Introduction
Premature ovarian failure (POF), also known as
primary ovarian insufficiency, is a hypergonadotropic disorder
characterized by the exhaustion of ovary reserve before the age of
40 years (1). Patients with POF
suffer from amenorrhea or oligomenorrhea, low estrogen level, high
follicle-stimulating hormone (FSH) level, infertility and clinical
manifestations associated with perimenopause (2). In clinical practice, POF is diagnosed
by the elevated serum level of FSH and amenorrhea (≥4 months)
(3). POF is one of the major causes
of female infertility and affects 1/1,000 infertile females
worldwide (4). In China, ~3% of
females of childbearing age will develop POF and subsequent
infertility before the age of 40 years (5). POF causes economic burden and mental
stress to the patients' families (5).
Despite the efforts made in POF treatment, such as
estrogen therapy, infertility remains difficult to treat,
especially in patients diagnosed with severe forms of the disease
(6-8).
Genetic mutations, such as PTEN mutations, are frequently observed
in POF patients and play central roles in the occurrence and
development of this disease (9,10).
Apart from protein-coding genes, non-coding RNAs (ncRNAs),
including long ncRNAs [lncRNAs (>200 nucleotides in length)]
also serve crucial roles in diverse biological processes, including
in the development of POF, such as cell proliferation and apoptosis
(11,12). However, the function of most lncRNAs
in POF is still unknown (11,12).
The lncRNA nuclear enriched abundant transcript 1 (NEAT1) is
involved in different types of cancer, including ovarian and oral
cancer, and regulates cancer cell behaviors, for example through
inhibition of cell apoptosis (13,14). A
previous study reported that NEAT1 could inhibit hydrogen peroxide
induced cardiomyocyte apoptosis (15). Cell apoptosis is known to promote
the progression of POF (4,9,10).
Preliminary RNA-seq analysis from our laboratory suggested altered
expression of NEAT1 in POF (data not shown). The present study
therefore hypothesized that NEAT1 may also participate in POF. The
present study aimed to determine the role of NEAT1 in POF.
Materials and methods
Subjects
A total of 60 patients with spontaneous (not induced
by chemotherapy, radiation or surgery) POF (age range, 21-39 years;
mean age, 28.1±4.6 years) that were admitted at The Sixth
Affiliated Hospital of Sun Yat-sen University (Guangzhou, China)
between December 2016 and December 2018 were enrolled in the
present study. All patients were diagnosed with POF based on their
FSH level. Patients with FSH level >30 IU/l (measured twice at 4
weeks interval) were included in the study. Patients with POF who
had other clinical complications, including other types of ovarian
disorders, such as endometriosis, ovarian cysts and ovarian
cancers, were excluded from this study. No therapy was initiated
prior to admission. The control group consisted of 60 healthy women
(age range, 21-39 years; mean age, 28.2±4.4 years) who were also
enrolled at the aforementioned hospital between December 2016 and
December 2018. These healthy women had suspected ovarian lesions
that were removed during ovarian biopsy. All participants provided
their written informed consent and the study was approved by the
Ethics Committee of The Sixth Affiliated Hospital of Sun Yat-sen
University (Guangzhou, China).
Tissues, cell lines and culturing
Ovarian biopsy was performed on ovaries of all
patients and healthy women to collect ovarian tissue samples
(0.08-0.12 g) from each participant. The two normal Chinese hamster
ovary cell lines Lec8 and CHO (American Type Culture Collection)
were used in the present study. Cells were cultured in ovarian
epithelial cell medium (OEpiCM; ScienCell Research Laboratories,
Inc.) supplemented with antibiotic-antimycotic (10,000 U/ml
penicillin, 10,000 µg/ml streptomycin and 25 µg/ml amphotericin B
from Thermo Fisher Scientific, Inc. (cat. no. 15240112; 100X) at
37˚C in a humidified incubator containing 5% CO2.
Transient cell transfections
NEAT1 and p53 expression vectors were constructed
using the pcDNA3 vector (Sangon, Biotech Co., Ltd.) as a backbone.
Negative control small interfering (si)RNA
(5'-UGGUACGAUGUGGACACGACC-3') and NEAT1 siRNA
(5'-ACAAUGCCACCGUUAAUUUGAC-3') were provided by Shanghai GenePharma
Co., Ltd. Lipofectamine 2000® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to transfect 10 nM NEAT1
expression vector, 10 nM p53 expression vector, 10 nM empty pcDNA3
vector (negative control, NC), 45 nM NEAT1 siRNA or 45 nM negative
control siRNA (NC) into 105 cells per well Lec8 or CHO
cells at 37˚C in a 6-well cell culture plate. Untransfected cells
were used as control cells (C). Cells were harvested 24 h
post-transfection for subsequent experimentation.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from ovarian tissues (0.05
g) or Lec8 and CHO cells (106) using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific Inc.). Following
DNase I digestion, cDNA was synthesized using a QuantiTect reverse
transcription kit (Qiagen China Co., Ltd.) at the following thermal
conditions: 55˚C for 20 min and 80˚C for 20 min. KAPA SYBR FAST
qPCR Master Mix (Kapa Biosystems; Roche Diagnostics Co., Ltd.) was
used to prepare all qPCR mixtures. 18S rRNA or GAPDH were used as
endogenous controls to detect the expression of NEAT1 and p53 mRNA,
respectively. PCR reaction conditions were: 95˚C for 1 min,
followed by 95˚C for 10 sec and 58˚C for 50 sec. The
2-ΔΔCq method (16) was used to normalize data. Three
replicates were conducted for each experiment. Primer sequences
were: 5'-CTTCCTCCCTTTAACTTATCCATTCAC-3' (forward) and
5'-CTCTTCCTCCACCATTACCAACAATAC-3' (reverse) for NEAT1;
5'-AGAGTCTATAGGCCCACCCC-3' (forward) and 5'-GCTCGACGCTAGGATCTGAC-3'
(reverse) for p53; 5'-GTCTCCTCTGACTTCAACAGCG-3' (forward) and
5'-ACCACCCTGTTGCTGTAGCCAA-3' (reverse) for GAPDH;
5'-CTACCACATCCAAGGAAGCA-3' (forward) and
5'-TTTTTCGTCACTACCTCCCCG-3' (reverse) for 18S rRNA.
Cell apoptosis assay
Lec8 and CHO cells were collected 24 h following
transfection. Cells were cultured in fresh medium (OEpiCM) for a
further 48 h at 37˚C. After washing with PBS, cells
(106) were mixed with 500 µl of Annexin binding buffer
(5X; Thermo Fisher Scientific, Inc.). Subsequently, 5 µl FITC
labeled Annexin-V and 5 µl propidium iodide solution was added to
the cells and incubated for 15 min in the dark at room temperature.
A FACSCalibur flow cytometer (BD Biosciences) was used to detect
apoptotic cells. Data were analyzed using FCSalyzer version 0.9.17
(SourceForge; https://sourceforge.net/projects/fcsalyzer/files/Version%200.9.17-alpha/).
Western blotting
Lec8 and CHO cells (106 cells) collected
24 h after transfection were mixed with 0.1 ml ice-cold RIPA buffer
(Sangon Biotech Co., Ltd.) for total protein extraction. BCA assay
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to determine
protein concentration. Proteins (40 µg) were separated by 10%
SDS-PAGE and transferred onto PVDF membranes. Membranes were
blocked using 5% non-fat milk at room temperature for 2 h.
Membranes were incubated with primary antibodies against GAPDH
(1:1,500; cat. no. ab9485; Abcam) and p53 (1:1,500; cat. no.
ab31333; Abcam) at 4˚C overnight. Subsequently, the membranes were
incubated with IgG-horseradish peroxidase secondary antibody
(1:1,000; goat anti rabbit; cat. no. MBS435036; MyBioSource, Inc.)
at room temperature for 2 h. Bands were detected using enhanced
chemiluminescence substrate (Merck KGaA). Relative expression level
was normalized to endogenous control GAPDH using ImageJ version
1.46 (National Institutes of Health).
Statistical analyses
Data are presented as the mean ± SD values of three
independent experiments. Unpaired t-test was used to compare
differences between two groups. ANOVA followed by the Tukey's post
hoc test was used for multiple comparison. Associations were
analyzed using linear regression. P<0.05 was considered to
indicate a statistically significant difference.
Results
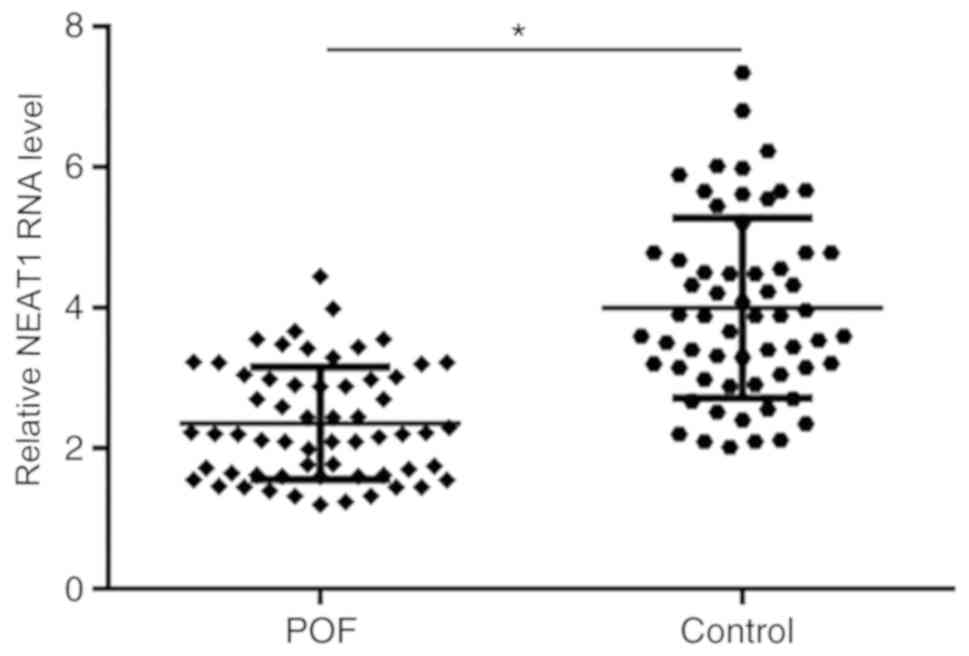
NEAT1 expression is downregulated in
ovarian tissues of patients with POF
NEAT1 expression in ovarian tissues of patients from
both the POF and control groups was detected using RT-qPCR. The
results demonstrated that expression level of NEAT1 was
significantly decreased in patients with POF compared with healthy
controls (Fig. 1).
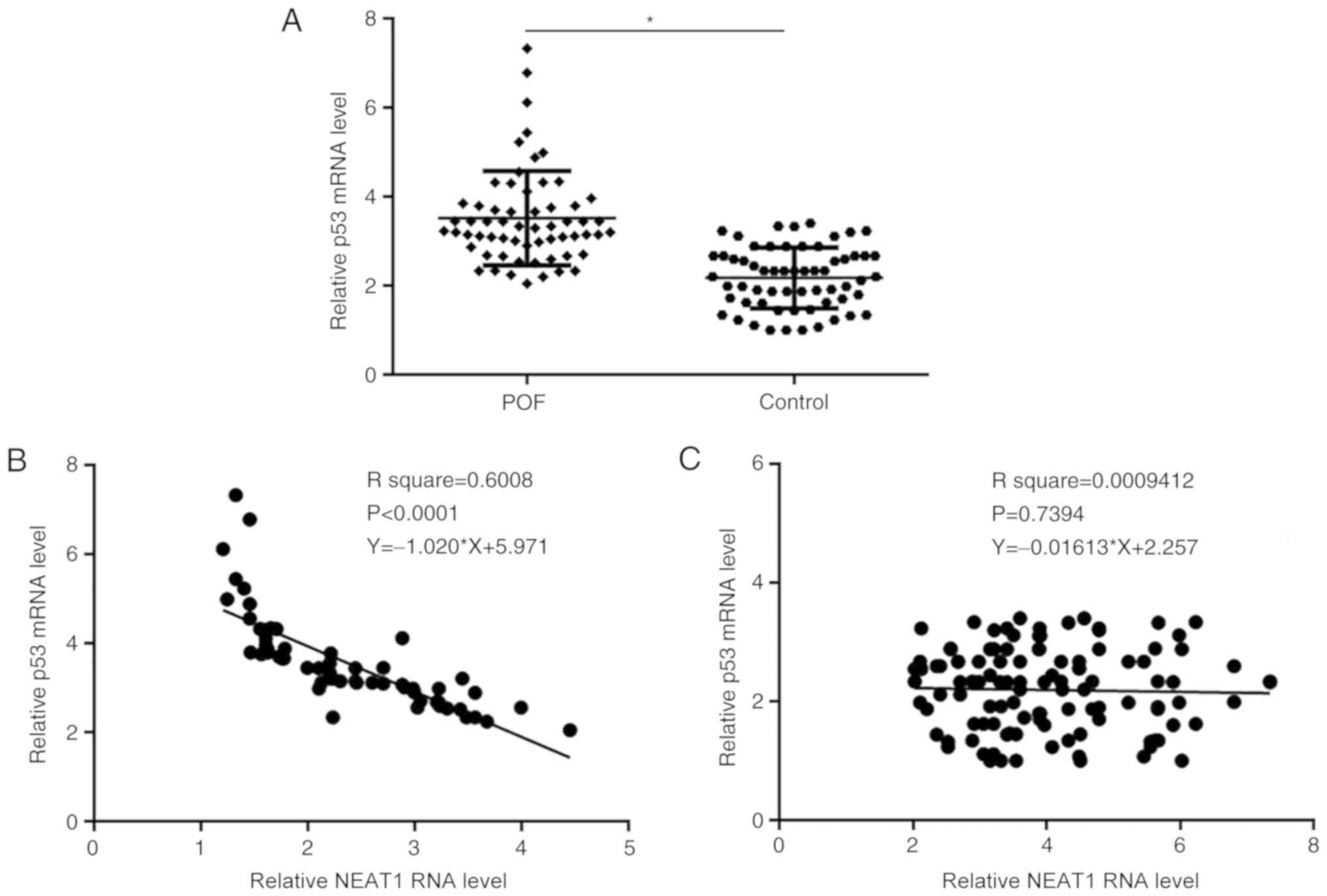
p53 mRNA expression is inversely
associated with NEAT1 expression in patients with POF
RT-qPCR was used to detect the expression level of
p53 in ovarian tissues of patients from both the POF and control
groups. The mRNA expression level of p53 was significantly
increased in the POF group compared with the control group
(Fig. 2A). Furthermore, association
between p53 mRNA and NEAT1 expression was analyzed using linear
regression. The results demonstrated that p53 and NEAT1 expression
levels were inversely associated in the POF group (Fig. 2B). However, p53 and NEAT1 expression
levels were not associated in the control group (Fig. 2C).
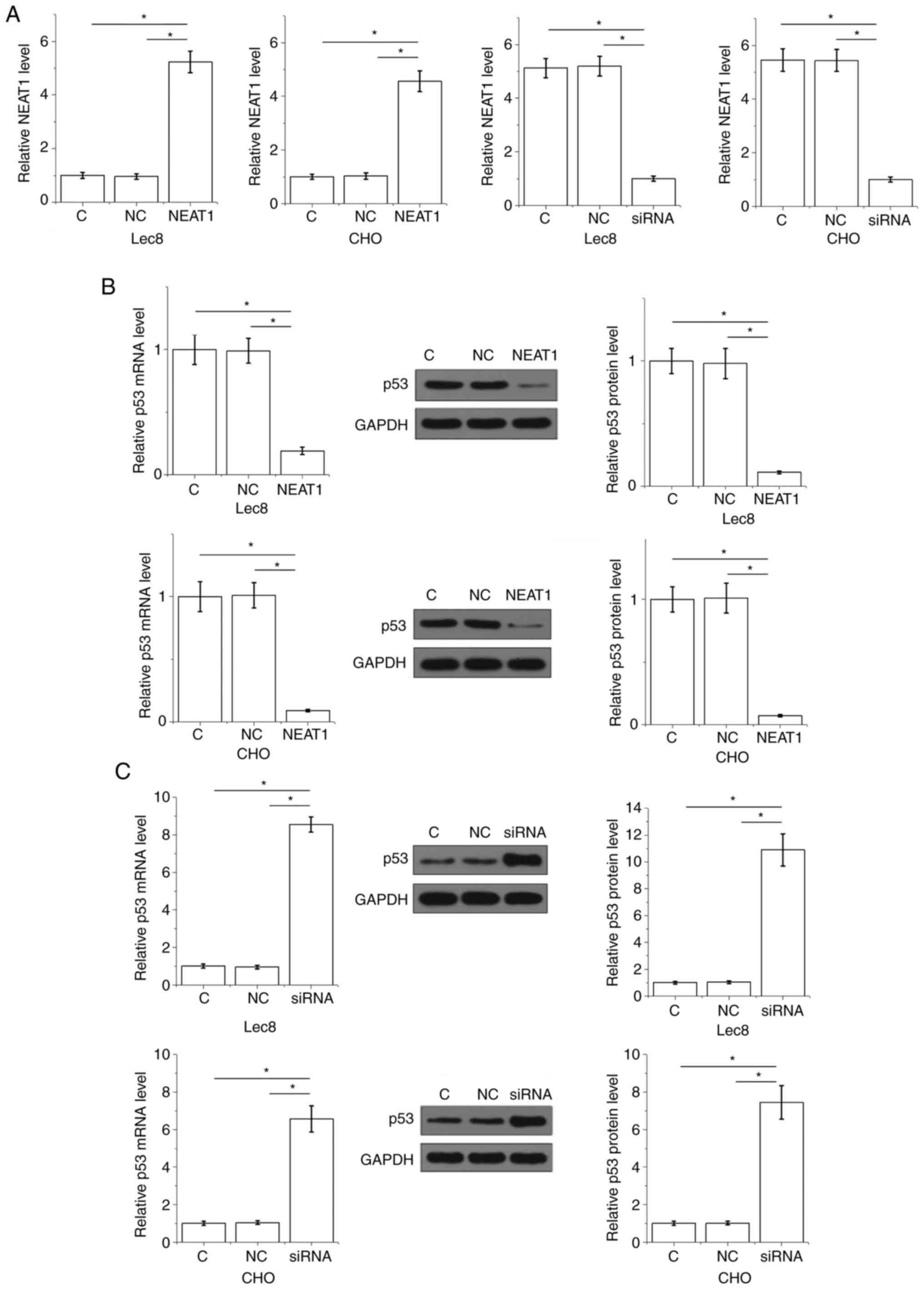
NEAT1 negatively regulates p53 in Lec8
and CHO cells
NEAT1 expression vector and siRNA were transfected
into Lec8 and CHO cells. At 24 h following transfection, expression
of NEAT1 was significantly altered in both cell lines compared with
the C and NC groups (Fig. 3A).
Furthermore, cells overexpressing NEAT1 demonstrated significantly
downregulated mRNA and protein levels of p53 (Fig. 3B). Cells that had NEAT 1 silenced
demonstrated significantly upregulated mRNA and protein levels of
p53 (Fig. 3C).
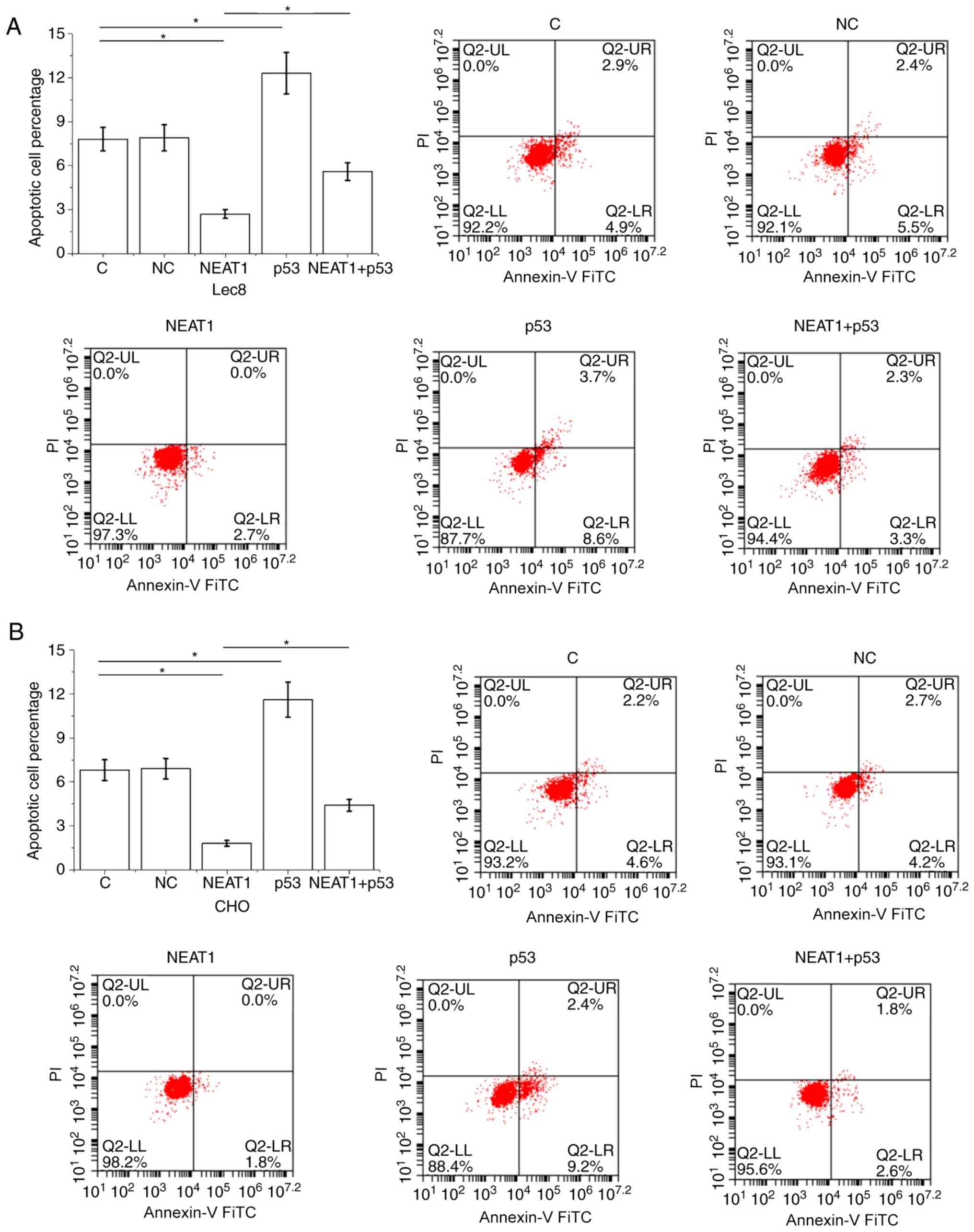
NEAT1 inhibits Lec8 and CHO cell
apoptosis through p53
Overexpression of p53 was also achieved 24 h
following transfection (Fig. S1).
Compared with the C and NC controls, overexpression of NEAT1
inhibited Lec8 (Fig. 4A) and CHO
(Fig. 4B) cell apoptosis.
Overexpression of p53 promoted Lec8 and CHO cell apoptosis. In
addition, overexpression of p53 attenuated the effects of
overexpressing NEAT1 on cell apoptosis.
Discussion
NEAT1 expression pattern and its role in POF was
investigated in the present study. The results demonstrated that
NEAT1 was downregulated in ovarian tissues from patients with POF,
and that NEAT1 downregulated p53 and subsequently ovarian cell
apoptosis.
Cell apoptosis in ovarian tissues promotes POF
(17). As a key player in the
regulation of cell apoptosis, p53 has been implicated in POF
(18,19). In a rat model of POF, Liu et
al (18) demonstrated the
activation of p53. Another study also observed upregulated p53 in
the female rat POF model (20). The
present study demonstrated that p53 expression was upregulated in
ovarian tissues from patients with POF compared with healthy women.
In addition, increased apoptotic rates of two normal ovary cell
lines were observed following p53 overexpression. The present study
further indicated the involvement of p53 in POF.
NEAT1 has been indicated to be a transcriptional
target of p53(21). Although
previous studies have characterized the functionality of multiple
lncRNAs in POF, the interaction between lncRNAs and p53 in POF
remains unclear (18,19). In the present study, an inverse
association between NEAT1 and p53 expression was found in patients
with POF. In addition, NEAT1 negatively regulated the expression of
p53 in normal ovary cells. This observation is possibly due to
disease specific patterns of p53 expression. p53 has been suggested
to be downregulated in ovarian cancer and to suppress cell
apoptosis but is suggested to be upregulated in POF to induce cell
apoptosis (18,19,21).
NEAT1 may serve as a microRNA sponge in pathological processes,
such as the apoptosis of cardiomyocytes (15). Future work may explore the possible
involvement of additional miRNAs in mediating the regulation of p53
expression by NEAT1.
It has been reported that NEAT1 can inhibit cancer
cell proliferation and promote cancer cell apoptosis in ovarian
cancer (13). However, in the
present study, NEAT 1 inhibited apoptosis of normal ovarian cell
apoptosis following NEAT1 overexpression. NEAT1 may therefore serve
opposing roles in different types of disease originating from the
same site.
This study is limited by the lack of in vivo
animal model experiments. We will further confirm our conclusions
by performing animal model experiments in our future studies.
In conclusion, NEAT1 was downregulated in POF
tissues and overexpression of NEAT1 in ovarian cells inhibited cell
apoptosis by downregulating p53.
Supplementary Material
Figure S1. Confirmation of p53
overexpression in Lec8 and CHO cells after transient transfections
using reverse transcription.quantitative PCR.
*P<0.05. C, untransfected cells; NC, negative control
(empty pcDNA3 vector).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML and JP performed the experiments, clinical
research, data analysis and wrote the manuscript. ZZ conceived and
designed the study, performed literature research and reviewed the
manuscript for important intellectual content. All authors have
read and approved the manuscript.
Ethics approval and consent to
participate
All participants were informed of the experimental
details. This study was approved by the Ethics Committee of The
Sixth Affiliated Hospital of Sun Yat-sen University (Guangzhou,
China; approval no. SAHSYU2016101725655) and all the procedures
were performed in accordance with the Declaration of Helsinki. Each
patient provided signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goswami D and Conway GS: Premature ovarian
failure. Hum Reprod Update. 11:391–410. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kovanci E and Schutt AK: Premature ovarian
failure: Clinical presentation and treatment. Obstet Gynecol Clin
North Am. 42:153–161. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nelson LM: Clinical practice. Primary
ovarian insufficiency. N Engl J Med. 360:606–614. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu T, Qin W, Huang Y, Zhao Y and Wang J:
Induction of estrogen-sensitive epithelial cells derived from
human-induced pluripotent stem cells to repair ovarian function in
a chemotherapy-induced mouse model of premature ovarian failure.
DNA Cell Biol. 32:685–698. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wu X, Cai H, Kallianpur A, Li H, Yang G,
Gao J, Xiang YB, Ji BT, Tang Y, Zheng W and Shu XO: Impact of
premature ovarian failure on mortality and morbidity among Chinese
women. PLoS One. 9(e89597)2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kawamura K, Kawamura N and Hsueh AJ:
Activation of dormant follicles: A new treatment for premature
ovarian failure? Curr Opin Obstet Gynecol. 28:217–222.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jankowska K: Premature ovarian failure.
Prz Menopauzalny. 16:51–56. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen Y, Tang H, Wang L, He J, Guo Y, Liu
Y, Liu X and Lin H: Fertility enhancement but premature ovarian
failure in esr1-deficient female zebrafish. Front Endocrinol
(Lausanne). 9(567)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chapman C, Cree L and Shelling AN: The
genetics of premature ovarian failure: Current perspectives. Int J
Womens Health. 7:799–810. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bilgin EM and Kovanci E: Genetics of
premature ovarian failure. Curr Opin Obstet Gynecol. 27:167–174.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Engreitz JM, Ollikainen N and Guttman M:
Long non-coding RNAs: Spatial amplifiers that control nuclear
structure and gene expression. Nat Rev Mol Cell Biol. 17:756–770.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhao W and Dong L: Long non-coding RNA
HOTAIR overexpression improves premature ovarian failure by
upregulating Notch-1 expression. Exp Ther Med. 16:4791–4795.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ding N, Wu H, Tao T and Peng E: NEAT1
regulates cell proliferation and apoptosis of ovarian cancer by
miR-34a-5p/BCL2. Onco Targets Ther. 10:4905–4915. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu X, Shang W and Zheng F: Long
non-coding RNA NEAT1 promotes migration and invasion of oral
squamous cell carcinoma cells by sponging microRNA-365. Exp Ther
Med. 16:2243–2250. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hong Y, Huasheng L, Lie L, Dongli C and
Qianhuan Z: Long noncoding RNA NEAT1 sponges miR125a5p to suppress
cardiomyocyte apoptosis via BCL2L12. Mol Med Rep. 19:4468–4474.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen W, Xu X, Wang L, Bai G and Xiang W:
Low expression of Mfn2 Is associated with mitochondrial damage and
apoptosis of ovarian tissues in the premature ovarian failure
model. PLoS One. 10(e0136421)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu TE, Zhang L, Wang S, Chen C and Zheng
J: Tripterygium glycosides induce premature ovarian failure in rats
by promoting p53 phosphorylation and activating the
serine/threonine kinase 11-p53-p21 signaling pathway. Exp Ther Med.
10:12–18. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Levine AJ, Tomasini R, McKeon FD, Mak TW
and Melino G: The p53 family: Guardians of maternal reproduction.
Nat Rev Mol Cell Biol. 12:259–265. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Liu Y, Li YJ, Wang N, Liu Z, Yang H, Liu Y
and Wang F: The expression changes of p53 and p21 in female rats of
premature ovarian failure in fluorosis. Chongqing Medicine.
47:1712–1715. 2018.
|
|
21
|
Idogawa M, Ohashi T, Sasaki Y, Nakase H
and Tokino T: Long non-coding RNA NEAT1 is a transcriptional target
of p53 and modulates p53-induced transactivation and
tumor-suppressor function. Int J Cancer. 140:2785–2791.
2017.PubMed/NCBI View Article : Google Scholar
|