Introduction
Coronavirus disease 2019 (COVID-19) is an infectious
disease, caused by the new coronavirus which was first observed in
Wuhan, China in December 2019. On the 11th of March 2020, the World
Health Organization (WHO) declared this outbreak as a pandemic. As
of the 8th of July 2020, more than 11.5 million people have been
confirmed to have contracted the virus and more than half a million
have died due to complications of the disease (1). The clinical symptoms of COVID-19 are
non-specific and in most of the cases include fever, cough, fatigue
and dyspnea (2). Obesity (3), chronic cardiovascular diseases
(4) and smoking habits (5) have also been reported to contribute to
the deterioration of the disease. Toxic stressors in urban
environments could have played a role in deteriorating the immune
system of the local population forming the basis for the spreading
of COVID-19 disease (6). In a
similar context, Tsatsakis et al (7) explored the association among
human-induced pollutants found in greenhouse gases, the effect on
the immune system and other environmental aspects related to the
COVID-19 pandemic. Early diagnosis is important not only for prompt
treatment planning but also for isolation of patients in order to
prevent spreading the virus to the community. Currently, reverse
transcription-polymerase chain reaction (RT-PCR) represents the
gold-standard for diagnosing COVID-19. However, testing with RT-PCR
shows limited sensitivity (SN) which, adding to the shortage of
testing kits and the increased waiting time for results, increase
the screening burden and delays the isolation procedure (8,9). Thus,
the scientific community has been searching alternative protocols
for timely and accurate diagnosis. X-ray and chest computed
tomography (CT) imaging could be used as a reliable and rapid
approach for COVID-19 screening (9,10).
Both methods have the potential to depict COVID-19 related chest
abnormalities, with ground-glass lung opacification and
consolidation showing bilateral and subpleural or diffuse
distribution representing the cardinal findings (9,11-16).
Although, the image acquisition is easy and fast, interpretation
can be challenging and time-consuming, especially for inexperienced
and subspecialized medical professionals. In order to eliminate
such drawbacks, the scientific community has been shifting its
focus towards developing automated tools for the analysis of
imaging data. In this context, several artificial intelligence (AI)
methods have been developed to provide a prediction for the disease
and preliminary severity assessment. Tsiknakis et al
(17) proposed an Interpretable
Convolutional Neural Network (CNN) based on transfer learning for
predicting COVID-19 against viral and bacterial pneumonia and
normal cases based on more than 400 X-ray images, achieving an area
under curve (AUC) of 100%. Apostolopoulos and Mpesiana (18) also developed a CNN for predicting
COVID-19 from almost 1,500 X-ray images, achieving an accuracy
(ACC) of 96.78%, SN of 98.66% and specificity (SPC) of 96.46%.
However, compared to X-ray images, CT scans provide a more detailed
overview of the internal structure of lung parenchyma due to the
lack of overlapping tissues. Thus, recent research has focused on
the development of effective AI methods based on CT scans. Li et
al (19) developed a 3D CNN for
predicting COVID-19 against community acquired pneumonia (CAP) from
3D CT scans. Their model was trained on a fairly large dataset of
4,356 CT scans from 3,322 patients, 1,296 of which referred to
COVID-19 positive patients, and achieved SN of 90%, SPC of 96% and
an AUC equal to 95% regarding the COVID-19 class. Their proposed
model consists of several identical ResNet50 models, one for each
CT image. The feature maps of all the backbone models were combined
through a max pooling layer, which was followed by a dense layer
for the final ternary classification. The main downside of such an
approach is that only one ground truth label per exam is available,
and not one for each layer of the CT scan. Optimally, for achieving
a finer and detailed network performance, each CT layer should be
graded individually. In this way, the model would benefit from the
inter-CT-layer relations of the 3D input, as well as from the
extended ground truth information. Wang et al (20) proposed a CNN model which was first
trained on CT scans with cancer from 4,106 patients in order to
learn lung features. Subsequently, they fine-tuned the model on a
COVID-19 multi-centric CT dataset (709 patients). Their model was
validated on 4 independent datasets from different clinical
centers. Their approach performed well in identifying COVID-19
against bacterial or other viral pneumonia cases, achieving an AUC
of 87%, SN of 80.39% and SPC of 76.61% against bacterial pneumonia
and AUC of 86%, SN of 79.35% and SPC of 71.43% against viral
pneumonia. In addition, they stratified the patients into high and
low risk groups, in order to propose a hospital-stay time schedule.
Zhang et al (21) utilized
another network to segment the lung regions, before applying the
classification network in order to discard the background and
irrelevant regions within the CT scan. Their proposed pipeline
achieved an ACC of 91.2%, SN of 94.03%, SPC of 88.46% and an AUC of
96.1% for predicting COVID-19 on a prospective Chinese dataset,
while similar results were achieved on two other prospective
Chinese datasets. Subsequently, the model was applied on a dataset
from Ecuador, ACC of 84.11%, SN of 86.67%, SPC of 82.26% and AUC of
90.5%. In addition, they evaluated the effect of drug treatment on
lesion size and volume changes using their AI model. Finally, they
compared their model to 8 junior and 4 mid-senior radiologists, for
predicting COVID-19 on an independent dataset which was annotated
by 4 independent senior radiologists. The model outperformed the
junior radiologists, while its performance was comparable to that
of the mid-senior ones. Ardakani et al (22) utilized 10 pre-trained convolutional
networks as the backbone of their classifier, achieving the best
AUC score of 99.4%, SN of 100%, SPC of 99% and ACC of 99.5%, for
distinguishing COVID-19 pneumonia from other types of pneumonia
(viral and bacterial). At the same time, the performance of a
radiologist was moderate, achieving much lower results, i.e., AUC
of 87.3%, SN of 89%, SPC of 83% and ACC of 86%. However, their
dataset included a limited amount of data, namely 1,020 CT slices
for 108 COVID-19 positive patients and 86 pneumonia positive
patients. Additionally, plenty of unpublished scientific preprints
have been available on open databases, claiming high accuracy, SN
and SPC scores (23-29),
for predicting COVID-19 against other pneumonia types or healthy
patients based on deep learning models.
In this study, we propose an extensive pipeline for
automatic COVID-19 screening against other types of pneumonia
(i.e., viral and bacterial) from CT scans, utilizing lung
segmentation for increasing the accuracy. Some key innovations of
this study can be summarized in the integration of
multi-institutional and open-access data from a variety of scanners
and imaging protocols retrieved from online repositories in formats
such as DICOM or Portable network Graphics (png), the development
of a deep learning lung segmentation model for multiple CT window
settings and finally a deep learning model for differentiating
COVID-19 from CAP. This study introduces a state-of-the-art deep
learning model for lung segmentation on slices with a variety of CT
window settings (DSC 99.6%) and an image analysis deep model
trained with multi-institutional data for differentiating COVID-19
from CAP (AUC 96.1%).
Materials and methods
Dataset
The proposed analysis was performed using open data
of confirmed COVID-19 and widely available online cases. In
particular, the patient cohort consisted of 3 datasets (30-32)
with 1,266 COVID-19 and 586 pneumonia cases stratified on a
patient-basis resulting in 5,109 CT slices. Furthermore, slices
characterized other than COVID-19 or pneumonia such as normal were
discarded. The curated individual slices were collected from
patients in hospitals from São Paulo in Brazil (30) and open repositories such as medRxiv
(https://www.medrxiv.org/) and bioRxiv (https://www.biorxiv.org/) (31). Additionally, these datasets were
made available as stand alone 8-bit image files with unknown
compression methodology, no metadata information available and
various CT window settings rendering segmentation and
classification tasks with traditional methods challenging. In
contrast, the dataset (32)
consisted of 20 cases with COVID-19 pixel-based annotated regions
of interest in meta-image form (NifTi files) from the open
repositories of Radiopedia (http://radiopedia.org) and Coronacases (http://coronacases.org). This multi-institutional
collection of CT examinations lead to high variability in spacing,
pixel array size and windowing type across the examined cases.
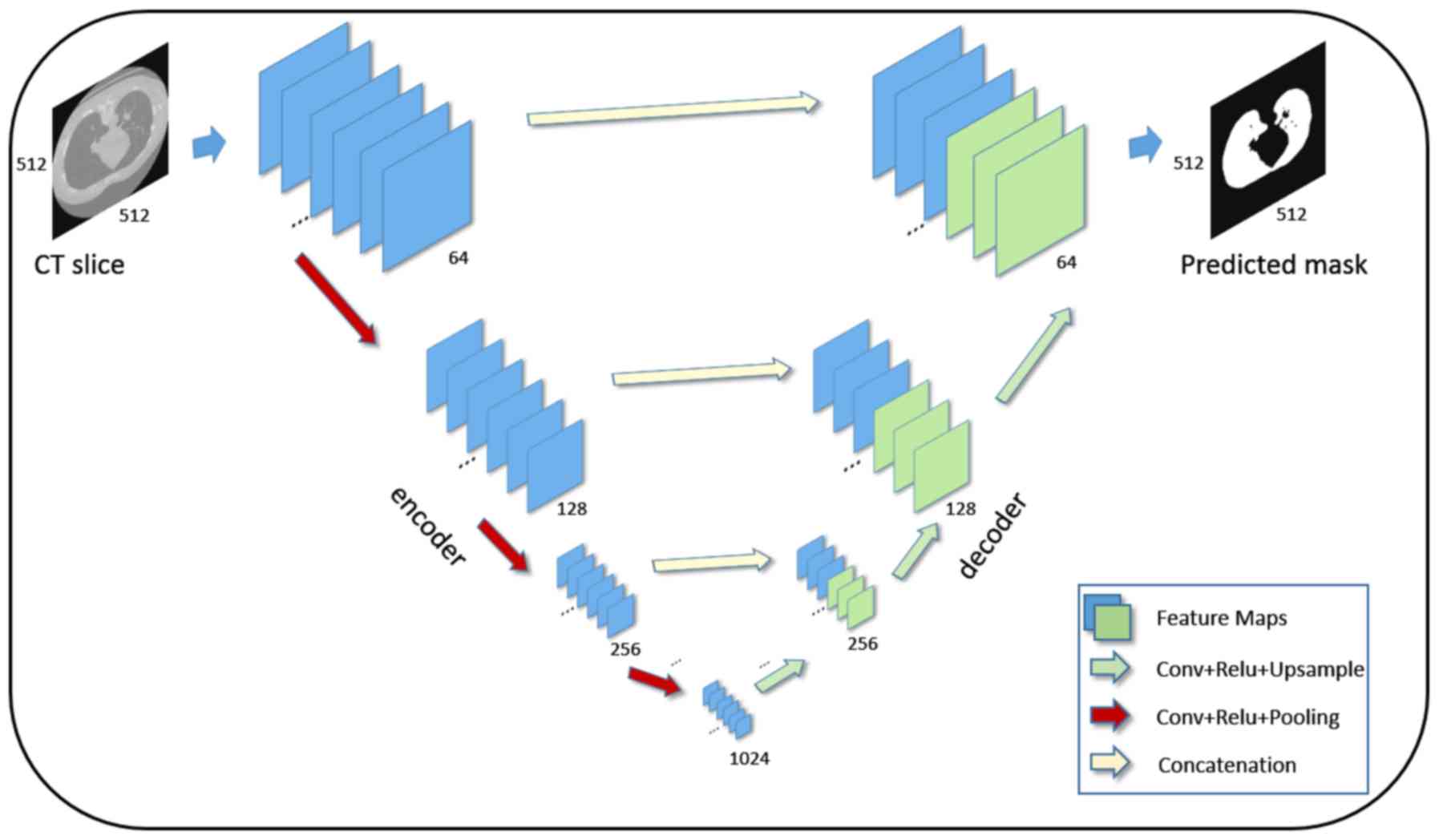
Lung segmentation
U-Net (33) is a
fully-convolutional architecture comprising multiple consecutive
convolutional, pooling layers in the encoder part and
convolutional, upsampling layers in the decoder part. The deep
network can accurately match incoming CT examination slices to
their corresponding segmentation mask. This process is learned from
data with known segmentation masks through the back-propagation of
the similarity error during the training phase. The Lung Image
Database Consortium-Image Database Resource Initiative (LIDC-IDRI)
(34) dataset was used for training
and evaluating the deep learning segmentation model. The ground
truth masks for lung segmentation were extracted by a
fully-automated Hounsfield Units (HU) based algorithm (35). A subset of the 1,018 scans with
98,433 CT slices was used for model convergence. A detailed view of
the architecture is depicted in (Fig.
1).
Pre-processing
The image resolution of the examined patient cohort
varied from 148x61 to 1,637x1,225 pixels introducing a significant
limitation for the deep learning analysis. Each slice was resized
with a linear interpolation technique targeting 512x512 pixels to
match the required dimension of input layer of the U-Net
segmentation network. The slices of the examined cohort were
segmented with the custom U-Net and normalized to achieve pixel
values with unit variance and zero mean prior to the deep learning
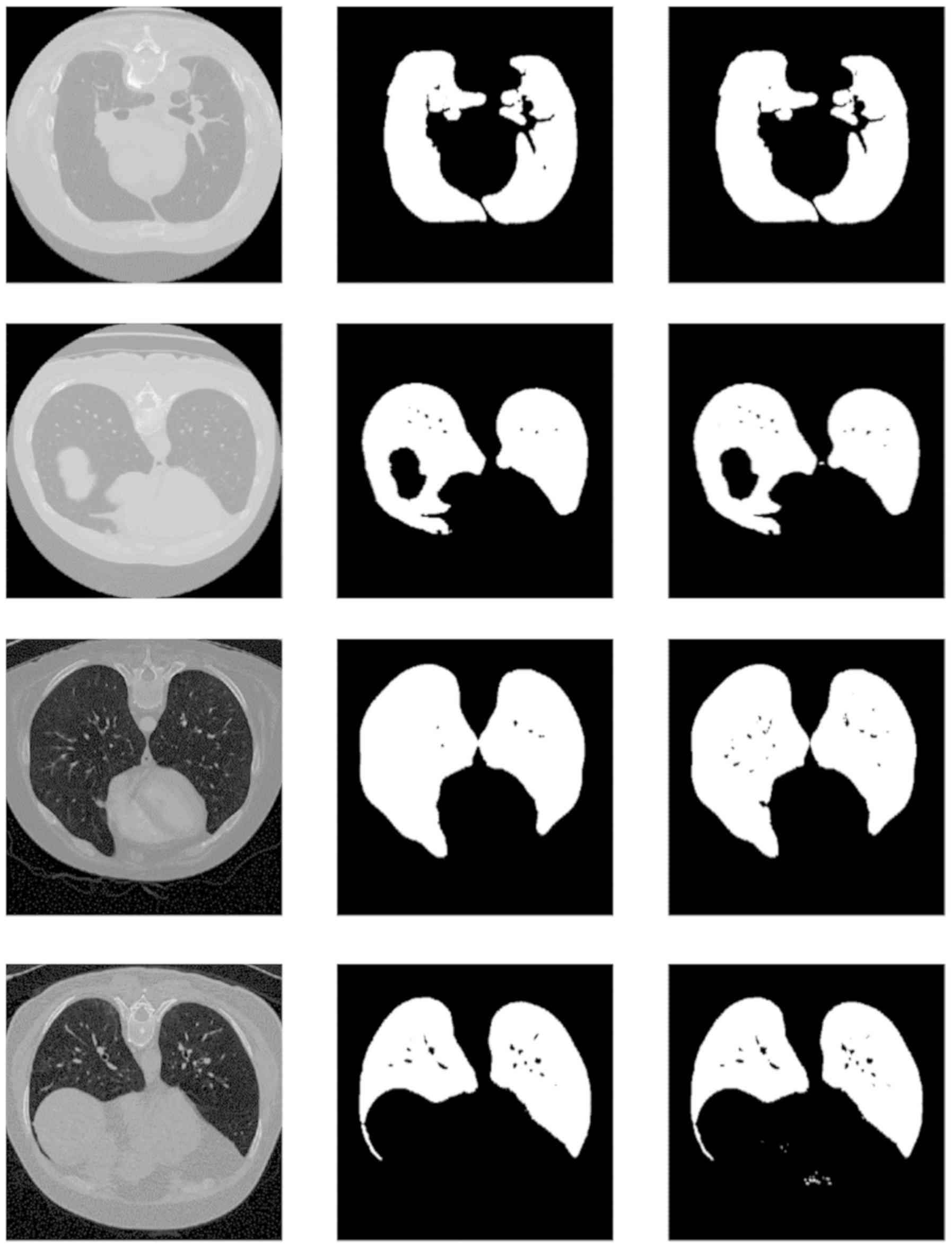
analysis. In Fig. 2 the testing set
ground truth masks versus predicted masks are presented revealing a
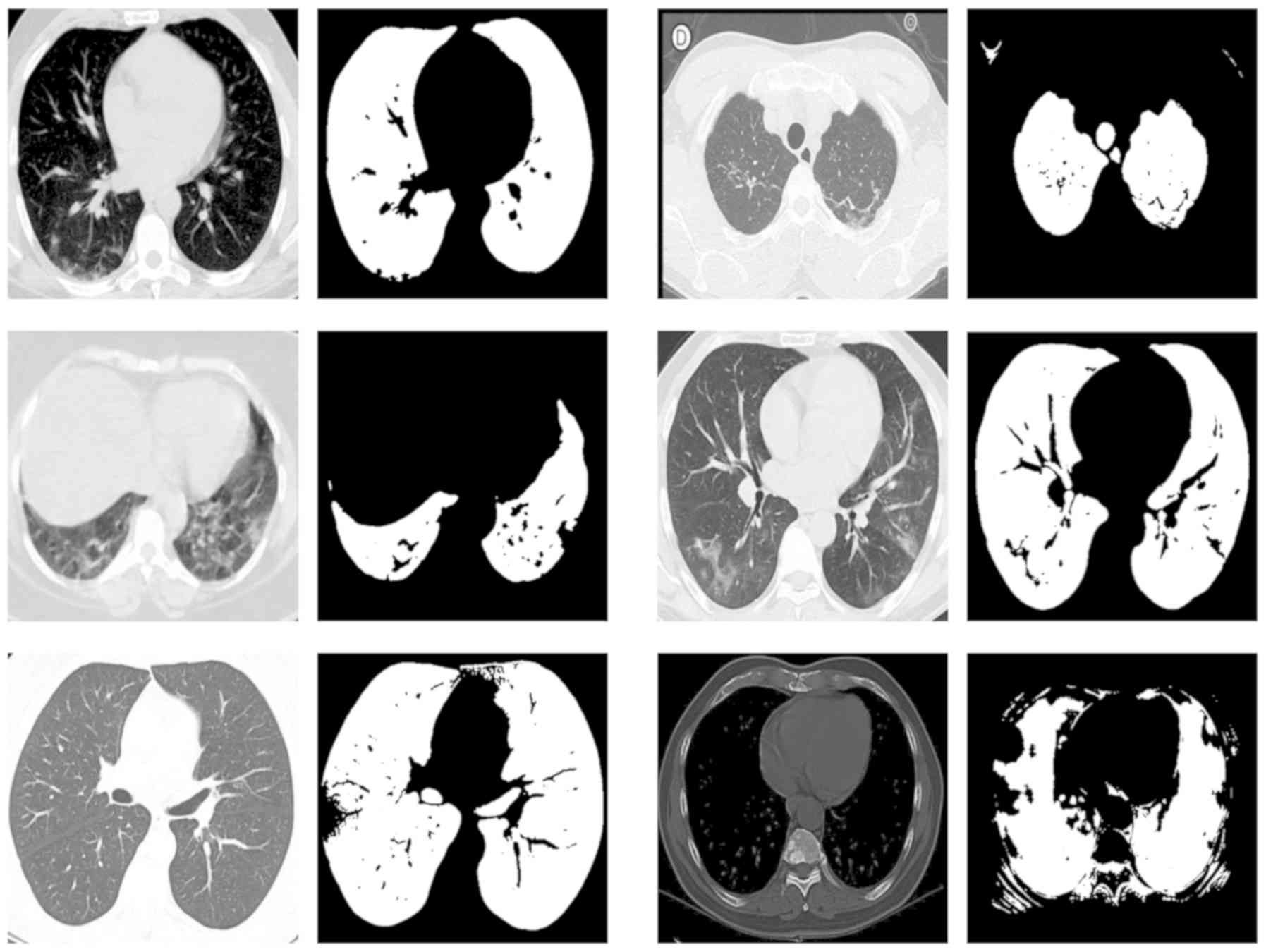
highly performing segmentation model and in Fig. 3 a randomly selected set of predicted
segmentation masks from the COVID-19 dataset are demonstrated.
Despite the aforementioned variability in pixel array dimensions
and CT window settings the proposed methodology establishes minimal
segmentation error.
Deep architecture analysis
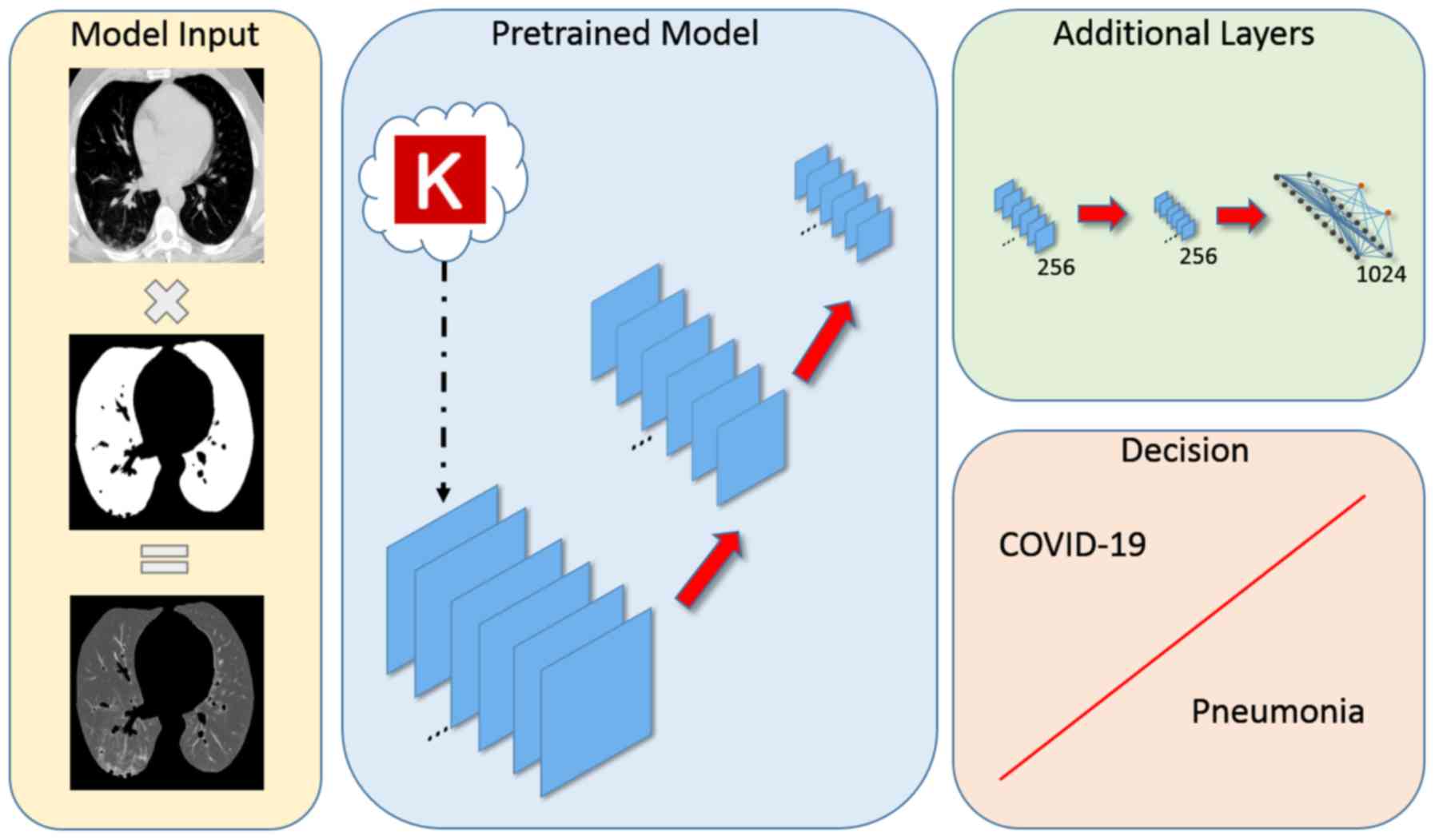
A transfer learning approach was used for improving
the convergence and fine-tuning process based on CT slices of the
examined cohort by adapting the inner representation of the
pre-trained model to the targeted COVID-19 versus CAP analysis. Two
additional convolutional layers with 256 filters each and a neural
network with 1,000 neurons were appended in the original
pre-trained model as depicted in Fig.
4. The source code and the detailed hyperparameter
configuration files are provided online (https://github.com/trivizakis/ct-covid-analysis).
Performance evaluation
The evaluation was assessed by following metrics of
ACC, SN, SPC and precision (PR):
Results
A 4-fold cross-validation process was performed for
splitting the dataset in the convergence and the testing set. The
convergence set was further randomly split into the training (80%)
and validation set (20%) for applying the fine-tuning and
hyperparameter optimization process respectively. The testing folds
remained unseen throughout the analysis to assess the performance
of the proposed deep learning model. A custom U-Net for lung
parenchyma segmentation was trained and evaluated on a total set of
109,370 LIDC-IDRI CT slices with ground truth segmentation masks
calculated on a HU basis by an automated algorithm. The U-Net
converged after 5 epochs of training and achieved a performance of
Dice Similarity Coefficient of 99.55% in the testing set providing
a reliable and accurate segmentation model as evident by the
randomly selected examples presented in Fig. 3. Although, perfect segmentation
masks cannot be obtained for the analysis dataset due to the
variability in image quality; the majority of pathological pixels
are present in the final segmented image despite some false
negative pixels. Several pre-trained models were examined including
VGG (36), Inception (37), NASNet (38), DenseNet (39) and MobileNet (40) with additional convolutional and
neural network layers as depicted in Fig. 4. The analysis architecture was
trained on average for 16 epochs before early-stopping. Most of
pre-trained models preformed comparably with an AUC up to 93% and
the highest performance (AUC 96.1%) was achieved with VGG-19 as the
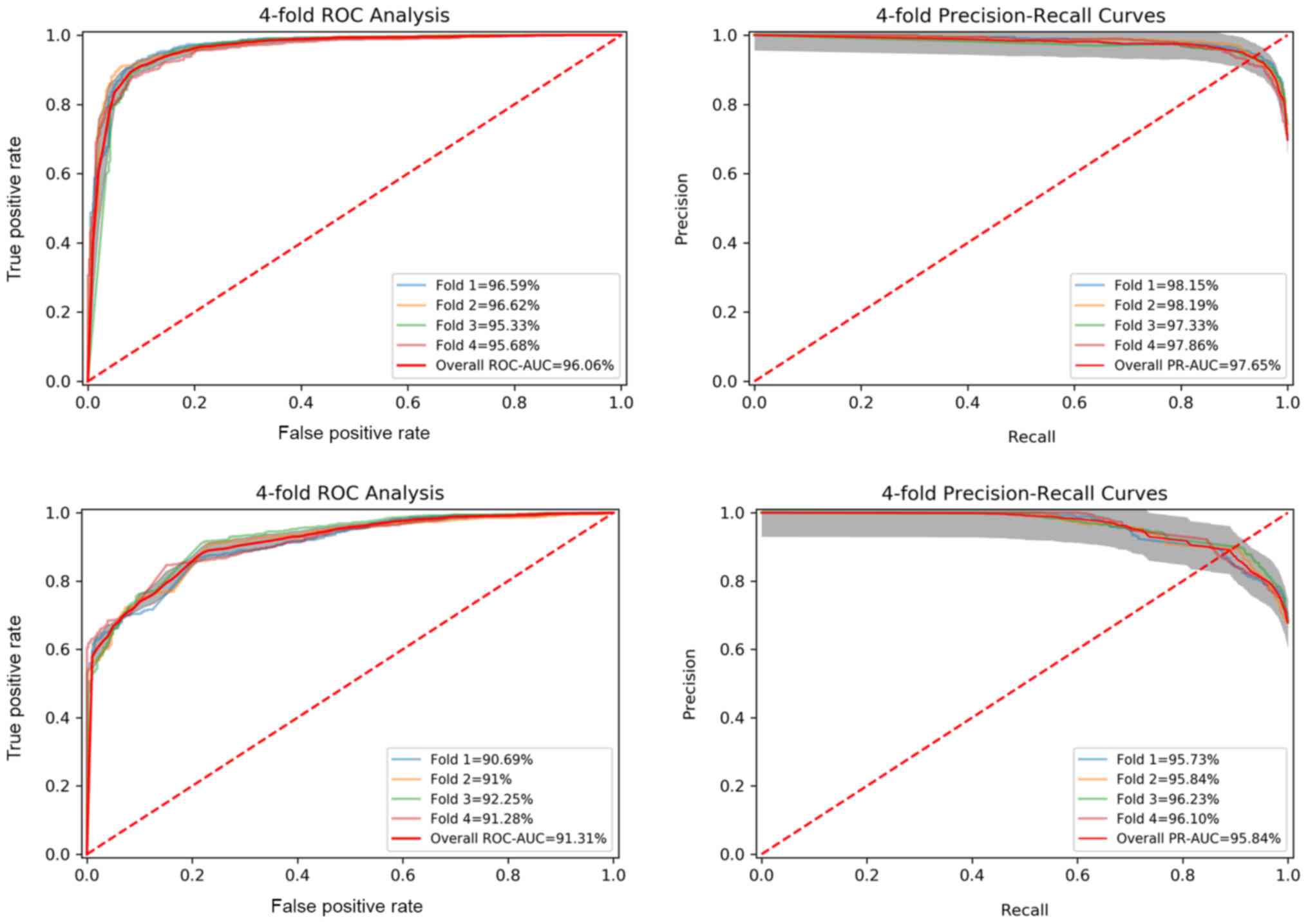
backend pre-trained network. The receiver operating characteristic
(ROC) and PR-recall curves of the best model are presented in
Fig. 5 (top) outlining the
robustness and constancy of the examined analysis. The proposed
deep learning architecture with a robust pre-processing protocol
including lung segmentation and image standardization outperforms
the baseline model (up-to AUC 91.3%) with similar architecture but
with unsegmented CT slices and the current literature (Table I) with similar experimental
protocols.
 | Table IPerformance of the proposed analysis
in comparison to the current peer-reviewed research with similar
end-points. |
Table I
Performance of the proposed analysis
in comparison to the current peer-reviewed research with similar
end-points.
| Type % | ACC | SN | SPC | AUC | Patients, no.
(training/testing) | CT Slices, no.
(training/testing) |
|---|
| Proposed with
segmentation | 91.1 | 92.0 | 87.5 | 96.1 | COVID-19: 894/372
CAP: 414/172 | COVID-19: 2,047/853
CAP: 976/406 |
| Proposed no lung
segmentation | 85.1 | 88.8 | 76.8 | 91.3 | | |
| Li et al
(19) | - | 90.0 | 96.0 | 95.0 | COVID-19: 400/68
CAP: 1396/155 Non-pneumonia: 1,173/130 | COVID-19: 1,165/127
CAP: 1,560/175 Non-pneumonia: 1,193/132 |
| Wang et al
(20) | - | 80.4 | 76.6 | 87.0 | All: 709/557 | - |
| Zhang et al
(21) | 91.2 | 94.0 | 88.5 | 96.0 | COVID-19: 752 CAP:
797 Non-pneumonia: 697 | All: 444.034 |
| Ardakani et
al (22) | 99.5 | 100.0 | 99.0 | 99.4 | COVID-19: 108
Non-COVID-19: 86 | COVID-19: 510
Non-COVID-19: 510 Training/testing (All): 816/102 |
| Harmon et al
(42) | 90.8 | 84.0 | 93.0 | 94.8 | COVID-19: 451/276
Non-COVID-19: 533/1,011 | 3D classification
model |
Discussion
The proposed analysis integrates deep learning lung
segmentation for removing irrelevant organs/landmarks and transfer
learning for discriminating between COVID-19 and other types of
pneumonia from CT imaging data with multiple window settings. The
segmentation model was trained on openly available CT data
(LIDC-IDRI) providing state-of-the-art performance even in datasets
such as the examined COVID-19 (Fig.
3) from various institutes with different CT scanners, no DICOM
metadata available and variability in image quality. Thus, the
transfer learning-based analysis was focused only on the lung
region resulting in higher performance (AUC 96.1%) significantly
advancing the baseline of 91.3% without segmented CT slices of the
examined dataset. The fact that during inference, suspicious or
disputable CT slices selected for analysis by the deep model should
have been identified by an expert radiologist from the raw CT
volume, might be considered as a limitation since a fully automated
diagnosis is not possible. That said, as presented in Table I, the proposed methodology
outperforms the current literature (19-21)
in terms of AUC performance. In particular, the deep model proposed
by Zhang et al (21)
demonstrates similar performance with the present analysis but the
examined CT slices were manually segmented on lesion basis before
classification (COVID-19 versus common pneumonia versus normal
patients). Ardakani et al (22) claims a model with an AUC performance
of 99.4%, however, it utilizes a different experimental protocol
than the examined herein, in which patches (60 by 60 pixels) were
extracted by an experienced radiologist for the analysis requiring
additional and time-consuming labor from a clinical practice
perspective.
In conclusion, the present study is addressing
limitations of other efforts (22,27,41)
where the analysis was applied on raw CT data with more demanding
preprocessing phase and uniform data quality. This is further
highlighted by the increased performance stability demonstrated by
the low prediction variability among deep models of each fold in
both ROC (standard deviation 0.55%) and PR (standard deviation
0.34%) curves as depicted in Fig. 5
(top).
Acknowledgements
Not applicable.
Funding
Part of this study was financially supported by the
Stavros Niarchos Foundation within the framework of the project
ARCHERS (‘Advancing Young Researchers' Human Capital in Cutting
Edge Technologies in the Preservation of Cultural Heritage and the
Tackling of Societal Challenges’).
Availability of data and materials
Not applicable.
Authors' contributions
ET, NT and KM conceived and designed the study. ET,
NT and KM researched the literature, performed analysis and
interpretation of data and drafted the manuscript. EEV, GZP, AHK,
NP, DAS, DS and AT critically revised the article for important
intellectual content and assisted in the literature search for this
article. All authors agree to be accountable for all aspects of the
work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated,
and finally approved the version of the manuscript to be
published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
All the other authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO): WHO
Coronavirus Disease (COVID-19) Dashboard. WHO, Geneva, 2020.
https://covid19.who.int/.
Accessed August 1, 2020.
|
|
2
|
World Health Organization (WHO): Report of
the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19).
WHO, Geneva, 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf.
Accessed February 28, 2020.
|
|
3
|
Petrakis D, Margină D, Tsarouhas K, Tekos
F, Stan M, Nikitovic D, Kouretas D, Spandidos DA and Tsatsakis A:
Obesity a risk factor for increased COVID-19 prevalence, severity
and lethality (Review). Mol Med Rep. 22:9–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Docea AO, Tsatsakis A, Albulescu D,
Cristea O, Zlatian O, Vinceti M, Moschos SA, Tsoukalas D, Goumenou
M, Drakoulis N, et al: A new threat from an old enemy: Re-emergence
of coronavirus (Review). Int J Mol Med. 45:1631–1643.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Farsalinos K, Niaura R, Le Houezec J,
Barbouni A, Tsatsakis A, Kouretas D, Vantarakis A and Poulas K:
Editorial: Nicotine and SARS-CoV-2: COVID-19 may be a disease of
the nicotinic cholinergic system. Toxicol Rep. 7:658–663.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kostoff RN, Briggs MB, Porter AL,
Hernández AF, Abdollahi M, Aschner M and Tsatsakis A: The
under-reported role of toxic substance exposures in the COVID-19
pandemic. Food Chem Toxicol: Aug 14, 2020 (Epub ahead of
print).
|
|
7
|
Tsatsakis A, Petrakis D, Nikolouzakis TK,
Docea AO, Calina D, Vinceti M, Goumenou M, Kostoff RN, Mamoulakis
C, Aschner M, et al: COVID-19, an opportunity to reevaluate the
correlation between long-term effects of anthropogenic pollutants
on viral epidemic/pandemic events and prevalence. Food Chem
Toxicol. 141(111418)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xie X, Zhong Z, Zhao W, Zheng C, Wang F
and Liu J: Chest CT for typical coronavirus disease 2019 (COVID-19)
pneumonia: Relationship to negative RT-PCR testing. Radiology.
296:E41–E45. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W,
Tao Q, Sun Z and Xia L: Correlation of chest CT and RT-PCR testing
for coronavirus disease 2019 (COVID-19) in China: A Report of 1014
cases. Radiology. 296:E32–E40. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fang Y, Zhang H, Xie J, Lin M, Ying L,
Pang P and Ji W: Sensitivity of chest CT for COVID-19: comparison
to RT-PCR. Radiology. 296:E115–E117. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen N, Zhou M, Dong X, Qu J, Gong F, Han
Y, Qiu Y, Wang J, Liu Y, Wei Y, et al: Epidemiological and clinical
characteristics of 99 cases of 2019 novel coronavirus pneumonia in
Wuhan, China: A descriptive study. Lancet. 395:507–513.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Holshue ML, DeBolt C, Lindquist S, Lofy
KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural
A, et al: Washington State 2019-nCoV Case Investigation Team: First
case of 2019 novel coronavirus in the United States. N Engl J Med.
382:929–936. 2020.
|
|
13
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J,
Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics
of 138 hospitalized patients with 2019 novel coronavirus-infected
pneumonia in Wuhan, China. JAMA. 323:1061–1069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong
Y, Ren R, Leung KSM, Lau EHY, Wong JY, et al: Early transmission
dynamics in Wuhan, China, of novel coronavirus-infected pneumonia.
N Engl J Med. 382:1199–1207. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chung M, Bernheim A, Mei X, Zhang N, Huang
M, Zeng X, Cui J, Xu W, Yang Y, Fayad ZA, et al: CT imaging
features of 2019 novel coronavirus (2019-NCoV). Radiology.
295:202–207. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tsiknakis N, Trivizakis E, Vassalou EE,
Papadakis GZ, Spandidos DA, Tsatsakis A, Sánchez-García J,
López-González R, Papanikolaou N, Karantanas AH, et al:
Interpretable artificial intelligence framework for COVID-19
screening on chest X-rays. Exp Ther Med. 20:727–735.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Apostolopoulos ID and Mpesiana TA:
Covid-19: automatic detection from X-ray images utilizing transfer
learning with convolutional neural networks. Phys Eng Sci Med.
43:635–640. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li L, Qin L, Xu Z, Yin Y, Wang X, Kong B,
Bai J, Lu Y, Fang Z, Song Q, et al: Using artificial intelligence
to detect COVID-19 and community-acquired pneumonia based on
pulmonary CT: Evaluation of the diagnostic accuracy. Radiology.
296:E65–E71. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang S, Zha Y, Li W, Wu Q, Li X, Niu M,
Wang M, Qiu X, Li H, Yu H, et al: A fully automatic deep learning
system for COVID-19 diagnostic and prognostic analysis. Eur Respir
J. 56(2000775)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang K, Liu X, Shen J, Li Z, Sang Y, Wu
X, Zha Y, Liang W, Wang C, Wang K, et al: Clinically applicable AI
system for accurate diagnosis, quantitative measurements, and
prognosis of COVID-19 pneumonia using computed tomography. Cell.
181:1423–1433.e11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ardakani AA, Kanafi AR, Acharya UR, Khadem
N and Mohammadi A: Application of deep learning technique to manage
COVID-19 in routine clinical practice using CT images: Results of
10 convolutional neural networks. Comput Biol Med.
121(103795)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Song Y, Zheng S, Li L, Zhang X, Zhang X,
Huang Z, Chen J, Zhao H, Jie Y, Wang R, et al: Deep learning
enables accurate diagnosis of novel coronavirus (COVID-19) with CT
images. medRxiv: doi: https://doi.org/10.1101/2020.02.23.20026930.
|
|
24
|
Zheng C, Deng X, Fu Q, Zhou Q, Feng J, Ma
H, Liu W and Wang X: Deep learning-based detection for COVID-19
from chest CT using weak label medRxiv: doi: https://doi.org/10.1101/2020.03.12.20027185.
|
|
25
|
Gozes O, Frid-Adar M, Greenspan H,
Browning PD, Zhang H, Ji W, Bernheim A and Siegel E: Rapid AI
development cycle for the coronavirus (COVID-19) pandemic: Initial
results for automated detection and patient monitoring using deep
learning CT image analysis. arXiv:2003.05037.
|
|
26
|
Shan F, Gao Y, Wang J, Shi W, Shi N, Han
M, Xue Z, Shen D and Shi Y: Lung infection quantification of
COVID-19 in CT images with deep learning. arXiv:2003.04655.
|
|
27
|
Kassani SH, Kassasni PH, Wesolowski MJ,
Schneider KA and Deters R: Automatic detection of coronavirus
disease (COVID-19) in x-ray and CT images: A machine learning-based
approach. arXiv:2004.10641.
|
|
28
|
Hu S, Gao Y, Niu Z, Jiang Y, Li L, Xiao X,
Wang M, Fang EF, Menpes-Smith W, Xia J, et al: Weakly supervised
deep learning for COVID-19 infection detection and classification
from CT images. IEEE Access. 8:118869–118883. 2020.
|
|
29
|
Chen X, Yao L and Zhang Y: Residual
attention U-Net for automated multi-class segmentation of COVID-19
chest CT images. arXiv:2004.05645.
|
|
30
|
Soares E, Angelov P, Biaso S, Froes MH and
Abe DK: SARS-CoV-2 CT-scan dataset: A large dataset of real
patients CT scans for SARS-CoV-2 identification. medRxiv: doi:
https://doi.org/10.1101/2020.04.24.20078584.
|
|
31
|
Yang X, He X, Zhao J, Zhang Y, Zhang S and
Xie P: COVID-CT-Dataset: A CT scan dataset about COVID-19.
arXiv:2003.13865.
|
|
32
|
Ma J, Ge C, Wang Y, An X, Gao J, Yu Z,
Zhang M, Liu X, Deng X, Cao S, et al: COVID-19 CT lung and
infection segmentation dataset. Zenodo: http://doi.org/10.5281/zenodo.3757476.
|
|
33
|
Ronneberger O, Fischer P and Brox T:
U-net: Convolutional networks for biomedical image segmentation.
In: Lecture Notes in Computer Science (including subseries Lecture
Notes in Artificial Intelligence and Lecture Notes in
Bioinformatics). Vol 9351. Springer Verlag, pp234-241, 2015.
|
|
34
|
Armato III S, McLennan G, Bidaut L,
McNitt-Gray M, Meyer C, Reeves A, Zhao B, Aberle D, Henschke C,
Clarke L, et al: The Lung Image Database Consortium (LIDC) and
Image Database Resource Initiative (IDRI): a completed reference
database of lung nodules on CT scans. Med Phys. 38:915–931.
2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
gitHub: wanwanbeen/ct_lung_segmentation:
Robust segmentation of lung and airway in CT scans. https://github.com/wanwanbeen?tab=repositories.
Updated November 29, 2017.
|
|
36
|
Simonyan K and Zisserman A: Very deep
convolutional networks for large-scale image recognition.
arXiv:1409.1556.
|
|
37
|
Szegedy C, Vanhoucke V, Ioffe S, Shlens J
and Wojna Z: Rethinking the inception architecture for computer
vision. In: Proceedings of the 2016 IEEE Conference on Computer
Vision and Pattern Recognition (CVPR), Las Vegas, NV, pp2818-2826,
2016.
|
|
38
|
Zoph B, Vasudevan V, Shlens J and Le QV:
Learning transferable architectures for scalable image recognition.
arXiv:1707.07012v4.
|
|
39
|
Huang G, Liu Z, van der Maaten L and
Weinberger KQ: Densely connected convolutional networks.
arXiv:1608.06993.
|
|
40
|
Sandler M, Howard A, Zhu M, Zhmoginov A
and Chen LC: MobileNetV2: Inverted Residuals and Linear
Bottlenecks. The IEEE Conference on Computer Vision and Pattern
Recognition (CVPR), pp4510-4520, 2018.
|
|
41
|
He X, Yang X, Zhang S, Zhao J, Zhang Y,
Xing E and Xie P: Sample-Efficient Deep Learning for COVID-19
Diagnosis Based on CT Scans medRxiv: doi: https://doi.org/10.1101/2020.04.13.20063941.
|
|
42
|
Harmon SA, Sanford TH, Xu S, Turkbey EB,
Roth H, Xu Z, Yang D, Myronenko A, Anderson V, Amalou A, et al:
Artificial intelligence for the detection of COVID-19 pneumonia on
chest CT using multinational datasets. Nat Commun.
11(4080)2020.PubMed/NCBI View Article : Google Scholar
|