Introduction
Tumour heterogeneity has been considered as an
obstacle for curing cancers. Among the hierarchically organized
tumours, exist a small portion of cancer cells that display stem
cell properties, which are termed cancer stem cells (CSCs)
(1). CSCs are essential for cancer
development, with self-renewal, differentiation, tumorigenesis and
chemoresistance properties (1,2).
Breast cancer is the most frequently occurring
cancer in women, causing the highest number of cancer-related
deaths in females worldwide (3). In
2018, 15% of all cancer-related deaths in females were caused by
breast cancer (4,5). Breast cancer stem cells (BCSCs), which
can be identified as cluster of differentiation
(CD)44+CD24- cells, are thought to be
responsible for the origin, metastasis and drug resistance of
breast cancer (6). Understanding
the regulatory mechanism underlying BCSCs may be beneficial for
developing improved therapeutic strategies for breast cancer.
MicroRNAs (miRNAs/miRs) are a class of small,
non-coding RNAs that are ~20 nucleotides in length (7,8). By
binding to the 3'-untranslated region of target mRNAs by
complementary base-pairing, miRNAs can induce target mRNA
degradation and translation suppression, thereby negatively
regulating gene expression (7,8). miRNA
dysregulation has been reported in a number of different types of
cancer (7). miRNAs serve important
regulatory roles in cancer initiation and development, affecting
the fate of CSCs (8-10).
miRNAs Let-7(11), miR-200c
(12,13), miR-93(14), miR-203(15) and mi-600(16) have been reported to modulate the
properties of BCSCs. The fate of other CSCs, such as liver
(17), prostate (18), colorectal (19,20)
and glioblastoma (21) CSCs, has
also been linked to miRNAs.
miR-376c-3p has been implicated in signalling
pathways that modulate cancer procession. miR-376c-3p has been
identified as a cancer suppressor in head and neck squamous cancer
via targeting of RUNX family transcription factor 2 (RUNX2) and
suppressing RUNX2-mediated metastasis (22). Moreover, miR-376c-3p negatively
regulates gastric tumour growth by modulating the expression of
BCL2 associated agonist of cell death (BAD) and Smad4(23). miR-376c-3p also suppresses cell
proliferation and induces apoptosis in oral squamous cancer cells
and neuroblastoma cells by targeting homeobox B7(24) and Cyclin D1(25), respectively. However, another study
reported that miR-376c-3p facilitates hepatocellular carcinoma
progression by inhibiting AT-rich interaction domain 2 expression
(26). miR-367c-3p was also
reported to enhance cell viability and inhibit apoptosis in
colorectal cancer cells (27).
Ras-related protein Rab-2A(RAB2A) is a small GTPase
that has been identified as an oncogene in breast cancer (28,29).
The present study suggested that miR-376c-3p was downregulated in
breast cancer by conducting a miRNA microarray analysis. RAB2A was
predicted as a target of miR-376c-3p via online software
TargetScan. Moreover, the expression level of miR-376c-3p was
negatively correlated with the expression level of RAB2A in breast
cancer tumours. Further investigation demonstrated that miR-376c-3p
inhibited BCSC fate and properties by targeting RAB2A.
Materials and methods
Tumour samples
A total of 60 paired breast tumour samples and
adjacent non-tumorous tissues (obtained 3-4 cm from the macroscopic
tumor) were obtained from patients (all female) with breast cancer
who underwent surgical resection at Shanghai Ninth People's
Hospital from March 2013 to December 2015 (Table SI). Adjacent breast tissues were
confirmed as non tumoral by conventional histopathological
analysis. All patients had not been diagnosed with additional
co-morbidities or other types of cancer. The clinic pathological
characteristics of the patients are presented in Table SI. Written informed consent was
obtained from all participants. The present study was approved by
the Ethics Committee of Shanghai Ninth People's Hospital (approval
no. 2016-147-T96).
Cell lines
Human BCSCs were isolated and maintained as
previously described (30,31). Briefly, human breast tumour cells
were digested with collagenase and strained using a 40-µm filter.
CD44+CD24- cells were further sorted via
fluorescence-activated cell sorting using anti-CD44 (cat. no.
75122; 1:100) and anti-CD24 (cat. no. 68390; 1:330) antibodies
(each, Cell Signaling Technology, Inc.). BCSCs were maintained as
spheres in ultralow attachment flasks in serum-free DMEM-F12
(Gibco; Thermo Fisher Scientific, Inc.; cat. no. 11320033)
supplemented with 2% B27 (cat. no. 17504044;Gibco; Thermo Fisher
Scientific, Inc.), 10 ng/ml basic fibroblast growth factor (cat.
no. PHG0266; Gibco; Thermo Fisher Scientific, Inc.), 20 ng/ml
epidermal growth factor (cat. no. PHG0311; Gibco; Thermo Fisher
Scientific, Inc.), 5 µg/ml insulin (cat. no. I8830; Beijing
Solarbio Science & Technology Co., Ltd.) and 1%
penicillin-streptomycin (cat. no. 10378016; Gibco; Thermo Fisher
Scientific, Inc.). The rest of the breast cancer cells (all cells
other than CD44+CD24- cells) were
characterized as non-BCSCs and maintained in the same conditions as
BCSCs. All cells were maintained at 37˚C with 5%
CO2.
293T cells, Lenti-X 293T cells (cat. no. 632180;
Clontech Laboratories, Inc.) and MCF7 cells were maintained in DMEM
(cat. no. C11995500; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (cat. no. 30044333; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin at 37˚C with 5%
CO2.
Plasmids, small interfering (si)RNAs
and transfection
RAB2B expressing plasmid (pcDNA-RAB2A) was
constructed by inserting the RAB2A coding sequence into vector
pcDNA 3.1 (cat. no. V79020; Invitrogen; Thermo Fisher Scientific,
Inc.). pcDNA 3.1 was used as the negative control for pcDNA-RAB2A.
miR-376c-3p mimic (cat. no. miR10000720-1-5), negative control (NC)
mimic (cat. no. miR1N0000001-1-5), si-RAB2A#1 (cat. no.
siB150325183848-1-5), si-RAB2A#2 (cat. no. siB150325183922-1-5), NC
siRNA (cat. no. siN0000001-1-5), miR-376c-3p inhibitor (cat. no.
miR20000720-1-5) and NC inhibitor (cat. no. miR2N0000002-1-5) were
purchased from Guangzhou RiboBio Co., Ltd. BCSCs in 6-well plates
(1x105 cells/well) were transfected with 1 µg plasmids
and 5 µl of siRNAs (20 µM) using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. At 48 h post-transfection, cells were
harvested for subsequent experimentation.
Database
miRNA profile data of breast cancer samples and
healthy tissue samples were downloaded from the Gene Expression
Omnibus (GEO) database (dataset no. GSE44124; www.ncbi.nlm.nih.gov/gds). The data were analyzed to
identify differentially expressed miRNAs in breast cancer compared
with healthy samples. Targets of miR-376c-3p were predicted using
online software TargetScan (http://www.targetscan.org/vert_72/).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from tissue samples and
BCSCs using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. To
assess miR-376c-3p expression levels, the TaqMan MicroRNA Assay kit
(cat. no. 4427975; Assay ID: 002122; Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used for reverse transcription and
qPCR according to the manufacturer's protocol (primer sequences
were withheld by the supplier). For RT-qPCR analysis of RAB2A mRNA,
total RNA was reversed transcribed into cDNA using the GoScript
Reverse Transcription system (Promega Corporation). Subsequently,
qPCR was performed using FastStart Universal Master Mix (Roche) and
a StepOnePlus real-time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The thermocycling conditions were as
follows: 40 cycles of denaturation at 94˚C for 30 sec, annealing at
58˚C for 30 sec and extension at 72˚C for 60 sec. The following
primers were used for qPCR: RAB2A forward,
5'-AGTTCGGTGCTCGAATGATAAC-3' and reverse,
5'-AATACGACCTTGTGATGGAACG-3'; GAPDH forward,
5'-TGCACCACCAACTGCTTAGC-3' and reverse,
5'-GGCATGGACTGTGGTCATGAG-3'; and U6 forward,
5'-CTCGCTTCGGCAGCACA-3' and reverse, 5'-AACGCTTCACGAATTTGCGT-3'.
Samples were quantified using the 2-∆∆Cq method
(32). miRNA and mRNA expression
levels were normalized to the internal reference genes U6 and
GAPDH, respectively.
Western blotting
Total protein was extracted using RIPA buffer
(Beyotime Institute of Biotechnology), determined using BCA Protein
Assay kit (Beijing Solarbio Science & Technology Co., Ltd.),
and 20 µg of total protein was separated via 12% SDS-PAGE and
transferred to PVDF membranes (EMD Millipore). After blocking with
5% non-fat milk for 1 h at room temperature, the membranes were
incubated with the following primary antibodies at 4˚C overnight:
RAB2A (cat. no. ab154729; 1:1,000; Abcam), Ki-67 (cat. no. 9449;
1:1,000; Cell Signalling Technology, Inc.) SOX2 (cat. no. 14962;
1:1,000; Cell Signalling Technology, Inc.), OCT4 (cat. no. 2890;
1:1,200; Cell Signalling Technology, Inc.) and β-actin (cat. no.
3700; 1:1,000; Cell Signalling Technology, Inc.). Subsequently, the
membranes were washed with TBST buffer (0.1% Tween-20 in TBS) and
incubated with the following HRP-conjugated secondary antibodies at
room temperature for 1 h: Anti-rabbit IgG (cat. no. 7074; 1:1,000;
Cell Signalling Technology, Inc.) and Anti-mouse IgG (cat. no.
7076; 1:1,000; Cell Signalling Technology, Inc.). Protein bands
were visualized using Pierce ECL Western Blotting substrate (Thermo
Fisher Scientific, Inc.; cat. no. 32209). Bands were analysed using
Image J1.50i software (National Institutes of Health).
Immunohistochemistry (IHC)
Tissues were fixed in 4% paraformaldehyde for 16 h
at room temperature. After fixation, tissue blocks were embedded in
paraffin, sliced into 5 µM sections and affixed onto the slide.
After deparaffinising and antigen retrieval, sections were blocked
with blocking buffer [0.3% Triton X-100 and 5% bovine serum albumin
(Beijing Solarbio Science & Technology Co., Ltd.) in PBS] for 1
h at room temperature and then incubated with the following
antibodies overnight at 4˚C: RAB2A (cat. no. ab154729; 1:500;
Abcam), Ki-67 (cat. no. 9449; 1:500; Cell Signalling Technology,
Inc.) SOX2 (cat. no. 14962; 1:300) and OCT4 (cat. no. 2890;
1:1,200; Cell Signalling Technology, Inc.). After washing with PBST
(0.1% Tween-20 in PBS), the slides were incubated with a diluted
HRP-conjugated secondary antibody (Anti-rabbit IgG; cat. no. 7074;
1:1,000; Anti-mouse IgG; cat. no. 7076; 1:1,000; each, Cell
Signalling Technology, Inc.) for 1 h at room temperature. After
washing 5 times using PBST, DAB was added to the slide for colour
developing. The slides were observed and photographed using an
Olympus IX71 inverted microscope (magnification, x400; Olympus
Corporation).
Luciferase reporter assay
The RAB2A 3'-untranslated region (UTR) fragment
containing putative the binding site for miR-376c-3p was amplified
by PCR from BCSC cDNA and inserted downstream of a luciferase gene
in pmirGLO vector (Promega Corporation; cat. no. E1330). PCR primer
sequences were as follows: Forward,
5'-GCCTCGAGGATTTGTTTGCCTTAATGAATAC-3' and reverse,
5'-GCTCTAGAGAGCAGTACCTGTCCTAGTTGCC-3'. The thermo cycling
conditions were as follows: 30 cycles of denaturation at 94˚C for
30 sec, annealing at 56˚C for 30 sec and extension at 72˚C for 60
sec. Phusion™ High-Fidelity DNA Polymerase was utilized for PCR
(Thermo Fisher Scientific, Inc.; cat. no. F530S). The seed-sequence
mutation was generated by site-directed point mutagenesis. 293T
cells (5x104) in a 24-well plate were co-transfected
with 50 ng wild-type (WT) RAB2A-3'UTR or mutated (MUT) RAB2A-3'UTR
and 20 pMol of miR-376c-3p mimic or NC-mimic. At 48 h
post-transfection, luciferase activity was measured using
Dual-luciferase reporter assay system (Promega Corporation; cat.
no. E1910) according to the manufacturer's protocol. Renilla
luciferase activity was used for normalization.
Mammosphere formation assay
At 48 h post-transfection, cells were harvested and
adjusted to 1x103 cells/ml in complete medium. Cell
suspension (1 ml/well) was plated into an ultra-low-attachment
12-well plate (Corning, Inc.) and cultured for 14 days at 37˚C.
Mammospheres were imaged and counted using an IX71 microscope
(Olympus Corporation; magnification, x400).
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay (Sigma-Aldrich; Merck KGaA) was
performed according to the manufacturer's protocol. Briefly, at 48
h post-transfection, cells were trypsinzed and seeded into a
96-well plate (3x103 cells/well). After culture for 3
days at 37˚C, 10 µl CCK-8 reagent was added to each well and
incubated at 37˚C for 2 h. The absorbance of each well was measured
at a wavelength of 450 nm using Enspire Multimode Plate Reader
(PerkinElmer, Inc.).
Colony formation assay
At 48 h post-transfection, cells were trypsinized
and seeded into a 12-well plate (5x102 cells/well).
After culture for 10-12 days at 37˚C, colonies were stained with
0.01% crystal violet for 15 min at room temperature and
photographed. The colonies were defined when visible by eye and
counted using ImageJ1.50i software (National Institutes of
Health).
Invasion assays
Invasion assays were performed using a HTS
Transwell-24 system (Corning, Inc.). The Transwell inserts were
coated with Matrigel (BD Biosciences) for 20 min at room
temperature. At 48 h post-transfection, cells were collected after
trypsin digestion. Subsequently, cells (1x105) in 200 µl
serum-free medium were plated into the upper chamber. Medium (300
µl) supplemented with 10% FBS was plated into the lower chamber.
After incubation for 24 h at 37˚C, non-invading cells on the upper
surface of the inserts were gently removed with a cotton swab.
Invading cells were fixed with 50% methanol for 10 min at room
temperature and stained with 0.01% crystal violet for 15 min at
room temperature. Invasive cells were imaged using an Olympus IX71
inverted microscope (Olympus Corporation; magnification, x400) and
analysed using ImageJ1. 50i software (National Institutes of
Health).
Lentivirus
To construct pLVX-miR-376c-3p, the following
oligonucleotides were designed: miR376c-Top,
5'-GAACATAGAGGAAATTCCACGTTTCAAGAGAACGTGGAATTTCCTCTATGTTTTTTTT-3';
miR376c-Bottom,
5'-AAAAAAAACATAGAGGAAATTCCACGTTCTCTTGAAACGTGGAATTTCCTCTATGTTC-3'.
The oligonucleotides were annealed (72˚C for 2 min, 37˚C for 2 min,
25˚C for 2 min and stored on ice) and then cloned into pLVX-shRNA1
(Clontech Laboratories, Inc.; cat. no. 632177) according to the
manufacturer's protocol. To generate the lentivirus,
5x105 Lenti-X 293T cells (Clontech Laboratories, Inc.;
cat. no. 632180) were seeded into a 10-cm dish 20 h prior to
transfection. A total of 15 µl Lenti-X HT Packaging Mix (Clontech
Laboratories, Inc.; cat. no. 631247) and 3 µg pLVX-miR-376c-3p
(pLVX-shRNA1 vector as control) were transfected into Lenti-X 293T
cells using lipofectamine®3000 according to
manufacturer's protocol. At 48 h post-transfection, the
lentivirus-containing supernatants were harvested, filtered through
0.45 µm filters, and stocked in aliquots at -80˚C.
Transduction of letivirus
BCSCs (5x105 cells) were seeded into
10-cm dishes 20 h prior to transduction. Lentivirus stock was
diluted 3 folds using fresh medium for BCSC culture. BCSC culture
supernatant was replaced with 5 ml of viral supernatant in the
presence of 4 µg/ml polybrene (Sigma-Aldrich; Merck KGaA; cat. no.
TR-1003-G). After incubating at 37˚C for 6 h, the viral supernatant
was replaced with fresh medium. At 48 h after supernatant
replenishment, cells were split 1:5 into BCSC culture medium
containing 1 µg/ml puromycin (Gibco; Thermo Fisher Scientific,
Inc.; cat. no. A1113803). After selection for 5 days, cells were
harvested for inoculation.
Xenograft mouse model
A total of 12 female NOD/SCID mice (age, 4-6 weeks;
weight: 18-22 g) were purchased from Shanghai Laboratory Animal
Center. The mice were randomly separated into two groups: i) One
group were injected with BCSCs infected with lentivirus vector; and
ii) the other group were injected with cells infected with
lentivirus expressing miR-376-3p. BCSCs (5x105) mixed
with Matrigel (1:1) were injected into the mouse mammary fat pad.
Tumour volume was measured on 18, 21, 24, 27 and 30 days
post-inoculation. On day 30 post-inoculation, mice were
anesthetized in a chamber containing 2.5% isoflurane in oxygen
prior to sacrifice by cervical dislocation. Subsequently, the
tumours were excised, weighed and subjected to IHC. The present
study was approved by the Animal Care and Use Committee of Shanghai
Ninth People's Hospital Affiliated to Shanghai Jiao Tong University
School of Medicine (Approval no. 2016-147-T96).
Statistical analysis
All experiments were repeated at least three times.
Data are presented as the mean ± standard deviation. Statistical
analyses were conducted using GraphPad Prism software (version 6.0;
GraphPad Software, Inc.). Comparisons between two groups were
analysed using the Student's t-test. Comparisons among multiple
groups were analysed using one-way ANOVA followed by Bonferroni's
test. The correlation between RAB2A and miR-376c-3p was assessed by
conducting Pearson's correlation analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
Aberrant expression of miR-376c-3p and
RAB2A in breast cancer
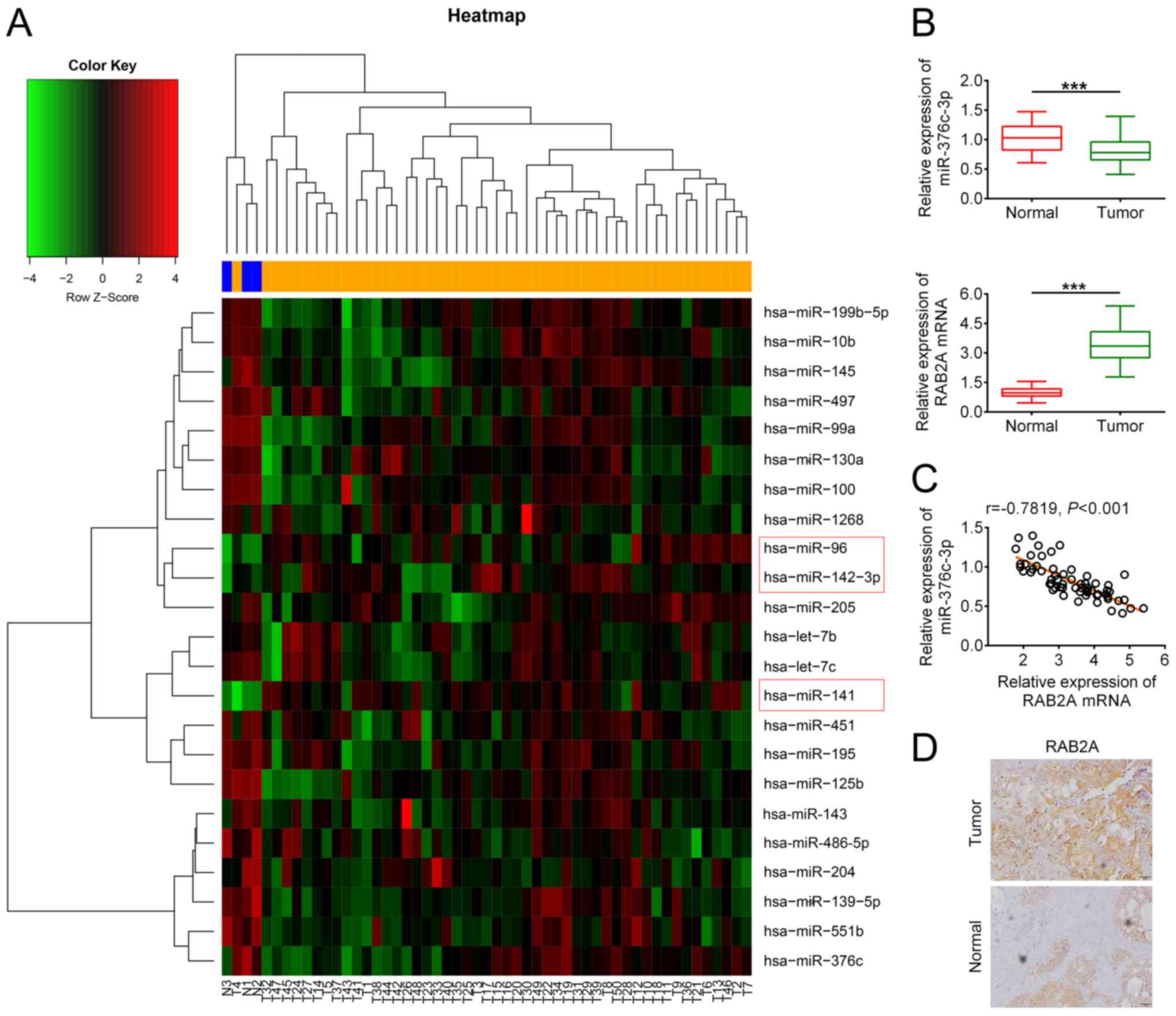
To identify miRNAs that participate in breast cancer
tumorigenesis, the miRNA profile data of breast cancer tumours and
adjacent non-cancerous tissues were obtained from the GEO database.
Bioinformatics analysis identified 20 downregulated and 3
upregulated miRNAs in breast cancer tumours (Fig. 1A). Among the miRNAs, miR-376c-3p has
been reported to participate in the development of several types of
cancer (22-27),
and is downregulated in breast cancer (33-36).
However, how miRNAs affect breast cancer progression is not
completely understood. Therefore, miR-376c-3p was selected for
further investigation in the present study. A total of 60 paired
breast cancer tumour samples and adjacent non-cancerous tissues
were obtained from patients with breast cancer who underwent
surgical resection. The RT-qPCR results indicated that the
expression level of miR-376c-3p in breast cancer tumour tissues was
significantly decreased compared with healthy tissues (Fig. 1B). The correlation analysis
indicated that low miR-376c-3p expression was significantly
correlated with lymph node metastasis (P=0.002; Table SI), which was consistent with
previous studies that demonstrated that miR-376c-3 served as a
cancer suppressor (22-25).
Among the potential targets of miR-376c-3ppredicted using online
software TargetScan (http://www.targetscan.org/vert_72/), RAB2A has been
reported to be involved in breast cancer development (28,29).
mRNA expression levels of RAB2A were significantly higher in breast
cancer tissues compared with healthy tissues (Fig. 1B). Moreover, the expression level of
miR-376c-3p was negatively correlated with the expression level of
RAB2A in breast cancer tumours (Fig.
1C). The IHC results suggested that the protein level of RAB2A
in breast cancer tumors was markedly higher compared with healthy
tissues (Fig. 1D). Overall, the
results indicated that miR-376c-3p was downregulated in breast
cancer, and RAB2A may be regulated by miR-376c-3p in breast
cancer.
Dysregulation of miR-376c-3p and RAB2A
in BCSCs
To investigate the role of miR-376c-3p in the
tumorigenesis of breast cancer, CD44+CD24-
cells were isolated from breast cancer tumours (Fig. 2A), which were characterized as
BSCSs. Stem cell-associated proteins OCT4 and SOX2 were expressed
at significantly higher levels in BCSCs compared with MCF7 breast
cancer cells (Fig. 2B). Breast
cancer cells (MCF7, non-BCSCs and BCSCs) expressed significantly
lower levels of miR-376c-3p and significantly higher levels of
RAB2A compared with MCF-10A normal breast epithelial cells
(Fig. 2C). The western blotting
results indicated that the protein expression level of RAB2A in
BCSCs was significantly higher compared with normal cells, MCF7
cells and non-BCSCs (Fig. 2D). The
dysregulation of miR-376c-3p and RAB2A in breast cancer tumours
suggested they may serve a role in BCSCs.
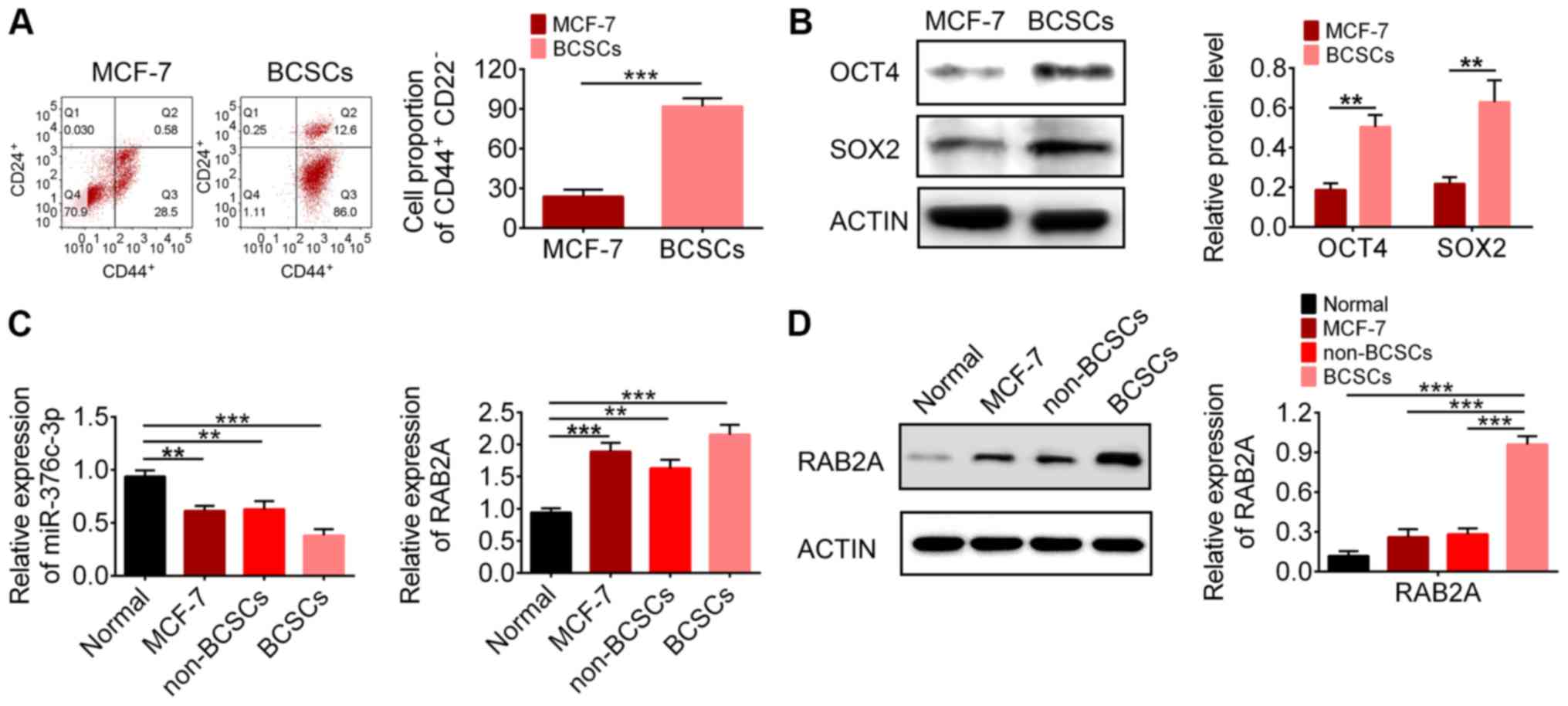 | Figure 2Expression levels of miR-376c-3p and
RAB2A in BCSCs. (A) Flow cytometry analysis of the proportion of
CD44+CD24-cells. (B) Protein expression levels of stem cell markers
OCT4 and SOX2 in MCF-7 cells and BCSCs. (C) Reverse
transcription-quantitative PCR analysis of miR-376c-3p and RAB2A
expression levels in MCF-10A cells, MCF-7 cells, non-BCSCs and
BCSCs. (D) Protein expression levels of RAB2A in MCF-10A cells,
MCF-7 cells, non-BCSCs and BCSCs. **P<0.01 and
***P<0.001. miR, microRNA; RAB2A, Ras-related protein
Rab-2A; BCSC, breast cancer stem cell; CD, cluster of
differentiation; OCT4, octamer-binding transcription factor 4;
SOX2, SRY-box transcription factor 2. |
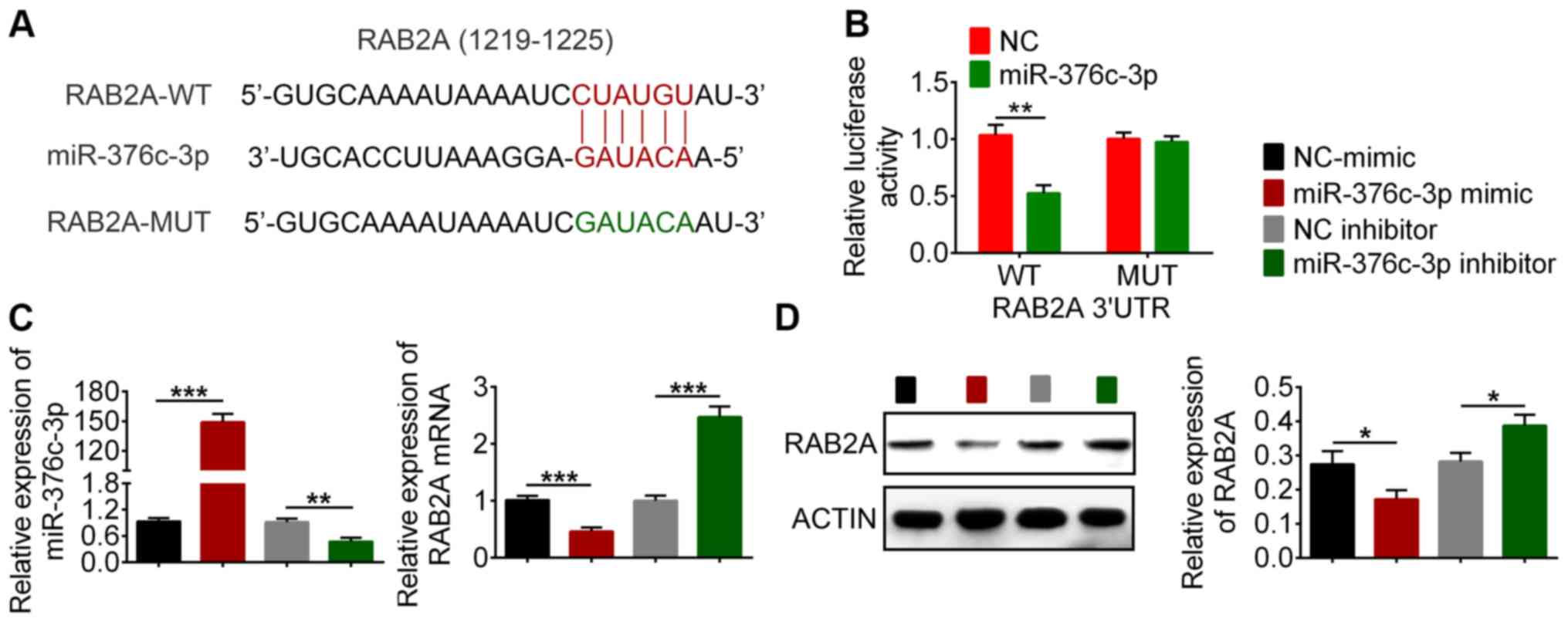
miR-376c-3p targets RAB2A mRNA for
degradation
The predicted targeted sequence of RAB2A by
miR-376c-3p is presented in Fig.
3A. To verify the interaction between RAB2A and miR-376c-3p,
luciferase reporter constructs were constructed by inserting the
3'UTR sequences of RAB2A downstream of a luciferase gene. 293T
cells were co-transfected with the reporter plasmid and miR-376c-3p
mimic or NC-mimic. The results indicated that miR-376c-3p mimic
significantly reduced the luciferase activity of WT RAB2A-3'UTR
compared with NC-mimic (Fig. 3B).
By contrast, miR-376c-3p mimic did not significantly alter the
luciferase activity of MUT RAB2A-3'UTR compared with NC-mimic
(Fig. 3B), indicating the
specificity of the target sequence. The effect of miR-376c-3p on
endogenous RAB2A expression in BCSCs was also examined. RAB2A was
expressed at lower levels in miR-376c-3p mimic-transfected cells
compared with the NC-mimic group, but expressed at higher levels in
miR-376c-3p inhibitor-transfected cells compared with the NC
inhibitor (Fig. 3C). The western
blotting results indicated that RAB2A protein expression was
decreased in miR-376c-3p mimic-transfected BCSCs compared with the
NC-mimic group, and increased in miR-376c-3p inhibitor-transfected
cells compared with the NC inhibitor group (Fig. 3D). Collectively, the results
demonstrated that miR-376c-3p targeted RAB2A and regulated RAB2A
expression.
miR-376c-3p reduces stem cell
population and downregulates stem cell regulatory proteins by
targeting RAB2A
The observation that miR-376c-3p was downregulated
in BCSCs implied that miR-376-3p may regulate BCSC fate. To
determine the effect of miR-376c-3p on BCSCs, BCSCs were
transfected with miR-376-3p mimic and the expression of the BCSC
surface markers CD44 and CD24 was examined via flow cytometry.
Compared with NC-mimic, miR-376c-3p mimic reduced the proportion of
CD44+CD24- cells from 95.6 to 38.2% (Fig. 4A), indicating a decrease in BCSCs.
To prove functional relevance of RAB2A regulation by miR-376c-3p,
cells were co-transfected with RAB2A-expressing plasmid pcDNA-RAB2A
and miR-376c-3p mimic. Expression of RAB2A in
pcDNA-RAB2A-transfected BCSCs is presented in Fig. S2. RAB2A overexpression reversed
miR-376c-3p-mediated loss of BCSCs. Furthermore, the expression of
stem cell regulatory genes OCT4 and SOX2 in miR-376c-3p-transfected
BCSCs was assessed by western blotting. Compared with NC-mimic,
OCT4 and SOX2 expression levels were significantly decreased by
miR-376c-3p mimic, which was reversed by RAB2A overexpression
(Fig. 4B). In addition, compared
with NC inhibitor, miR-376c-3p inhibitor significantly increased
the expression of OCT4 and SOX2, which was reversed by RAB2A siRNA
(Figs. 4C and S1). si-RAB2A#1 was used in Figs. 4 and 5, as it demonstrated a more prominent
silencing effect. Collectively, the results demonstrated that
miR-376c-3p reduced the stem cell population and downregulated stem
cell regulatory proteins by targeting RAB2A.
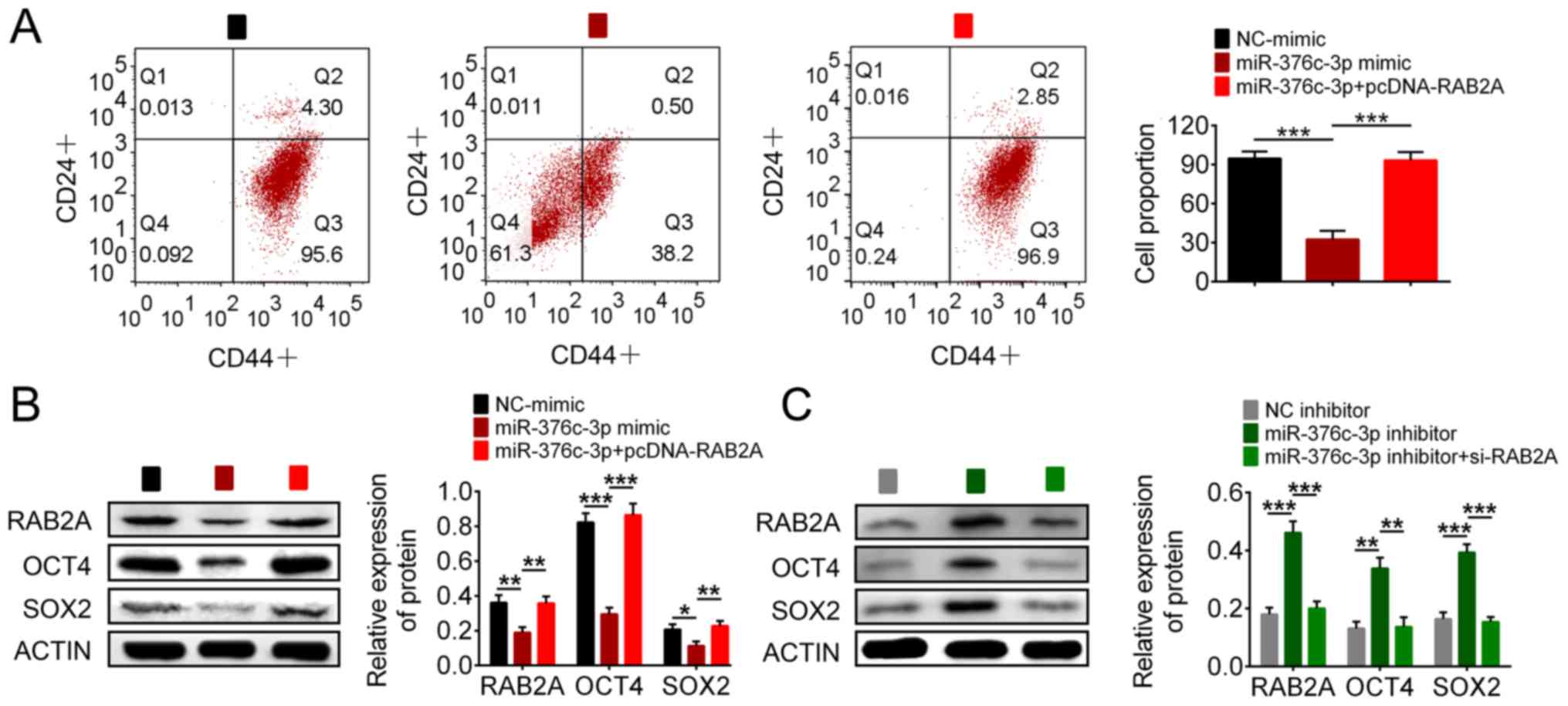 | Figure 4miR-376-3p reduces the stem cell
population and downregulates stem cell regulatory proteins by
targeting RAB2A. (A) Flow cytometry analysis of the proportion of
CD44+CD24-cells. The effect of (B) miR-376c-3p mimic, pcDNA-RAB2A,
(C) miR-376c-3p inhibitor and si-RAB2A on RAB2A, OCT4 and SOX2
expression levels. *P<0.05, **P<0.01
and ***P<0.001. miR, microRNA; RAB2A, Ras-related
protein Rab-2A; CD, cluster of differentiation; si, small
interfering RNA; OCT4, octamer-binding transcription factor 4;
SOX2, SRY-box transcription factor 2; NC, negative control. |
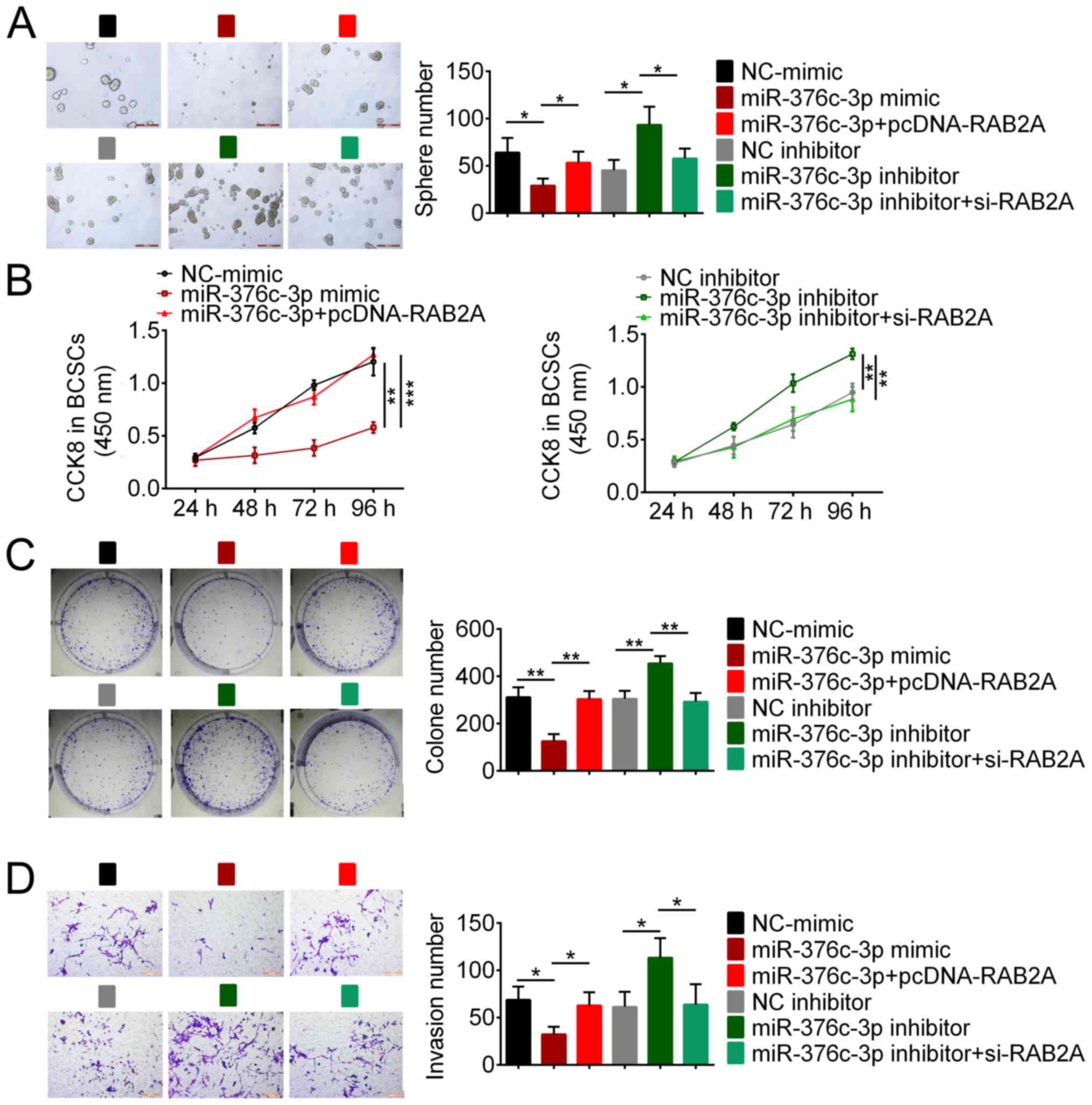
miR-376c-3p regulates BCSC tumorigenic
properties via RAB2A
To further explore the role of miR-376c-3p in
tumorigenesis, gain- and loss-of-function experiments were
conducted by transfecting BCSCs with miR-376c-3p mimic or
miR-376c-3p inhibitor. A mammosphere assay was performed to assess
the effect of miR-376c-3p on BCSC self-renewal. miR-376c-3p mimic
significantly reduced the sphere number compared with NC-mimic,
whereas miR-376c-3p inhibitor significantly increased the sphere
number compared with NC inhibitor, which suggested that miR-376c-3p
had an inhibitory effect on BCSC self-renewal (Fig. 5A). Subsequently, CCK-8 and colony
formation assays were conducted to examine the effect of
miR-376c-3p on cell proliferation. The results indicated that
miR-376c-3p mimic significantly inhibited BCSC proliferation
compared with NC-mimic, whereasmiR-376c-3p inhibitor significantly
enhanced BCSC proliferation compared with NC inhibitor (Fig. 5B and C). Moreover, BCSC invasion was examined
using a cell invasion assay. miR-376c-3p overexpression
significantly inhibited BCSC invasion compared with NC-mimic,
whereas miR-376c-3p knockdown significantly enhanced BCSC invasion
compared with NC inhibitor (Fig.
5D). RAB2A overexpression reversed miR-376c-3p mimic-induced
effects and RAB2A knockdown reversed miR-376c-3p inhibitor-induced
effects (Fig. 5A-D), indicating
that miR-376c-3p targeted RAB2A to inhibit the tumorigenic
properties of BCSCs.
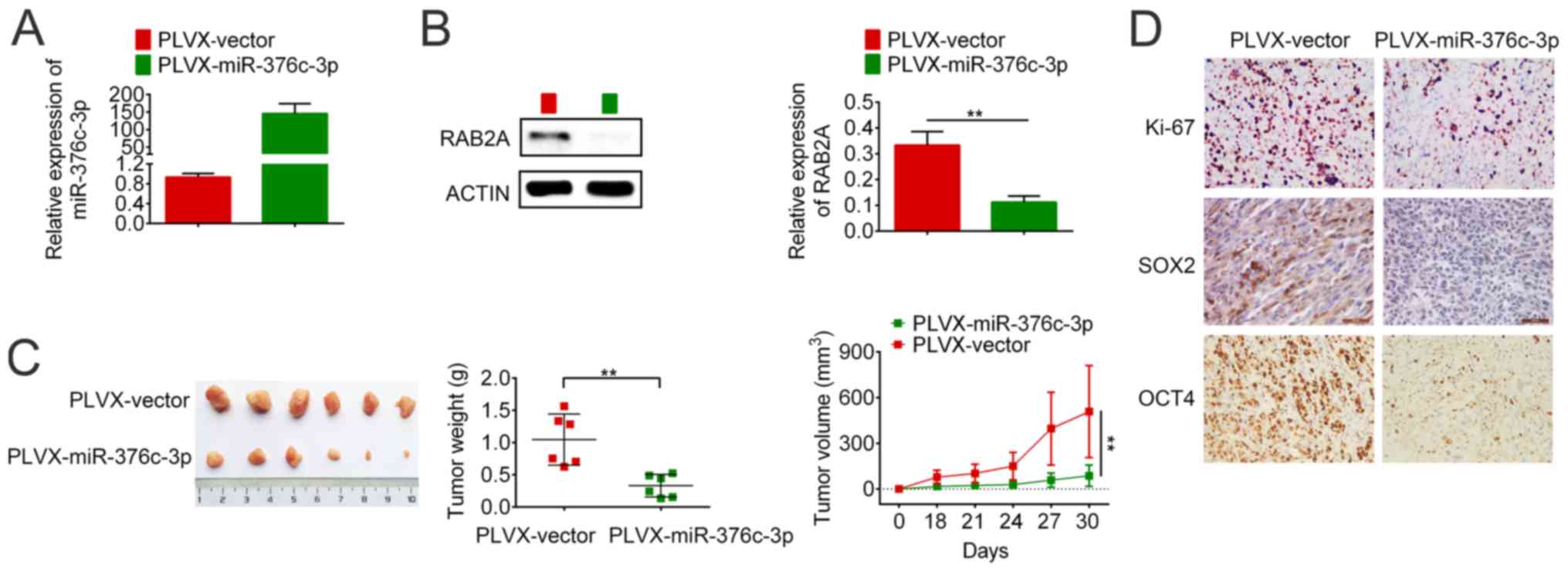
miR-376c-3p overexpression inhibits
breast cancer growth in a mouse xenograft model
The effect of miR-376c-3p overexpression on tumour
formation in a mouse model was assessed. The expression levels of
miR-376c-3p and RAB2A were measured in lentivirus-infected BCSCs
(Fig. 6A and B). RAB2A expression was significantly
downregulated in PLVX-miR-376c-3p-infected BCSCs compared with
PLVX-vector-infected BCSCs (Fig.
6B). Subsequently, the infected cells were inoculated into the
mammary fat pad of 4-6 week old female NOD/SCID mice and tumour
growth was monitored for 30 days. On day 30 post-inoculation, the
mice were sacrificed and the tumours were excised. The tumour
volume and weight in the miR-376c-3p group was significantly
reduced compared with the vector group (Fig. 6C). The expression levels of Ki-67,
SOX2 and OCT4 in the tumours of the miR-376c-3p group were markedly
lower compared with the vector group (Fig. 6D). The results demonstrated that
miR-376c-3p overexpression inhibited breast cancer growth in a
mouse xenograft model.
Discussion
miRNAs are common gene regulators in cellular
signalling pathways that are implicated in cancer progression
(7,8). A number of miRNAs have been reported
to regulate BCSC stemness (6,37).
let-7 regulates BCSC self-renewal and differentiation by targeting
ras homolog family member Hand high mobility group AT-hook
2(11). miR-200c serves a role in
regulating BCSC stemness by targeting BMI1 proto-oncogene, polycomb
ring finger and programmed cell death 10 (12,13).
miR-93 expression depletes the BCSC population and inhibits tumor
development (14). miR-600 targets
SCD1 and inhibits WNT signalling, leading to a reduction in BCSC
self-renewal (16).
The present study identified 23 differentially
expressed miRNAs in breast cancer tissues compared with healthy
tissues by conducting miRNA microarray analysis. miR-376c-3p, which
was downregulated in breast cancer tissues and BCSCs, was selected
for further study. By conducting bioinformatics analysis and
validation assays, RAB2A was identified as a target of miR-376c-3p.
The results also indicated that miR-376c-3p and RAB2A were
dysregulated in BCSCs, suggesting they may serve roles in
regulating BCSC stemness. By analysing CD44 and CD24 surface marker
expression levels, the results indicated that miR-376c-3p
overexpression depleted the BCSC population. miR-376c-3p mimic also
downregulated the expression of stem cell regulatory genes OCT4 and
SOX2 compared with NC-mimic. miR-376c-3p-mediated effects were
reversed by RAB2A overexpression, indicating the functional
interaction between RAB2A and miR-376c-3p in BCSCs. Furthermore,
functional assays indicated that, compared with NC-mimic,
miR-376c-3p mimic inhibited BCSC self-renewal, proliferation and
invasion, which was reversed by RAB2A overexpression. In
vivo experiments were conducted in a mouse xenograft model that
suggested miR-376c-3p overexpression inhibited breast cancer growth
compared with the vector group. Collectively, the results indicated
that miR-376c-3p served a role in regulating BCSC stemness by
targeting RAB2A.
RAB2A is a small GTPase that serves an essential
role in the membrane transport between ER and Golgi (28). Luo et al (28) reported that RAB2A also functions as
an oncogene in breast cancer viagenomic profiling analysis.
Bioinformatics analysis indicated that RAB2A is aberrantly
activated in human cancer and associated with poor prognosis
(28). Mechanistically, RAB2A
interacts with and prevents the inactivation of ERK1/2, leading to
upregulation of zinc finger E-box binding homeobox 1 and nuclear
translocation of β-catenin (28).
Abnormal activation of RAB2A ultimately promotes BCSC expansion and
tumorigenicity (28). In another
study, Kajiho et al (29)
demonstrated that RAB2A regulates the trafficking of matrix
metallopeptidase 14 and E-cadherin, thereby enhancing breast cancer
invasion. The study also indicated that elevated expression of
RAB2A is strongly linked to metastatic recurrence of breast cancer
in patients, which suggests that RAB2A may serve as a useful
prognosis biomarker in breast cancer. RAB2A was also reported to be
required for autophagosome clearance in breast cancer cells
(38). In the present study, the
results indicated that RAB2A expression was regulated by
miR-376c-3p, and the miRNA/target axis modulated BCSC fate and
properties. miR-376c-3p also targets other genes, such as
RUNX2(22), BAD and Smad4(23); therefore, whether these genes affect
BCSC stemness requires further investigation.
In summary, the present study indicated that
miR-376c-3p was associated with regulating BCSC stemness. Compared
with NC-mimic, miR-376c-3p overexpression reduced the
CD44+CD24- population and downregulated stem
cell regulatory genes. Additionally, miR-376c-3p overexpression
inhibited BCSC self-renewal, proliferation and invasion, and
suppressed tumour growth in a mouse xenograft model.miR-376c-3p
performed the aforementioned biological functions by targeting
RAB2A. The present study identified a potential mechanism
underlying BCSC stemness regulation and suggested that miR-376c-3p
may serve as a therapeutic target for breast cancer.
Supplementary Material
Figure S1. Expression of RAB2A in
siRNA.transfected BCSCs transfected with siRNAs. BCSCs were
transfected with siRNA targeting RAB2A.targeting siRNAs (si.RAB2A#1
and si.RAB2A#2) or NC siRNA. At 48 h post.transfection, the
expression of RAB2A was analysed via (A) reverse
transcription.quantitative PCR and (B) western blotting. si.RAB2A#1
was used in Figs. 4 and 5. **P<0.01 and
***P<0.001. RAB2A, Ras.related protein Rab.2A; BCSC,
breast cancer stem cell; siRNA, small interfering RNA; NC, negative
control.
Figure S2. Expression of RAB2A in
pcDNA.RAB2A.transfected BCSCs. BCSCs were transfected with
pcDNA-RAB2A or pcDNA3.1. At 48 h post-transfection, RAB2A
expression was assessed via western blotting.
***P<0.001. RAB2A, Ras-related protein Rab-2A; BCSC,
breast cancer stem cell.
Table SI. Association between
miR-376c-3p expression and the clinicopathological characteristics
of patients (all female).
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ and YC conceived and designed the study. MZ
analyzed and interpreted the data. WP and BT performed the
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shanghai Ninth People's Hospital (approval no.
2016-147-T96). Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kreso A and Dick JE: Evolution of the
cancer stem cell model. Cell Stem Cell. 14:275–291. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Visvader JE and Lindeman GJ: Cancer stem
cells: Current status and evolving complexities. Cell Stem Cell.
10:717–728. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Richie RC and Swanson JO: Breast cancer: A
review of the literature. J Insurance Med. 35:85–101.
2003.PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shimono Y, Mukohyama J, Nakamura S and
Minami H: MicroRNA regulation of human breast cancer stem cells. J
Clin Med. 5(2)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Peng Y and Croce CM: The role of MicroRNAs
in human cancer. Signal Transduct Target Ther.
1(15004)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Takahashi RU, Miyazaki H and Ochiya T: The
role of microRNAs in the regulation of cancer stem cells. Front
Genet. 4(295)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Garg M: Emerging role of microRNAs in
cancer stem cells: Implications in cancer therapy. World J Stem
Cells. 7:1078–1089. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong
C, Huang Y, Hu X, Su F, Lieberman J and Song E: Let-7 regulates
self renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shimono Y, Zabala M, Cho RW, Lobo N,
Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, et al:
Downregulation of miRNA-200c links breast cancer stem cells with
normal stem cells. Cell. 138:592–603. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Feng ZM, Qiu J, Chen XW, Liao RX, Liao XY,
Zhang LP, Chen X, Li Y, Chen ZT and Sun JG: Essential role of
miR-200c in regulating self-renewal of breast cancer stem cells and
their counterparts of mammary epithelium. BMC Cancer.
15(645)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu S, Patel SH, Ginestier C, Ibarra I,
Martin-Trevino R, Bai S, McDermott SP, Shang L, Ke J, Ou SJ, et al:
MicroRNA93 regulates proliferation and differentiation of normal
and malignant breast stem cells. PLoS Genet.
8(e1002751)2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Taube JH, Malouf GG, Lu E, Sphyris N,
Vijay V, Ramachandran PP, Ueno KR, Gaur S, Nicoloso MS, Rossi S, et
al: Epigenetic silencing of microRNA-203 is required for EMT and
cancer stem cell properties. Sci Rep. 3(2687)2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
El Helou R, Pinna G, Cabaud O, Wicinski J,
Bhajun R, Guyon L, Rioualen C, Finetti P, Gros A, Mari B, et al:
miR-600 Acts as a Bimodal switch that regulates breast cancer stem
cell fate through WNT signaling. Cell Rep. 18:2256–2268.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma S, Tang KH, Chan YP, Lee TK, Kwan PS,
Castilho A, Ng I, Man K, Wong N, To KF, et al: miR-130b promotes
CD133(+) liver tumor-initiating cell growth and self-renewal via
tumor protein 53-induced nuclear protein 1. Cell Stem Cell.
7:694–707. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Natu Med. 17:211–215.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Bu P, Chen KY, Chen JH, Wang L, Walters J,
Shin YJ, Goerger JP, Sun J, Witherspoon M, Rakhilin N, et al: A
microRNA miR-34a-regulated bimodal switch targets Notch in colon
cancer stem cells. Cell Stem Cell. 12:602–615. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hwang WL, Jiang JK, Yang SH, Huang TS, Lan
HY, Teng HW, Yang CY, Tsai YP, Lin CH, Wang HW and Yang MH:
MicroRNA-146a directs the symmetric division of Snail-dominant
colorectal cancer stem cells. Nat Cell Biol. 16:268–280.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Wang H, Sun T, Hu J, Zhang R, Rao Y, Wang
S, Chen R, McLendon RE, Friedman AH, Keir ST, et al: miR-33a
promotes glioma-initiating cell self-renewal via PKA and NOTCH
pathways. J Clin Invest. 124:4489–4502. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Chang WM, Lin YF, Su CY, Peng HY, Chang
YC, Lai TC, Wu GH, Hsu YM, Chi LH, Hsiao JR, et al: Dysregulation
of RUNX2/Activin-A Axis upon miR-376c downregulation promotes lymph
node metastasis in head and neck squamous cell carcinoma. Cancer
Res. 76:7140–7150. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tu L, Zhao E, Zhao W, Zhang Z, Tang D,
Zhang Y, Wang C, Zhuang C and Cao H: Hsa-miR-376c-3p regulates
gastric tumor growth both in vitro and in vivo. Biomed Res Int.
2016(9604257)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang K, Jin J, Ma T and Zhai H:
MiR-376c-3p regulates the proliferation, invasion, migration, cell
cycle and apoptosis of human oral squamous cancer cells by
suppressing HOXB7. Biomed Pharmacother. 91:517–525. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bhavsar SP, Lokke C, Flaegstad T and
Einvik C: Hsa-miR-376c-3p targets Cyclin D1 and induces G1-cell
cycle arrest in neuroblastoma cells. Oncol Lett. 16:6786–6794.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang Y, Chang W, Chang W, Chang X, Zhai S,
Pan G and Dang S: MicroRNA-376c-3p Facilitates human hepatocellular
carcinoma progression via repressing at-rich interaction domain 2.
J Cancer. 9:4187–4196. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang YH, Fu J, Zhang ZJ, Ge CC and Yi Y:
LncRNA-LINC00152 down-regulated by miR-376c-3p restricts viability
and promotes apoptosis of colorectal cancer cells. Am J Transl Res.
8:5286–5297. 2016.PubMed/NCBI
|
|
28
|
Luo ML, Gong C, Chen CH, Hu H, Huang P,
Zheng M, Yao Y, Wei S, Wulf G, Lieberman J, et al: The Rab2A GTPase
promotes breast cancer stem cells and tumorigenesis via Erk
signaling activation. Cell Rep. 11:111–124. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kajiho H, Kajiho Y, Frittoli E,
Confalonieri S, Bertalot G, Viale G, Di Fiore PP, Oldani A, Garre
M, Beznoussenko GV, et al: RAB2A controls MT1-MMP endocytic and
E-cadherin polarized Golgi trafficking to promote invasive breast
cancer programs. EMBO Reps. 17:1061–1080. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lin X, Chen W, Wei F, Zhou BP, Hung MC and
Xie X: POMC maintains tumor-initiating properties of tumor
tissue-derived long-term-cultured breast cancer stem cells. Int J
Cancer. 140:2517–2525. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lin X, Chen W, Wei F, Zhou BP, Hung MC and
Xie X: Nanoparticle delivery of miR-34a Eradicates
Long-term-cultured breast cancer stem cells via targeting C22ORF28
directly. Theranostics. 7:4805–4824. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Huo D, Clayton WM, Yoshimatsu TF, Chen J
and Olopade OI: Identification of a circulating microRNA signature
to distinguish recurrence in breast cancer patients. Oncotarget.
7:55231–55248. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang L, Chen Y, Wang H, Zheng X, Li C and
Han Z: miR-376a inhibits breast cancer cell progression by
targeting neuropilin-1 NR. OncoTargets Ther. 11:5293–5302.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
van Schooneveld E, Wildiers H, Vergote I,
Vermeulen PB, Dirix LY and Van Laere SJ: Dysregulation of microRNAs
in breast cancer and their potential role as prognostic and
predictive biomarkers in patient management. Breast Cancer Res.
17(21)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cuk K, Zucknick M, Heil J, Madhavan D,
Schott S, Turchinovich A, Arlt D, Rath M, Sohn C, Benner A, et al:
Circulating microRNAs in plasma as early detection markers for
breast cancer. Int J Cancer. 132:1602–1612. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fan X, Chen W, Fu Z, Zeng L, Yin Y and
Yuan H: MicroRNAs, a subpopulation of regulators, are involved in
breast cancer progression through regulating breast cancer stem
cells. Oncol Lett. 14:5069–5076. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lőrincz P, Tóth S, Benkő P, Lakatos Z,
Boda A, Glatz G, Zobel M, Bisi S, Hegedűs K, Takáts S, et al: Rab2
promotes autophagic and endocytic lysosomal degradation. J Cell
Biol. 216:1937–1947. 2017.PubMed/NCBI View Article : Google Scholar
|