Introduction
The apnea of premature infants is an interruption of
breathing for >15 sec and is accompanied by hypoxia or
bradycardia, which is a risk factor for the damage to a developing
brain (1,2). Although the apnea usually resolves by
the time the infant reaches 36-37 weeks of age (3), the incidence of the disease is higher
in the neonates born at 30-31 weeks (4). Within clinical practice, apnea is
classified into three types: Central, obstructive and mixed, and
the mixed type accounts for 50% of apnea events (5). The pathogenesis of the apnea of
prematurity is unclear. However, previous studies have indicated
the impairment of neuronal development and the inability to control
breathing in preterm infants (6,7).
The management of the apnea of prematurity involves
a combination of two major therapies, a pharmacological treatment
and the supply of O2, which is necessary for normal body
function (1,3,6).
Although caffeine citrate and aminophylline have been the primary
treatments of infant apnea within clinical practice (8), a comparison of the efficacy and safety
of both drugs in the treatment of apnea still remains to be
performed, particularly for those who underwent different
strategies of O2 supply. It has been reported that
continuous positive airway pressure and nasal intermittent positive
pressure ventilation are safe and effective in improving the
respiratory function and decreasing the bradycardia (9,10).
Despite the widespread use of mechanical ventilation to treat
hypoxia (1), there is considerable
uncertainty regarding the performance of these two drugs in the
treatment of apnea in the infants receiving different strategies of
O2 delivery.
The current study was performed to investigate the
efficacy and safety of caffeine and aminophylline in the treatment
of the apnea of premature infants under different conditions of
O2 delivery. The results of the present study indicated
that caffeine is more potent than aminophylline in improving the
efficacy of the O2 therapy and reducing the incidence of
complications.
Materials and methods
Premature neonates and inclusion
criteria
A cohort of 120 premature infants with apnea, who
received caffeine or aminophylline therapy from January to December
2017 at the First People's Hospital of Zhengzhou City, were
retrospectively included in the present study. The treatment with
either caffeine or aminophylline was at the discretion of the
physician. These hospitalized infants included 63 males and 57
females with a birth weight between 500 and 1,250 g. The
demographic characteristics of the infants are presented in
Tables I-III. The present study was approved by the Ethics
Committee of the First People's Hospital of Zhengzhou City
(Zhengzhou, China; approval no. 2017-16).
The infants selected for the current retrospective
study fulfilled the following criteria: i) All infants born after
<34 weeks of gestation were diagnosed with apnea (the mixed type
accounted for ~75% of all the types of apnea); ii) a stay of ≥24 h
in hospital occurred; iii) the apnea of prematurity was solely
treated with either caffeine or aminophylline under varying
conditions of oxygen (O2) delivery; iv) there were no
contraindications to either invasive or noninvasive ventilation;
and v) there were no complex congenital malformations occurring in
airways, chromosomal abnormalities or inherited metabolic
diseases.
Drug treatment and supplemental
O2 delivery
The doses of caffeine (Shanghai Yuduo Biotechnology
Co., Ltd.) and aminophylline (Shanghai Yuduo Biotechnology Co.,
Ltd.) used to treat the apnea were selected according to previous
studies (11,12). In the present study, 77 infants with
apnea received an intravenous (IV) injection of caffeine at a first
dose of 20 mg/kg followed by a maintenance dose of 10 mg/kg per day
after birth until the 34th week from the gestation of mother. The
other 43 infants were treated by IV administration of aminophylline
at a first dose of 5 mg/kg followed by a maintenance dose of 2.5
mg/kg twice per day after birth until the 34th week from the
gestation of mother.
The efficacy and safety of the drugs applied for the
treatment of apnea were analyzed with regard to the different types
of mechanical ventilation and O2 delivery devices, which
were selectively used according to the O2 requirements
of the infants, the reliability of the O2 supply, the
convenience of the therapeutic application and the patients'
consent. The overall goal of O2 administration is to
maintain an adequate tissue oxygenation while minimizing the
cardiopulmonary load. The infants with apnea started to receive
O2 therapy prior to pharmacotherapy. The various forms
of supplemental O2 delivery included: i) Invasive
mechanical ventilation via an endotracheal tube to provide
continuous positive airway pressure (CPAP); ii) non-invasive
mechanical ventilation via a nasal mask to provide nasal
intermittent positive pressure ventilation (NIPPV) or nasal CPAP
(NCPAP); and iii) A hood mask or a nasal cannula used to deliver
O2 directly into the nostrils of the infant.
Ventilator settings
The tidal volume (4-6 ml/kg) and respiratory rate
(30-40 times/min) were used to calculate the minute ventilation.
The sensitivity setting was used to adjust the level of negative
pressure required to trigger the SLE5000 infant ventilator (SLE
Ltd.). The peak inspiratory (PIP) and end-expiratory pressure
settings for all premature infants were adjusted to 16-28 and 5-6
cmH2O, respectively, according to previous reports
(13,14). The pressure settings were adjusted
according to the result of the arterial blood gas analysis
performed by i-STAT®1 analyzer (LumiraDx, Ltd.), which
was routinely checked at 15-30-min intervals until the
O2 saturation (>95%) and the acid-base balance (pH
7.35-7.45) was achieved in a normal range. The levels of the peak
inspiratory and expiratory pressures were initially designed to be
slightly <16 and 5 cmH2O in the mode of NIPPV or
NCPAP, respectively (15).
Criteria for drug treatment and
withdrawal
The indications for caffeine and aminophylline
treatment included: i) Appearance of apnea in premature infants or
infants remaining at high risk of apnea; and ii) Independent of the
types of the mechanical ventilation that would be used, the drug
therapy would start within 24 h of disconnecting the
ventilator.
The indications for drug withdrawal were the
following: i) Infants being free of apnea for at least 7 days, or
whom the gestational age reached 34 weeks; and ii) no mechanical
ventilation required for 7 days; and iii) serious adverse effects
arising from the combined use of the drugs and the mechanical
ventilation, such as the slow development of the nerve system,
brain and other organs.
Criteria for the efficacy of caffeine
and aminophylline in the treatment of apnea
The efficacy of caffeine and aminophylline in the
treatment of infants with apnea was assessed according to the
frequency of recurrent apneic episodes and of O2
delivery devices replaced by an invasive ventilation, the
alterations in the duration and concentration of inhaled
O2. The efficacy was evaluated according to the
following: i) Ease of apnea within 48 h after administering the
drugs; ii) apnea episodes of <2 times/day associated with a
normal breathing rhythm; iii) no alteration in the frequency of
apnea episodes within 48 h after administering the drugs; iv)
recurrence of apnea (a time interval of ≥3 days between the first
and second apnea episode).
Complications of caffeine- and
aminophylline-treated preterm infants
The complications were diagnosed according to the
outcomes of clinical and laboratory examinations, including chest
or abdominal radiography, echocardiogram, growth of abnormal blood
vessels in the area of retina and brain ultrasound (16-20).
The complications presented in the current study primarily included
patent ductus arteriosus (PDA), bronchopulmonary dysplasia (BPD),
necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP)
and intraventricular hemorrhage (IVH). The incidence of these
complications was calculated as a percentage of the population
proportion in the caffeine- and aminophylline-treated infants,
respectively.
Statistical analysis
The results are presented as the mean ± standard
deviation or a percentage (%) of the population, as appropriate.
The statistical analysis was performed using SPSS software version
17.0 (SPSS, Inc). The comparisons between the variables of the
infants treated with caffeine and aminophylline were made using the
unpaired Student's t-test. The χ2 test was used to
analyze the differences between the population proportions observed
in these two treatment approaches. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of drugs on preterm infants
receiving invasive mechanical ventilation
Certain premature infants (n=40) with apnea required
a therapeutic intervention of invasive mechanical ventilation
according to the severity of their breathing problem. The effects
of caffeine and aminophylline on the ventilated infants were
examined according to the frequency of recurrent apneic episodes
and of the invasive ventilation required additionally after
disconnecting the ventilator, the duration of O2
inhalation and the changes in PIP. The results are presented in
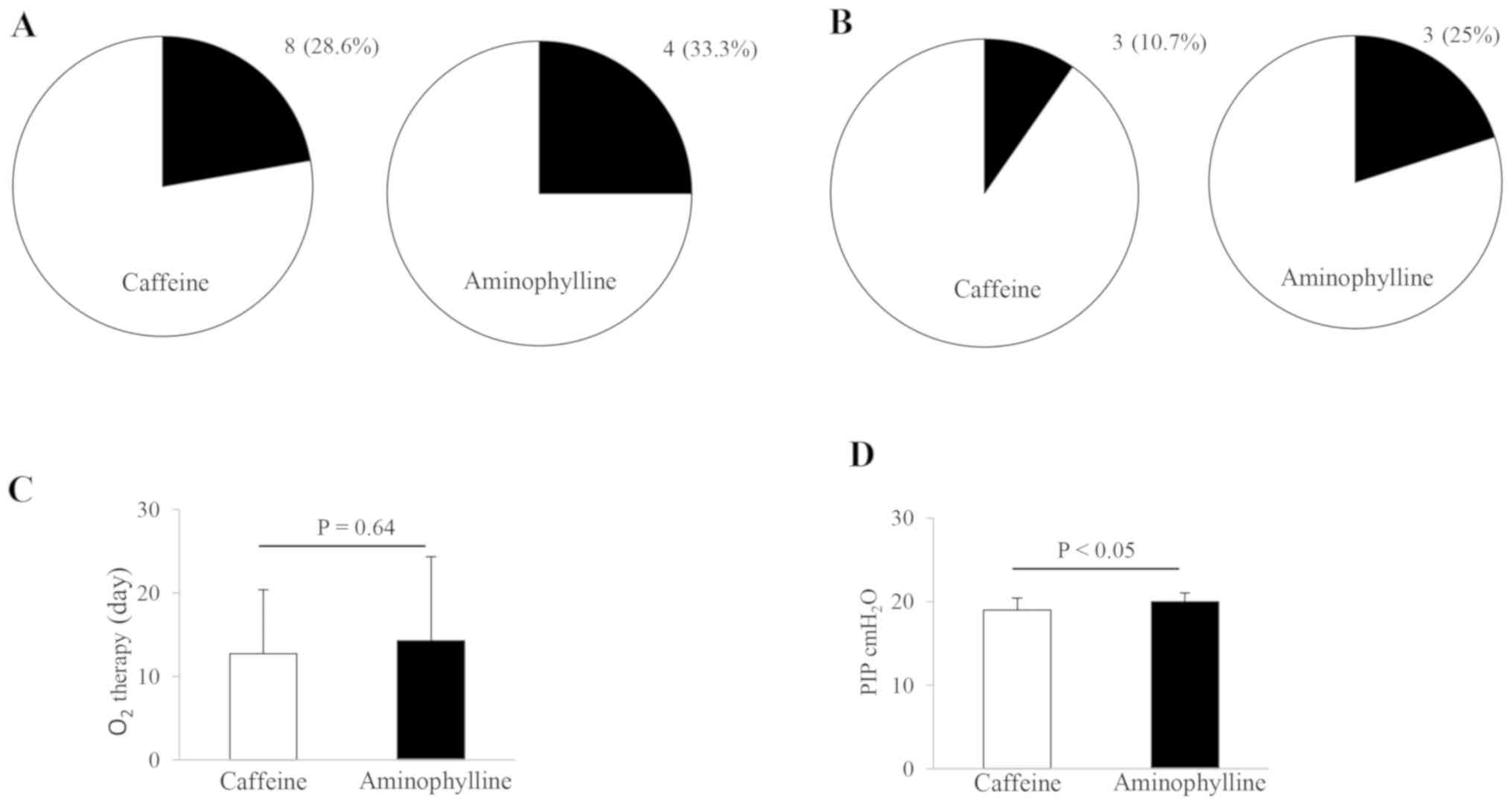
Fig. 1. The recurrence of apnea and
the frequency of ventilator replacement was similar in the infants
treated with both drugs. The population proportion of caffeine- and
aminophylline-treated infants (n=28 and 12) reached 28.6% (8/28)
and 33.3% (4/12) in terms of the recurrent episodes of apnea, and
10.7% (3/28) and 25% (3/12) in terms of the ventilator reconnection
(Fig. 1A and B). There were no statistically significant
differences in the aforementioned comparisons between these two
therapies. In subsequent analysis, the duration of the
O2 inhalation therapy was similar in caffeine-treated
infants compared with aminophylline-treated infants (Fig. 1C). The mean time of O2
inhalation was 12.71±7.66 and 14.25±10.09 days in caffeine- and
aminophylline-treated infants, respectively. Although the duration
of O2 inhalation in caffeine-treated infants was
slightly shorter than in aminophylline-treated infants, no
statistical difference was observed between these two treatment
approaches. The alterations in PIP, which were regulated by the
ventilator, were also examined in the treated infants. The
treatment with caffeine resulted in a small but significant decline
in the PIP level compared with aminophylline treatment, in the
ventilated infants (P<0.05; Fig.
1D). The mean PIP value was 18.96±1.45 and 20.00±1.04
cmH2O in caffeine- and aminophylline-treated infants,
respectively. In contrast to the duration of O2 therapy,
there was a statistically significant difference in the alteration
of the PIP levels between these two treatment options
(P<0.05).
Effects of drugs on preterm infants
receiving noninvasive mechanical ventilation
Certain premature infants (n=40) with apnea required
a supplemental O2 delivery via an NIPPV or NCPAP mode,
which provides a steady pressure to the rear of the nose that is
transmitted to the lungs, aiding the infant to breathe more
conveniently. The efficacy of the drugs in the treatment of
infantile apnea was evaluated in the ventilated infants according
to the frequency of recurrent apneic episodes and of invasive
ventilation used as alternative to non-invasive ventilation, as
well as the duration and the concentration of inhaled
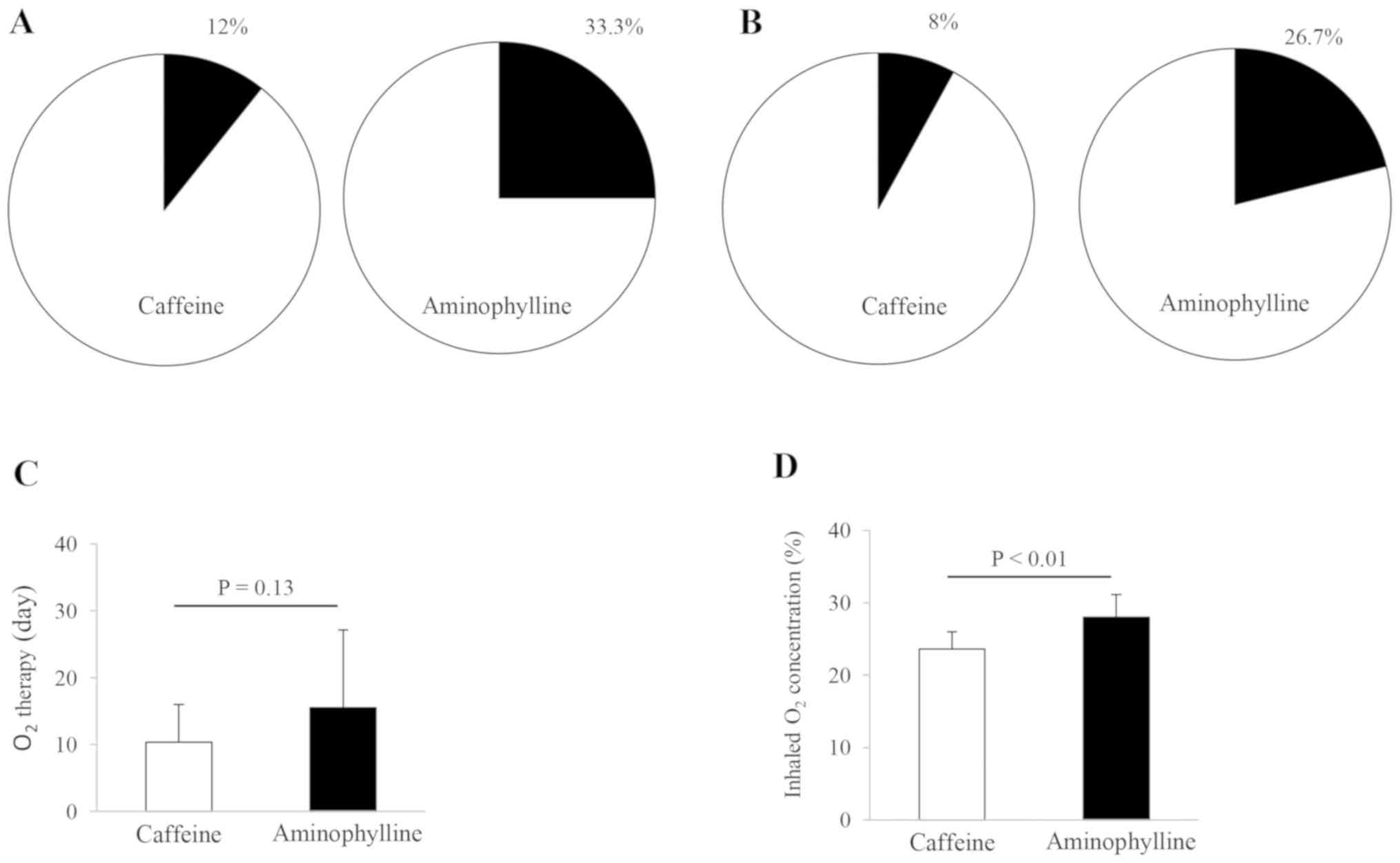
O2. The results are presented in Fig. 2. The treatment with either caffeine
or aminophylline did not result in apparent alterations in the
incidence of recurrent apnea and of the requirement of invasive
ventilation. The population proportion of caffeine- and
aminophylline-treated infants (n=25 and 15, respectively) reached
12% (3/25) and 33.3% (5/15) in the recurrent apnea incidents
(Fig. 2A) and 8% (2/25) and 26.7%
(4/15) in the incidents of the infants receiving invasive
ventilation (Fig. 2B),
respectively. There were no significant differences in these
clinical outcomes between the two therapies (Fig. 2A and B). In order to clarify the effects of
O2 therapy, the duration and concentration of the
inhaled O2 was examined in the ventilated infants. The
average values of the duration and concentration of the
O2 supply were 10.40±5.60 days and 23.60±2.38% in
caffeine-treated infants, respectively, and 15.53±11.06 days and
28.00±3.16% in aminophylline-treated infants, respectively. The
duration of O2 intake in caffeine-treated infants was 6
days shorter than that in aminophylline-treated infants (Fig. 2C). However, no statistically
significant difference was observed in the duration of the
O2 supply between these two treatment options. The
inhaled O2 concentration in the aminophylline-treated
infants was significantly higher than in the caffeine-treated
infants (P<0.01; Fig. 2D).
Effects of drugs on preterm infants
receiving a hood mask or a nasal cannula
Certain premature infants (n=40) with apnea received
O2 therapy through a hood mask or a nasal cannula. The
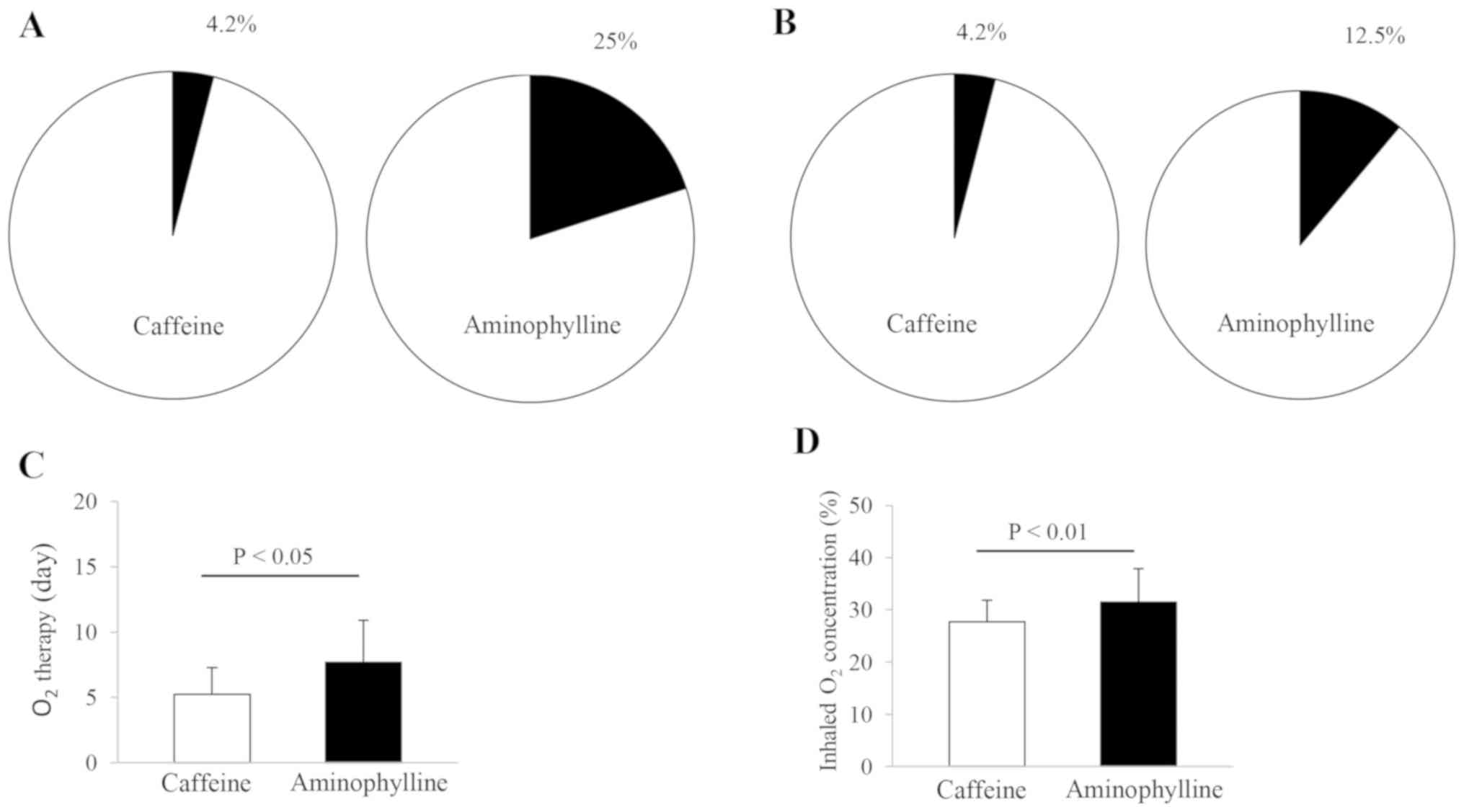
results are presented in Fig. 3.
The population proportion of caffeine- and aminophylline-treated
infants (n=24 and 16) reached 4.2% (1/24) and 25% (4/16),
respectively, in the recurrence of apnea and 4.2% (1/24) and 12.5%
(2/16), respectively, in the frequency of infants requiring an
invasive ventilation (Fig. 3A and
B). No statistically significant
differences were observed in the aforementioned observations
between these two therapies. In a subsequent analysis, the duration
and concentration of the inhaled O2 in caffeine-treated
infants were reduced compared with aminophylline-treated infants
(Fig. 3C and D). The mean values of the duration and
concentration of inhaled O2 were 5.21±2.06 days and
27.75±4.08% in caffeine-treated infants and 7.69±3.20 days and
31.50±6.35% in aminophylline-treated infants, respectively.
Statistically significant differences were observed in the duration
and concentration of the inhaled O2 between these two
treatment approaches (P<0.05 and P<0.01, respectively).
Efficacy and safety of caffeine and
aminophylline in the treatment of apnea
A total of 120 premature infants were selected for
the general assessment of the therapeutic efficacy and safety of
caffeine and aminophylline in the current retrospective study. The
incidence of infants with recurrent apneic episodes and
complications following the suspension of drug therapy was assessed
under the overall criterium of adequate O2 supply. The
recurrence rate of apnea was lower in caffeine-treated compared
with aminophylline-treated infants. The proportion of the infants
treated with caffeine and aminophylline (n=77 and 43, respectively)
reached 14.3% (11/77) and 32.6% (14/43) in the recurrent incidents,
respectively. A statistically significant difference was observed
in the incidents between these two treatment approaches
(P=0.033).
The risk of complications was also examined in the
infants treated with caffeine and aminophylline. The population
proportion of the infants with complications in the treatment
groups of caffeine and aminophylline was displayed as 5.2% (4/77)
and 23.2% (10/43) in PDA, 3.9% (3/77) and 18.6% (8/43) in BPD, 0%
(0/77) and 2.3% (1/43) in NEC, 2.6% (2/77) and 2.3% (1/43) in ROP,
and 14.3% (11/77) and 16.3% (7/43) in IVH, respectively (Table IV). The risks of NEC, ROP and IVH
were similar in the infants treated with caffeine and
aminophylline. However, the incidence of PDA and BPD in the
caffeine-treated infants was lower than that in the
aminophylline-treated infants, with statistically significant
differences observed in the occurrence of these complications
between these two treatment approaches (P<0.05).
 | Table IVComparison of efficacy and safety
between caffeine and aminophylline. |
Table IV
Comparison of efficacy and safety
between caffeine and aminophylline.
| Complication | Caffeine, n=77 | Aminophylline,
n=43 | P-value |
|---|
| Recurrent event of
apnea (%) | 11 (14.3) | 14 (32.6) | 0.033 |
| Patent ductus
arteriosus (%) | 4 (5.2) | 10 (23.2) | 0.006 |
| Bronchopulmonary
dysplasia (%) | 3 (3.9) | 8 (18.6) | 0.016 |
| Necrotizing
enterocolitis (%) | 0 (0) | 1 (2.3) | 0.358 |
| Retinopathy of
prematurity (%) | 2 (2.6) | 1 (2.3) | 1.000 |
| Intraventricular
hemorrhage (%) | 11 (14.3) | 7 (16.3) | 0.794 |
Discussion
The effects of drugs on the recurrence of apnea, the
invasive mechanical ventilation replacement, the duration of
O2 inhalation and the alterations in the PIP levels, to
the best of our knowledge, were analyzed for the first time in the
current retrospective study. The results indicated that the
efficacy of caffeine in the treatment of apnea in premature infants
with an invasive respiratory support was similar to that of
aminophylline. However, a lower PIP level was observed in the
ventilated infants of the caffeine-treated group, suggesting that
treatment with caffeine may result in an increased O2
delivery into the infant's lungs via decreasing the airway
resistance. Caffeine and aminophylline have typically been
prescribed as the first-line drugs for treating apnea of premature
infants since they function as respiratory stimulants to increase
the minute ventilation, and as neural stimulants to drive the
diaphragm contraction and the respiratory muscle function (4,21,22).
The results of the current study also demonstrated that in contrast
to aminophylline, caffeine may be more effective than aminophylline
in in infants treated with mechanical ventilation or O2
delivery. The decrease in the PIP level in the ventilated infants
may be attributed to the broader therapeutic range and the longer
half-life of caffeine in the plasma compared with aminophylline
(23,24). As PIP is the highest pressure level
provided to the lungs during inhalation, it was hypothesized that
the low pressure occurred as an additional effect of delivering
adequate O2 to the lungs of caffeine-treated infants. In
addition, it is worth noting that a low PIP level protects the
premature lungs from damage originating from increased
O2 exposure (25).
Therefore, the invasive strategy is considered the preferred option
to treat the apnea of prematurity.
Although both invasive and non-invasive mechanical
ventilation are used for treating premature infants with
respiratory insufficiency (26),
the latter is increasingly being employed in neonatal units and
appears to be safe and efficient when used by specialists (27). The non-invasive respiratory support
can be accomplished in a variety of ways; however, to the best of
our knowledge, none of them have yet been demonstrated to be
superior to invasive ventilation in the management of the apnea of
prematurity. NIPPV or NCPAP was introduced as an alternative to
invasive mechanical ventilation for treating preterm infants with
breathing problems (15). The
results of the current study revealed that caffeine treatment did
not reduce the duration of O2 inhalation and the
recurrence rate of apnea in the ventilated infants. However, the
treatment of the infants with caffeine decreased the level of
inhaled O2 compared with aminophylline treatment,
suggesting that caffeine was superior to aminophylline in improving
the efficacy of supplemental O2 under the condition of
delivering O2 via NIPPV or NCPAP. Furthermore, it may be
presumed that caffeine was more efficient than aminophylline in
maintaining the levels of O2 saturation, as
O2 desaturation appeared in infants with mixed apnea
(28). This effect is apparently
associated with the pharmacological action of caffeine in the
infants, since the drug has a broader therapeutic index, including
increasing the respiratory rate and minute ventilation via
stimulating the respiratory center and improving the sensitivity of
the central medullary areas to hypercapnia (29,30).
Premature infants who experience frequent bouts of apnea may be
mechanically ventilated to facilitate their breathing. NCPAP and
NIPPV, as primary modes of non-invasive mechanical ventilation,
have widely been employed as an alternative to invasive ventilation
for the early management of infant apnea (27). Both NCPAP and NIPPV provide steady
pressure to the rear of the nose via the ventilator, which is
transmitted to the neonatal lungs and counteracts the collapsing
lung symptoms, thus facilitating gas exchange (31). Therefore, these two modes of
assisted ventilation have been increasingly earning acceptance in
the treatment of neonatal apnea (32,33).
Given that the efficacy of both drugs in the treatment of infantile
apnea was based on the levels of supplemental O2
required for the infants, it has been suggested that using the
non-invasive techniques may be considered as a preferable option
for the infants treated with caffeine.
The effects of caffeine and aminophylline on the
recurrent episodes of apnea, the invasive ventilation required and
the duration and concentration of the inhaled O2 were
examined in premature infants receiving O2 via a hood
mask or a nasal cannula. The results indicated that the duration
and concentration of the inhaled O2, which were required
for the caffeine-treated infants, were shorter (2.5 days) and lower
(4%) than those for the aminophylline-treated infants.
Statistically significant differences were observed in the duration
and the concentration of the inhaled O2 between these
two therapies. Based on these findings, it was concluded that
caffeine may not only accomplish the supply of the body tissues
with adequate O2 via the devices, but also prevent any
inconvenience for the infants undergoing endotracheal intubation
and mechanical ventilation. Premature infants are at high risk of
developing breathing problems, as their lungs do not produce
sufficient amounts of surfactants, which keep the lung alveoli open
(34). Therefore, selecting a
method of supplemental O2 delivery should be carefully
considered, since in certain cases the artificial breathing machine
may result in lung problems, such as BPD (35). Since the majority of the infants in
the current retrospective study presented with mixed apnea, which
is a combination of central with obstructive apnea (21), a method of efficient O2
delivery to the infants' lungs is of particular importance for the
management of the pediatric population. Although the application of
CPAP is an approach that is used to treat both obstructive and
mixed apnea (36), its efficacy in
central apnea still remains to be clarified (7). The results of the current study
indicated that these simple methods can also provide sufficient
O2 to the patients with mixed apnea. Furthermore, the
therapy was not perturbed when feeding was required. Caffeine
improved the effects of the pediatric O2 therapy via
stimulating the central nervous system to reduce the apnea episodes
and improving the respiratory muscle strength to unblock an
obstruction if the airway collapses (37,38).
Overall, the results of the current study revealed that the
efficiency of these devices used to deliver O2 was
superior to both the invasive and non-invasive neonatal mechanical
ventilation in the present retrospective cohort study. Therefore,
it is suggested that the application of these O2
delivery devices should be the primary option for an O2
supply in the treatment of the infants with apnea, since this
method of supplying O2 would be more practical and
economical in developing countries.
A general assessment of the performance of caffeine
and aminophylline in the treatment of apnea was accomplished in
premature infants requiring ventilatory support or O2
delivery devices. The results indicated that a population
proportion with recurrent episodes of apnea following withdrawal of
drug therapy reached to 11 and 14% in the caffeine- and
aminophylline-treated infants, respectively, indicating that the
efficacy of caffeine was superior to that of aminophylline in
treating the apnea of prematurity, under the three conditions of
delivering O2. Compared with infants treated using
aminophylline, the proportion of the population with PDA and BPD
was reduced in the caffeine-treated infants by 6 and 5%,
respectively, in a total population of 120 cases. Based on these
findings, it was concluded that the overall performance of caffeine
in the treatment of apnea was superior to that of aminophylline in
the pediatric population requiring ventilatory support and
O2 delivery devices. Caffeine is one of the most
frequently prescribed medications in the neonatal intensive care
(39). As a respiratory stimulant,
caffeine can reduce the recurrent episodes of apnea in premature
infants (4,40). The results of the present study also
indicated that the incidence of complications was comparable to
previous reports, in which caffeine therapy reduced the risks of
PDA and BPD in preterm infants with a low birth weight or who were
born at <29 weeks gestation (41,42).
Currently, the efficacy and safety of caffeine and aminophylline
are not fully elucidated in infants receiving O2 via
mechanical ventilation and O2 delivery devices. The
present study provided novel insights regarding the impact of both
drugs on the infants, as they require different strategies of
O2 supply. As the incidence of the recurrent apneic
episodes and complications decreases in infants treated with
caffeine, it is hypothesized that these benefits of the medication
may reduce the financial burden on their families thanks to the
reduced requirements for hospitalization and further medical
care.
The limitations of the current study primarily
include the small sample size and the lack of information regarding
the long-term efficacy of caffeine in the treatment of apnea.
Severe apnea affects the neurodevelopmental outcome, and caffeine
improves the neural respiratory drive to reduce the apnea via
multiple physiological and pharmacological mechanisms (43). Therefore, a subsequent study is
required to assess the long-term efficacy of caffeine in protecting
the cerebral function from neurodevelopmental impairment.
In conclusion, the present study indicated that
caffeine had numerous advantages over aminophylline in the
treatment of apnea under varying conditions of O2
supply. Caffeine had high efficacy in the treatment of apnea
primarily via improving the efficiency of O2 therapy and
reducing the risk of complications in the pediatric population.
Acknowledgements
Not applicable.
Funding
The current study was funded by the Key Project of
Science and Technology of Henan Province (grant no.
182102310421).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CYZ, DJL and SDH conceived and designed the study.
SG, XYL, BZ and LHA collected and analyzed the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First People's Hospital of Zhengzhou City
(Zhengzhou, China; approval no. 2017-16). Informed consent was
obtained from all participants' guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schmidt B, Roberts RS, Davis P, Doyle LW,
Barrington KJ, Ohlsson A, Solimano A and Tin W: Caffeine for Apnea
of Prematurity Trial Group. Caffeine therapy for apnea of
prematurity. N Engl J Med. 354:2112–2121. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pillekamp F, Hermann C, Keller T, von
Gontard A, Kribs A and Roth B: Factors influencing apnea and
bradycardia of prematurity-implications for neurodevelopment.
Neonatology. 91:155–161. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ducrocq S, Biran-Mucignat V, Boelle PY,
Lebas F, Baudon JJ and Gold F: Apnea of prematurity: Risk factors
and ambulatory treatment with caffeine citrate. Arch Pediatr.
13:1299–1304. 2006.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
4
|
Martin RJ, Abu-Shaweesh JM and Baird TM:
Apnoea of prematurity. Paediatr Respir Rev. 5 (Suppl A):S377–S382.
2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Siu A and James A: Apnea of prematurity
pharmacotherapy. US Pharm. 35:HS2–HS6. 2010.
|
|
6
|
Janvier A, Khairy M, Kokkotis A, Cormier
C, Messmer D and Barrington KJ: Apnea is associated with
neurodevelopmental impairment in very low birth weight infants. J
Perinatol. 24:763–768. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhao J, Gonzalez F and Mu D: Apnea of
prematurity: From cause to treatment. Eur J Pediatr. 170:1097–1105.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Henderson-Smart DJ and De Paoli AG:
Methylxanthine treatment for apnoea in preterm infants. Cochrane
Database Syst Rev: CD000140, 2010.
|
|
9
|
Pantalitschka T, Sievers J, Urschitz MS,
Herberts T, Reher C and Poets CF: Randomised crossover trial of
four nasal respiratory support systems for apnoea of prematurity in
very low birthweight infants. Arch Dis Child Fetal Neonatal Ed.
94:F245–F248. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lemyre B, Davis PG and de Paoli AG: Nasal
intermittent positive pressure ventilation (NIPPV) versus nasal
continuous positive airway pressure (NCPAP) for apnea of
prematurity. Cochrane Database Syst Rev: CD002272, 2002.
|
|
11
|
Koch G, Datta AN, Jost K, Schulzke SM, van
den Anker J and Pfister M: Caffeine citrate dosing adjustments to
assure stable caffeine concentrations in preterm neonates. J
Pediatr. 191:50–56.e1. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schoen K, Yu T, Stockmann C, Spigarelli MG
and Sherwin CM: Use of methylxanthine therapies for the treatment
and prevention of apnea of prematurity. Paediatr Drugs. 16:169–177.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Brown MK and DiBlasi RM: Mechanical
ventilation of the premature neonate. Respir Care. 56:1298–1313.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Reyes ZC, Claure N, Tauscher MK, D'Ugard
C, Vanbuskirk S and Bancalari E: Randomized, controlled trial
comparing synchronized intermittent mandatory ventilation and
synchronized intermittent mandatory ventilation plus pressure
support in preterm infants. Pediatrics. 118:1409–1417.
2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shi Y, Tang S, Zhao J and Shen J: A
prospective, randomized, controlled study of NIPPV versus nCPAP in
preterm and term infants with respiratory distress syndrome.
Pediatric Pulmonology. 49:673–678. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Arlettaz R: Echocardiographic evaluation
of patent ductus arteriosus in preterm infants. Front Pediatr.
5(147)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gien J and Kinsella JP: Pathogenesis and
treatment of bronchopulmonary dysplasia. Curr Opin Pediatr.
23:305–313. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Coursey CA, Hollingsworth CL, Wriston C,
Beam C, Rice H and Bisset G III: Radiographic predictors of disease
severity in neonates and infants with necrotizing enterocolitis.
AJR Am J Roentgenol. 193:1408–1413. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fierson WM: AMERICAN ACADEMY OF PEDIATRICS
Section on Ophthalmology; AMERICAN ACADEMY OF OPHTHALMOLOGY;
AMERICAN ASSOCIATION FOR PEDIATRIC OPHTHALMOLOGY AND STRABISMUS;
AMERICAN ASSOCIATION OF CERTIFIED ORTHOPTISTS. Screening
examination of premature infants for retinopathy of prematurity.
Pediatrics. 142(e20183061)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
O'Shea TM, Allred EN, Kuban KC, Hirtz D,
Specter B, Durfee S, Paneth N and Leviton A: ELGAN Study
Investigators. Intraventricular hemorrhage and developmental
outcomes at 24 months of age in extremely preterm infants. J Child
Neurol. 27:22–29. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Stokowski LA: A primer on Apnea of
prematurity. Adv Neonatal Care. 5:155–170; quiz 171-174.
2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Aranda JV, Beharry K, Valencia GB,
Natarajan G and Davis J: Caffeine impact on neonatal morbidities. J
Matern Fetal Neonatal Med. 23:20–23. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Henderson-Smart DJ and Steer PA: Caffeine
versus theophylline for apnea in preterm infants. Cochrane Database
Syst Rev. 20(CD000273)2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mueni E, Opiyo N and English M: Caffeine
for the management of apnea in preterm infants. Int Health.
1:190–195. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Donn SM and Sinha SK: Minimising
ventilator induced lung injury in preterm infants. Arch Dis Child
Fetal Neonatal Ed. 91:F226–F230. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rocha G, Soares P, Gonçalves A, Silva AI,
Almeida D, Figueiredo S, Pissarra S, Costa S, Soares H,
Flôr-de-Lima F and Guimarães H: Respiratory care for the ventilated
neonate. Can Respir J. 2018(7472964)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Garg S and Sinha S: Non-invasive
ventilation in premature infants: Based on evidence or habit. J
Clin Neonatol. 2:155–159. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Deschamp A and Daftary A: A newborn infant
with oxygen desaturation during sleep. Chest. 151:e17–e20.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dobson NR and Patel RM: The role of
caffeine in non-invasive respiratory support. Clin Perinatol.
43:773–782. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Abdel-Hady H, Nasef N, Shabaan AE and Nour
I: Caffeine therapy in preterm infants. World J Clin Pediatr.
4:81–93. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Owen LS, Manley BJ, Davis PG and Doyle LW:
The evolution of modern respiratory care for preterm infants.
Lancet. 389:1649–1659. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kieran EA, Walsh H and O'Donnell CP:
Survey of nasal continuous positive airways pressure (NCPAP) and
nasal intermittent positive pressure ventilation (NIPPV) use in
Irish newborn nurseries. Arch Dis Child Fetal Neonatal Ed.
96(F156)2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lemyre B, Laughon M, Bose C and Davis PG:
Early nasal intermittent positive pressure ventilation (NIPPV)
versus early nasal continuous positive airway pressure (NCPAP) for
preterm infants. Cochrane Database Syst Rev.
12(CD005384)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chakraborty M and Kotecha S: Pulmonary
surfactant in newborn infants and children. Breathe. 9:476–488.
2013.
|
|
35
|
Jobe AH: The new bronchopulmonary
dysplasia. Curr Opin Pediatr. 23:167–172. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Muza RT: Central sleep apnoea-a clinical
review. J Thorac Dis. 7:930–937. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bancalari E: Caffeine for apnea of
prematurity. N Engl J Med. 354:2179–2181. 2006.
|
|
38
|
Kassim Z, Greenough A and Rafferty GF:
Effect of caffeine on respiratory muscle strength and lung function
in prematurely born, ventilated infants. Eur J Pediatr.
168:1491–1495. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Clark RH, Bloom BT, Spitzer AR and
Gerstmann DR: Reported medication use in the neonatal intensive
care unit: Data from a large national data set. Pediatrics.
117:1979–1987. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Stevenson DK: On the Caffeination of
Prematurity. N Engl J Med. 357:1967–1968. 2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
April J, Ekta P, Kyle C, Jreisat C,
Kampanatkosol R and Sajous C: Does early caffeine therapy effect
patent ductus arteriosus outcomes in very low birth weight infants?
Acad J Ped Neonatol. 4(555696)2017.
|
|
42
|
Lodha A, Entz R, Synnes A, Creighton D,
Yusuf K, Lapointe A, Yang J and Shah PS: investigators of the
Canadian Neonatal Network (CNN) and the Canadian Neonatal Follow-up
Network (CNFUN). Early caffeine administration and
neurodevelopmental outcomes in preterm infants. Pediatrics.
143(e20181348)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bairam A, Uppari N, Mubayed S and Joseph
V: An overview on the respiratory stimulant effects of caffeine and
progesterone on response to hypoxia and apnea frequency in
developing rats. Adv Exp Med Biol. 860:211–220. 2015.PubMed/NCBI View Article : Google Scholar
|