Introduction
Bone marrow mesenchymal stem cells are known to have
multi-lineage differentiation potential and are used for various
therapeutic purposes (1). Bone
morphogenetic protein 2 (BMP-2) has been widely applied for the
regeneration of destructed bone (2). More recently, BMP-2 has been applied
for osteogenic differentiation of stem cells (3). Application of BMP-2 enhanced in
vitro mineralization (4).
Moreover, it has been reported that type 2 diabetes mellitus may
impair differentiation of bone marrow mesenchymal stem cells. BMP-2
has promoted osteogenesis of bone marrow mesenchymal stem cells in
type 2 diabetic rats through the Wnt signaling pathway (5).
The traditional three-dimensional cultures have been
of great interest because they can mimic in vivo conditions
(6). The three-dimensional culture
system has produced higher cell survival for stem cells (7) and has a better capability to maintain
inherent cell characteristics (8).
Three dimensional spheres made from bone marrow-derived stem cells
were able to differentiate into various types of cells (9). More recently, three-dimensional human
bone marrow mesenchymal stem cells have been evaluated osteogenesis
(10). The three-dimensional
culture technique had the advantage of enhanced differentiation of
stem cells into osteoblasts when compared with a two-dimensional
culture technique (11). This study
was performed to evaluate the effects of BMP-2 on proliferation,
osteogenic potential, and protein expression using cell spheres
composed of bone marrow mesenchymal stem cells and to analyze the
feasibility of the stem cell spheroid in tissue regeneration.
Materials and methods
Cell spheres using bone marrow
mesenchymal stem cells
Ethical approval was granted regarding the present
study through the Institutional Review Board of Seoul St Mary's
Hospital, College of Medicine, the Catholic University of Korea
(number: KC18SESI0083), and all experiments were carried out
following the relevant guidelines. Human bone marrow mesenchymal
stem cells (BMSCs, Catholic MASTER cells) were obtained from the
Catholic Institute of Cell Therapy (CIC, Seoul, Korea). Isolation
and propagation of the BMSCs were performed following previously
reported methods (12). CIC
verified that all samples showed >90% positive CD 73 and CD 90
expression. We seeded the cells on a culture dish. We removed the
cells that were not attached to the dish. We refreshed the culture
medium every 2 or 3 days, and grew the cells in the incubator with
95% O2 and 5% CO2 at 37˚C.
Fig. 1 shows an
overview of the study's design. We used commercially available
concave microwells (H389600, StemFIT 3D; MicroFIT) to fabricate
stem cell spheres. We loaded a total of 1x106 cells in
each well and evaluated the cell response. We treated cell spheres
made of bone marrow mesenchymal stem cells with BMP-2 at
predetermined concentrations of 0, 10 and 100 ng/ml. We evaluated
the morphological characteristics using an inverted microscope
(Leica DM IRM, Leica Microsystems). Morphological evaluation of the
spheres was conducted on days 1, 3, 7, 14, and 21.
Determination of cellular
viability
We cultured stem cell spheres in osteogenic media.
We used the commercially available two-color assay based on plasma
membrane integrity and esterase activity (Live/Dead Kit assay,
Molecular Probes) for qualitative analysis of the stem cell spheres
on days 1, 3, 5, and 7. We also performed a quantitative cellular
viability test using an assay kit based on water-soluble
tetrazolium salt (Cell Counting Kit-8). Experiments were carried
out in triplicate.
Level of alkaline phosphatase activity
and calcium deposition
The level of alkaline phosphatase activity and an
anthraquinone dye assay for calcium deposit evaluation were used to
assess osteogenic differentiation on day 14(13). Alkaline phosphatase activity is
reported to an early marker of osteogenic differentiation (14). Highest alkaline phosphatase activity
in preosteoblast was noted on day 14(15). We obtained cell spheres grown on
culture plates with osteogenic media. We used a commercially
available kit (K412-500, BioVision, Inc.) for the evaluation of
level of alkaline phosphatase activity.
We used an anthraquinone dye assay for calcium
deposit evaluation to assess osteogenic differentiation on days 7,
14, and 21. We stained the stem cell spheres with Alizarin Red S
for 30 min at room temperature after washing and fixation
procedures. The quantification of the bound dyes was performed on
day 21. The assays were performed three times.
Evaluation of Runx2 and Col1 by
quantitative polymerase chain reaction
We harvested the cells on day 7. We isolated total
RNA using purification (Thermo Fisher Scientific, Inc.); RNA was
used as a template for reverse transcription using SuperiorScript
II reverse transcriptase (Invitrogen; Thermo Fisher Scientific,
Inc.), and we determined quantities were within ratios of
absorbance at 260 and 280 nm spectrophotometrically (ND-2000,
Thermo Fisher Scientific, Inc.) on day 7.
We detected mRNA expression by quantitative
polymerase chain reaction. We designed the sense and antisense
primers based on GenBank. The primer sequences were in the
followings: Runx2 forward 5': AATGATGGTGTTGACGCTGA-3'; reverse 5':
TTGATACGTGTGGGATGTGG-3'; Col1 forward 5':
CCAGAAGAACTGGTACATCAGCAA-3', reverse 5': CGCCATACTCGAACTGGAATC-3',
β-actin forward 5': TGGCACCCAGCACAATGAA-3', and reverse 5':
CTAAGTCATAGTCCGCCTAGAAGCA-3'. Normalization was performed by
applying β-actin as a housekeeping gene. The experiments were
performed in three times.
Statistical analysis
We presented the results as means ± standard
deviations of the data. Shapiro-Wilk test were performed to conduct
the tests of normality and we checked the Levene's tests to
evaluate the equal of variances. We performed two-way analysis of
variance to evaluate the effects of concentration and time points.
We tested the differences among groups by applying one-way analysis
of variance with Tukey's post hoc test (SPSS 12 for Windows, SPSS
Inc.). The significance level was set at P<0.05.
Results
Formation of cell spheres with human
bone marrow-derived stem cells
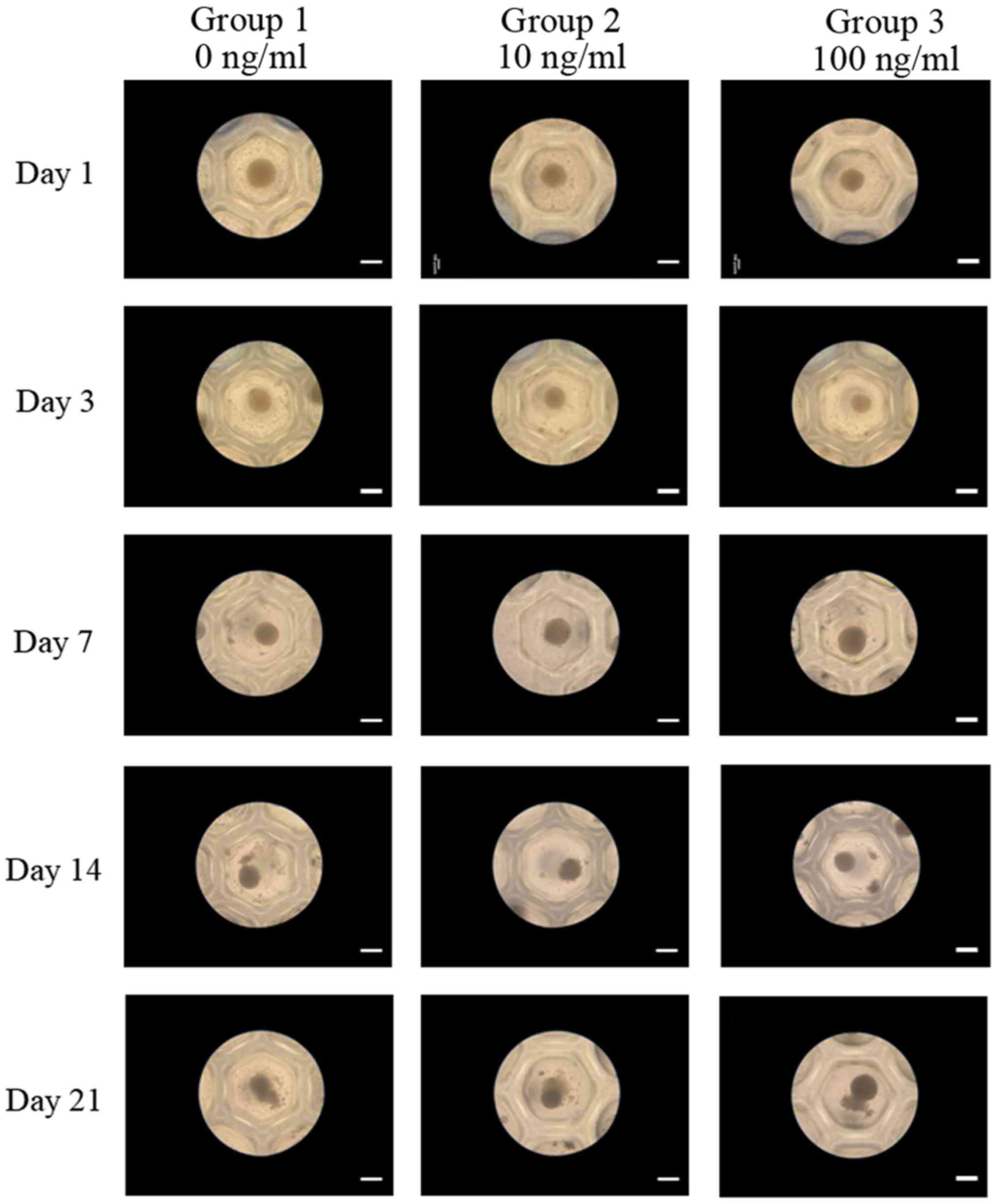
We noticed a spherical shape of stem cell aggregates
on day 1 (Fig. 2). No significant
morphological change of the cell spheres cultured in osteogenic
media was observed with the addition of BMP-2. Shape on days 3, 7,
14, and 21 is shown in Fig. 2, and
no noticeable changes were noted with longer incubation time.
Cellular viability
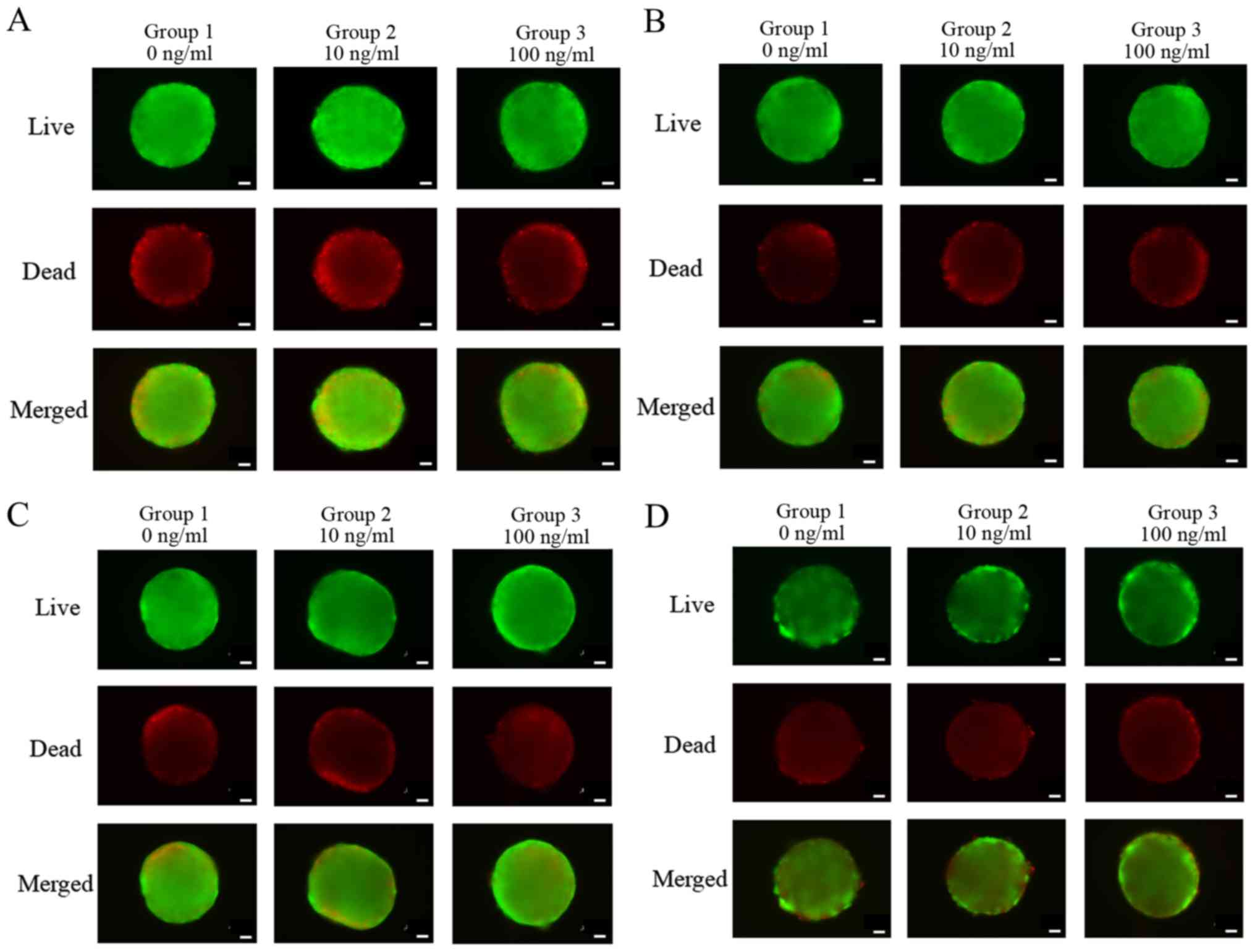
The qualitative results on the viability of cell
spheres were analyzed using a Live/Dead Kit assay at day 1, as
shown in Fig. 3A. In all cases, we
noticed that most of the cells in the spheres presented green
fluorescence, indicating live cells. We did not see any noticeable
changes with longer incubation time (Fig. 3B-D).
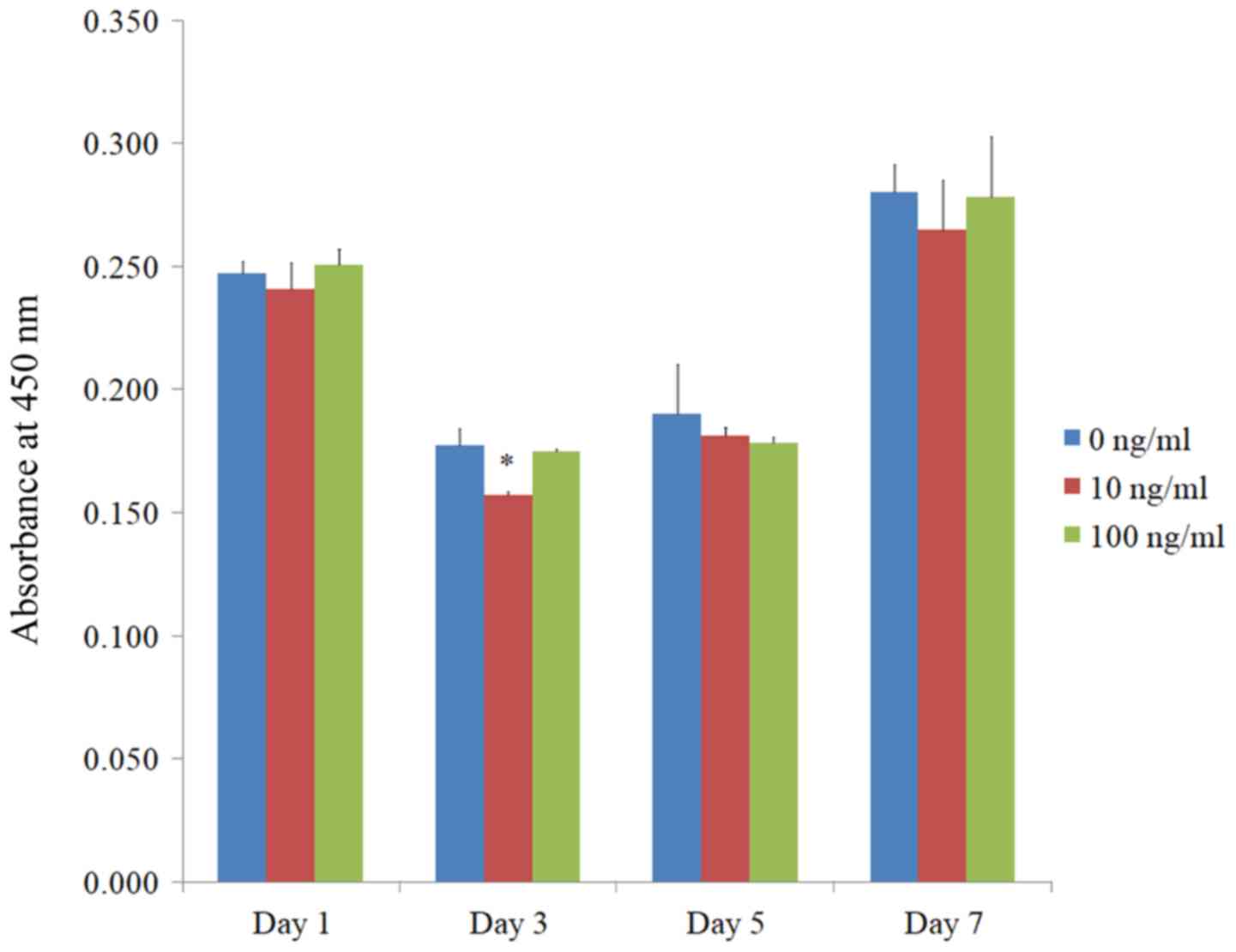
The quantitative value for cellular viability on
days 1, 3, 5, and 7 is shown in Fig.
4. The relative cell viability assay values were 100.0±1.9,
97.3±4.4, and 101.3±2.6% for BMP-2 at 0, 10, and 100 ng/ml on day
1, respectively (P>0.05).
Level of alkaline phosphatase activity
and calcium deposition
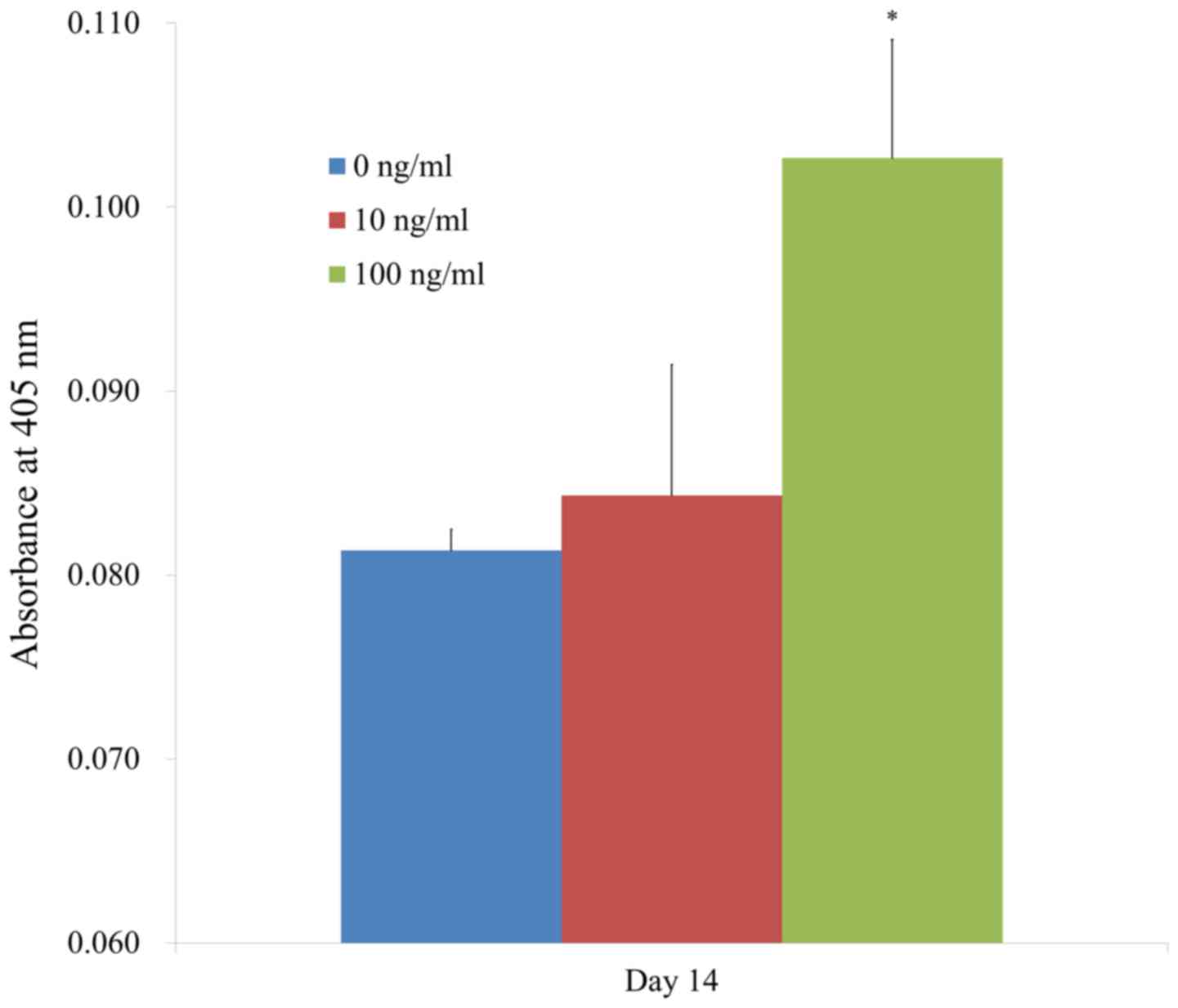
The results of the alkaline phosphatase activity
assays on day 14 are shown in Fig.
5. The absorbance values at 405 nm at day 14 for BMP-2 at 0,
10, and 100 ng/ml were 0.081±0.001, 0.084±0.007, and 0.103±0.006,
respectively. There were significantly higher values for BMP-2
groups at 100 ng/ml concentration when compared with the control
group (P<0.05).
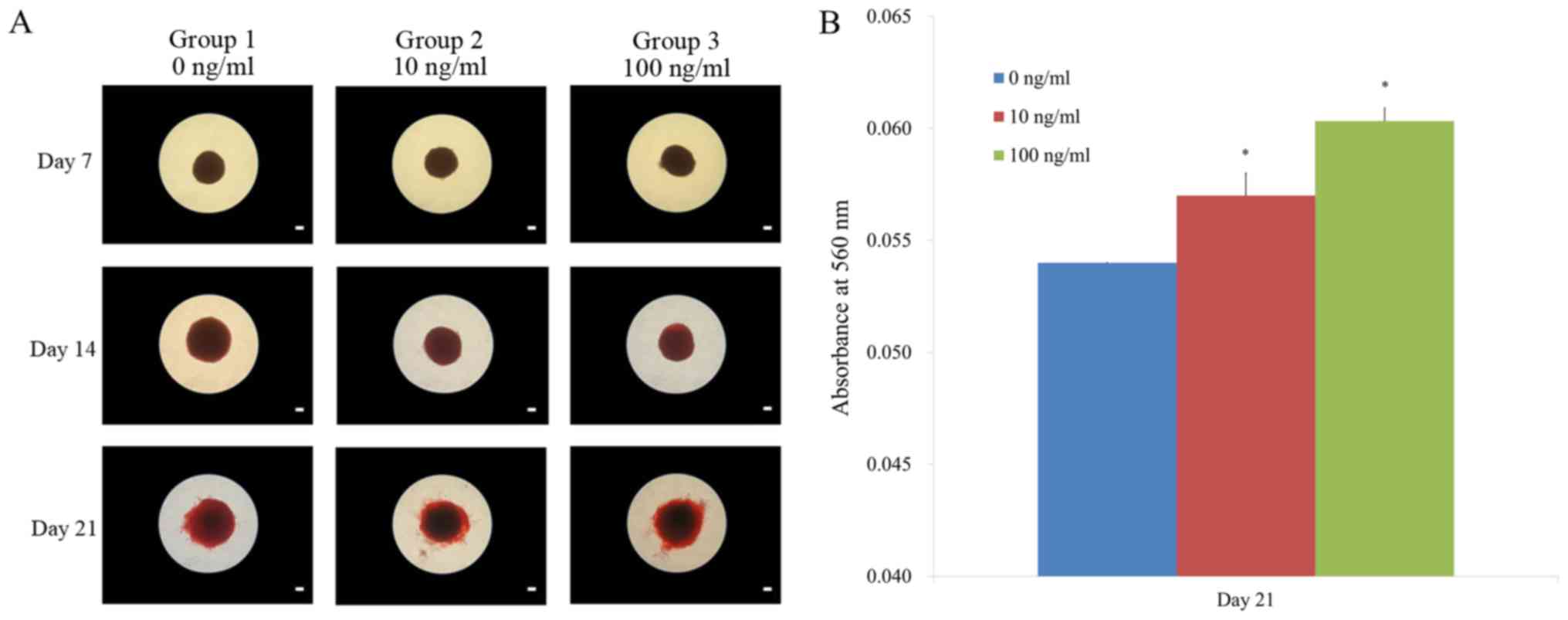
The results of the mineralization assay at days 7,
14, and 21 are shown in Fig. 6A.
Mineralized extracellular deposits were evenly noted in each group.
The quantification results showed that there were significantly
higher values for BMP-2 groups at 100 ng/ml concentration when
compared with the control (Fig. 6B;
P<0.05).
Evaluation of Runx2 and Col1 by
quantitative polymerase chain reaction
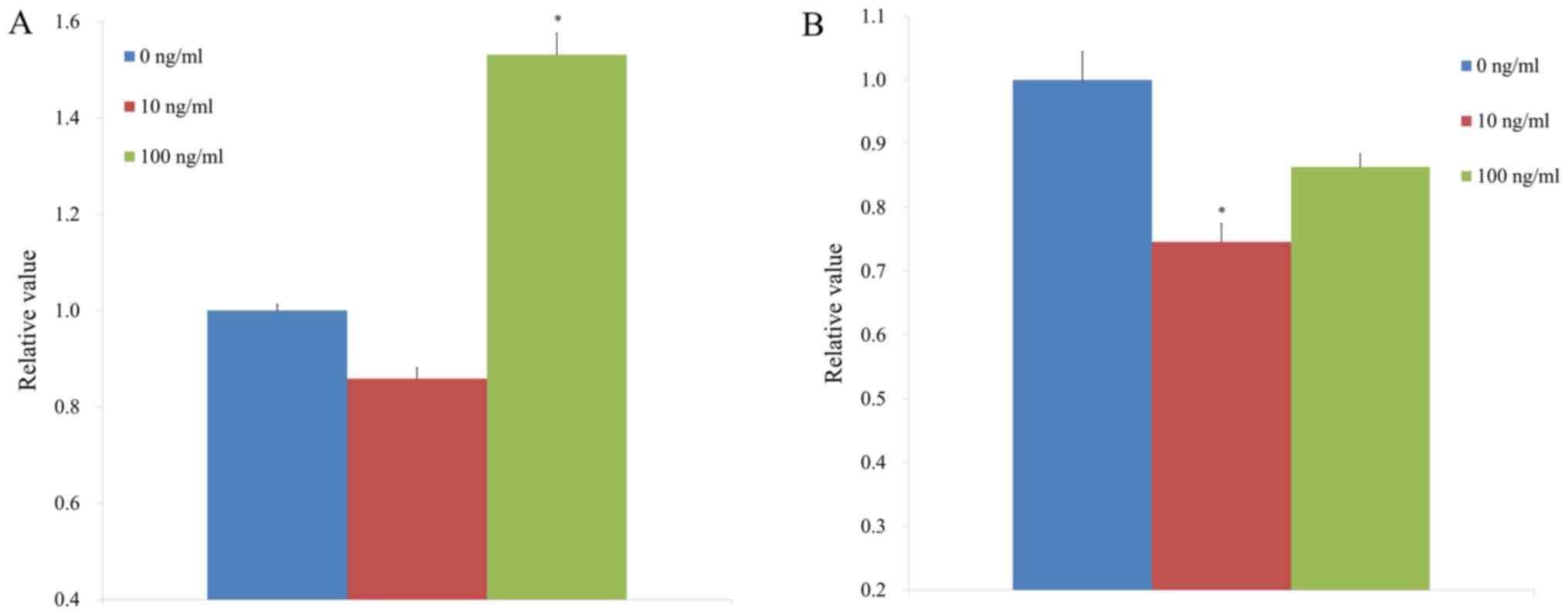
We saw significantly higher expression of mRNA
levels of Runx2 using quantitative polymerase chain reactions in
BMP-2 groups at 100 ng/ml concentration when compared with the
control (P<0.05, Fig. 7A). The
results showed that application of BMP-2 at a concentration of 100
ng/ml did not produce significant changes in Col1 expression when
compared with the control (P>0.05, Fig. 7B).
Discussion
This report discusses the effects of BMP-2 on
cellular viability and osteogenic differentiation using cell
spheres of stem cells. Our study showed that the application of
BMP-2 increased alkaline phosphatase activity and increased Runx2
at 100 ng/ml.
Bone marrow mesenchymal stem cells may enhance bone
regeneration through secretion of growth factors or through direct
differentiation into bone cells (16). The results of this study suggest
combination therapy using stem cells and BMP-2 with a
three-dimensional approach. The combined approach of
osteoconductive scaffolds and osteoinductive growth factors may
have synergistic effects on osteogenesis (17). In a previous report, the application
of a combined approach with bone marrow mesenchymal stem cells,
BMP-2 and hydroxyapatite led to successful treatment of
critical-sized defects in human beings (18). Bone marrow stem cells were loaded
into a hydrogel system by enzyme-catalyzed crosslinking, and the
addition of bone BMP-2 led to the improvement of osteogenic
potential (19). Moreover,
transient transfection of stem cells with BMP-2 expressing plasmids
was applied. The transfected cells were loaded into a
three-dimensional hydrogel system, and higher expression of
osteogenic transcription factor was achieved (20).
The effects of concentration of BMP-2 were evaluated
in previous studies (21-25).
It was reported that BMP-2 in concentrations of 0.1, 0.5, 1, 10,
50, 100, and 300 ng/ml was evaluated for osteoblast
differentiation, and a dose of 50 ng/ml or above of BMP-2 was
effective (21). Various
concentrations of BMP-2, including 20, 50, and 100 ng/ml, were
applied, and it was suggested that 50 ng/ml of BMP-2 may be optimal
(22). The addition of 60 ng/ml
BMP-2 exhibited enhanced osteoblast differentiation by regulating
the expression of phospho-Smad1/5/8(23). In rat animal models, BMP-2 in a
concentration of 50 ng/ml increased the radiographic density of
bone defects (25). However,
preconditioning of mesenchymal stem cells with lower concentrations
of 10 and 20 ng/ml of BMP-2 enhanced osteogenic differentiation
(24). In a previous report, the
application of BMP-2 significantly increased gene expression of
Runx2 and Col1(24). In this study,
the highest expression of Runx2 was noted at 100 ng/ml; however, no
significant change of Col1 expression was noted at 100 ng/ml.
Moreover, a previous report also showed that BMP-2 induced osterix
expression, but it was independent of Runx2 expression (26). In a previous report, the application
of BMP-2 resulted in increased bone sialoprotein production
(27). The overall effects of BMP-2
or maximal effective dose of BMP-2 may show differences because of
differences in types of cells, culturing condition, and culturing
period (28).
In this study, stem cell spheres were made without
using a scaffold and their morphology was maintained up to the
experimental time points. Scaffolds made of animal-derived or
chemical biomaterials are widely utilized for stem cell-based
tissue regeneration (29). More
recently, advances in three-dimensional printing technology have
made it possible to utilize scaffold-free spheroid-based
bioprinting (30). It seems that an
exogenous scaffold-free approach may have the advantage of
long-term safety (29). A previous
report used a scaffold-free structure with a mixture of cell types
using three-dimensional printing technology, and the larger amount
of stem cells resulted in greater strength (31).
The effects of stem cell spheres treated with BMP-2
may be further enhanced. In a previous study, researchers
sequentially applied BMP-2 and basic fibroblast growth factor to
achieve synergistic promotion of osteogenesis (32). Similarly, retinoic acid has been
shown to increase the effect of BMP-2 on osteogenic differentiation
of stem cells (33). BMP-2 and
Forkhead c2 synergistically enhance osteogenic differentiation and
mineralization of stem cells (34).
Moreover, mesenchymal stem cells were treated with endothelial
cells encapsulated in collagen/fibrin hydrogels to increase stem
cell functionality (35).
Collectively, this study shows that the application
of BMP-2 increases alkaline phosphatase activity and Runx2
expression in stem cell spheres at 100 ng/ml. This research
suggested that the use of BMP-2 enhanced the differentiation of
stem cell spheres, which was demonstrated by increased alkaline
phosphatase activity and Runx2 expression. Future studies may be
warranted for the use of BMP-2 with stem cell spheroids for various
models, including in vivo bony defect models in the calvaria
and mandibles. The osteogenic effects of stem cell spheroids with
the different dosages of BMP-2 can be evaluated
histomorphometrically by analyzing the amount of bone
formation.
Acknowledgements
The authors would like to acknowledge the Catholic
Institute of Cell Therapy (Seoul, Korea) for providing Catholic
MASTER Cells.
Funding
The present study was supported by the Korean
government National Research Foundation (grant no.
2020R1A2C4001624) and the Research Fund of Seoul St. Mary's
Hospital, Catholic University of Korea.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SKM, MK and JBP conceived and designed the
experiments of the present study, analyzed the data, and wrote and
reviewed the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the Institutional
Review Board of Seoul St Mary's Hospital, College of Medicine,
Catholic University of Korea (approval no. KC18SESI0083).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu H, Wei LK, Jian XF, Huang J, Zou H,
Zhang SZ and Yuan GH: Isolation, culture and induced
differentiation of rabbit mesenchymal stem cells into osteoblasts.
Exp Ther Med. 15:3715–3724. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fan J, Im CS, Cui ZK, Guo M, Bezouglaia O,
Fartash A, Lee JY, Nguyen J, Wu BM, Aghaloo T and Lee M: Delivery
of phenamil enhances BMP-2-induced osteogenic differentiation of
adipose-derived stem cells and bone formation in calvarial defects.
Tissue Eng Part A. 21:2053–2065. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lee YH, Lee BW, Jung YC, Yoon BI, Woo HM
and Kang BJ: Application of alginate microbeads as a carrier of
bone morphogenetic protein-2 for bone regeneration. J Biomed Mater
Res B Appl Biomater. 107:286–294. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wongwitwichot P and Kaewsrichan J:
Osteogenic differentiation of mesenchymal stem cells is impaired by
bone morphogenetic protein 7. Adv Med Sci. 62:266–272.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Qian C, Zhu C, Yu W, Jiang X, Zhang F and
Sun J: Bone morphogenetic protein 2 promotes osteogenesis of bone
marrow stromal cells in type 2 diabetic rats via the Wnt signaling
pathway. Int J Biochem Cell Biol. 80:143–153. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Qiao Y, Xu Z, Yu Y, Hou S, Geng J, Xiao T,
Liang Y, Dong Q, Mei Y, Wang B, et al: Single cell derived spheres
of umbilical cord mesenchymal stem cells enhance cell stemness
properties, survival ability and therapeutic potential on liver
failure. Biomaterials. 227(119573)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rosenzweig M, Pykett M, Marks DF and
Johnson RP: Enhanced maintenance and retroviral transduction of
primitive hematopoietic progenitor cells using a novel
three-dimensional culture system. Gene Ther. 4:928–936.
1997.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang W, Zhuang A, Gu P, Zhou H and Fan X:
A review of the three-dimensional cell culture technique:
Approaches, advantages and applications. Curr Stem Cell Res Ther.
11:370–380. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brboric A, Vasylovska S, Saarimäki-Vire J,
Espes D, Caballero-Corbalan J, Larfors G, Otonkoski T and Lau J:
Characterization of neural crest-derived stem cells isolated from
human bone marrow for improvement of transplanted islet function.
Ups J Med Sci. 124:228–237. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kabiri M, Kul B, Lott WB, Futrega K,
Ghanavi P, Upton Z and Doran MR: 3D mesenchymal stem/stromal cell
osteogenesis and autocrine signalling. Biochem Biophys Res Commun.
419:142–147. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Naito H, Yoshimura M, Mizuno T, Takasawa
S, Tojo T and Taniguchi S: The advantages of three-dimensional
culture in a collagen hydrogel for stem cell differentiation. J
Biomed Mater Res A. 101:2838–2845. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jeong CH, Kim SM, Lim JY, Ryu CH, Jun JA
and Jeun SS: Mesenchymal stem cells expressing brain-derived
neurotrophic factor enhance endogenous neurogenesis in an ischemic
stroke model. Biomed Res Int. 2014(129145)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee H and Park JB: Dimethyl sulfoxide
leads to decreased osteogenic differentiation of stem cells derived
from gingiva via Runx2 and collagen I expression. Eur J Dent.
13:131–136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee JM, Kim MG, Byun JH, Kim GC, Ro JH,
Hwang DS, Choi BB, Park GC and Kim UK: The effect of biomechanical
stimulation on osteoblast differentiation of human jaw
periosteum-derived stem cells. Maxillofac Plast Reconstr Surg.
39(7)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yazid MD, Ariffin SHZ, Senafi S, Razak MA
and Wahab RMA: Determination of the differentiation capacities of
murines' primary mononucleated cells and MC3T3-E1 cells. Cancer
Cell Int. 10(42)2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Futrega K, Mosaad E, Chambers K, Lott WB,
Clements J and Doran MR: Bone marrow-derived stem/stromal cells
(BMSC) 3D microtissues cultured in BMP-2 supplemented osteogenic
induction medium are prone to adipogenesis. Cell Tissue Res.
374:541–553. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hu S, Chen H, Zhou X, Chen G, Hu K, Cheng
Y, Wang L and Zhang F: Thermally induced self-agglomeration 3D
scaffolds with BMP-2-loaded core-shell fibers for enhanced
osteogenic differentiation of rat adipose-derived stem cells. Int J
Nanomedicine. 13:4145–4155. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dilogo IH, Phedy P, Kholinne E, Djaja YP,
Fiolin J, Kusnadi Y and Yulisa ND: Autologous mesenchymal stem cell
implantation, hydroxyapatite, bone morphogenetic protein-2, and
internal fixation for treating critical-sized defects: A
translational study. Int Orthop. 43:1509–1519. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Y, Chen H, Zhang T, Zan Y, Ni T, Cao
Y, Wang J, Liu M and Pei R: Injectable hydrogels from
enzyme-catalyzed crosslinking as BMSCs-laden scaffold for bone
repair and regeneration. Mater Sci Eng C Mater Biol Appl.
96:841–849. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Paidikondala M, Kadekar S and Varghese OP:
Innovative strategy for 3D transfection of primary human stem cells
with BMP-2 expressing plasmid DNA: A clinically translatable
strategy for ex vivo gene therapy. Int J Mol Sci.
20(56)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kato S, Kawabata N, Suzuki N, Ohmura M and
Takagi M: Bone morphogenetic protein-2 induces the differentiation
of a mesenchymal progenitor cell line, ROB-C26, into mature
osteoblasts and adipocytes. Life Sci. 84:302–310. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kim SY, Kim YK, Kim KS, Lee KB and Lee MH:
Enhancement of bone formation on LBL-coated Mg alloy depending on
the different concentration of BMP-2. Colloids Surf B
Biointerfaces. 173:437–446. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Park JB: Combination of simvastatin and
bone morphogenetic protein-2 enhances the differentiation of
osteoblasts by regulating the expression of phospho-Smad1/5/8. Exp
Ther Med. 4:303–306. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lysdahl H, Baatrup A, Foldager CB and
Bünger C: Preconditioning human mesenchymal stem cells with a low
concentration of BMP2 stimulates proliferation and osteogenic
differentiation in vitro. Biores Open Access. 3:278–285.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yarygin NV, Parshikov MV, Prosvirin AA,
Gur'ev VV, Govorov MV, Bosykh VG, Akatov VS and Chekanov AV: Effect
of morphogenetic protein BMP-2 on X-ray density of bone defect in
the experiment. Bull Exp Biol Med. 168:574–577. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lee MH, Kwon TG, Park HS, Wozney JM and
Ryoo HM: BMP-2-induced Osterix expression is mediated by Dlx5 but
is independent of Runx2. Biochem Biophys Res Commun. 309:689–694.
2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rickard DJ, Sullivan TA, Shenker BJ, Leboy
PS and Kazhdan I: Induction of rapid osteoblast differentiation in
rat bone marrow stromal cell cultures by dexamethasone and BMP-2.
Dev Biol. 161:218–228. 1994.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee H, Son J, Yi G, Koo H and Park JB:
Cellular viability and osteogenic differentiation potential of
human gingiva-derived stem cells in 2D culture following treatment
with anionic, cationic, and neutral liposomes containing
doxorubicin. Exp Ther Med. 16:4457–4462. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yasui Y, Ando W, Shimomura K, Koizumi K,
Ryota C, Hamamoto S, Kobayashi M, Yoshikawa H and Nakamura N:
Scaffold-free, stem cell-based cartilage repair. J Clin Orthop
Trauma. 7:157–163. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ong CS, Yesantharao P, Huang CY, Mattson
G, Boktor J, Fukunishi T, Zhang H and Hibino N: 3D bioprinting
using stem cells. Pediatr Res. 83:223–231. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Takeoka Y, Matsumoto K, Taniguchi D,
Tsuchiya T, Machino R, Moriyama M, Oyama S, Tetsuo T, Taura Y,
Takagi K, et al: Regeneration of esophagus using a scaffold-free
biomimetic structure created with bio-three-dimensional printing.
PLoS One. 14(e0211339)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kang W, Liang Q, Du L, Shang L, Wang T and
Ge S: Sequential application of bFGF and BMP-2 facilitates
osteogenic differentiation of human periodontal ligament stem
cells. J Periodontal Res. 54:424–434. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cruz ACC, Cardozo FTGS, Magini RS and
Simões CMO: Retinoic acid increases the effect of bone
morphogenetic protein type 2 on osteogenic differentiation of human
adipose-derived stem cells. J Appl Oral Sci.
27(e20180317)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang W, Zhang X, Li J, Zheng J, Hu X, Xu
M, Mao X and Ling J: Foxc2 and BMP2 induce osteogenic/odontogenic
differentiation and mineralization of human stem cells from apical
papilla. Stem Cells Int. 2018(2363917)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Han K, Ko Y, Park YG and Park JB:
Associations between the periodontal disease in women before
menopause and menstrual cycle irregularity: The 2010-2012 Korea
national health and nutrition examination survey. Medicine
(Baltimore). 95(e2791)2016.PubMed/NCBI View Article : Google Scholar
|