Introduction
Alzheimer's disease (AD) is one of the most common
neurodegenerative disorders in the elderly (1). AD is characterized by learning and
memory dysfunction, cognitive impairment and personality changes
(2). Moreover, this neurological
disease affects >30 million individuals worldwide and this
number is expected to double by the year 2030(3). A main cause of neurodegeneration in AD
is increased production and accumulation of amyloid β (Aβ)
(4). Aβ changes its conformation to
form aggregates, which are eventually deposited as senile plaques;
the pathological hallmark of AD in the brain (5). The aggregated state of Aβ is of great
importance in the induction of its toxic effects and Aβ is also
implicated in increased free radical production, which in turn
induces neuronal damage (6).
Oxidative stress caused by overproduction of free radical
transforms non-aggregated Aβ to aggregated Aβ and Aβ itself is also
a source of free radicals (7).
Consequently, oxidative stress induced by free radicals has been
proposed to be a major contributing factor in neuronal dysfunction
and AD (8). Thus, reducing Aβ
neurotoxicity is one of the key strategies in improving AD outcomes
and there are several studies that support this approach as a valid
therapeutic strategy in treatment of AD pathogenesis (9-11).
Black rice (BR), Oryza sativa L. var.
japonica, is predominantly grown and consumed in Korea,
Japan and China (12). BR is known
to serve several beneficial roles in mitigating the effects of
pathological conditions such as cardiovascular disease, cancer and
inflammation (13-15).
Previous studies have also reported that the anthocyanins from BR,
including cyanidin, malvidin and peonidin, have anti-oxidative,
anti-bacterial and anti-cancer activities (16). Research on BR has mainly focused on
its anthocyanin pigment, which has been shown to exert a positive
effect in preventing arterial aging and lowering blood pressure
(17). It has also been revealed
that anthocyanin inhibits cholesterol absorption and reduces
oxidative stress in cellular models (18,19).
Previous studies on the beneficial effects of BR and its
constituents have focused on the prevention of cancer and
arteriosclerosis (13,14,20,21).
However, to the best of our knowledge, the protective effect of BR
in AD remain unknown and whether consumption of BR improves
cognitive impairment is yet to be elucidated. Therefore, the aim of
the present study was to investigate the protective activity of BR
extracts on cognition and memory impairment in an in vivo AD
model induced by Aβ25-35.
Materials and methods
Reagents
Malondialdehyde (MDA) was purchased from Sigma
Aldrich (Merck KGaA). NaCl was purchased from Bio Basics, Inc.
Thiobarbituric acid (TBA) was provided by Lancaster Synthesis Ltd.
Phosphoric acid and 1-butanol were acquired from Samchun Pure
Chemical Co., Ltd. Methanol (MeOH) was purchased from Duksan Pure
Chemicals Co., Ltd.
Preparation of MeOH extracts of
BR
BR was obtained from Jeon-ju National Agricultural
Cooperative Federation Gongpanjang. Whole BR was washed with water
and dried at 55˚C for 24 h and ground to powder. Then, 10 g BR
powder was refluxed in 200 ml MeOH for 24 h at room temperature and
vacuum-filtered through a Whatman no. 4 filter paper (pore size, 4
µm; Whatman; Cytiva). This was repeated three times and duration of
each cycle was 24 h. The extract was concentrated using a rotary
evaporator at 34˚C. The final yield of this extraction was 3.7%
(w/w). The dried extract was stored in a deep freezer at -80˚C
until further use. The extract was dissolved in PBS for the
subsequent experiments.
Animals and experimental
protocols
The animal protocol used in this study was reviewed
and approved by the Pusan National University-Institutional Animal
Care and Use Committee (approval no. PNU-2010-000142) on the
Ethical Procedures and Scientific Care of Laboratory Animals. A
total of 50 male ICR mice (age, 5 weeks; Orient Bio, Inc.; weight,
25-27 g) were housed in plastic cages at 20±2˚C, 50±10% humidity
and 12 h light/dark cycle with ad libitum access to food and
water. ICR mice were divided into four groups (n=8/group) as
follows: Normal (0.9% NaCl injection + PBS), control
(Aβ25-35 injection + PBS), BR 50 (Aβ25-35
injection + BR MeOH extract 50 mg/kg/day) and BR 100
(Aβ25-35 injection + BR MeOH extract 100 mg/kg/day).
There were no significant differences in body weight among the
groups, which helped to eliminate physical differences due to body
weight variation. The normal and control groups were orally
administered 100 µl of PBS (n=8/group) and the BR 50 and BR 100
groups were orally administered BR extract at doses of 50 and 100
mg/kg/day for 14 days (n=8/group) via oral gavage. All the
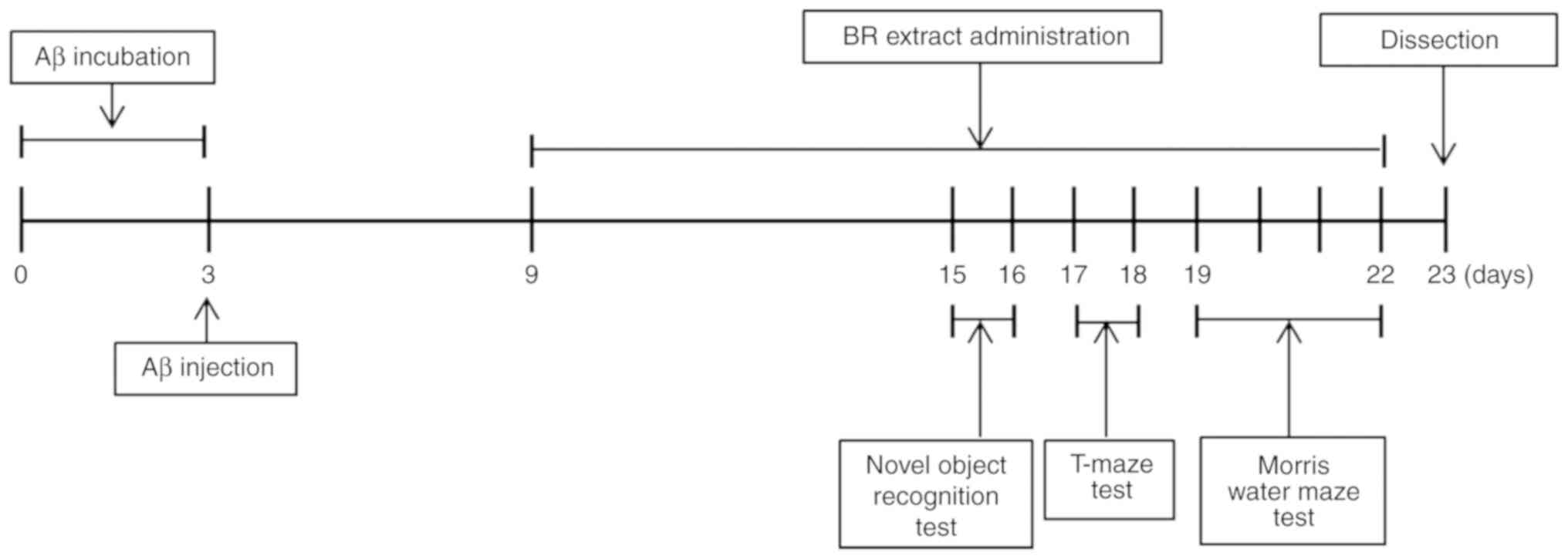
experimental and behavioral procedures are presented in Fig. 1.
Development of the
Aβ25-35-induced mouse model
To induce aggregation, Aβ25-35 (Sigma
Aldrich; Merck KGaA) was solubilized at a concentration of 5 nmol
in 0.9% NaCl and incubated at 37˚C for 3 days. Non-aggregated
Aβ25-35 was dissolved in 0.9% NaCl at same concentration
and incubated at 37˚C for 10 min. Mice were anesthetized with a
mixture of Zoletil 50® (30 mg/kg) and Rompun (10 mg/kg)
to reduce unnecessary pain. When Rompun (xylazine) is added to the
Zoletil, this combination provides rapid induction, immobilization,
good muscle relaxant and smooth recovery from anesthesia; thus, the
Zoletil/Rompun mixture has been commonly used to anesthetize both
wild animals and small laboratory animals (22-24). To ensure the
animals were fully anesthetized, the pedal withdrawal reflex was
assessed by pinching the skin between the toes and any toxic or
side effect, such as muscle tremors, cardiac or respiratory arrest
were not observed. Aggregated Aβ25-35 was dissolved in
saline solution (5 µl) and injected into the right ventricle using
a 10 µl Hamilton microsyringe (Hamilton Company) fitted with a 26
gauge-needle, with the following stereotaxic coordinates from the
Bregma (anteroposterior: -0.2 mm; mediolateral: +1.0 mm;
dorsoventral: -0.22 mm; speed 1 µl/min). The volume of the
injection was 5 µl (5 nmol/mouse) (25). In the preliminary study, to
establish the AD model, mice underwent the same procedures and same
volume (5 µl) of aggregated Aβ25-35 or non-aggregated
Aβ25-35 (n=5, 5 nmol/mouse) were injected into the
bregma. Mice in saline group was injected with 5 µl of 0.9% NaCl.
After 3 days of injection, mice were scarified and measured the
levels of MDA concentrations, as described in ‘Measurement of
lipid peroxidation’. After establishment of AD model, mice in
control and BR groups were given with aggregated Aβ25-35
(5 µl). In the normal group, mice were injected with saline (5 µl)
instead of Aβ25-35. At day 6 post-Aβ25-35
injection, BR 50 and 100 groups of mice were orally administered BR
extract (50 and 100 mg/kg/day, respectively) via oral gavage once a
day for 14 days. The normal and control groups were administered
PBS for 14 days.
Novel object recognition test
Tasks were carried out in mice following 12 days of
Aβ25-35 injection and each mouse underwent one trial/day
for 2 days. The object recognition test was performed in a
black-painted square apparatus (40x30x20 cm), as described
previously (26). A training
session was performed using two identical objects (plastic
bottles). The objects were placed at a fixed distance within a
square field. The mice were placed at the center of the square
field and the number of touch or sniffs each object was recorded
for 10 min. After 24 h, the mice were placed back into the same
field for the test session, in which one of the objects used in the
training session was replaced with a new object (a differently
shaped plastic bottle). The mice were allowed to explore freely for
10 min and the number of touch or sniffs of each object was
manually recorded by two experienced independent observers who were
blind to the groups (27). Object
recognition ability (%) was calculated by comparing the number of
touch or sniff for the old object and new object. All scores in
behavioral tests were counted using the replay function in the
digital camcorder mounted above the apparatus.
T-maze test
The T-maze test was conducted in mice following 14
days of Aβ25-35 injections and each mouse was underwent
one trial/day for 2 days (28). The
apparatus was T-shaped and the walls and bottom of the maze were
equipped with a black square board (length of start and goal stems,
50 cm; width, 13 cm; height, 20 cm). The T-maze used in the current
study consisted of a start box, a left arm and a right arm with a
block door that could be separated. On the first day, each animal
was placed at the start box and the number of right arm entries was
recorded during a 10 min period (training session, one trial per
day). The mice were placed back into the same apparatus 24 h after
the training session and allowed to explore freely for 10 min. The
number of left or right arm entries was manually recorded by two
experienced independent observers (test session, one trial per
day). Space perceptive ability (%) was expressed as a ratio of the
number of entries into either the right (old route) or left arms
(new route) over the total number of arms entries (29). All scores in behavioral tests were
counted using the replay function in the digital camcorder mounted
above the apparatus.
Morris water maze test
The Morris water maze test was performed in mice
after 16 days of Aβ25-35 injection using a previous
procedure established by Morris (30) with slight modification. The
apparatus used in this study consisted of a dark plastic circular
pool (diameter, 80 cm; surrounded by a 40 cm high wall), which was
divided into quadrants with four visual cues on the walls to
provide navigation. Milk powder was added into the pool to make the
water opaque. The water temperature was maintained at 22±1˚C. A
platform (diameter, 8 cm) was placed at 1 cm below surface of the
water in one of the pool's quadrants. The position of the platform
did not change during the training sessions. In total, three
training trials per day were conducted for 3 days. In training
trials, the mice were randomly placed in the water facing the pool
wall and allowed to swim for a maximum of 60 sec. The latency time
to find the platform was recorded. The mice that found the platform
were allowed to rest on the platform for 15 sec. If a mouse did not
reach the platform within 60 sec, it was guided to the platform and
allowed to rest for 15 sec, before being returned to the cage.
Then, 1 day after finishing the training trials (day 4), a probe
trial was performed.
After completion of the probe trial of the Morris
water maze task, a secondary test was conducted by removing the
platform. The mice were placed in the pool and allowed to swim for
60 sec and the time spent in the target quadrant where the platform
had been in the training trails was recorded. For the tertiary
test, the time to reach the platform was recorded in transparent
water. For the secondary and tertiary tests, only one trial was
conducted for each mouse. At the end of each trial, all mice were
dried and returned to the home cage. The time to reach the platform
and the time spent exploring the target quadrant by the animals
were recorded manually using a stopwatch and all scores in
behavioral tests were counted using the replay function in the
digital camcorder mounted above the apparatus.
Measurement of lipid peroxidation
To evaluate MDA levels in the brain, liver and
kidneys, mice were anesthetized using CO2 gas and
sacrificed under controlled chamber-replacement rate of 30%
(chamber volume per minute) as previously reported (31,32).
Death was confirmed by observation of the loss of the postural
reflex and visible cessation of breathing. The brain, liver and
kidneys were isolated immediately and placed on ice for 20 min. The
dissected tissues were weighed and stored at -80˚C. The tissues
were homogenized (12,000 x g; 15 min at 4˚C) in saline solution.
The supernatant was collected and mixed with 1% phosphoric acid and
0.67% TBA, which was then heated at 100˚C for 45 min. After cooling
on ice, 2 ml 1-butanol was added and the samples were centrifuged
(1,150 x g; 10 min at 4˚C). The absorbance values for each
supernatant were measured at 535 and 520 nm wavelength using a
microplate reader. The yield of lipid peroxidase was calculated
using an MDA standard curve (33).
Nitric oxide (NO) scavenging
activity
The NO concentrations in the brain, liver and kidney
tissues were determined according to a previously described method
by Schmidt et al (34).
Briefly, 150 µl tissue homogenate was mixed with 130 µl distilled
water and then 20 µl mixed solution was added to the same amount of
1% sulfanilamide in 5% phosphoric acid and 0.1% N-(1-naphthyl)
ethylene-diamide dihydrochloride solution. The mixture was
incubated at 37˚C for 30 min and the absorbance value was detected
at 540 nm using microplate reader. The yield of NO production was
calculated with a standard curve of NaNO2 content.
Statistical analysis
Statistical significance was determined using
one-way ANOVA followed by Tukey's post-hoc analysis performed using
SPSS version 23 software (IBM Corp.). In the T-maze and novel
object recognition test, the perceptive ability between training
and test sessions were compared using a paired Student's t-test
performed with SAS 9.4 software (SAS Institute, Inc.). Data are
presented as the mean ± SD. P<0.05 was considered to indicate a
statistically significant difference. Each experiment was performed
once.
Results
Establishment of injection of an AD
mouse model
To establish the ideal conditions for the injection
of Aβ25-35 into the cerebral tissues of mice, a
preliminary study was performed. The MDA levels of groups injected
with Aβ25-35 3 days post-injection were significantly
elevated compared with the control group and the group injected
non-incubation Aβ25-35 (Table I). These results were used to
demonstrate that this method could reliably produce an
Aβ25-35-induced AD mouse model.
 | Table IEffect of injection of
Aβ25-35 peptide in mice brain on lipid peroxidation. |
Table I
Effect of injection of
Aβ25-35 peptide in mice brain on lipid peroxidation.
| Group | MDA (nmol/mg
protein) |
|---|
| Saline | 2.57±0.1 |
| Non-aggregated
Aβ25-35 peptide | 2.80±0.4 |
| Aggregated
Aβ25-35 peptide |
3.75±0.7a |
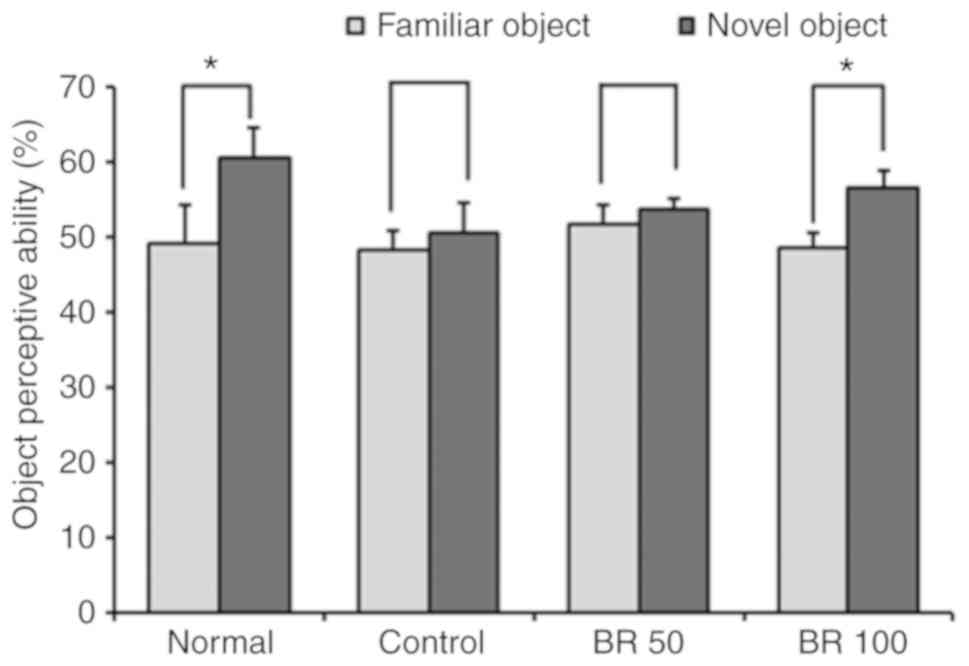
Effect of BR extract on the object
recognition test
The same two objects were explored during training
session and then a test session was conducted 24 h after the
training session. In the testing session, one of the familiar
objects was replaced with a novel object. The normal group
demonstrated a higher number of touches for the novel object
compared with the familiar object, showing 49.12 and 60.54% for
familiar object and novel object, respectively (Fig. 2). However, the control group
injected with Aβ25-35 had no significant preference for
either the familiar or novel object, while the 100 mg BR
administered group had a significantly increased preference for the
novel object compared with the familiar object, 48.57 (familiar
object) and 56.56% (novel object), respectively. These results
indicated that administration of BR (100 mg) extract protected
against object recognition impairment induced by
Aβ25-35.
Effect of BR extract on the T-maze
test
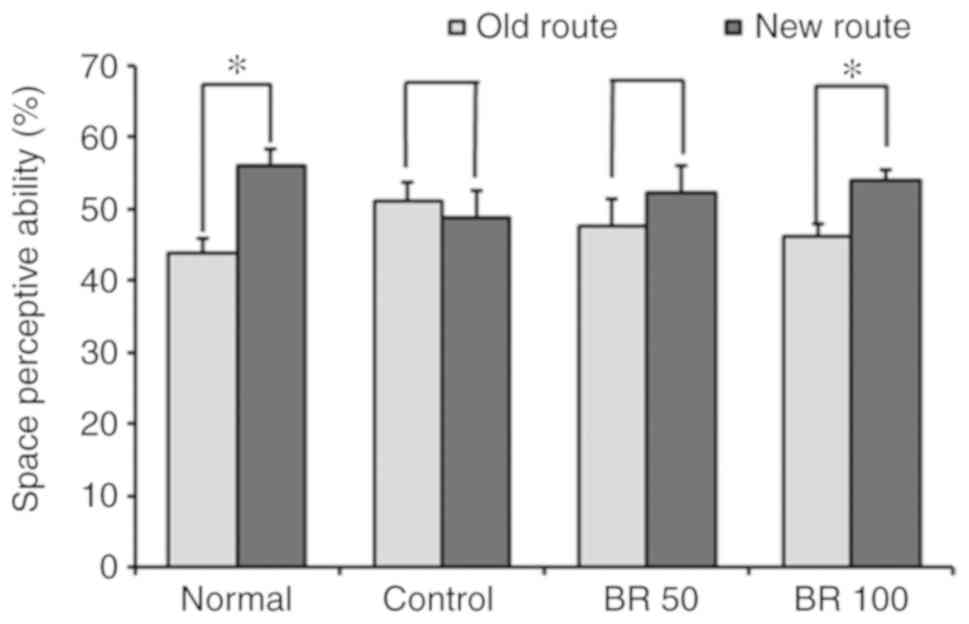
To investigate the protective effect of BR extract
on cognitive dysfunction from Aβ25-35 toxicity, a T-maze
test was conducted (Fig. 3). The
normal group approached the old and new routes at a rate of 43.94
and 56.06%, respectively, indicating a higher number of entries
into the new route compared with the old route. However, the
Aβ25-35-injected control group exhibited no significant
differences in the number of entries between old and new route,
with rates of entries at 51.17 and 48.83%, respectively. However,
mice in the BR 50 group did not exhibit significant differences
between the old and new routes (47.66 and 52.34%, respectively),
the administration of 100 mg BR significantly increased the rate of
the new route entries compared with the old route, with 46.21 and
53.79% for the old and new routes, respectively. These results
demonstrated that the oral administration of 100 mg BR protected
spatial cognition impairments in mice induced by
Aβ25-35.
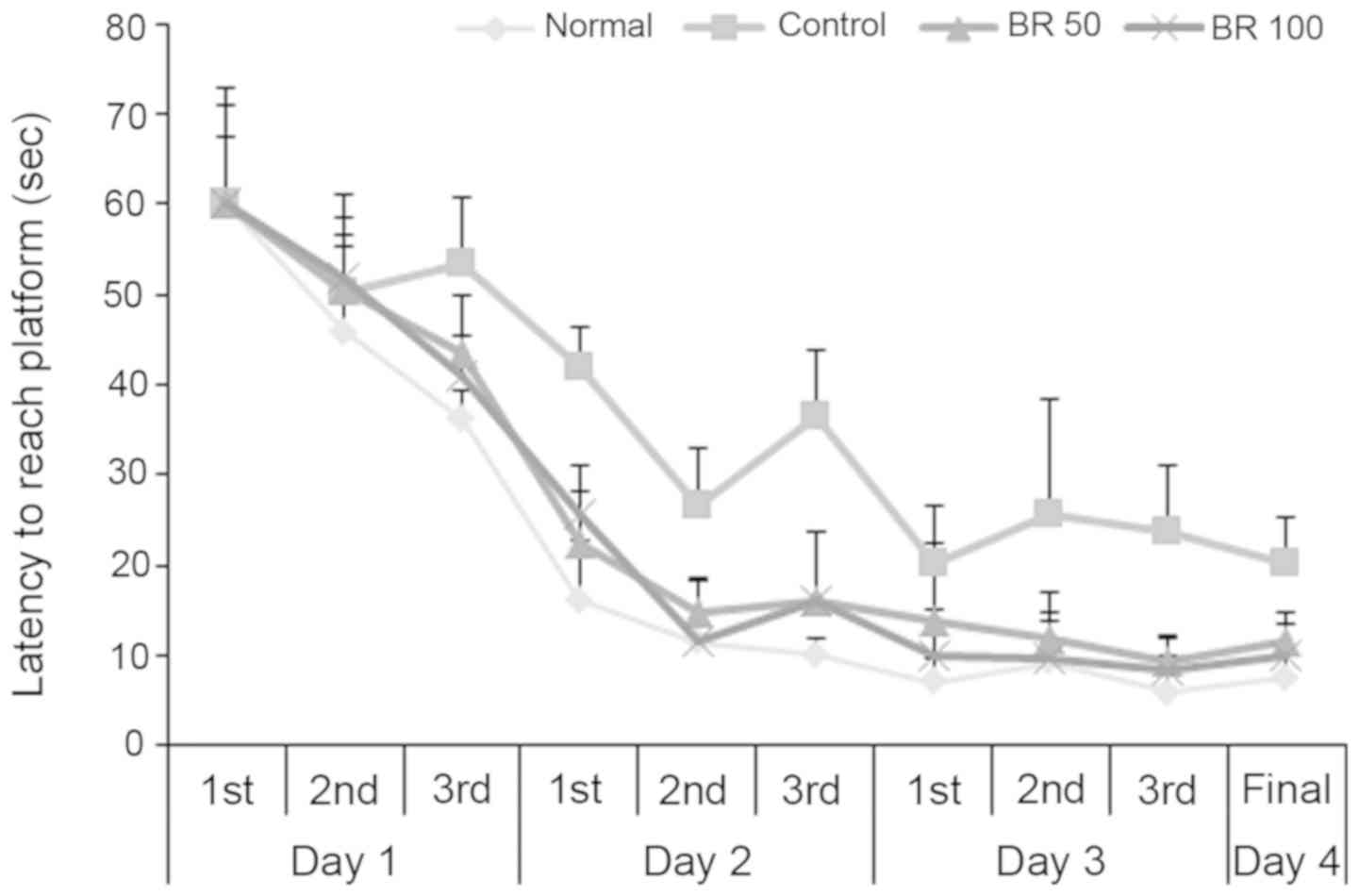
Effect of BR extract on the Morris
water maze test
To assess the protective effect of BR extracts on
spatial learning and memory impairment following Aβ25-35
injection, a Morris water maze test was performed. The
time taken to reach the platform was recorded consecutively during
the test period. It was found that it took less time to reach the
platform in all experimental groups as training time increased.
However, the control group injected with Aβ25-35
recorded a time of 20.25 sec at the final test, which indicated a
relatively small decrease compared with the normal group record of
7.60 sec (Fig. 4). The groups
administered with 50 and 100 mg BR extract recorded 11.67 and 10.00
sec at the final test, respectively, demonstrating a reduced
latency time compared with the control group. There was no
significant difference in the mean latency time when locating the
exposed platform among the experimental groups (Fig. 5). However, when the platform was
hidden, it took longer for the control group mice to find the
platform compared with the normal and BR extract-administered
groups. Thus, these results suggested that the differences in the
time taken to locate platform in the experimental groups were
related to memory ability rather than visual or physical
abilities.
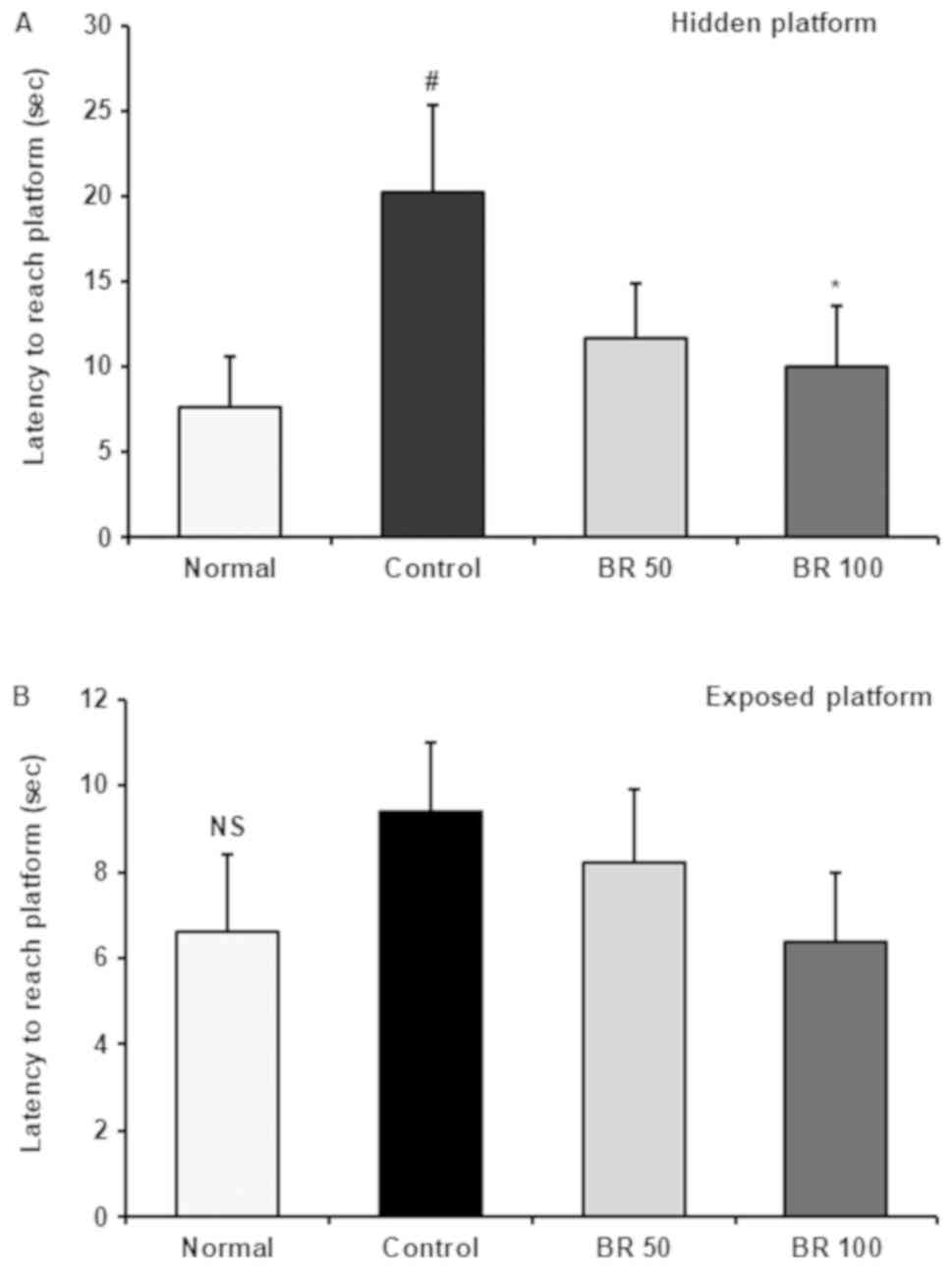 | Figure 5Latency to reach the hidden and
exposed platform in Morris water maze test. Time to find (A) hidden
and (B) exposed platform was recorded on final test day in water
maze test. Data are presented as the mean ± SD.
#P<0.05 vs. Normal, *P<0.05 vs.
Control, by Tukey's multiple range test. NS, no significance.
Normal, 0.9% NaCl injection + oral administration of PBS; Control,
Aβ25-35 injection + oral administration of PBS; BR 50,
Aβ25-35 injection + oral administration of BR MeOH
extract (50 mg/kg/day); BR 100, Aβ25-35 injection + oral
administration of BR MeOH extract (100 mg/kg/day). Aβ, amyloid β;
BR, black rice; MeOH, methanol. |
Inhibitory effect of BR extract on
lipid peroxidation in the tissues
The results of inhibitory effect of BR extract from
lipid peroxidation in tissues are presented in Table II. The MDA concentration of normal
group in brain was 21.22 nmol/mg protein, while the control group
injected with Aβ25-35 had a notably higher MDA level at
69.10 nmol/mg protein. However, the MDA values in the BR extract 50
and 100 mg groups were significantly reduced (51.52 and 46.88
nmol/mg protein, respectively), suggesting that BR extract was
exerting a protective effect against lipid peroxidation in the
brain.
 | Table IIProtective activity of BR from lipid
peroxidation in mice brain induced by Aβ25-35. |
Table II
Protective activity of BR from lipid
peroxidation in mice brain induced by Aβ25-35.
| | MDA (nmol/mg
protein) |
|---|
| Sample | Brain | Kidney | Liver |
|---|
| Normal | 21.22±4.22 | 21.85±5.19 | 2.59±0.77 |
| Control |
69.1±5.49a |
37.29±5.6a |
6.18±1.41a |
| BR 50 |
51.52±7.22b |
23.99±3.66b |
3.71±0.63b |
| BR 100 |
46.88±8.48b |
17.64±4.44b |
3.34±0.73b |
The results from the measurement of the kidney MDA
levels identified that the control group treated with
Aβ25-35 had 37.29 nmol/mg protein, which was higher
compared with the normal group (21.85 nmol/mg protein). However,
the administration of BR 50 and 100 mg inhibited lipid peroxidation
in the kidney, with levels of 23.99 and 17.64 nmol/mg protein,
respectively.
The MDA concentration in the liver of the control
group was 6.18 nmol/mg protein, which was 2.4 times higher compared
with the normal group (2.59 nmol/mg protein). However, 50 and 100
mg of BR extract-administered group had significantly lower MDA
values compared with the control group, with 3.71 and 3.34 nmol/mg
protein, respectively. These results indicated that administration
of BR extract protected lipid peroxidation induced by
Aβ25-35 in brain, kidney and liver.
Effect of BR extract on NO production
in the tissues
Table III presents
the scavenging effect of BR extract from NO generation induced by
Aβ25-35 in tissues. The NO level of the normal group was
27.49 nmol/mg protein, while that of the control group was
significantly increased to 63.68 nmol/mg protein. However, the
groups administered with 50 and 100 mg BR extract demonstrated
lower NO levels compared with the control group, 37.02 and 27.79
nmol/mg protein, respectively.
 | Table IIIEffect of oral administration of BR
on Aβ25-35 induced nitric oxide formation in organ. |
Table III
Effect of oral administration of BR
on Aβ25-35 induced nitric oxide formation in organ.
| | NaNO2
(µmol/mg protein) |
|---|
| Sample | Brain | Kidney | Liver |
|---|
| Normal | 27.49±2.76 | 22.99±3.65 | 29.53±3.89 |
| Control |
63.68±4.93a |
40.35±5.48a |
58.03±5.65a |
| BR 50 |
37.02±4.89b | 33.28±5.20 |
39.77±4.38b |
| BR 100 |
27.79±3.01b | 31.71±4.05 |
34.23±4.43b |
The NO levels in the kidney, (normal group, 22.99
nmol/mg protein; control group, 40.35 nmol/mg protein; BR 50 mg
group, 33.28 nmol/mg protein; BR 100 mg group, 31.71 nmol/mg
protein) and the liver (normal group, 9.53 nmol/mg protein; control
group, 58.03 nmol/mg protein; BR 50 mg group, 39.77 nmol/mg
protein; BR 100 mg group, 34.23 nmol/mg protein) were found to
follow a similar pattern. Collectively, these findings suggested
that supplementation of BR extract can inhibit
Aβ25-35-induced NO formation in the brain, kidney and
liver.
Discussion
AD is one of the most common age-dependent
neurological disorders, affecting mental function, memory and other
cognitive dysfunction, resulting in changes in personality and
behavior (35). Deposition of Aβ
plaques in the brain is the most important risk factor for the
development of AD (36). Previous
studies have reported that acute or continuous injections of Aβ
into the brain of mice can cause neurodegeneration and impair
learning and memory abilities (37,38).
Therefore, the AD mouse model induced by Aβ25-35 is
widely used to study the pathology and screen therapeutics against
AD. Aβ25-35 is the core fragment of full-length Aβ and
exerts several of the characteristics of full-length Aβ peptides,
including the neurotoxic properties described in patients with AD
(39). According to previous study,
Aβ25-35 is more toxic compared with the full-length
peptide and often causes oxidative damage more rapidly than
full-length Aβ (40). Moreover, the
injection of Aβ25-35 into the brain leads to learning
and memory dysfunction via the deposition and dissemination of Aβ
in the cortex and hippocampus of mice (41). The aggregation of Aβ also induces
oxidative stress via the overproduction of free radicals and Aβ
transforms itself from its non-aggregated to its aggregated form
(7). Thus, the
Aβ25-35-injected mice model is an effective method of
examining functional improvements and pathological effects.
Furthermore, the inhibition of Aβ accumulation and attenuation of
oxidative stress are important strategies in the treatment of AD.
Therefore, efforts to identify dietary supplements with antioxidant
activities to help prevent AD have attracted increased attention in
recent years.
A previous study revealed that BR extract (125 and
250 mg/kg body weight) did not significantly influence liver
function as demonstrated by the non-significant changes in the
serum levels of alanine aminotransferase and aspartate
transaminase, which are enzymatic bio-marker for liver toxicity
(42). BR extract is also rich in
polyphenols with anthocyanins, which have no toxic effects at doses
≤20 mg/kg/day in rat and 25 mg/kg/day in mice (43). Previous studies have reported that
bread containing BR contributes to the reduction of Aβ peptide
concentrations in the plasma of aged mice (44). In addition, BR and its constituent,
cyanidin, have been shown to attenuate Aβ-induced neuronal cell
death via modulation of the mitochondrial death pathway in SK-N-SH
cells (45). Anthocyanin, a major
component of BR, prevents Aβ-induced neurotoxicity by inhibiting
reactive oxygen species production and regulating Ca2+
homeostasis (46). Moreover,
anthocyanin has been revealed to block β-secretase activation,
which is a key enzyme in Aβ production (46). However, to the best of our
knowledge, there is limited evidence of BR efficacy against
Aβ-induced cognitive impairment and oxidative damage in
vivo. Therefore, the present study investigated the
neuroprotective effects of BR extract on cognitive dysfunction in
an Aβ25-35-induced AD mice model.
It has been previously demonstrated that neither the
reversed nor scrambled peptide of Aβ 25-35 can induce
neurodegenerative changes in animal brain (47,48).
Therefore, to establish the incubation method for Aβ, a preliminary
study was performed in mice. It was found that injection of
Aβ25-35 into brain after aggregation at 37˚C for 3 days
led to lipid peroxidation. The MDA level of groups injected with
aggregated Aβ25-35 was significantly elevated compared
with the control and non-incubated Aβ25-35 injected
groups. Thus, the present study injected Aβ25-35 after 3
days of incubation at 37˚C to investigate the protective effect of
BR against AD-associated memory impairment.
Previous studies have reported that BR has
anti-oxidant, anti-inflammatory and anti-hyperlipidemic activities
(49,50) Furthermore, anthocyanins, such as
cyanidin and malvidin, from BR have been shown to serve a
protective role in numerous pathological conditions via their
induction of superoxide dismutase and catalase (51). However, to the best of our
knowledge, studies on the protective effect of BR against aging and
aging-related degenerative diseases including AD have not been
performed. In the current study, BR extracts significantly improved
the cognitive impairments induced by Aβ25-35 in the
T-maze test, object recognition test and Morris water maze test. A
T-maze test is used to evaluate the short-term memory of mice
(52), while a novel object
recognition test is used to obtain information on the amnesiant
potential of functional substances (29). Moreover, since patients with AD
exhibit deficits in object recognition, this task is considered as
a useful tool to investigate learning ability and memory function
in animal models (53). In the
novel object recognition test, the exploration of a previously seen
object and a novel object is measured and used as an index of
memory performance (54). The
present results indicated that cognitive dysfunction was observed
in the Aβ25-35-induced mice, as demonstrated by the lack
of preference for the new routes and objects compared with the
familiar route and object. However, groups administered BR extracts
had significantly increased preference for new routes and objects
compared with the familiar route and object, suggesting BR can
protect the impairment of learning and memory function against
Aβ25-35.
The Morris water maze test is well-known for the
assessment of spatial cognition ability and long-term memory
(55). In training trials, the
latency of mice administered BR was significantly shortened by
training repeatedly for 3 days compared with control group mice.
Furthermore, the groups that were BR exhibited a considerable
decrease in the time to reach the platform compared with the
control group in the final test (day 4), indicating that the mice
administered with BR extract could recognize the location of the
platform, even when it was removed. In addition, the time to reach
the exposed platform was not significantly different among the
groups, while the time was shorter in BR treated group compared
with the control group when the platform was hidden. These
experimental results indicated that BR exerts a protective effect
against cognitive impairment induced by Aβ25-35 and this
effect is not related to the visual or exercise abilities.
Lipid peroxidation is widespread in the AD brain and
is a marker for oxidative stress (56). Previous studies have revealed that
lipid peroxidation is an important mechanism for neurodegeneration
in AD and Aβ causes lipid peroxidation in the brain (57-59).
Moreover, the injection of Aβ25-35 into the brain of
mice leads to notable increases in MDA levels in the hippocampus,
indicating that Aβ25-35 results in lipid peroxidation
(57-59). NO is
involved in neuronal death in AD and other neurodegenerative
disorders (60). Furthermore, NO
can generate peroxynitrite via its reaction with
O2-, which induces various chemical reactions
producing compounds such as nitrotyrosine (61). It has also been reported that the
overproduction of nitrotyrosine is correlated with increased levels
of cerebral Aβ and NO-mediated oxidative damage in the brain
contributes to the neurotoxicity and cognitive impairments
(62). Cleavage of the Aβ precursor
protein (APP), one of the most abundant proteins present in central
nervous system, can produce Aβ (63). APP is ubiquitously expressed in
muscle, epithelial and several circulating cells (64,65).
Furthermore, deposition of Aβ is detectable in the brain and
several other tissues, including the skin, intestine and other
organs, and Aβ is circulated in the blood and cerebrospinal fluid
(66,67). Accumulation of Aβ is also strongly
associated with oxidative stress, leading to pathological
conditions in the peripheral tissues. For instance, our previous
studies revealed that the injection of Aβ25-35
significantly elevated the levels of MDA and NO in the brain and
liver of mice (68-72).
In the present study, groups administered with BR extract had
significantly reduced MDA and NO contents in the brain, liver and
kidney. Moreover, the protective effect of BR extract against lipid
peroxidation and NO production was greatest in the brain.
Collectively, these findings suggested that BR supplementation may
exert a positive effect on cognitive improvement by attenuating
oxidative stress induced by Aβ25-35.
A limitation with the present study was that only
cognitive improvement by oral administration of BR extract was
observed. This may be associated with attenuation of oxidative
stress in vivo model. However, the molecular mechanisms by
which BR extract ameliorates Aβ-induced cognitive deficit through
anti-oxidative pathway remains unclear. In addition, the present
study emphasized the effect of BR MeOH extract; however, active
compounds, including anthocyanins, were not examined. Therefore,
characterizing the specific active compound and elucidating the
mechanism of action, which responsible for learning and memory
improvement property of BR, should be investigated further.
In conclusion, it was demonstrated that
supplementation with BR extracts resulted in improved cognitive
function, as indicated by behavioral tests in the
Aβ25-35-induced AD mouse model. BR administration also
significantly inhibited the generation of MDA and NO in the brain,
kidney and liver following Aβ25-35 injection. Although
further studies are required to evaluate the underlying mechanism
involved in the neuroprotective effects of BR on Aβ-induced
cognitive impairment and oxidative damage, BR may have a role as a
protective agent against Aβ-induced learning and memory impairment,
which may be mediated by attenuating oxidative stress.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AYL, JMC and SHS were responsible for data
acquisition, analysis and interpretation. AYL wrote the manuscript
and prepared the figures and tables. YAL participated in the design
of the study and assisted in certain experiments. AYL and YAL were
responsible for the critical revision of the manuscript. EJC was
responsible for the research creation and design, interpretation of
data and critical revision of the manuscript for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The experimental procedures were approved and
permitted using the guidelines established by the Pusan National
University Institutional Animal Care and Use Committee (approval
no. PNU-2010-000142).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fratiglioni L and Qiu C: Prevention of
common neurodegenerative disorders in elderly. Exp Gerontol.
44:46–50. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Praticó D: Alzheimer's disease and oxygen
radicals: New insights. Biochem Pharmacol. 63:563–567.
2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Maddison DC and Giorgini F: The kynurenine
pathway and neurodegenerative disease. Semin Cell Dev Biol.
40:134–141. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Selkoe DJ: Soluble oligomers of the
amyloid β-protein impair synaptic plasticity and behavior. Behav
Brain Res. 192:106–113. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Millucci L, Raggiaschi R, Franceschini D,
Terstappen G and Santucci A: Rapid aggregation and assembly in
aqueous solution of Aβ (25-35) peptide. J Biosci. 34:293–303.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pike CJ, Walencewicz AJ, Glabe CG and
Cotman CW: In vitro aging of β-amyloid protein causes
peptide aggregation and neurotoxicity. Brain Res. 563:311–314.
1991.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dyrks T, Dyrks E, Hartmann T, Masters C
and Beyreuther K: Amyloidogenicity of beta A4 and beta A4-bearing
amyloid protein precursor fragments by metal-catalyzed oxidation. J
Biol Chem. 267:18210–18217. 1992.PubMed/NCBI
|
|
8
|
Reddy PH: Amyloid precursor
protein-mediated free radicals and oxidative damage: Implications
for the development and progression of Alzheimer's disease. J
Neurochem. 96:1–13. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rubio-Perez JM, Albaladejo MD, Zafrilla P,
Vidal-Guevara ML and Morillas-Ruiz JM: Effects of an antioxidant
beverage on biomarkers of oxidative stress in Alzheimer's patients.
Eur J Nutr. 55:2105–2116. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nabavi SF, Braidy N, Orhan IE, Badiee A,
Daglia M and Nabavi SM: Rhodiola rosea L. and Alzheimer's disease:
From farm to pharmacy. Phytother Res. 30:532–539. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Kong S and Lee J: Antioxidants in milling
fractions of black rice cultivars. Food Chem. 120:278–281.
2010.
|
|
13
|
Salgado JM, Oliveira AG, Mansi DN,
Donado-Pestana CM, Bastos CR and Marcondes FK: The role of black
rice (Oryza sativa L.) in the control of
hypercholesterolemia in rats. J Med Food. 13:1355–1362.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen XY, Zhou J, Luo LP, Han B, Li F, Chen
JY, Zhu YF, Chen W and Yu XP: Black rice anthocyanins suppress
metastasis of breast cancer cells by targeting RAS/RAF/MAPK
pathway. Biomed Res Int. 2015(414250)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Limtrakul P, Yodkeeree S, Pitchakarn P and
Punfa W: Suppression of inflammatory responses by black rice
extract in RAW 264.7 macrophage cells via downregulation of NF-κB
and AP-1 signaling pathways. Asian Pac J Cancer Prev. 16:4277–4283.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen PN, Kuo WH, Chiang CL, Chiou HL,
Hsieh YS and Chu SC: Black rice anthocyanins inhibit cancer cells
invasion via repressions of MMPs and u-PA expression. Chem Biol
Interact. 163:218–229. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jennings A, Welch AA, Fairweather-Tait SJ,
Kay C, Minihane AM, Chowienczyk P, Jiang B, Cecelija M, Spector T,
Macgregor A and Cassidy A: Higher anthocyanin intake is associated
with lower arterial stiffness and central blood pressure in women.
Am J Clin Nutr. 96:781–788. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yao SL, Xu Y, Zhang YY and Lu YH: Black
rice and anthocyanins induce inhibition of cholesterol absorption
in vitro. Food Funct. 4:1602–1608. 2016.
|
|
19
|
Sangkitikomol W, Tencomnao T and
Rocejanasaroj A: Antioxidant effects of anthocyanins-rich extract
from black sticky rice on human erythrocytes and mononuclear
leukocytes. Afr J Biotechnol. 9:8222–8229. 2010.
|
|
20
|
Wang LS and Stoner GD: Anthocyanins and
their role in cancer prevention. Cancer Lett. 269:281–290.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xia M, Ling WH, Ma J, Kitts DD and
Zawistowski J: Supplementation of diets with the black rice pigment
fraction attenuates atherosclerotic plaque formation in
apolipoprotein E deficient mice. J Nutr. 133:744–751.
2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Latagliata EC, Lo lacono L, Chiacchierini
G, Sancandi M, Rava A, Oliva V and Puglisi-Allegra S: Single
prazosin infusion in prelimbic cortex fosteres extinction of
alphetamine-induced conditioned place preference. Front Pharmacol.
8(530)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Turnbull MT, Boskovic Z and Coulson EJ:
Acute Down-regulation of BDNF signaling does not replicate
exacerbated amyloid-β levels and cognitive impairment induced by
cholinergic basal forebrain lesion. Front Mol Neurosci.
11(51)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Stahl K, Rahmani S, Prydz A, Skauli N,
MacAulay N, Mylonakou MN, Torp R, Skare Ø, Berg T, Leergarard TB,
et al: Targeted deletion of the aquaglyceroporin AQP9 is protective
in a mouse model of Parkinson's disease. PLoS One.
13(e0194896)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Laursen SE and Belknap JK:
Intracerebroventricular injections in mice: Some methodological
refinements . J Pharmacol Methods. 16:355–357. 1986.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bevins RA and Besheer J: Object
recognition in rats and mice: A one-trial non-matching-to-sample
learning task to study ‘recognition memory’. Nat Protoc.
1:1306–1311. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bertaina-Anglade V, Enjuanes E, Morillon D
and Drieu la Rochelle C: The object recognition task in rats and
mice: A simple and rapid model in safety pharmacology to detect
amnesic properties of a new chemical entity. J Pharmacol Toxicol
Methods. 54:99–105. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Montgomery KC: A test of two explanations
of spontaneous alternation. J Comp Physiol Psychol. 45:287–293.
1952.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Spowart-Manning L and Van Der Staay FJ:
The T-maze continuous alternation task for assessing the effects of
putative cognition enhancers in the mouse. Behav Brain Res.
151:37–46. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Morris R: Developments of a water-maze
procedure for studying a spatial learning in the rat. J Neurosci
Methods. 11:47–60. 1984.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Creamer-Hente MA, Lao FK, Dragos ZP and
Waterman LL: Sex- and Strain-related differences in the stress
response of mice to CO2 euthanasia. J Am Assoc Lab Anim Sci.
57:513–519. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Moody CM, Chua B and Weary DM: The effect
of carbon dioxide flow rate on the euthanasia of laboratory mice.
Lab Anim. 48:298–304. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Schmidt HH, Warner TD, Nakane M,
Förstermann U and Murad F: Regulation and subcellular location of
nitrogen oxide synthases in RAW264.7 macrophages. Mol Pharmacol.
41:615–624. 1992.PubMed/NCBI
|
|
35
|
Poling A, Morgan-Paisley K, Panos JJ, Kim
EM, O'Hare E, Cleary JP, Lesné S, Ashe KH, Porritt M and Baker L:
Oligomers of the amyloid-beta protein disrupt working memory:
Confirmation with two behavioral procedures. Behav Brain Res.
193:230–234. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bush AI: The metallobiology of Alzheimer's
disease. Trends Neurosci. 26:207–214. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Maurice T, Lockhart BP and Privat A:
Amnesia induced in mice by centrally administered beta-amyloid
peptides involves cholinergic dysfunction. Brain Res. 706:181–193.
1996.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yamada K, Nitta A, Saito T, Hu J and
Nabeshima T: Changes in ciliary neurotrophic factor content in the
rat brain after continuous intracerebroventricular infusion of
beta-amyloid (1-40) protein. Neurosci Lett. 201:155–158.
1995.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Olariu A, Tran MH, Yamada K, Mizuno M,
Hefco V and Nabeshima T: Memory deficits and increased emotionality
induced by beta-amyloid (25-35) are correlated with the reduced
acetylcholine release and altered phorbol dibutyrate binding in the
hippocampus. J Neural Transm (Vienna). 108:1065–1079.
2001.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hervás-Aguilar A, Puebla-Jiménez L,
Burgos-Ramos E, Aguado-Llera D and Arilla-Ferreiro E: Effects of
single and continuous administration of amyloid β-peptide (25-35)
on adenylyl cyclase activity and the somatostatinergic system in
the rat frontal and parietal cortex. Neurosciences. 135:181–190.
2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Stepanichew MY, Zdobnava IM, Zarubenko II,
Moiseeva YV, Lazareva NA, Onufriev MV and Gulyaeva NV:
Amyloid-beta(25-35)-induced memory impairments correlate with cell
loss in rat hippocampus. Physiol Behav. 80:647–655. 2004.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Al-Jameel SS and Al-Namshan MM: Protective
effect of black rice extract on the functional status of lver and
hepatic stellate cell against toxicity induced by ethanol. J Indian
Chem Soc. 94:213–220. 2017.
|
|
43
|
Wallace TC and Giusti MM: Anthocyanins.
Adv Nutr. 6:620–622. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Nakamura S, Hara T, Joh T, Kobayashi A,
Yamazaki A, Kasuga K, Ikeuchi T and Ohtsubo K: Effects of
super-hard rice bread blended with black rice bran on amyloid β
peptide production and abrupt increase in postprandial blood
glucose levels in mice. Biosci Biotechnol Biochem. 81:323–334.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Badshah H, Kim TH and Kim MO: Protective
effect of anthocyanins against amyloid beta-induced neurotoxicity
in vivo and in vitro. Neurochem Int. 80:51–59. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Thummayot S, Tocharus C, Pinkaew D,
Biwatpinyo K, Sringarm K and Tocharus J: Neuroprotective effect of
purple rice extract and its constituent against amyloid
beta-induced neuronal cell death in SK-N-SH cells. Neurotoxicology.
45:149–158. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Maurice T, Lockhart BP, Su TP and Privat
A: Reversion of beta 25-35-amyloid peptide-induced amnesia by NMDA
receptor-associated glycine site agonists. Brain Res. 731:249–253.
1996.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sun MK and Alkon DL: Impairment of
hippocampal CA1 heterosynaptic transformation and spatial memory by
beta-amyloid (25-35). J Neurophysiol. 87:2441–2449. 2002.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Choi SP, Kang MY and Nam SH: Inhibitory
activity of pigmented rice bran extract to the allergic
inflammation in basophilic cell line and peritoneal mast cells. J
Korean Soc Appl Biol Chem. 48:315–321. 2005.
|
|
50
|
Ling WH, Cheng QX, Ma J and Wang T: Red
and black rice decrease atherosclerotic plaque formation and
increase antioxidant status in rabbits. J Nutr. 131:1421–1426.
2001.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Chiang AN, Wu HL, Yeh HI, Chu CS, Lin HC
and Lee WC: Antioxidant effects of black rice extract through the
induction of superoxide dismutase and catalase activities. Lipids.
41:797–803. 2006.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gerlai R: A new continuous alternation
task in T-maze detects hippocampal dysfunction in mice. A strain
comparison and lesion study. Behav Brain Res. 95:91–101.
1998.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Caterini F, Della Sala S, Spinnler H,
Stangalino C and Tumbull OH: Object recognition and object
orientation in Alzheimer's disease. Neurophysicology. 16:146–155.
2002.
|
|
54
|
Mumby DG, Gaskin S, Glenn MJ, Schramek TE
and Lehmann H: Hippocampal damage and exploratory preferences in
rats: Memory for objects, places and contexts. Learn Mem. 9:49–57.
2002.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Vorhees CV and Williams MT: Morris water
maze: Procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Butterfield DA, Castegna A, Lauderback CM
and Drake J: Evidence that amyloid beta-peptide-induced lipid
peroxidation and its sequelae in Alzheimer's disease brain
contribute to neuronal death. Neurobiol Aging. 23:655–664.
2002.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Praticò D, Uryu K, Leight L, Trojanoswki
JQ and Lee VM: Increased lipid peroxidation precedes amyloid plaque
formation in an animal model of Alzheimer amyloidosis. J Neurosci.
21:4183–4187. 2001.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Montine TJ, Neely MD, Quinn JF, Beal MF,
Markesbery WR, Roberts LJ and Morrow JD: Lipid peroxidation in
aging brain and Alzheimer's disease. Free Radic Biol Med.
33:620–626. 2002.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Butterfield DA and Lauderback CM: Lipid
peroxidation and protein oxidation in Alzheimer's disease brain:
Potential causes and consequences involving amyloid
beta-peptide-associated free radial oxidative stress. Free Radic
Biol Med. 32:1050–1060. 2002.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Lu P, Mamiya T, Lu LL, Mouri A, Niwa M,
Hiramatsu M, Zou LB, Nagai T, Ikejima T and Nabeshima T: Silibinin
attenuates amyloid beta(25-35) peptide-induced memory impairments:
Implication of inducible nitric-oxide synthase and tumor necrosis
factor-Alpha in mice. J Pharmacol Exp Ther. 331:319–326.
2009.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Brwon GC: Nitric oxide and neuronal death.
Nitric Oxide. 23:153–165. 2010.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Pacher P, Beckman JS and Liaudet L: Nitric
oxide and peroxynitrite in health and disease. Physiol Rev.
87:315–424. 2007.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Selkoe DJ: Alzheimer's disease: A central
role for amyloid. J Neuropathol Exp Neurol. 53:438–447. 1994.
|
|
64
|
Gardella JE, Gorgone GA, Newman P,
Frangione B and Gorevic PD: Characterization of Alzheimer amyloid
precursor protein transcripts in platelets and megakaryocytes.
Neurosci Lett. 138:229–232. 1992.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Mattson MP, Furukawa K, Bruce AJ, Mark RJ
and Blanc EM: Calcium homeostasis and free radical metabolism as
convergence points in the pathophysiology of dementia. In: Wasco W
and Tanzi RE, (eds) Molecular Mechanisms of Dementia. New York,
pp103-143, 1995.
|
|
66
|
Mattson MP, Begley JG, Mark RJ and
Furukawa KA: Abeta25-35 induces rapid lysis of red blood cells:
Contrast with Abeta1-42 and examination of underlying mechanisms.
Brain Res. 771:147–153. 1997.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Yasojima K, McGeer EG and McGeer PL:
Relationship between beta amyloid peptide generating molecules and
neprilysin in Alzheimer disease and normal brain. Brain Res.
919:115–121. 2001.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Miklossy J, Qing H, Radenovic A, Kis A,
Vileno B, Làszló F, Miller L, Martins RN, Waeber G, Mooser V, et
al: Beta amyloid and hyperphosphorylated tau deposits in the
pancreas in type 2 diabetes. Neurobiol Aging. 31:1503–1515.
2010.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Smith MA, Sayre LM, Monnier VM and Perry
G: Radical ageing in Alzheimer's disease. Trends Neurosci.
18:172–176. 1995.
|
|
70
|
Choi JY, Cho EJ, Lee HS, Lee JM, Yoon YH
and Lee S: Tartary buckwheat improves cognition and memory function
in an in vivo amyloid-β-induced Alzheimer model. Food Chem Toxicol.
53:105–111. 2013.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Lee AY, Yamabe N, Kang KS, Kim HY, Lee S
and Cho EJ: Cognition and memory function of Taraxacum
coreanum in an in vivo amyloid-β-induced mouse model of
Alzheimer's disease. Arch Biol Sci. 66:1357–1366. 2014.
|
|
72
|
Choi JY, Lee JM, Lee DG, Cho S, Yoon YH,
Cho EJ and Lee S: The n-Butanol fraction and rutin from tartary
buckwheat improve cognition and memory in an in vivo model of
amyloid-β-induced Alzheimer's disease. J Med Food. 18:631–641.
2015.PubMed/NCBI View Article : Google Scholar
|