Introduction
The thymus is relatively large at birth and is
important for fetal growth and immunity. Previous studies indicate
that thymus is a mediator of the associations between fetal
undernutrition and atopic and autoimmune disease in infancy and
adulthood (1,2). Therefore, quantitative analysis of the
thymus is important during fetal development.
Ultrasonography (US) is considered the first choice
for monitoring fetal growth. Measurement of fetal thymus
contributes to our understanding on the function of the immune
system in the fetus. However, in cases of maternal obesity or
oligohydramnios with an unfavorable plane of view, US is not the
best for visualizing the fetal thymus (3). In addition, the thymus is located at a
position behind the upper portion of the sternum, which cannot be
clearly displayed by US. According to previous studies (4-9),
the proper antenatal US visualization of thymus is dependent on
normal maternal body habitus and amniotic fluid volume. Currently,
three-dimensional (3D) US presenting more data in an accurate
manner has been used in clinical practice, but its extensive
application is hampered due to its disadvantages including motion
artifacts and fetal irritability. There are also disputes in the
validity of US, particularly the reproducibility and accuracy
(3).
Currently, fetal magnetic resonance imaging (MRI)
has been widely accepted as a complementary modality to US in
prenatal diagnosis (10,11) However, it is still a challenge to
identify abnormalities, especially in the middle trimester when the
fetus is comparatively small and pulse artifacts may present.
Furthermore, in the later stages of fetal development, the thymus
cannot be easily observed due to changes of fetal position and
overlapping of limbs (10). Thus
far, extensive studies have been carried out on the development of
the fetus using post-mortem MRI (12-14).
The major advantages of this method are excellent resolution and
stable image quality as it is not restricted in its scanning field
and sequences (15,16). In brief, the anatomical structure
and precise scalar dimensions of the fetal thymus can be clearly
displayed in the MRI.
The present study investigated the anatomical
microstructure, features and signals of the fetal thymus by 3.0T
FS-T2 weighted turbo spin echo (TSE) sequences. It provided imaging
evidence for the evaluation of early-stage development of fetal
thymus. In addition, the T2-weighted 3D sequences and the
three-dimensional processing may contribute to the establishment of
reference ranges for the fetal thymus.
Materials and methods
Specimen selection
The present study was carried out with approval of
the Ethical Committee of School of Medicine, Shandong University
(approval no. 2012033). Written informed consent was obtained from
the guardian(s) of each patient. Clinical information was collected
from maternal and neonatal medical records by one investigator
blinded to the prenatal findings.
A total of 78 fetal specimens, 16-39 weeks'
gestational age (GA), were collected from Shandong Provincial
Hospital. The specimens were obtained from medically indicated
abortions, spontaneous abortions, fetal deaths or stillbirths, as
well as premature deaths. The inclusion criteria were: i) maternal
pregnancy record demonstrated an absence of fetal chromosomal
abnormality, stressful uterine conditions, genetic disease or
diabetes; and, ii) US findings of the fetus during pregnancy and
the findings of the post-mortem MRI examination of the specimen
indicated no anatomical abnormality. The exclusion criteria were:
Multiple pregnancies, maternal pregnancy records showing a
documented fetal chromosomal abnormality, presence of stressful
intrauterine conditions, a family history of maternal genetic
disease or a history of inflammation, sepsis or stressor (16-19).
A total of 64 specimens met the inclusion criteria.
As the femur length is more accurate for the determination of the
GA than biparietal diameter, head circumference or foot length
(20), the femur length of the
fetuses was measured using MRI. The GA was obtained according to
the morphometric criteria proposed by Guihard-Costa et al
(14).
3.0T MR scanning
Post-mortem MRI was performed within 48 h of
mortality. Fetal specimens were imaged with T2-weighted 3D
sequences and FS-T2 weighted TSE sequences, using a Siemens Skyra
3.0T system (Siemens Healthineers). A head and neck joint coil was
used for the scanning of the thymus. For the T2-weighted 3D
sequences, the repetition time was 13.95 msec, echo time was 5.2
msec, the matrix was 512x512, voxel size was 0.4x0.4x0.4 mm and the
number of excitations was 2. For the FS-T2-weighted TSE sequences,
the repetition time was 6,160 msec, echo time was 60 msec, the
matrix was 640x446, the voxel size was 0.3x0.3x1.5 mm and the
number of excitations was 4. The scanning time was approximately 20
min. The field of view and the distance factor were adjusted
according to the circumference of the chest to produce a
signal-to-noise ratio of <1.0.
3D reconstruction
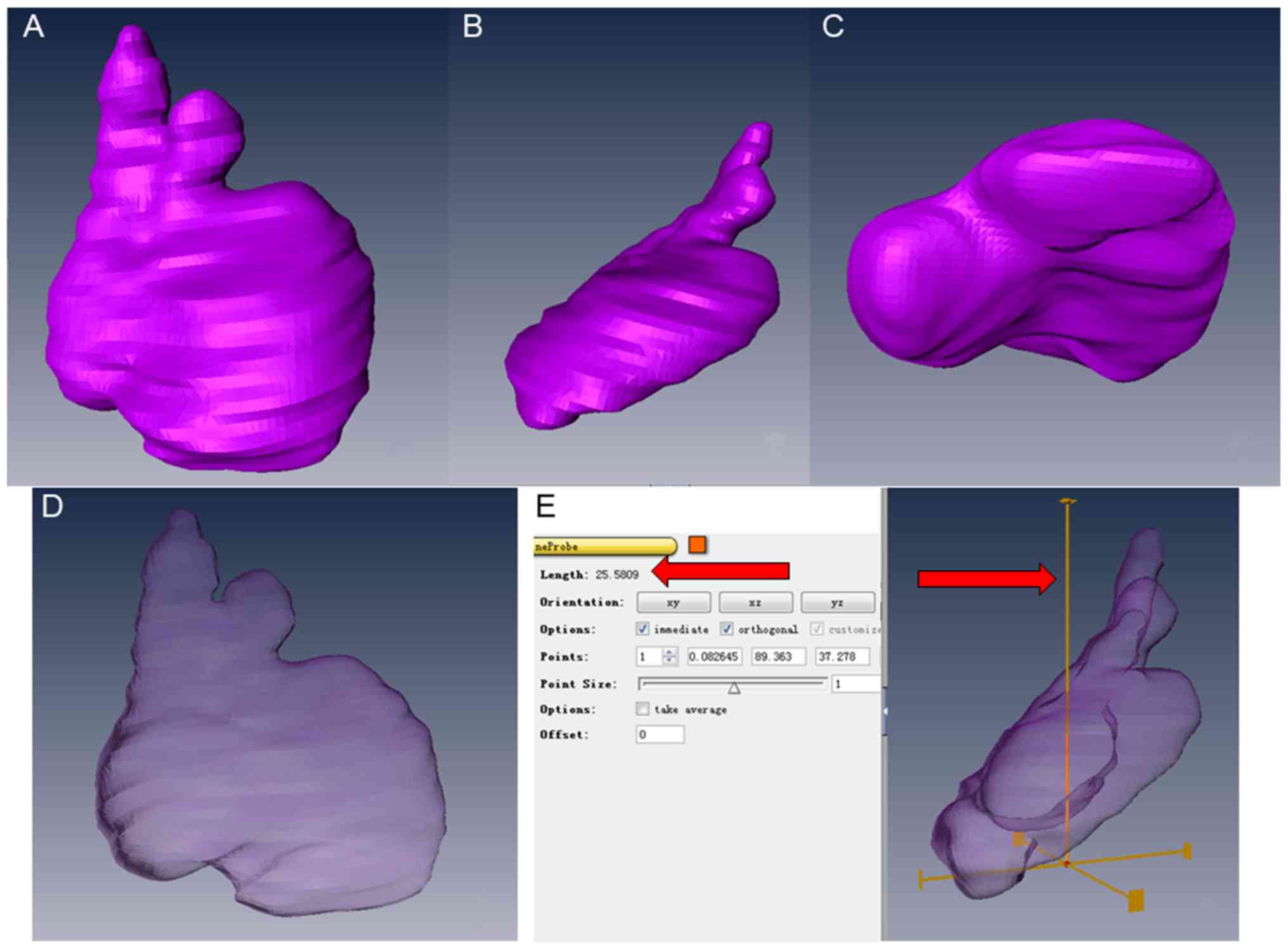
The 3D sequences of the thymus were measured using
Amira 5.4 software (Thermo Fisher Scientific, Inc.). Initially, the
T2-weighted 3D sequence images were aligned using Amira 5.4
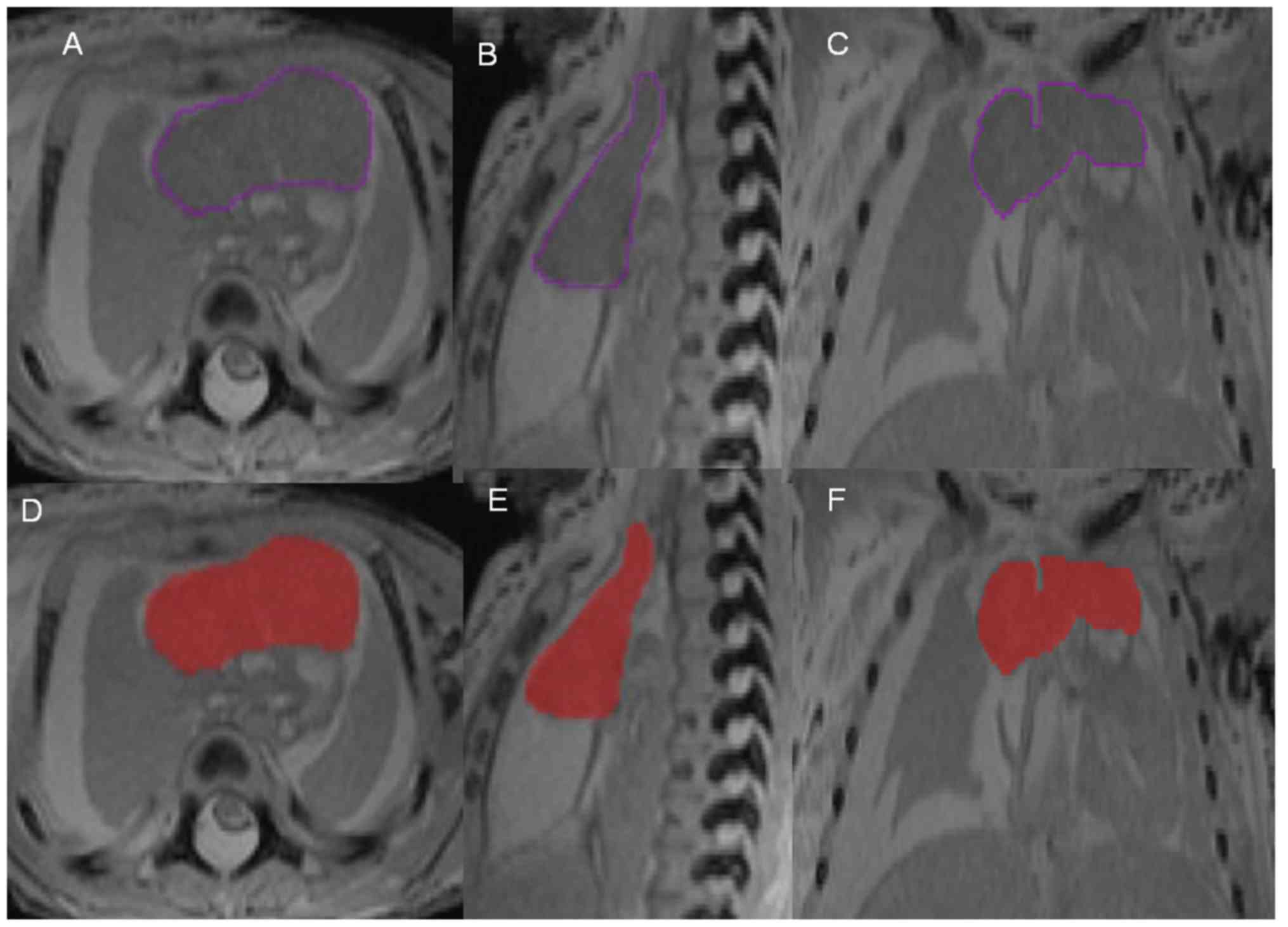
software. Subsequently, the silhouette of the fetal thymus was
artificial marked with purple lines on each image in the
transverse, sagittal and coronal planes (Fig. 1A-C). After tracing, the 3D software
system automatically filled the area with red color for correction
(Fig. 1D-F). Later, the 3D
visualization was built automatically. After the model had been
obtained, the anteroposterior diameter (TA), thymus width (TW) and
thymus height (TH) were measured. They were recorded as the longest
measurements of the three axes of the fetal thymus, respectively.
Finally, the surface area and volume of the thymus were obtained
automatically. All the results for thymus glands in the present
study were measured three times and the average was calculated. To
check the reproducibility of the manual segmentation, the thymus
was segmented manually twice simultaneously by two experienced
pediatric radiologists to obtain an average value. The time
interval between each round of manual segmentation was ≥1 week.
Determination of thymus height and
width and anteroposterior diameter
TH, defined as the maximum distance between the base
and apex of the thymus, was determined as was TW, defined as the
maximum distance of the thymus along the horizontal plane and TA,
defined as the longest distance perpendicular to the height and
width of the thymus. The thymus was manually segmented twice
simultaneously by two experienced anatomists to obtain an average
value to check the reproducibility of manual segmentation.
Statistical analysis
The Student's t-test was employed, with GA as the
confounding variable, to analyze the effect of gender on different
measurements of the fetal thymus. The relationship between each
measurement and GA was evaluated using regression analysis. The
linear regression model was used and significant correlations were
defined between data in presence of R2 values of
>0.6. Statistical analyses were performed using SPSS 17.0
software (SPSS Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Location and morphology of the fetal
thymus
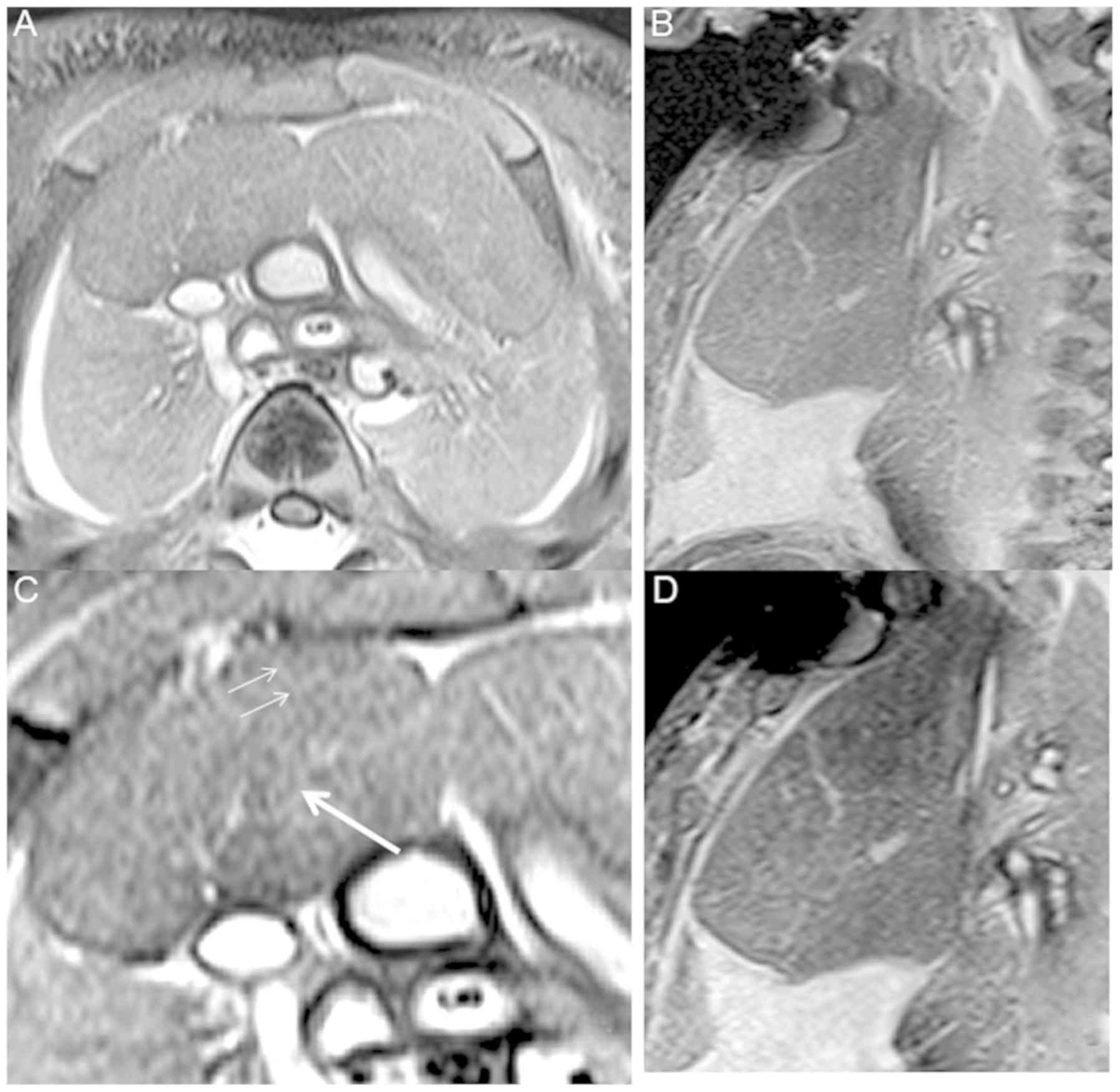
The thymus at 16 weeks' GA was clearly demonstrated.
In the images of the FS-T2 TSE sequences, the thymus appeared
heterogeneous with moderate signal intensity surrounded by many
portions of thymus cortex with lower signal intensity (Fig. 2). The thymus was visualized more
clearly in FS-T2 weighted MRI compared with other sequences. It is
the major anterior component of the superior mediastinum lying
immediately posterior to the manubrium of the sternum. The upper
limit of the thymus reached the thyroid gland. The lower portion
typically extended into the anterior mediastinum over the
pericardial sac. The majority of the contour of axial images was
roughly square, in which an asymmetric ‘X’ configuration was
evident in the coronal image. A signal of low intensity was
observed at the edge of the thymus, while a high intensity signal
was noted between the thymus and the lung. The interlobular septum
was clearly shown, which was full of liquid and displayed a linear
high signal. After 3D reconstruction, the shape of the thymus was
successfully obtained (Fig. 3).
TA, TW, TH, TS and volume (TV) of the
thymus
The original measurements including TA, TW, TH, TS
and TV of the thymus at a 16-39 week GA are given in Table I.
 | Table IThe original measurements of the fetal
thymus (n=64). |
Table I
The original measurements of the fetal
thymus (n=64).
| GA | TA | TW | TH | TS | TV | GA | TA | TW | TH | TS | TV |
|---|
| 16 | 0.49 | 0.85 | 0.79 | 1.39 | 0.11 | 25 | 1.68 | 2.45 | 2.49 | 15.48 | 3.10 |
| 16 | 0.44 | 0.64 | 0.51 | 0.83 | 0.06 | 26 | 2.11 | 3.28 | 2.40 | 17.68 | 3.24 |
| 17 | 0.72 | 0.72 | 1.01 | 2.80 | 0.16 | 26 | 2.23 | 3.64 | 2.77 | 23.32 | 3.59 |
| 17 | 0.39 | 0.90 | 0.67 | 0.94 | 0.05 | 27 | 1.71 | 1.81 | 2.25 | 10.56 | 1.70 |
| 19 | 0.58 | 1.03 | 1.28 | 2.97 | 0.28 | 27 | 1.68 | 3.34 | 3.00 | 20.96 | 3.13 |
| 19 | 0.77 | 1.06 | 1.56 | 3.30 | 0.34 | 28 | 2.14 | 3.61 | 2.92 | 21.06 | 4.22 |
| 20 | 0.56 | 1.41 | 1.40 | 3.13 | 0.21 | 28 | 1.50 | 2.20 | 2.59 | 13.21 | 3.06 |
| 20 | 0.84 | 1.69 | 1.06 | 3.80 | 0.35 | 28 | 1.97 | 2.26 | 2.56 | 12.12 | 2.08 |
| 20 | 0.76 | 2.18 | 1.55 | 5.45 | 0.51 | 28 | 1.40 | 2.15 | 0.99 | 6.07 | 0.64 |
| 20 | 0.68 | 1.19 | 1.09 | 2.28 | 0.21 | 28 | 2.37 | 3.39 | 2.48 | 19.74 | 4.32 |
| 21 | 1.40 | 1.76 | 1.00 | 4.88 | 0.48 | 28 | 1.65 | 2.82 | 3.10 | 15.29 | 2.57 |
| 21 | 1.10 | 1.21 | 1.74 | 4.85 | 0.56 | 28 | 1.86 | 2.52 | 3.04 | 16.65 | 2.69 |
| 22 | 0.87 | 1.82 | 1.82 | 7.80 | 1.05 | 29 | 1.78 | 1.36 | 2.12 | 7.75 | 1.29 |
| 22 | 0.72 | 2.28 | 0.75 | 7.81 | 0.92 | 29 | 2.63 | 4.14 | 3.02 | 32.00 | 6.60 |
| 22 | 1.14 | 1.69 | 1.59 | 4.43 | 0.34 | 29 | 2.20 | 2.05 | 2.43 | 14.13 | 2.29 |
| 22 | 1.12 | 1.58 | 2.17 | 6.37 | 0.62 | 30 | 2.18 | 3.37 | 3.37 | 31.60 | 8.43 |
| 22 | 1.00 | 2.14 | 2.28 | 7.69 | 1.04 | 30 | 2.25 | 3.95 | 3.53 | 33.38 | 6.57 |
| 22 | 1.10 | 2.03 | 1.63 | 7.55 | 1.25 | 30 | 1.56 | 2.11 | 2.65 | 9.36 | 0.92 |
| 23 | 1.19 | 1.66 | 1.50 | 5.02 | 0.60 | 31 | 2.10 | 3.31 | 2.47 | 18.50 | 3.88 |
| 23 | 1.41 | 1.95 | 1.82 | 7.07 | 0.85 | 32 | 2.13 | 3.75 | 2.50 | 27.51 | 4.35 |
| 23 | 1.18 | 1.97 | 1.56 | 6.35 | 0.56 | 34 | 2.12 | 2.38 | 2.70 | 14.03 | 2.37 |
| 24 | 1.02 | 2.12 | 1.85 | 8.20 | 0.82 | 34 | 2.11 | 2.88 | 3.74 | 22.46 | 5.83 |
| 24 | 1.54 | 2.43 | 1.83 | 9.98 | 1.08 | 34 | 2.71 | 5.07 | 4.02 | 39.88 | 9.06 |
| 24 | 1.76 | 3.12 | 1.88 | 12.18 | 1.80 | 35 | 2.87 | 4.62 | 2.79 | 36.40 | 9.90 |
| 24 | 1.88 | 2.46 | 2.33 | 12.86 | 1.94 | 35 | 3.27 | 3.04 | 2.34 | 22.17 | 4.23 |
| 24 | 1.66 | 2.42 | 2.60 | 11.30 | 1.61 | 36 | 2.52 | 3.88 | 3.76 | 26.70 | 4.80 |
| 24 | 1.19 | 1.54 | 2.15 | 6.67 | 0.93 | 36 | 2.91 | 4.93 | 4.62 | 45.31 | 11.90 |
| 24 | 0.67 | 1.44 | 1.64 | 3.63 | 0.31 | 38 | 2.74 | 4.14 | 3.79 | 37.14 | 12.21 |
| 25 | 1.42 | 2.36 | 2.14 | 9.16 | 1.08 | 38 | 2.67 | 3.06 | 3.55 | 31.77 | 8.37 |
| 25 | 1.45 | 2.47 | 2.40 | 11.23 | 1.69 | 38 | 3.25 | 4.58 | 4.52 | 52.07 | 12.04 |
| 25 | 1.01 | 1.56 | 2.39 | 7.45 | 0.94 | 39 | 3.23 | 4.32 | 3.70 | 48.55 | 12.80 |
| 25 | 2.00 | 3.32 | 2.86 | 17.29 | 2.93 | 39 | 3.83 | 4.48 | 3.96 | 42.08 | 12.79 |
There were 33 male fetuses. The TA, TW, TH, TS and
TV were 1.69±0.85 cm, 2.67±1.21 cm, 2.41±1.01 cm, 17.04±13.83
cm2 and 3.31±3.52 cm3. There were 31 female
fetuses. The TA, TW, TH, TS and TV were 1.66±0.77 cm, 2.37±0.99 cm,
2.24±0.91 cm, 13.84±11.79 cm2 and 2.94±3.69
cm3. No statistical differences were observed between
the TA, TW, TH, TS and TV in the male and female fetuses
(P>0.05; Table II).
 | Table IIThe measurements of thymus between
sex groups. |
Table II
The measurements of thymus between
sex groups.
| Groups | N | TA | TW | TH | TS | TV |
|---|
| Male | 33 | 1.69±0.85 | 2.67±1.21 | 2.41±1.01 | 17.04±13.83 | 3.31±3.52 |
| Female | 31 | 1.66±0.77 | 2.37±0.99 | 2.24±0.91 | 13.84±11.79 | 2.94±3.69 |
| t-value | | 0.164 | 1.068 | 0.742 | 0.994 | 0.413 |
| P-value | | 0.121 | 0.079 | 0.642 | 0.132 | 0.799 |
The linear equations of the data and GA were as
follows: TA=0.123xGA-1.574; TW=0.152xGA-1.515; TH=0.139xGA-1.353;
TS=1.871xGA-34.145; and TV=0.508xGA-10.342.
The slopes of the lines (Fig. 4) differed for different
measurements. That for TS was the steepest (1.871), followed by
that for TV (0.508), TW (0.152), TH (0.139) and TA (0.123). From
the slope of the lines, it could be concluded that the measurements
were developing at a different speed. The increase of thymus
surface area and volume was the fastest, followed by its width. The
TA increase was the slowest. However, linear regression analysis
indicated the strongest correlation between TA and GA
(R2=0.844). Additionally, GA was correlated with TS
(R2=0.764), TH (R2=0.753), TV
(R2=0.729) and TW (R2=0.675), respectively
(Fig. 4).
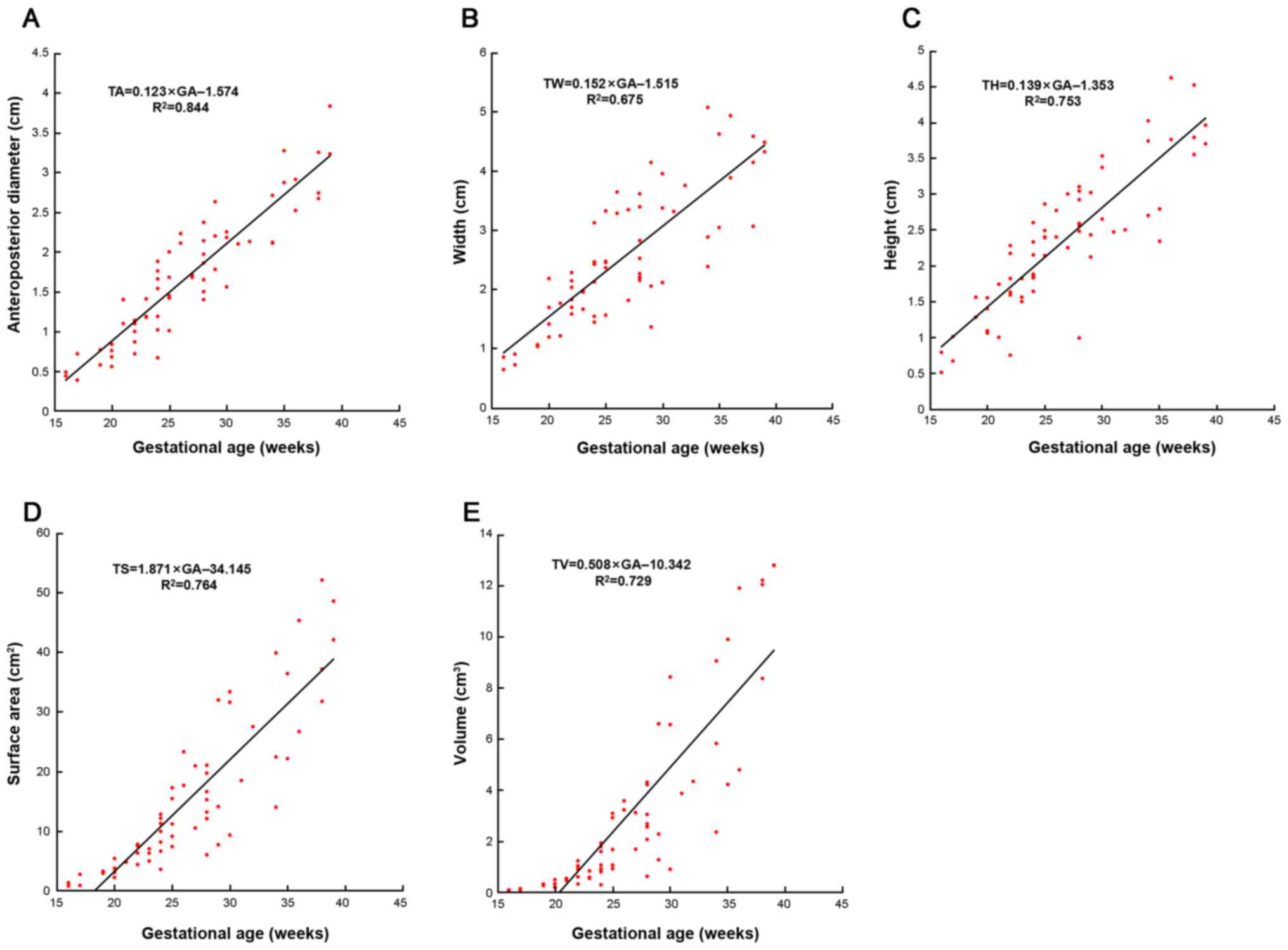 | Figure 4The statistical results between all
the measurements and GA. The scattergrams, best-fit equations and
correlation coefficients (R2) of (A) TA, (B) TW, (C) TH, (D) TS,
(E) TV and GA. All the measurements linearly increase with GA. Each
symbol represents a single fetus. GA, gestational age; TA,
anteroposterior diameter; TW, thymus width; TH, thymus height; TS,
thymus. |
Discussion
The majority of the imaging studies on the fetal
thymus have been focused on the prenatal ultrasonography, however,
the structures of the intrauterine thymus could not be clearly
displayed by US (3,4,6,8,9).
In a previous study, Leon-Luis et al (10) determined that the trans diameter and
circumference of the fetal thymus (at 21-34 weeks GA) demonstrated
no statistical differences among 17 cases using prenatal US and
MRI. This study first reported the feasibility of MRI for the
monitoring of fetal thymus. Nevertheless, the selected sequences
could not effectively present the microanatomical structures of the
thymus and the sample size was relatively small. Damodaram et
al (21) utilized the T2
weighted single shot TSE sequence to compare the organ size and
total fetal volume of the fetus (n=20) with intrauterine growth
retardation and those (n=19) with normal development. This study
revealed that turbo MRI contributed to the prenatal monitoring, but
it lacked of real volume data for each organ. In addition, the
microanatomical structures and normal ranges of the thymus were not
mentioned.
Compared with in vivo diagnosis, post-mortem
MRI (PMMRI) seems to be more reliable as it is free from the
influence of maternal organs, pulse of arteries and movements of
the fetus. Kang et al (11)
proposed that 3T PMMRI was superior to post-mortem US for all
anatomical regions except for the spine, particularly for fetuses
of GA ≥20 weeks and the brain in fetuses <20 weeks. PMMRI
remains the first line imaging investigation for perinatal autopsy.
In the present study, the FS-T2 weighted TSE sequences could
clearly display the morphological features and internal signals of
the thymus. As previously described, medullary development was
observed from a GA of 8 weeks and distinct cortical and medullary
compartments by 16 weeks (22,23).
Histology of the thymus has shown that the periphery of the lobuli
thymi is the cortex and the deep part is the medulla (24). However, the medulla is not
completely surrounded by the cortex (25). Previous studies have shown abundant
T cell progenitor cells in the cortex of the thymus (8,17). By
contrast, a small population of T cell progenitor cells were
noticed in the medulla. In addition, other cells types were noticed
in the medulla, such as thymic epithelial cells, neural
crest-derived mesenchymal cells, endothelial cells and dendritic
cells (11). This may help to
explain why the cortex and medulla can be clearly distinguished on
MRI.
It has been previously indicated that thymic size is
influenced by a series of hormones, including sex steroids and
those involve in the hypothalamic-pituitary-adrenal axes (26). It has been revealed that sex
hormones receptors are expressed in both thymocytes and TECs
(26,27). Castration of male rodents results in
significant enlargement of thymus (27,28).
Nevertheless, the data from the present study demonstrated that
there were no statistical differences between the gender and thymic
size, which was in line with a previous study (6) in which sonography was used to measure
the transverse diameter and perimeter of 59 normal thymuses and
demonstrated no effects of fetal gender on the thymus.
In prenatal MR imaging, in addition to morphological
subjective evaluation of structures, quantitative information may
be important in the diagnosis of anomalies. Graham et al
(13) suggested that the thymus was
morphologically complete at 16-20 gestational weeks upon
maturation. Li et al (29)
proposed that the volume of the thymus was more appropriate to
evaluate the size of the thymus. Cho et al (30) reported that it is difficult to
confirm the size and arrangement of the large blood vessels by
analyzing the anteroposterior diameter. Unlike previous studies
(29,30), the present study demonstrated that
the anteroposterior diameter of the thymus was more closely
associated with GA than other factors (R2=0.844).
Additionally, the majority of the measured values were lower than
the previous data obtained from 3D US (29). It is hypothesized that the data from
the present study can provide more accurate information about the
thymus because they are based on the high resolution of post-mortem
3.0T MRI.
The growth curve generated in the present study can
be considered as a model for clinical applications. Some studies
have indicated that 2D data obtained from US are less efficient
than 3D data in assessing the morphometry of the thymus (14,20,31).
For this reason, 2D technique could only reflect the size of the
thymus in a single dimension or plane, but 3D volume measurement is
more objective and reliable in reflecting the thymic size. The
present study, based on MRI, demonstrated that parameters measured
in 2D were as closely correlated with GA as that of 3D data.
The present study reported a new and reliable method
for the study of fetal thymus development and maturation combining
the image of thymus obtained by post-mortem 3.0T MRI scanning with
powerful 3D software. More intuitive and accurate morphological
data of thymus were obtained. The 3D reconstruction analysis is
superior to traditional pathological analysis in the following
aspects: First, 3D reconstruction analysis displayed the fetal
thymus surface and inner structures without excising the thymus,
which precluded the possibility of deformation by gravity during
excision. Second, arbitrary slices can be obtained by
postprocessing on ordinary computers. Third, accurate measurements
of each component can be easily and precisely obtained with the
thymus in its natural state. Therefore, Amira 5.4 software may be
useful for the research of irregular organs. It is hoped that it
will be applied to the monitoring of fetal thymus in uterus in the
future.
There are some limitations to the present study.
First, the range of GA was large, but the sample size of 64 fetuses
was small. To be exact, only two specimens at a GA of 31 weeks
(n=1) and 32 weeks (n=1) were included. No specimens at a GA of 18,
33 and 37 weeks were included, which may lead to bias to the
conclusion of the present study. Second, an undetected anomaly may
present in the selected specimens.
The present study is the first to use PMMRI and
Amira 5.4 software to measure the normal fetal thymus. PMMRI may
play a vital role in providing more anatomical detail than US and
in vivo fetal MRI. The results of the present study may
serve as a valuable reference for fetal MRI applied in vivo
in prenatal diagnosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 31371213) and the
National Natural Science Foundation of Shandong Province (grant no.
ZR2013HM091).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY and XL conceived and designed the experiments.
LY, JC, ZW, JD and HX performed the experiments. LY, LZ and XW
analyzed the data. LY and LZ contributed
reagents/materials/analysis tools. LY and XL wrote the manuscript.
YW and JC performed the collection and storage of fetuses. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was carried out with approval of
the Ethical Committee of School of Medicine, Shandong University
(approval no. 2012033). Written informed consent was obtained from
the guardian(s) of each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferguson AC: Prolonged impairment of
cellular immunity in children with intrauterine growth retardation.
J Pediatr. 93:52–56. 1978.PubMed/NCBI View Article : Google Scholar
|
|
2
|
McDade TW, Beck MA, Kuzawa CW and Adair
LS: Prenatal undernutrition and postnatal growth are associated
with adolescent thymic function. J Nutr. 131:1225–1231.
2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Felker RE, Cartier MS, Emerson DS and
Brown DL: Ultrasound of the fetal thymus. J Ultrasound Med.
8:669–673. 1989.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gamez F, De Leon-Luis J, Pintado P, Perez
R, Robinson JN, Antolin E, Ortiz-Quintana L and Santolaya-Forgas J:
Fetal thymus size in uncomplicated twin and singleton pregnancies.
Ultrasound Obstet Gynecol. 36:302–307. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Lamouroux A, Mousty E, Prodhomm O, Bigi N,
Le Gac MP, Letouzey V, De Tayrac R and Mares P: Absent or
hypoplastic thymus: A marker for 22q11.2 microdeletion syndrome in
case of polyhydramnios. J Gynecol Obstet Biol Reprod (Paris).
45:388–396. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
De Leon-Luis J, Gamez F, Pintado P,
Antolin E, Pérez R, Ortiz-Quintana L and Santolaya-Forgas J:
Sonographic measurements of the thymus in male and female fetuses.
J Ultrasound Med. 28:43–48. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Warncke K, Lickert R, Eitel S, Gloning KP,
Bonifacio E, Sedlmeier EM, Becker P, Knoop J, Beyerlein A and
Ziegler AG: Thymus growth and fetal immune responses in diabetic
pregnancies. Horm Metab Res. 49:892–898. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yinon Y, Zalel Y, Weisz B, Mazaki-Tovi S,
Sivan E, Schiff E and Achiron R: Fetal thymus size as a predictor
of chorioamnionitis in women with preterm premature rupture of
membranes. Ultrasound Obstet Gynecol. 29:639–643. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Zalel Y, Gamzu R, Mashiach S and Achiron
R: The development of the fetal thymus: An in utero sonographic
evaluation. Prenat Diagn. 22:114–117. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
De Leon-Luis J, Ruiz Y, Gamez F, Pintado
P, Oyelese Y, Pereda A, Ortiz-Quintana L and Santolaya-Forgas J:
Comparison of measurements of the transverse diameter and perimeter
of the fetal thymus obtained by magnetic resonance and ultrasound
imaging. J Magn Reson Imaging. 33:1100–1105. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kang X, Shelmerdine SC, Hurtado I,
Bevilacqua E, Hutchinson C, Mandalia U, Segers V, Cos Sanchez T,
Cannie MM, Carlin A, et al: Postmortem fetal imaging: A prospective
blinded comparison study of 2-dimensional ultrasound with MR
imaging. Ultrasound Obstet Gynecol. 53:229–238. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gordon J and Manley NR: Mechanisms of
thymus organogenesis and morphogenesis. Development. 138:3865–3878.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Graham A, Okabe M and Quinlan R: The role
of the endoderm in the development and evolution of the pharyngeal
arches. J Anat. 207:479–487. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guihard-Costa AM, Menez F and Delezoide
AL: Organ weights in human fetuses after formalin fixation:
Standards by gestational age and body weight. Pediatr Dev Pathol.
5:559–578. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu F, Zhang Z, Lin X, Teng G, Meng H, Yu
T, Fang F, Zang F, Li Z and Liu S: Development of the human fetal
cerebellum in the second trimester: A post mortem magnetic
resonance imaging evaluation. J Anat. 219:582–588. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang Z, Liu S, Lin X, Teng G, Yu T, Fang
F and Zang F: Development of fetal brain of 20 weeks gestational
age: Assessment with post-mortem magnetic resonance imaging. Eur J
Radiol. 80:e432–e439. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Di Naro E, Cromi A, Ghezzi F, Raio L,
Uccella S, D'Addario V and Loverro G: Fetal thymic involution: A
sonographic marker of the fetal inflammatory response syndrome. Am
J Obstet Gynecol. 194:153–159. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Meng H, Zhang Z, Geng H, Lin X, Feng L,
Teng G, Fang F, Zang F and Liu S: Development of the subcortical
brain structures in the second trimester: Assessment with 7.0-T
MRI. Neuroradiology. 54:1153–1159. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Z, Hou Z, Lin X, Teng G, Meng H,
Zang F, Fang F and Liu S: Development of the fetal cerebral cortex
in the second trimester: Assessment with 7T postmortem MR imaging.
AJNR Am J Neuroradiol. 34:1462–1467. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jelev L and Surchev L: Radial artery
coursing behind the biceps brachii tendon: Significance for the
transradial catheterization and a clinically oriented
classification of the radial artery variations. Cardiovasc
Intervent Radiol. 31:1008–1012. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Damodaram MS, Story L, Eixarch E, Patkee
P, Patel A, Kumar S and Rutherford M: Foetal volumetry using
magnetic resonance imaging in intrauterine growth restriction.
Early Hum Dev. 88 (Suppl 1):S35–S40. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chung L, Maestas DR Jr, Housseau F and
Elisseeff JH: Key players in the immune response to biomaterial
scaffolds for regenerative medicine. Adv Drug Deliv Rev.
114:184–192. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gordon J, Wilson VA, Blair NF, Sheridan J,
Farley A, Wilson L, Manley NR and Blackburn CC: Functional evidence
for a single endodermal origin for the thymic epithelium. Nat
Immunol. 5:546–553. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Farley AM, Morris LX, Vroegindeweij E,
Depreter ML, Vaidya H, Stenhouse FH, Tomlinson SR, Anderson RA,
Cupedo T, Cornelissen JJ and Blackburn CC: Dynamics of thymus
organogenesis and colonization in early human development.
Development. 140:2015–2026. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Von Gaudecker B: Functional histology of
the human thymus. Anat Embryol (Berf). 183:1–15. 1991.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hince M, Sakkal S, Vlahos K, Dudakov J,
Boyd R and Chidgey A: The role of sex steroids and gonadectomy in
the control of thymic involution. Cell Immunol. 252:122–138.
2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Olsen NJ, Olson G, Viselli SM, Gu X and
Kovacs WJ: Androgen receptors in thymic epithelium modulate thymus
size and thymocyte development. Endocrinology. 142:1278–1283.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Williams KM, Lucas PJ, Bare CV, Wang J,
Chu YW, Tayler E, Kapoor V and Gress RE: CCL25 increases
thymopoiesis after androgen withdrawal. Blood. 112:3255–3263.
2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li L, Bahtiyar MO, Buhimschi CS, Zou L,
Zhou QC and Copel JA: Assessment of the fetal thymus by two- and
three-dimensional ultrasound during normal human gestation and in
fetuses with congenital heart defects. Ultrasound Obstet Gynecol.
37:404–409. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Cho JY, Min JY, Lee YH, McCrindle B,
Hornberger LK and Yoo SJ: Diameter of the normal fetal thymus on
ultrasound. Ultrasound Obstet Gynecol. 29:634–638. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
El-Haieg DO, Zidan AA and El-Nemr MM: The
relationship between sonographic fetal thymus size and the
components of the systemic fetal inflammatory response syndrome in
women with preterm prelabour rupture of membranes. BJOG.
115:836–841. 2008.PubMed/NCBI View Article : Google Scholar
|