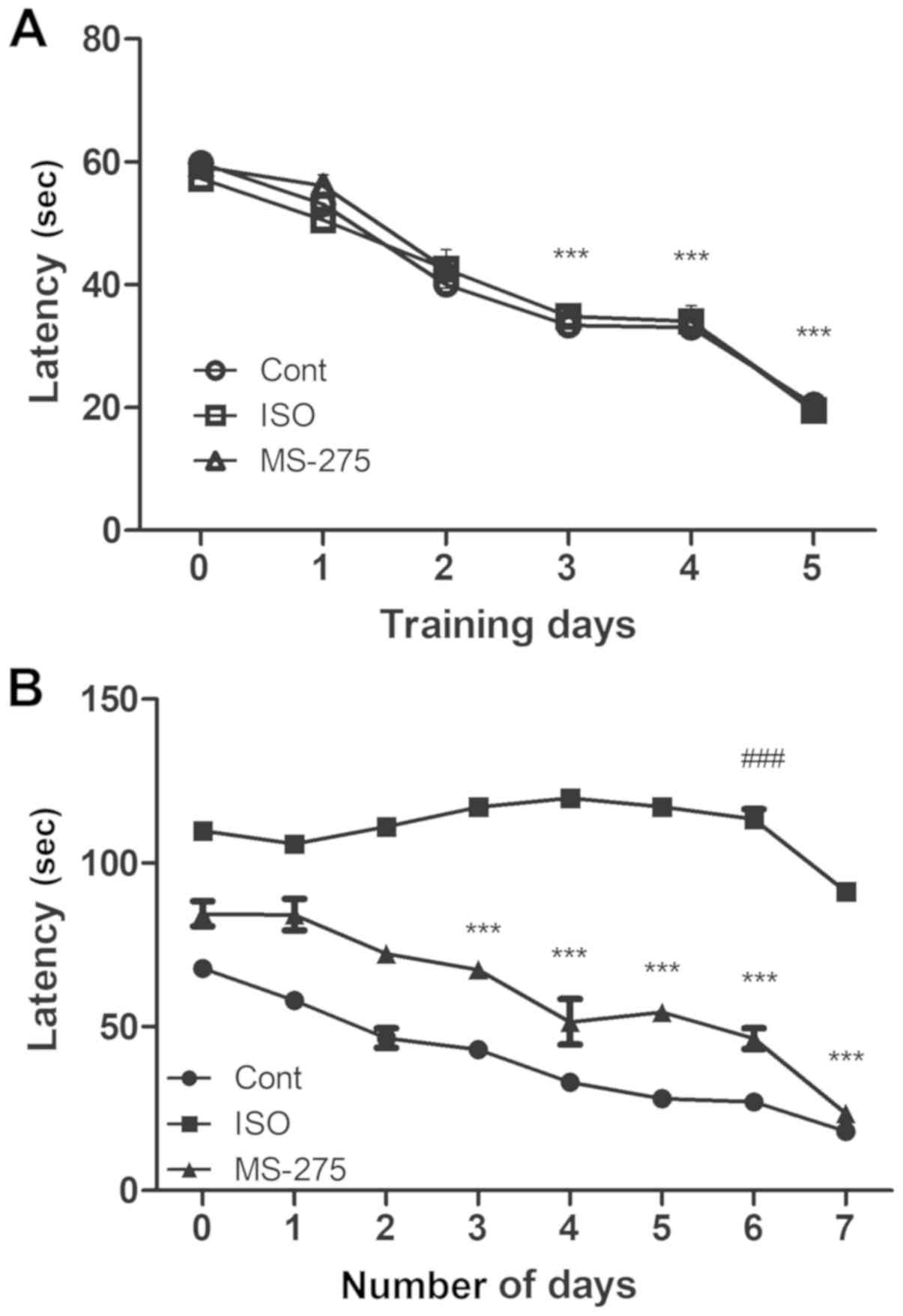
learning and memory in rodents (24). In the present study, aged rats in
each group could find the hidden platform prior to isoflurane
exposure following MWM training for 5 days. In comparison with the
latency on day 1, a significant decrease in latency was detected on
day 3 in all rats (P<0.001). On day 5 of training, the animals
in all three groups were able to find the hidden platform within 30
sec (Fig. 5A).
After the training phase, the rats either received
ISO or did not (serving as the Cont group). A third group was
treated with MS-275 prior to isoflurane exposure. On day 6 of
isoflurane exposure, the ISO group took a longer time (close to the
maximum time of 120 sec permitted for each animal) to find the
hidden platform, compared with the Cont group (113.4 sec;
P<0.001 vs. 27.1 sec in the Cont group; Fig. 5B). However, MS-275 treatment
significantly reduced the amount of time required to find the
platform submerged under the water surface compared with the ISO
group (46.4 sec; P<0.001). When the platform was raised on day 7
such that it was visible to the rats, the latency to find the
platform relatively reduced in all groups. Nonetheless, the MS-275
pre-treated group exhibited a significant cognitive recovery,
compared with the ISO group (23.5 and 91.3 sec, respectively;
P<0.001). This suggested that MS-275 pre-treatment had a
substantial role in alleviating cognitive decline after isoflurane
exposure.
Discussion
Isoflurane is a broadly used inhalation anesthetic
that has been reported to cause cognitive impairment in rodent
models as well as humans (1-5).
Although these previous studies suggested an association between
isoflurane exposure and neuro-inflammation, apoptosis and
mitochondrial dysfunction, the pathogenesis of isoflurane-induced
cognitive decline needs extensive investigation. Therefore, in the
present study, the effect of isoflurane exposure on neuronal
apoptosis as well as cognitive decline via epigenetic modulation of
MAPK signaling was investigated in aged rats.
Epigenetic modification regulates the transcription
of genes that are essential for the maintenance of memory processes
(6). Histone acetylation, one of
the most studied forms of epigenetic modification, is achieved by
maintaining a balance between histone acetyltransferases and HDACs.
Previous studies suggested that the deregulation of histone
acetylation in the hippocampus leads to cognitive decline via the
suppression of primary genes involved in learning and memory
(7-10).
In the present study, isoflurane exposure caused
epigenetic dysregulation with the subsequent disruption of neuronal
as well as cognitive function. In congruence with the hypothesis of
the present study, aged rats exposed to isoflurane exhibited
upregulation of class-I HDACs (HDAC1, -2, and -3), while HDAC4-11
showed low expression in the ISO group. Notably, HDAC8 did not
exhibit significant change in the expression following isoflurane
exposure, unlike the other class-I HDACs. This could be due to the
fact that, unlike HDACs 1-3, HDAC8 is not recruited to chromatin
through large multi-protein complexes and is completely active as a
standalone class-I HDAC (25).
Thus, the results of the present study suggested that isoflurane
exposure increased histone deacetylation, specifically via HDAC1-3,
which may result in a compromised epigenetic milieu.
Moreover, the present study also demonstrated the
restoration of histone acetylation using the HDACi MS-275, which is
specific to HDAC1-3. In contrast to other HDACi, MS-275 has a long
half-life and can enter the brain even at low doses, raising Ac-H3
levels in the brain (14). A recent
study demonstrated that MS-275 improved autism-induced synaptic and
social discrepancies through the epigenetic modulation of
associated genes, such as surface NMDARs and actin regulators
(27). Thus, MS-275 pre-treatment
was used to inhibit HDAC1-3 and study its effect on MAPK signaling
proteins, neuronal apoptosis and cognitive function following
isoflurane exposure.
Isoflurane predominantly affects the hippocampal
region of the brain and induces neurotoxicity, leading to severe
hippocampal lesion and an abnormal response to contextual fear
conditioning (28). In the present
study, MS-275 pre-treatment alleviated isoflurane-induced
neuro-apoptosis in the hippocampus of the aged rats. Furthermore,
MS-275 pre-treatment reduced isoflurane-induced levels of cleaved
caspase-3 (total caspase was not measured) and Bax protein, while
increasing Bcl-2 expression. A MWM test also demonstrated that
MS-275 pre-treatment prevented isoflurane-induced cognitive and
spatial memory impairments. A previous study demonstrated the
efficiency of MS-275 in enhancing memory and altering
depression-mediated behavior (29);
thus, the present study further supported the role of MS-275 in
isoflurane-induced cognitive impairment in aged rats. However,
cognitive decline was only evaluated by the MWM test, a
well-established behavioral test to evaluate spatial learning and
memory in rodents. Therefore, the present study would benefit from
future studies connecting neuronal architecture, synapse number and
neurotransmitters with other cognitive tests.
One of the critical objectives of the present study
was to investigate the association between the inhibition of HDAC1,
-2, and -3 with MAPK signaling protein expression following
isoflurane exposure. MS-275 altered the expression of MAPK
signalingproteinsp38, JNK, and ERK1/2, which may have accounted for
neuroprotection and cognitive recovery in aged rats exposed to
isoflurane. MS-275 suppressed p38 expression in E-11 cells, which
demonstrated its efficacy against rheumatoid arthritis (30). Another study demonstrated the
importance of highly selective class-I HDACi in the suppression of
nuclear ERK1/2 signaling (31).
These studies highlight the potential action of MS-275 on MAPK
signaling proteins.
The JNK signaling pathway is implicated in neuronal
apoptosis triggered by several brain injury stimuli, such as
ischemia/reperfusion and ethanol (32,33).
Activated JNK phosphorylates c-Jun (a nuclear substrate) and also
modulates the transcription of apoptosis-related genes, including
Bcl-2 family members that form part of the non-nuclear JNK pathway
(32). JNK activation has been
demonstrated to be involved in isoflurane-induced neuronal
apoptosis (34). The present study
suggested that MS-275 pre-treatment deactivated the JNK nuclear
pathway by preventing isoflurane-induced increase of
phosphorylation of JNK. Additionally, MS-275 also inhibited the JNK
non-nuclear pathway by preventing the isoflurane-induced increase
of Bax and downregulation of Bcl-2 expression, thereby reversing
the isoflurane-induced increase of caspase-3. These results
demonstrated the critical role of MAPK signaling in the
neuroprotection of MS-275 against the effects of isoflurane.
In summary, the present study demonstrated that
pre-treatment with the HDACi MS-275 alleviated isoflurane-induced
neuronal apoptosis and cognitive decline in aged rats. The present
study may also provide insight for future research involving direct
association of epigenetic programming and other neuro-inflammatory
pathways, such as nuclear factor-κB signaling following isoflurane
exposure in aging animals.
Supplementary Material
Table SI. Primer sequences used for
reverse transcription-quantitative PCR.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81671948) and The
Guidance Fund of Renmin Hospital of Wuhan University (grant no.
RMYD2018M18).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LH, HBF, HHC and ZYX conceptualized and designed the
experiments. LH, HBF, HHC and SLM performed the experiments. YPC,
YL and QTM performed the statistical analysis and provided
technical assistance for the study. ZYX revised the manuscript
critically and approved the final version for submission. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All the experiments in the present study were
approved by the local ethical committee of Renmin Hospital of Wuhan
University and performed according to the guidelines presented in
the Declaration of the National Institutes of Health Guide for Care
and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang B, Tian M, Zhen Y, Yue Y, Sherman J,
Zheng H, Li S, Tanzi RE, Marcantonio ER and Xie Z: The effects of
isoflurane and desflurane on cognitive function in humans. Anesth
Analg. 114:410–415. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kong F, Chen S, Cheng Y, Ma L, Lu H, Zhang
H and Hu W: Minocycline attenuates cognitive impairment induced by
isoflurane anesthesia in aged rats. PLoS One.
8(e61385)2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lin D and Zuo Z: Isoflurane induces
hippocampal cell injury and cognitive impairments in adult rats.
Neuropharmacology. 61:1354–1359. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cao Y, Li Z, Ma L, Ni C, Li L, Yang N, Shi
C and Guo X: Isoflurane-induced postoperative cognitive dysfunction
is mediated by hypoxia-inducible factor-1α-dependent
neuroinflammation in aged rats. Mol Med Rep. 17:7730–7736.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang S, Hu X, Guan W, Luan L, Li B, Tang
Q and Fan H: Isoflurane anesthesia promotes cognitive impairment by
inducing expression of β-amyloid protein-related factors in the
hippocampus of aged rats. PLoS One. 12(e0175654)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang TY and Meaney MJ: Epigenetics and
the environmental regulation of the genome and its function. Annu
Rev Psychol. 61:439–466, C1-C3. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zovkic IB, Guzman-Karlsson MC and Sweatt
JD: Epigenetic regulation of memory formation and maintenance.
Learn Mem. 20:61–74. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim S and Kaang BK: Epigenetic regulation
and chromatin remodeling in learning and memory. Exp Mol Med.
49(e281)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Peleg S, Sananbenesi F, Zovoilis A,
Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL,
Gogol-Doering A, Opitz L, et al: Altered histone acetylation is
associated with age-dependent memory impairment in mice. Science.
328:753–756. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Peixoto L and Abel T: The role of histone
acetylation in memory formation and cognitive impairments.
Neuropsychopharmacology. 38:62–76. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
MacDonald JL and Roskams AJ: Epigenetic
regulation of nervous system development by DNA methylation and
histone deacetylation. Prog Neurobiol. 88:170–183. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sun J, Sun J, Ming GL and Song H:
Epigenetic regulation of neurogenesis in the adult mammalian brain.
Eur J Neurosci. 33:1087–1093. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hsieh J and Eisch AJ: Epigenetics,
hippocampal neurogenesis, and neuropsychiatric disorders:
Unraveling the genome to understand the mind. Neurobiol Dis.
39:73–84. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Simonini MV, Camargo LM, Dong E, Maloku E,
Veldic M, Costa E and Guidotti A: The benzamide MS-275 is a potent,
long-lasting brain region-selective inhibitor of histone
deacetylases. Proc Natl Acad Sci USA. 103:1587–1592.
2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mousa A and Bakhiet M: Role of cytokine
signaling during nervous system development. Int J Mol Sci.
14:13931–13957. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Thomas GM and Huganir RL: MAPK cascade
signalling and synaptic plasticity. Nat Rev Neurosci. 5:173–183.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Peng S, Zhang Y, Zhang J, Wang H and Ren
B: ERK in learning and memory: A review of recent research. Int J
Mol Sci. 11:222–232. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sharma SK and Carew TJ: The roles of MAPK
cascades in synaptic plasticity and memory in Aplysia: Facilitatory
effects and inhibitory constraints. Learn Mem. 11:373–378.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Wang WY, Yang R, Hu SF, Wang H, Ma ZW and
Lu Y: N-stearoyl-L-tyrosine ameliorates sevoflurane induced
neuroapoptosis via MEK/ERK1/2 MAPK signaling pathway in the
developing brain. Neurosci Lett. 541:167–172. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bi C, Cai Q, Shan Y, Yang F, Sun S, Wu X
and Liu H: Sevoflurane induces neurotoxicity in the developing rat
hippocampus by upregulating connexin 43 via the JNK/c-Jun/AP-1
pathway. Biomed Pharmacother. 108:1469–1476. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ji M, Dong L, Jia M, Liu W, Zhang M, Ju L
and Yang J, Xie Z and Yang J: Epigenetic enhancement of
brain-derived neurotrophic factor signaling pathway improves
cognitive impairments induced by isoflurane exposure in aged rats.
Mol Neurobiol. 50:937–944. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
AVMA Guidelines for the Euthanasia of
Animals: Edition. American Veterinary Medical Association, 2013.
https://www.avma.org/resources-tools/avma-policies/avma-guidelines-euthanasia-animals.
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Δ Δ C(T)) Method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Vorhees CV and Williams MT: Assessing
spatial learning and memory in rodents. ILAR J. 55:310–332.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chakrabarti A, Oehme I, Witt O, Oliveira
G, Sippl W, Romier C, Pierce RJ and Jung M: HDAC8: A multifaceted
target for therapeutic interventions. Trends Pharmacol Sci.
36:481–492. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guo X, Ruan H, Li X, Qin L, Tao Y, Qi X,
Gao J, Gan L, Duan S and Shen W: Subcellular Localization of Class
I Histone Deacetylases in the Developing Xenopus tectum.
Front Cell Neurosci. 9(510)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ma K, Qin L, Matas E, Duffney LJ, Liu A
and Yan Z: Histone deacetylase inhibitor MS-275 restores social and
synaptic function in a Shank3-deficient mouse model of autism.
Neuropsychopharmacology. 43:1779–1788. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sanders RD, Xu J, Shu Y, Januszewski A,
Halder S, Fidalgo A, Sun P, Hossain M, Ma D and Maze M:
Dexmedetomidine attenuates isoflurane-induced neurocognitive
impairment in neonatal rats. Anesthesiology. 110:1077–1085.
2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Covington HE III, Maze I, LaPlant QC,
Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ III,
Wu EY, et al: Antidepressant actions of histone deacetylase
inhibitors. J Neurosci. 29:11451–11460. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Choo QY, Ho PC, Tanaka Y and Lin HS: The
histone deacetylase inhibitors MS-275 and SAHA suppress the p38
mitogen-activated protein kinase signaling pathway and chemotaxis
in rheumatoid arthritic synovial fibroblastic E11 cells. Molecules.
18:14085–14095. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ferguson BS, Harrison BC, Jeong MY, Reid
BG, Wempe MF, Wagner FF, Holson EB and McKinsey TA:
Signal-dependent repression of DUSP5 by class I HDACs controls
nuclear ERK activity and cardiomyocyte hypertrophy. Proc Natl Acad
Sci USA. 110:9806–9811. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Guan QH, Pei DS, Zhang QG, Hao ZB, Xu TL
and Zhang GY: The neuroprotective action of SP600125, a new
inhibitor of JNK, on transient brain ischemia/reperfusion-induced
neuronal death in rat hippocampal CA1 via nuclear and non-nuclear
pathways. Brain Res. 1035:51–59. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kapfhamer D, King I, Zou ME, Lim JP,
Heberlein U and Wolf FW: JNK pathway activation is controlled by
Tao/TAOK3 to modulate ethanol sensitivity. PLoS One.
7(e50594)2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li Y, Wang F, Liu C, Zeng M, Han X, Luo T,
Jiang W, Xu J and Wang H: JNK pathway may be involved in
isoflurane-induced apoptosis in the hippocampi of neonatal rats.
Neurosci Lett. 545:17–22. 2013.PubMed/NCBI View Article : Google Scholar
|