Introduction
Glomerular disease affects both kidneys (1), and is mainly caused by primary
glomerulopathy (2). Glomerular
disease is the main cause of chronic renal failure, which requires
dialysis or renal transplantation to improve the quality of life in
patients with end-stage renal disease (3). At present, renal biopsy is the main
strategy used to evaluate glomerulopathy (4). In very few and specific cases,
patients with poor renal function and a lower platelet count suffer
from severe serious bleeding following renal biopsy (5), and in such cases, renal biopsies are
not suitable. Thus, the identification of a novel method with which
to diagnose glomerular disease more conveniently and at an earlier
stage is urgently required in clinical practice, which may provide
information for clinical treatment. The current developments in
genomics, epigenetics, transcriptomics, proteomics and metabolomics
suggest that the introduction of novel technologies mat aid in the
identification of novel biomarkers for kidney disease; the
diagnosis of chronic kidney disease is usually based on the levels
of blood urea and serum creatinine (sCr); however, it has been
proven that sCr lacks a high predictive value (6). In the study by Ling et al
(7), urinary CD80 levels were used
as non-invasive diagnostic biomarkers, which is important for the
early diagnosis of nephrotic syndrome, suggesting that a better
biological indicator could be found.
Monocytes/macrophages are defense cells which
originate in mesoblasts and play important roles in immune and
non-immune functions (8). When
glomerulonephritis occurs, mononuclear/macrophage infiltration can
be found in renal tissue (9), which
reflects the degree of glomerular disease to a certain extent. CD68
is a cell-pulp glycoprotein with a relative molecular mass of
110,000, which is associated with lysosomal particles and is the
most reliable marker for macrophages (10). The transforming growth factor-β
(TGF-β) plays an important role in the pathophysiological processes
of tumor (11) and cardiovascular
disease (12) in a group of newly
discovered TGF-β superfamily that regulate cell growth and
differentiation. TGF-β1 is an important cytokine in the process of
glomerulosclerosis (13) and tubule
fibrosis (14), which can be
regarded as a marker of inflammation.
The aim of the present study was to determine
whether the concentrations of CD68 and TGF-β1 in patients with
glomerular disease can be used as clinical diagnostic indexes for
glomerular diseases, which may provide a reference for the
diagnosis and treatment of glomerular diseases.
Patients and methods
General materials
A total of 218 patients with glomerulopathy
diagnosed at Weifang People's Hospital from January, 2014 to March,
2017 were used as the study group, including 132 males and 86
females, aged 33 to 64 years. In addition, blood was collected from
100 healthy individuals who underwent a physical examination at the
same hospital and during the same time period. These healthy
individuals were enrolled as the control group, and included 66
males and 34 females, aged 25 to 65 years. The present study was
approved by the Ethics Committee of Weifang People's Hospital, and
all the research subjects have signed the informed consent
forms.
Inclusion and exclusion criteria
The inclusion criteria were as follows: The
diagnosis was made was in accordance with the criteria for the
diagnosis of glomerular disease (15); patients were treated at Weifang
People's Hospital, and were aged between 18 to 70 years; patients
were to have had a primary school or higher education; patients who
cooperated with the research protocol; patients had to be without
any serious diseases in other organs. All subjects or their
immediate families signed the informed consent forms.
The exclusion criteria were as follows: Death during
treatment; patients with respiratory or blood system diseases;
patients with mental diseases and speech dysfunction; pregnant or
lactating women; patients who had recently been treated with
immunosuppressive agents and hormone drugs.
Detection of CD68 and TGF-β1 in
serum
Peripheral blood samples were collected from the
subjects in the 2 groups in vacuum blood collection tubes in the
morning, and were separated into two parts. One part of the blood
was separated by centrifugation at 1,505 x g at 4˚C for 10 min. The
upper serum was put into the -80˚C refrigerator for testing. The
level of serum TGF-β1 was detected by ELISA in strict accordance
with the kit instructions (SND-H035; Chuzhou Shinuoda Biological
Technology Co., Ltd.).
The other part was for CD68 detection. Following the
dilution of an equal volume of phosphate-buffered saline solution,
peripheral blood mononuclear cells (PBMCs) were obtained by density
gradient centrifugation using human lymphocyte separation solution.
The concentration of the cells was adjusted to 2x106/ml
with RPMI-1640 medium (cat. no. CDLG-5404; ChunduBio). The cells
were inoculated in a 24-well culture plate and then added with the
stimulant, phorbol myristate acetate (50 µg/l), ionomycin (1
µmol/l) and the protein transport inhibitor, monensin (50 µg/l).
The mixture was mixed and cultured in a 5% CO2 cell
incubator at 37˚C for 4 h. The cells were collected and divided
into experimental tubes and control tubes equally. This was
followed by the addition of 4 µl FITC-labeled mouse anti-human CD68
monoclonal antibody (bs-20402r; Bioss), incubation at 4˚C in the
dark for 30 min, and washing twice with PBS. The fixative was
maintained at room temperature for 20 min, and then centrifuged at
1,505 x g at 4˚C. The supernatant was then discarded, and washed
twice with PBS. Subsequently, 1 ml of rupture agent (EY-24882;
Shanghai Institute of Biotechnology Co., Ltd.) was added to each
tube for cell puncturing to facilitate the entry of cytokine
monoclonal antibody (Shanghai Qunji Biological Technology Co.,
Ltd., MAB11128) into the cells. Following centrifugation 1,505 x g
at 4˚C for 10 min, the supernatant was discarded, and intracellular
cytokine staining at 4˚C for 30 min was performed. CD68-postivie
cells were detected by flow cytometry (BD FACSAria™ III sorter; BD
Biosciences).
Observation of renal injury index
The examination of renal injury indexes was observed
in the 2 groups. The indexes of renal injury included blood urea
nitrogen (BUN), serum creatinine (SCR), glycosylated albumin (GA),
glycosylated hemoglobin (HbA1c) and 24-h urinary protein
quantity.
Observation index
The clinical data of the patients were recorded and
analyzed, including renal injury indicators, such as blood urea
nitrogen (BUN), serum creatinine (SCR), uric acid (UA), 24-h urine
protein quantity, white blood cell count (WBC) and platelet count
(PC).
Statistical analysis
SPSS v24.0 software (Yuchuang Network Technology
Co., Ltd.) was used to calculate all experimental results.
Graphpad8 software (Softhead Inc.) was used to plot figures and
examine the results again. The counting data were represented in
the form of rate. The Chi-square test was used for comparison
between groups. The measurement data are expressed as the means ±
standard deviation. The t-test was used for comparisons between
groups. Repeated measures analysis of variance was used for
comparisons of multiple time points in the group, followed by
Bonferroni's correction. Correlation analysis was performed using
Spearman's correlation analysis. A value of P<0.050 was
considered to indicate a statistically significant difference
between groups.
Results
General characteristics of the 2
groups
No significant differences were observed in age,
sex, BMI, medical history, marital status, smoking history and
alcohol consumption history between the study group and the control
group (P>0.050), which proved that the 2 groups of patients were
comparable (Table I).
 | Table IComparison of the clinical data. |
Table I
Comparison of the clinical data.
| Index | Study group
(n=218) | Control group
(n=100) | χ2 or
test | P-value |
|---|
| Age (years) | 47.29±5.38 | 46.71±6.17 | 0.852 | 0.395 |
| Sex, n (%) | | | 0.867 | 0.352 |
|
Male | 132 (60.55) | 66 (66.00) | | |
|
Female | 86 (39.45) | 34 (34.00) | | |
| BMI
(kg/m2) | 23.47±1.81 | 23.34±1.93 | 0.582 | 0.561 |
| Medical history, n
(%) | | | | |
|
Hypertension | 48 (22.02) | 25 (25.00) | 0.347 | 0.557 |
|
Diabetes
mellitus | 45 (20.64) | 19 (19.00) | 0.009 | 0.927 |
|
Hyperlipemia | 37 (16.97) | 17 (17.00) | 0.162 | 0.688 |
| Marital status, n
(%) | | | 0.301 | 0.583 |
|
Married | 173 (79.36) | 82 (82.00) | | |
|
Unmarried | 45 (20.64) | 18 (18.00) | | |
| Smoking history, n
(%) | | | 0.284 | 0.594 |
|
Yes | 129 (59.17) | 56 (56.00) | | |
|
No | 89 (40.83) | 44 (44.00) | | |
| Alcohol
consumption, n (%) | | | 0.341 | 0.559 |
|
Yes | 121 (55.50) | 59 (59.00) | | |
|
No | 97 (44.50) | 41 (41.00) | | |
| Leukocytes
1x109/l) | 12.84±4.46 | 5.35±1.38 | 16.421 | <0.001 |
| Blood platelet
count (1x109/l) | 225.17±66.94 | 213.38±67.89 | 1.452 | 0.147 |
Comparison of the CD68 and TGF-β1
concentration in serum between the 2 groups
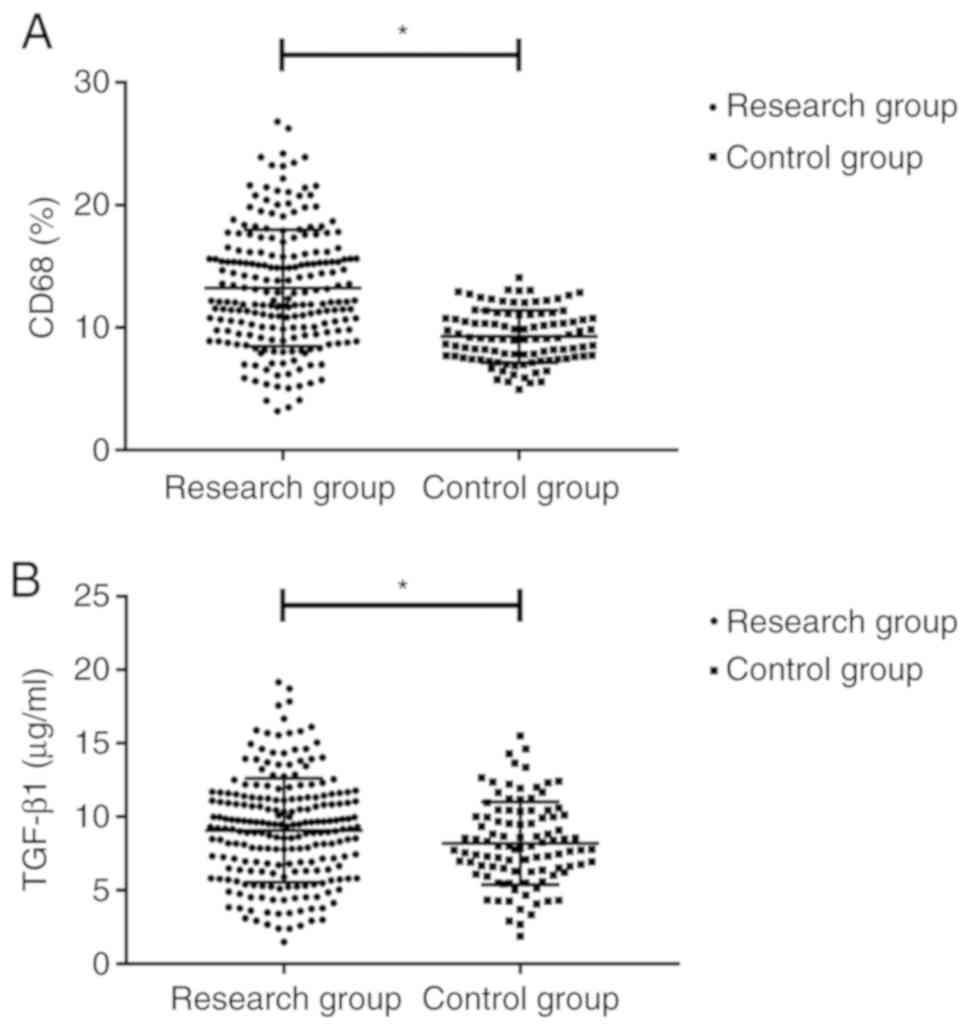
Compared with the control group, the expression of
CD68 in the study group was significantly increased (P<0.05).
The concentration of TGF-β1 in the study group was 12.36±4.41
pg/ml, which was higher than that in the control group (9.25±3.56
pg/ml) (P<0.05; Fig. 1).
Comparison of the renal injury index
concentration between the 2 groups
The levels of BUN, SCR and UA and the 24-h urinary
protein quantity in the study group were significantly higher than
those in the control group (P<0.05; Table II).
 | Table IIComparison of the concentrations of
renal injury indexes between the two groups. |
Table II
Comparison of the concentrations of
renal injury indexes between the two groups.
| Index | Study group
(n=218) | Control group
(n=100) | t value | P-value |
|---|
| BUN (mmol/l) | 13.39±3.52 | 4.36±2.48 | 23.141 | <0.001 |
| SCR (µmmol/l) | 521.49±24.38 | 76.29±25.23 | 149.521 | <0.001 |
| UA (µmol/l) | 481.46±31.85 | 279.39±29.49 | 53.753 | <0.001 |
| 24-h urinary
protein quantity (mg/24 h) | 190.38±19.48 | 76.39±15.49 | 51.512 | <0.001 |
Correlation analysis of CD68 and
TGF-β1 with renal injury indexes in the study group
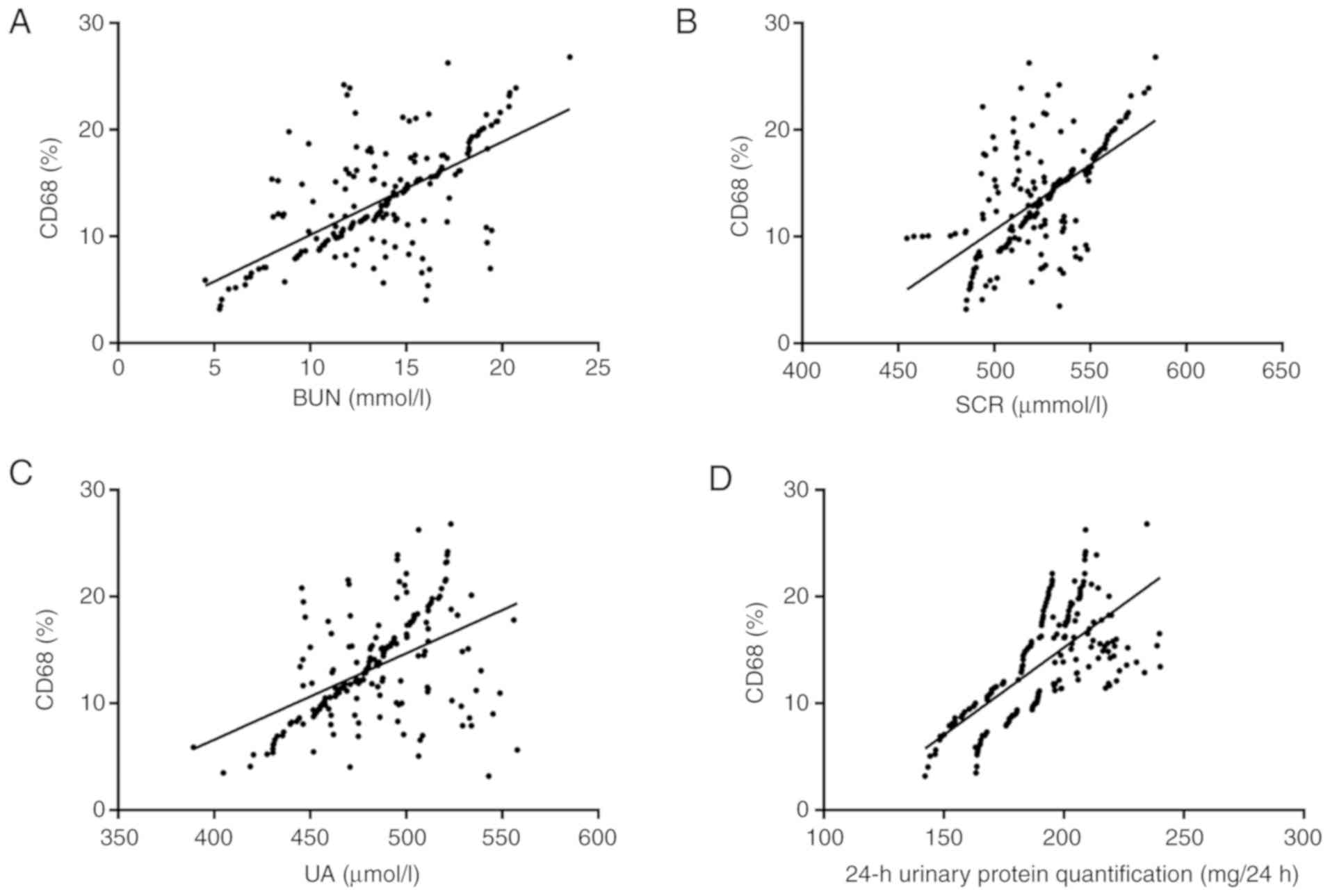
The serum levels of CD68 in the study group
positively correlated with the BUN (r=0.647; 95% CI, 0.56-0.72;
R2=0.419; P<0.001), SCR (r=0.605; 95% CI, 0.51-0.68;
R2=0.367; P<0.001) and UA (r=0.497; 95% CI,
0.39-0.59; R2=0.247; P<0.001) levels, and with the
24-h urinary protein quantity (r=0.697; 95% CI, 0.62-0.76;
R2=0.486; P<0.001; Fig.
2).
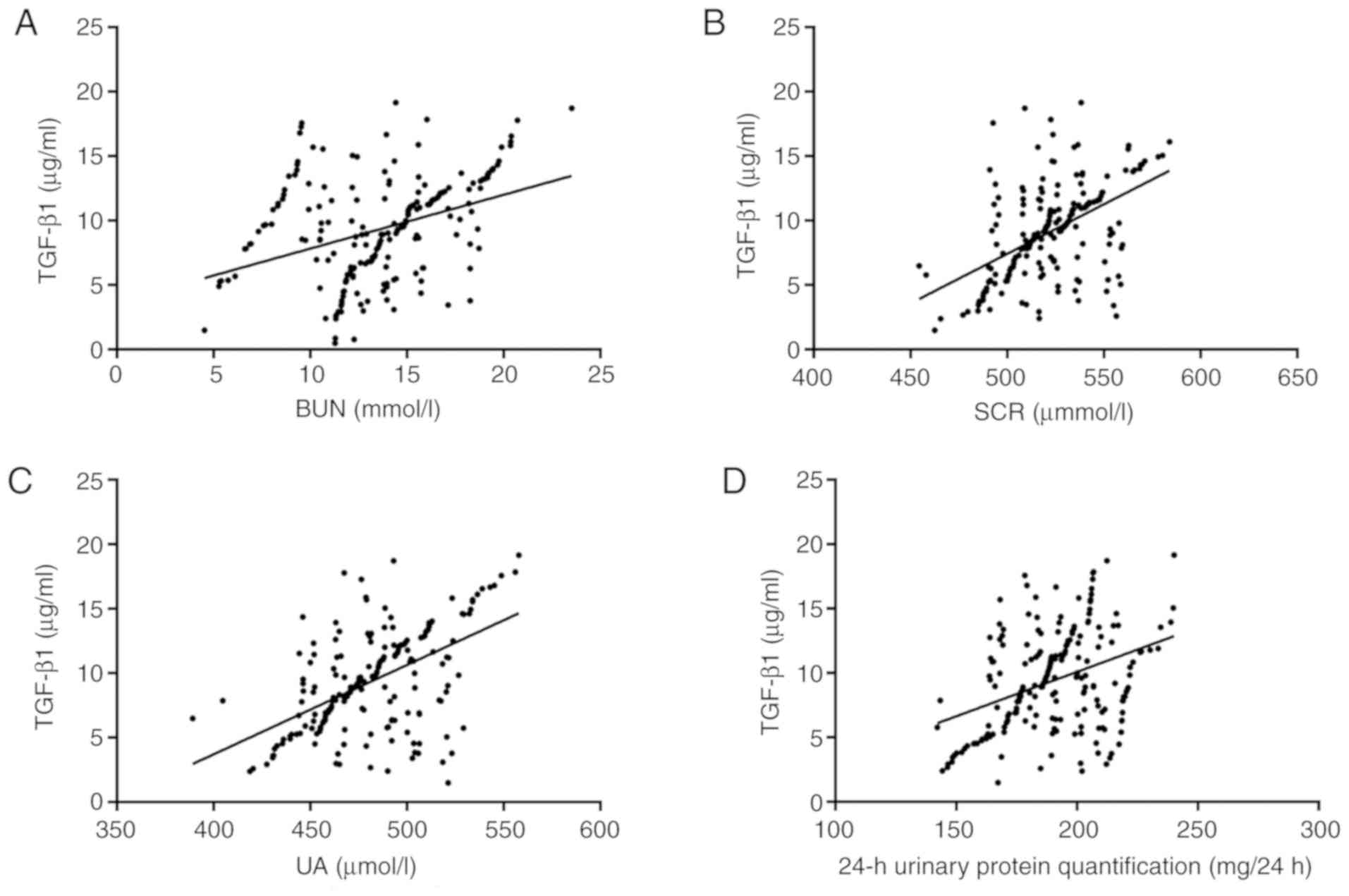
The level of TGF-β1 in serum of the study group also
positively correlated with the BUN (r=0.376, 95% CI, 0.26-0.48;
R2=0.142; P<0.001), SCR (r=0.513; 95% CI, 0.41-0.60;
R2=0.263; P<0.001) and UA (r=0.534; 95% CI,
0.43-0.62; R2=0.285; P<0.001) levels, and with the
24-h urinary protein quantity (r=0.379; 95% CI, 0.26-0.49;
R2=0.144; P<0.001; Fig.
3).
Diagnostic efficacy of CD68 and TGF-β1
on glomerular diseases
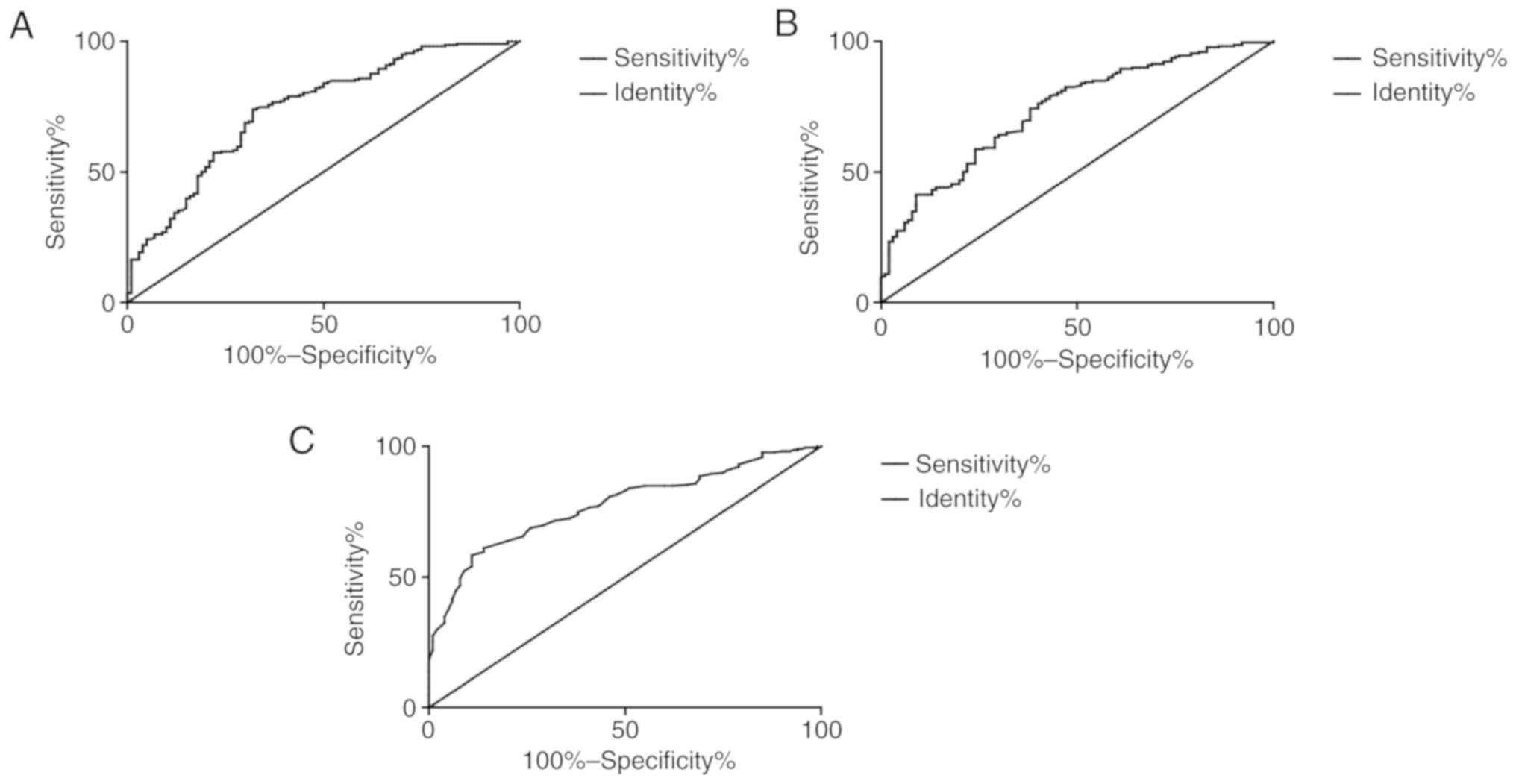
ROC curve analysis revealed that the area under the
curve of CD68 was 0.808, and the cut-off value was 8.606, with a
sensitivity of 63.76% and specificity of 89.00%. The area under the
curve of TGF-β1 was 0.738, and the cut-off value was 9.881 pg/ml,
with a sensitivity of 74.31% and specificity of 62.00%. The area
under the curve of CD68 and TGF-β1 combination was 0.866, and the
cut-off value was 0.308, with a sensitivity of 76.15% and
specificity of 84.00% (Fig. 4).
Univariate alysis of the association
between patient characteristics and renal function
According to the remission of renal function, the
patients were divided into the renal function remission group (139
cases, 63.76%) and renal function non-remission group (79 cases,
36.24%). The clinical data of the 2 groups were collected and
analyzed using by univariate analysis using the Chi-squared test or
t-test. It was found that there was no difference in sex, BMI,
medical history, smoking history and alcohol consumption history
between the 2 groups (P>0.05); however, a significant difference
was observed in clinical classification, and in the levels of CD68
and TGF-β1 between the 2 groups (P<0.05; Table III).
 | Table IIIUnivariate analysis. |
Table III
Univariate analysis.
| Factor | Renal function
remission group (n=139) | Renal function
non-remission group (n=79) | χ2 or
test | P-value |
|---|
| Age (years), n
(%) | | | 0.156 | 0.693 |
|
≥55 | 86 (61.87) | 51 (64.55) | | |
|
≥55 | 53 (38.13) | 28 (35.44) | | |
| Sex, n (%) | | | 0.002 | 0.962 |
|
Male | 84 (60.43) | 48 (60.76) | | |
|
Female | 55 (39.57) | 31 (39.24) | | |
| BMI
(kg/m2) | 23.55±1.34 | 23.46±1.50 | 0.456 | 0.648 |
| Medical history, n
(%) | | | | |
|
Hypertension | 25 (12.85) | 23 (29.11) | 3.633 | 0.057 |
|
Diabetes
mellitus | 29 (20.86) | 16 (20.25) | 0.011 | 0.915 |
|
Hyperlipemia | 24 (17.26) | 13 (16.46) | 0.023 | 0.878 |
| Smoking history, n
(%) | | | 1.486 | 0.223 |
|
Yes | 78 (56.12) | 51 (64.56) | | |
|
No | 61 (43.88) | 28 (35.44) | | |
| Alcohol
consumption, n (%) | | | 1.385 | 0.239 |
|
Yes | 73 (52.52) | 48 (60.75) | | |
|
No | 66 (47.48) | 31 (39.25) | | |
| Oliguresis | 15 (10.79) | 48 (60.75) | 61.213 | <0.001 |
| Clinical
classification | | | 44.321 | <0.001 |
|
Glomerular
nephritis | 61 (43.89) | 12 (15.99) | | |
|
Hematuria or
proteinuria | 49 (5.35) | 15 (18.99) | | |
|
Nephrotic
syndrome | 29 (2086) | 52 (65.82) | | |
| Leukocyte
(1x109/l) | 12.84±2.31 | 13.41±3.19 | 1.520 | 0.130 |
| Blood platelet
count (1x109/l) | 219.57±56.94 | 233.39±49.33 | 1.806 | 0.07 |
| CD68 | 7.06±1.48 | 8.39±2.70 | 4.701 | <0.001 |
| TGF-β1 | 11.09±3.81 | 12.78±3.25 | 3.315 | <0.001 |
Multivariate analysis of the prognosis
of renal function
The indicators with differences in univariate
analysis into the assignment (the assignment information is
presented in Table IV). The
results of multivariate logistic regression analysis with LR
revealed that oliguria, clinical classification, and the CD68 and
TGF-β1 levels were prognostic factors of renal function in patients
(P<0.05; Table V).
 | Table IVAssignment of factors. |
Table IV
Assignment of factors.
| Factor | Assignment |
|---|
| Oliguresis | Yes, 1; no, 0 |
| Clinical
classification | Glomerular
nephritis, 1; hematuria or proteinuria, 2; nephrotic syndrome,
3 |
| CD68 | Data are continuous
variables using the original data analysis |
| TGF-β1 | Data are continuous
variables using the original data analysis |
| Remission of renal
function | Remission, 1;
non-remission, 0 |
 | Table VMultifactor analysis. |
Table V
Multifactor analysis.
| | | | | | | 95% CI of Exp
(B) |
|---|
| Variable | B | SE | Wals | Sig. | Exp (B) | Lower limit | Upper limit |
|---|
| Oliguresis | 0.714 | 0.831 | 0.739 | 0.389 | 2.044 | 1.403 | 4.435 |
| Clinical
classification | | | | | | | |
| Glomerular
nephritis | 1.863 | 0.800 | 5.414 | 0.020 | 6.436 | 2.341 | 9.848 |
| Hematuria or
proteinuria | 2.013 | 0.933 | 4.62 | 0.031 | 7.491 | 4.184 | 12.826 |
| Nephrotic
syndrome | 1.913 | 0.542 | 11.693 | 0.002 | 6.781 | 5.261 | 9.327 |
| CD68 | 1.497 | 0.560 | 4.862 | 0.024 | 3.475 | 1.140 | 5.536 |
| TGF-β1 | 1.512 | 0.682 | 4.886 | 0.025 | 2.541 | 1.300 | 5.171 |
Discussion
Renal glomerular disease leads to earlier and
severer damage to glomerular function before it affects renal
tubular function (16). Glomerular
disease has a severe impact on the quality of life of patients, and
poses a heavy burden on the health care system (17). It is generally considered that the
immune mechanism is the initiating mechanism of glomerulopathy. On
this basis, inflammatory mediators (18) and complement activation (19) function together to induce the
occurrence and development of the disease. At present, the main
methods of the clinical diagnosis of glomerulopathy are the
observation of the clinical manifestations of patients and renal
biopsy. However, renal biopsy can cause side-effects, such as pain
and anxiety (20); thus, it is
crucial to evaluate the degree of renal injury by evaluating the
process of renal disease more conveniently and quickly.
The CD68 molecule is an important surface marker of
macrophages, which is often used for macrophage identification and
functional analysis (21). At
present, it has been confirmed that CD68 is closely related to
non-small cell lung cancer (22)
and colorectal adenoma (23);
however, there is a lack of research on the correlation between
CD68 and glomerulopathy. In the present study, the concentration of
CD68 in the serum of the study group and the control group revealed
that the concentration of CD68 in the study group was significantly
increased and positively correlated with the concentration of renal
injury indexes, suggesting that CD68 may be related to the
occurrence of glomerular diseases. Dias et al found that
CD68 was associated with the progression of chronic kidney disease
in 50 patients with proliferative LN by immunohistochemical
analysis (24). Guillén-Gómez et
al found that a large number of CD68-positive cells in the
kidneys were negatively associated with long-term renal function
(25), which was consistent with
the results of the present study. TGF-β is a 25 kDa homodimer
prototype of the protein family that regulate cell growth and
differentiation, which can stimulate and inhibit cell growth, and
is considered to be the main switch in the pathogenesis of organ
fibrosis (26). Renal fibrosis is a
common pathological feature of almost all types of chronic renal
diseases, which is closely related to the decline of renal function
(27). Renal fibrosis is mainly
caused by the formation of tissue scar by activating TGF-β1/Smads
signaling pathway (28). The
association between TGF-β1 and the process of glomerular disease is
not yet clear. In the present study, the concentration of TGF-β1 in
patients with glomerular disease was significantly higher than that
in healthy individuals and positively correlated with the
concentration of renal injury indexes, suggesting that TGF-β1 may
be a marker for determining the process of glomerular diseases. The
study by Fukuda et al (29)
demonstrated that urinary podocytes and TGF-β1 mRNA expression may
be used as markers for the progression and treatment of
anti-glomerular basement membrane nephritis, which to a certain
extent supports the present experimental results. In the previous
study by Ostermann et al (30), serum creatinine and urine output
were only signs of excretion function, and the abnormal performance
needed to be explained in the clinical context. In the case of slow
changes in creatinine and urine values, misleading or inaccurate
interpretation occurs, lacking sufficient sensitivity, and other
tools are required to diagnose acute kidney injury. As for the
complex pathological mechanisms involved in the development and
progression of glomerular disease, a combination of markers is
required to reflect all types of changes during this disease
process. In the present study, through ROC curve analysis, it was
found that the area under the CD68 curve was 0.808, and the area
under the TGF-β1 curve was 0.738. The area under the CD68 and
TGF-β1 combination curve was 0.866, and the cut-off value was
0.308, with a sensitivity of 76.15% and specificity of 84.00%. That
indicates that the CD68 and TGF-β1 combination can be used a
biomarker for the diagnosis of glomerular disease. At the same
time, according to the remission of renal function, the patients
were divided into the remission group and non-remission group. The
clinical classification, and CD68 and TGF-β1 levels were shown to
be independent risk factors that affect the response of renal
function through multivariate logistic regression analysis, which
suggests that the concentrations of CD68 and TGF-β1 can be used as
predictive indexes for renal function remission in patients with
glomerular diseases. Mehta et al (31), analyzed the association between the
plasma TGF-β level and chronic kidney disease in the elderly by
analyzing the data of 1,722 elderly subjects, and evaluated whether
the baseline TGF-β level could predict eGFR. It was found that the
level of TGF-β was independently associated with a lower eGFR in
cross-section analysis, but not with albuminuria.
In the present study, the subjects were selected
strictly according to the inclusion and exclusion criteria. No
significant differences were found in sex, age, BMI and medical
history between the study group and the control group, which
ensured the rigor and reliability of the study. In the present
study, the number of samples was small, and in the analysis of the
prognosis of the patients with glomerular disease, the follow-up
was not performed on the patients. The long-term prognosis was
unknown, which is a limitation to the present study. In future
research, it is necessary to extend the research time and expand
the sample size.
In conclusion, the present study demonstrates that
the increased concentrations of CD68 and TGF-β1 in patients with
glomerular disease are positively correlated with the indicators of
renal injury, and are expected to become clinical diagnostic
indicators of glomerular disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS and LH were responsible for ELISA. JS and LH
analyzed and interpreted the patient data. HS assisted with the
statistical analysis. JS wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Weifang People's Hospital. Patients who participated in this
research, signed the informed consent and had complete clinical
data. Signed written informed consents were obtained from the
patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Herrera-Caceres JO, Finelli A and Jewett
MAS: Renal tumor biopsy: Indicators, technique, safety, accuracy
results, and impact on treatment decision management. World J Urol.
37:437–443. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhu P, Zhou FD, Wang SX, Zhao MH and Wang
HY: Increasing frequency of idiopathic membranous nephropathy in
primary glomerular disease: A 10-yearrenal biopsy study from a
single Chinese nephrology centre. Nephrology (Carlton). 20:560–566.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Thakkar UG, Vanikar AV and Trivedi HL:
Stem cell therapy: An emerging modality in glomerular diseases.
Cytotherapy. 19:333–348. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lees JS, McQuarrie EP and Mackinnon B:
Renal biopsy: It is time for pragmatism and consensus. Clin Kidney
J. 11:605–609. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xu DM, Chen M, Zhou FD and Zhao MH: Risk
Factors for severe bleeding complications in percutaneous renal
biopsy. Am J Med Sci. 353:230–235. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Moledina DG, Hall IE, Thiessen-Philbrook
H, Reese PP, Weng FL, Schröppel B, Doshi MD, Wilson FP, Coca SG and
Parikh CR: Performance of serum creatinine and kidney injury
biomarkers for diagnosing histologic acute tubular injury. Am J
Kidney Dis. 70:807–816. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ling C, Liu X, Shen Y, Chen Z, Fan J,
Jiang Y and Meng Q: Urinary CD80 levels as a diagnostic biomarker
of minimal change disease. Pediatr Nephrol. 30:309–316.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee AH, Ledderose C, Li X, Slubowski CJ,
Sueyoshi K, Staudenmaier L, Bao Y, Zhang J and Junger WG: Adenosine
triphosphate release is required for toll-like receptor-induced
monocyte/macrophage activation, inflammasome signaling,
interleukin-1β production, and the host immune response to
infection. Crit Care Med. 46:e1183–e1189. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liang S, Cai J, Li Y and Yang R:
1,25-Dihydroxy-Vitamin D3 induces macrophage polarization to M2 by
upregulating T-cell Ig-mucin-3 expression. Mol Med Rep.
19:3707–3713. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chistiakov DA, Killingsworth MC,
Myasoedova VA, Orekhov AN and Bobryshev YV: CD68/macrosial in: Not
just a histochemical marker. Lab Invest. 97:4–13. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ooshima A, Park J and Kim SJ:
Phosphorylation status at Smad3 linker region modulates
transforming growth factor-β-induced epithelial-mesenchymal
transition and cancer progression. Cancer Sci. 110:481–488.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shi Q and Chen YG: The functional switch
of TGF-β signaling in breast cancer. Oncotarget. 10:1604–1605.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Trajceska L, Severova-Andreevska G,
Dzekova-Vidimliski P, Nikolov I, Selim G, Spasovski G,
Rambabova-Busletik I, Ristovska V, Grcevska L, Grcevska L and
Sikole A: Complica-tions and risks of percutaneous renal biopsy.
Open Access Maced J Med Sci. 7:992–995. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rakaee M, Busund LR, Jamaly S, Paulsen EE,
Richardsen E, Andersen S, Al-Saad S, Bremnes RM, Donnem T and
Kilvaer TK: Prognostic value of macrophage phenotypes in resectable
non-small cell lung cancer assessed by multiplex
immunohisto-chemistry. Neoplasia. 21:282–293. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Doi K, Nishida O, Shigematsu T, Sadahiro
T, Itami N, Iseki K, Yuzawa Y, Okada H, Koya D, Kiyomoto H, et al:
The Japanese clinical practice guideline for acute kidney injury
2016. Clin Exp Nephrol. 22:985–1045. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nester CM and Falk RJ: Introduction:
Glomerular disease update for the clinician. Clin J Am Soc Nephrol.
11:1662–1663. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ishigami J and Matsushita K: Clinical
epidemiology of infectious disease among patients with chronic
kidney disease. Clin Exp Nephrol. 23:437–447. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Conley SM, Abais JM, Boini KM and Li PL:
Inflammasome activation in chronic glomerular diseases. Curr Drug
Targets. 18:1019–1029. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Noris M and Remuzzi G: Glomerular diseases
dependent on complement activation, including atypical hemolytic
uremic syndrome, membranoproliferative glomerulonephritis, and C3
glomerulopathy: Core curriculum 2015. Am J Kidney Dis. 66:359–375.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Moledina DG, Cheung B, Kukova L, Luciano
RL, Peixoto AJ, Wilson FP, Alfano S and Parikh CR: A survey of
patient attitudes toward participation in biopsy-based kidney
research. Kidney Int Rep. 3:412–416. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang L, Zhang C, Zhang Z, Han B, Shen Z,
Li L, Liu S, Zhao X, Ye F and Zhang Y: Specific clinical and immune
features of CD68 in glioma via 1,024 samples. Cancer Manag Res.
10:6409–6419. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li Z, Maeda D, Yoshida M, Umakoshi M,
Nanjo H, Shiraishi K, Saito M, Kohno T, Konno H, Saito H, et al:
The intratumoral distribution influences the prognostic impact of
CD68-and CD204-positive macrophages in non-small cell lung cancer.
Lung Cancer. 123:127–135. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ngoh CLY, Wee BBK and Wong WK: Lumbar
Artery bleed as a complication of percutaneous renal biopsy and a
proposed workflow for massive bleeding. Case Rep Nephrol Dial.
8:268–276. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dias CB, Malafronte P, Lee J, Resende A,
Jorge L, Pinheiro CC, Malheiros D and Woronik V: Role of renal
expression of CD68 in the long-term prognosis of proliferative
lupus nephritis. J Nephrol. 30:87–94. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guillén-Gómez E, Dasilva I, Silva I, Arce
Y, Facundo C, Ars E, Breda A, Ortiz A, Guirado L, Ballarín JA, et
al: Early macrophage infiltration and sustained inflammation in
kidneys from deceased donors are associated with long-term renal
function. Am J Transplant. 17:733–743. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kang JH, Jung MY, Yin X, Andrianifahanana
M, Hernandez DM and Leof EB: Cell-penetrating peptides selectively
targeting SMAD3 inhibit profibrotic TGF-β signaling. J Clin Invest.
127:2541–2554. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Zeng F, Miyazawa T, Kloepfer LA and Harris
RC: ErbB4 deletion accelerates renal fibrosis following renal
injury. Am J Physiol Renal Physiol. 314:F773–F787. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-β/Smad signaling in
tissue fibrosis. Chem Biol Interact. 292:76–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fukuda A, Minakawa A, Sato Y, Iwakiri T,
Iwatsubo S, Komatsu H, Kikuchi M, Kitamura K, Wiggins RC and
Fujimoto S: Urinary podocyte and TGF-β1 mRNA as markers for disease
activity and progression in anti-glomerular basement membrane
nephritis. Nephrol Dial Transplant. 32:1818–1830. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ostermann M and Joannidis M: Acute kidney
injury 2016: Diagnosis and diagnostic workup. Crit Care.
20(299)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mehta T, Buzkova P, Kizer JR, Djousse L,
Chonchol M, Mukamal KJ, Shlipak M, Ix JH and Jalal D: Higher plasma
transforming growth factor (TGF)-β is associated with kidney
disease in older community dwelling adults. BMC Nephrol.
18(98)2017.PubMed/NCBI View Article : Google Scholar
|