Introduction
Multiple large-scale clinical studies have indicated
that, in the early stages of diabetes, strict regulation of blood
glucose levels may reduce the occurrence of diabetic vasculopathy
complications, including microangiopathy and macroangiopathy
(1). If blood glucose levels of
patients with diabetes are not strictly regulated in the long-term,
even in cases where levels are controlled in the future, chronic
blood vessel complications associated with diabetes may develop
(2). This phenomenon is termed as
metabolic memory or hyperglycemic memory. Various studies have
indicated that if long-term blood glucose levels of patients with
diabetes are not efficiently regulated, advanced glycation end
products (AGEs) are generated through a series of non-enzyme
glycations and lipid oxidations (2,3). This
phenomenon may be the primary reason for generating metabolic
memory. Previous results have indicated that AGEs are highly
correlated with severe degrees of diabetic macroangiopathy and
microangiopathy, but have no correlation with other glycated
proteins (4). Furthermore,
exogenous synthetic AGEs have been demonstrated to damage normal
vascular endothelial cells and result in various lesions (5).
Silent information regulator 1 (SIRT1) is a
conservative gene in mammalian cells that is located in endothelial
cells and belongs to the histone acetylation enzyme family
(6). A number of studies have
suggested that endonuclear SIRT1 has an important role in
maintaining genome stability, regulating cell energy metabolism,
lengthening cell survival and delaying cell aging (7). Previous results have revealed that
SIRT1 may relieve and decrease various cell functions, including
oxidative stress, inflammation and apoptosis, by regulating
endothelial cells, endothelial nitric oxide synthase, p53, Foxo
family components and endothelial cell regulation by regulating
angiotensin receptor II acetylation (8). During the inflammatory response, SIRT1
downregulates nuclear factor (NF)-κB subunit Rel/65 and B-cell
lymphoma associated X protein (Bax) activity and reduces the
generation of inflammatory factors and inhibits suppressor cells
(9). Furthermore, it has been
indicated that SIRT1 may have a role in inhibiting and promoting
cell protection in cardiovascular disease, diabetes mellitus, tumor
formation, aging and inflammation (9,10).
SIRT1 in sepsis are a few; however, SIRT1 is an important
inflammatory and anti-apoptotic factor.
Oxidative stress was first proposed by Seis in
1985(11). Oxidative stress refers
to the overproduction of oxides and limited anti-oxide generation
that results in unbalanced pro-oxidation and anti-oxidation, which
promotes tissue damage and affects various mechanisms (12). Reactive oxygen species (ROS) are a
primary source of oxidative stress and are associated with
peroxidation, as well as superoxide anion and free radical
generation (13). Additionally, ROS
are continuously generated during the metabolic activities of cells
(13). Under normal conditions, low
concentrations of ROS regulate the function of vascular cells and
are necessary for the maintenance of normal blood vessel functions
(14). However, in cases where ROS
are overproduced or not eliminated rapidly, cellular damage of
human tissues may occur, including the reduction of nitric oxide
activity, lipid oxidation and protein nitration (14). Multiple enzymes are involved in the
generation of ROS in vascular endothelial cells, including reduced
nicotinamide adenine dinucleotide phosphate (NAPDH), xanthine
oxidase, endothelial nitric oxide synthase, cyclooxyggenase-2
(COX-2) and lipoxygenase (15).
The primary component of Panax notoginseng
saponins (PNS) injections (Sanqi Panax Notoginseng) are
Panax notoginseng saponins (16). PNS are effective pharmacological
components of pseudo-ginseng (16).
PNS are widely applied in the clinic and are primarily used to
carotid artery disease, hemiplegia, sequelae of cerebrovascular
disease and chest congestion and pains (16). A previous study has shown that PNS
have anti-cerebral ischemic properties, improve blood rheological
parameters and microcirculation, reduce the fat composition in the
blood and alleviate free radical-induced cell injury (16). Furthermore, PNS are typically used
for curing ischemic cerebrovascular diseases and protecting
ischemic damage of nerve cells (16). In the present study, it was
investigated whether PNS alleviated AGE-induced apoptosis of
HUVECs.
Materials and methods
Reagents, cell culture and
treatment
Kaighn's modification of Ham's F-12 medium (F-12 K
medium), penicillin and streptomycin were purchased from
Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Fetal calf serum (FCS) was purchased from Gibco; Thermo Fisher
Scientific, Inc. Trypsin was obtained from Ameresco, Inc.
(Framingham, MA, USA). Human umbilical vein endothelial cells
(HUVECs) were purchased from Shanghai Cell Bank of Chinese Academy
of Sciences and maintained in F-12 K medium supplemented with 10%
FBS, 100 U/l penicillin and 10 mg/l streptomycin at 37˚C in an
atmosphere containing 5% CO2. PNS (95%) was purchased
from Yunnan Botanical Pharmaceutical Co., Ltd. Advanced glycation
end product (AGE-BSA) was prepared using D-glucose (Sigma-Aldrich;
Merck KGaA) and bovine serum albumin (Beyotime Institute of
Biotechnology)
Cell viability
To determine the cell viability, HUVEC cells, at
~85% confluency, were incubated with F-12 K medium containing 2%
FBS and 300 µg/ml AGE alone or AGE and PNS (0.05, 0.5 or 1 mg/ml)
for 48 h in a humidified atmosphere containing 5% CO2.
The control group consisted of HUVEC cells incubated with F-12 K
medium containing 2% FBS. Following a 4 h incubation period at 37˚C
with 50 µl 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT; 5 mg/ml; Invitrogen; Thermo Fisher Scientific, Inc.),
the medium was discarded and 150 µl of dimethyl sulfoxide
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added. Cell
viability was measured using a microplate reader (Model 550;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Apoptosis rate assay
HUVEC cells, at ~85% confluency, were incubated with
F-12 K medium containing 2% FBS and 300 µg/ml AGE or PNS (0.05, 0.5
or 1 mg/ml) for 48 h. Cells in the control group were treated with
PBS. Cells were washed twice with 4˚C phosphate-buffered saline and
resuspended in 100 µl binding buffer (Invitrogen; Thermo Fisher
Scientific, Inc.). Cell was fixed with 4% paraformaldehyde for 15
min at room temperature. Subsequently, cells were stained with 5 µl
Annexin V-fluorescein isothiocyanate (FITC) and 5 µl propidium
iodide (PI) for 15 min at room temperature in the dark. The
apoptotic rate was quantified using the Coulter Epics XL flow
cytometer (Beckman Coulter, Inc., Brea, CA, USA).
ELISA
HUVEC cells (1x106 cells/ml, 200 µl/ well
in 96 well plates) at a confluency of ~85% were incubated with F-12
K medium supplemented with 2% FBS and 300 µg/ml AGE alone or AGE
and PNS (0.05, 0.5 or 1 mg/ml) for 48 h in a humidified atmosphere
containing 5% CO2. The control group consisted of HUVEC
cells incubated with F-12 K medium containing 2% FBS at 37˚C. Cells
were lysed in ice-cold cell lysis buffer (Cell Signaling
Technology, Inc., Danvers, MA, USA) and protease cocktails (1:100;
Gibco; Thermo Fisher Scientific, Inc.). The supernatant was
collected to measure the contents of caspase-3 (cat. no. G015-1-3;
Nanjing Jiancheng Biology Engineering Institute, Nanjing, China),
monocyte chemoattractant protein-1 (MCP-1; cat. no. H115; Nanjing
Jiancheng Biology Engineering Institute), malondialdehyde (MDA;
cat. no. A003-1-2; Nanjing Jiancheng Biology Engineering Institute)
and sodium dismutase superoxide dismutase (SOD; cat. no. A001-3-2;
Nanjing Jiancheng Biology Engineering Institute) using an ELISA
reader (Start Fax 2100; Awareness Technology Inc., Fisher Bioblock
Scientific, Tournai, Belgium).
Western blotting
HUVEC cells were incubated with F-12 K medium
containing 2% FBS and 300 µg/ml AGE alone or AGE and PNS (control,
0.05, 0.5 or 1 mg/ml) for 48 h in a humidified atmosphere
containing 5% CO2. Control group was HUVEC cells
incubated with F-12 K medium containing 2% FBS at 37˚C. Cell was
lysed in ice-cold cell lysis buffer (Cell Signaling Technology,
Inc.) and protease cocktails. Protein concentration was determined
using a BCA protein assay kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Equal quantities of
protein (50 µg) were loaded and separated using SDS-PAGE (10% gels)
and transferred to polyvinylidene difluoride (PVDF) membranes (GE
Healthcare Life Sciences, Chalfont, UK). Following this, membranes
were blocked with 5% milk solution (Yili Group Co., Ltd.,
Neimenggu, China) in 0.1% Tris-buffered saline tween (TBS-T) for 2
h at room temperature. Target protein bands in the PVDF membranes
were probed with anti-silent information regulator 1 (SIRT1; cat.
no. sc-74465, 1:1,000, Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), anti-transforming growth factor (TGF)-β1 (cat. no. 3709;
1:1,000; Cell Signaling Technology Inc.), anti-inducible nitric
oxide synthase (iNOS, cat. no. 13120, 1:1,000, Cell Signaling
Technology, Inc.), anti-cyclooxygenase 2 and anti-GAPDH (cat. no.
5174, 1:1,000, Cell Signaling Technology, Inc.) at 4˚C overnight.
Following washing with TBS-T for 5 min for a total of three times,
PVDF membranes were incubated with Anti-rabbit IgG, HRP-linked
Antibody (cat. no. 7074; 1:5,000; Cell Signaling Technology Inc.)
for 1 h at 37˚C. Bands were detected using enhanced
chemiluminescence reagents (SuperSignal West Femto, Pierce; Thermo
Fisher Scientific, Inc.). Images were captured using a FluorChem
FC2 Imaging System (Alpha Innotech Corp., San Leandro, CA,
USA).
Statistical analysis
Data are indicated as mean ± standard error of the
mean using SPSS software (version 11.0; SPSS, Inc., Chicago, IL,
USA). Data were analyzed using one-way analysis of variance
analysis and Tukey's post hoc test for three groups or data were
analyzed using Student's t-test for two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
PNS promotes cell viability of
AGE-induced HUVECs
AGE-induced HUVECs were established to study the
effect of PNS on cell viability of AGE-induced HUVECs. Cell
viability of AGE-induced HUVECs was determined using the MTT assay.
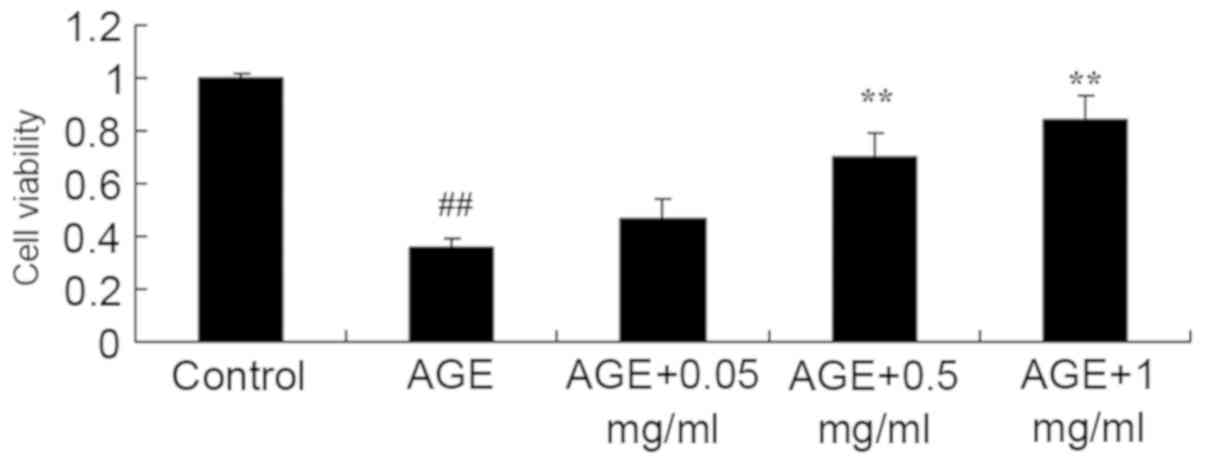
As indicated in Fig. 1, the cell
viability of AGE-induced HUVECs was significantly reduced compared
with that of the control (P<0.01). However, AGE-treated cells
with 0.5 or 1 mg/ml PNS exhibited significantly increased cell
viability of AGE-induced HUVECs compared with that of the
AGE-induced HUVECs model (P<0.01; Fig. 1).
PNS inhibits the apoptotic rate of
AGE-induced HUVECs
Annexin V-FITC/PI was performed to examine the
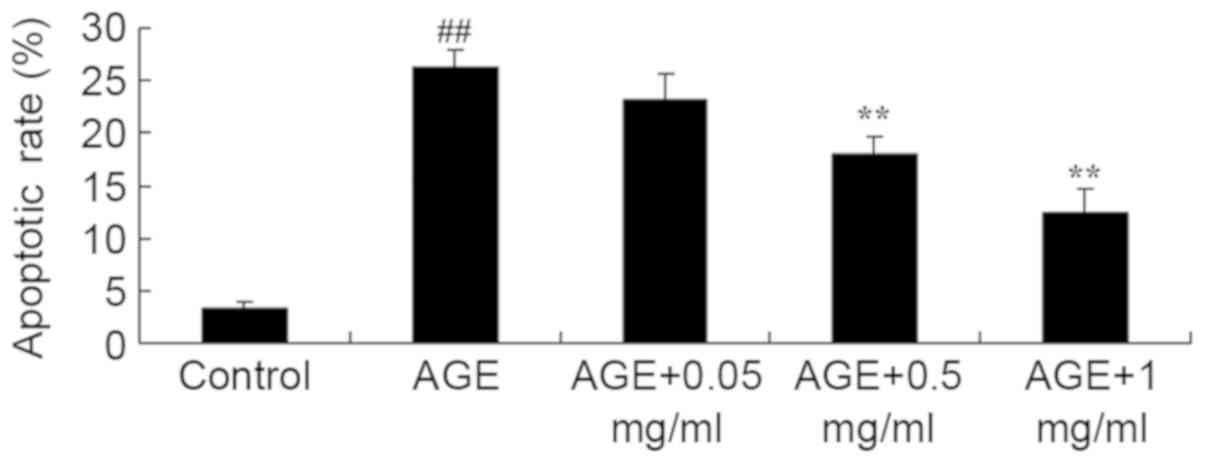
effect of PNS on the apoptotic rate in AGE-induced HUVECs. A
significant increase in the apoptotic rate was exhibited in
AGE-induced HUVECs compared with the control group (P<0.01;
Fig. 2). Compared with the
AGE-induced HUVECs model group, the apoptotic rate of AGE-induced
HUVECs was significantly decreased by 0.5 or 1 mg/ml PNS treatment
(P<0.01; Fig. 2).
PNS suppresses caspase-3 activity of
AGE-induced HUVECs
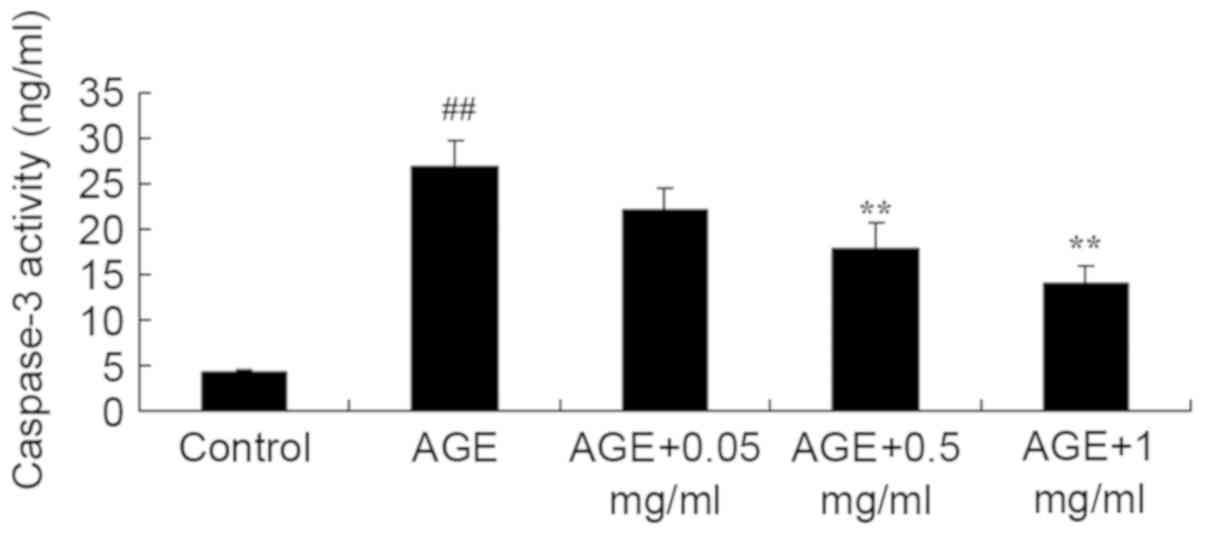
The effect of PNS on caspase-3 activity was
evaluated in AGE-induced HUVECs. AGE significantly induced
caspase-3 activity in HUVECs compared with that of the control
group (P<0.01; Fig. 3).
Following treatment with 0.5 or 1 mg/ml PNS, caspase-3 activity in
AGE-induced HUVECs was significantly reduced compared with the
AGE-induced HUVECs model (P<0.01; Fig. 3).
PNS suppresses MCP-1 and MDA
activities and increases SOD activity in AGE-induced HUVECs
The present study explored the protective effect of
PNS on AGE-induced HUVECs, specifically exploring the effect of PNS
on MCP-1, MDA and SOD. As indicated in Fig. 4, AGEs significantly increased the
levels of MCP-1 and MDA and decreased the levels of SOD in HUVECs
compared with control group (all P<0.01). However, treatment
with 0.5 or 1 mg/ml PNS significantly reduced the MCP-1 and MDA
levels and increased the levels of SOD in AGE-induced HUVECs
compared with the AGE-induced HUVECs model (P<0.01; Fig. 4).
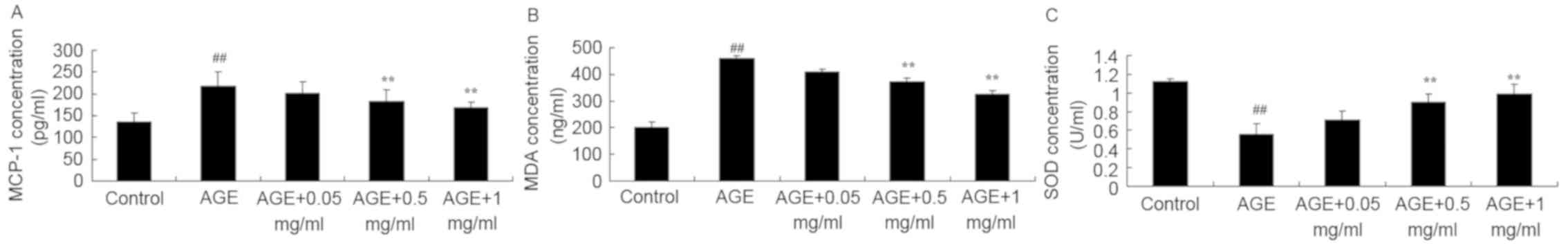 | Figure 4PNS suppresses MCP-1 and MDA activity
and increases SOD activity of AGE-induced HUVECs. PNS suppressed
(A) MCP-1 and (B) MDA activity and (C) increased SOD activity of
AGE-induced HUVECs. ##P<0.01 vs. Control group;
**P<0.01 vs. AGE group. Control, HUVECs without AGE
and PNS (Control group); AGE, AGE-induced HUVECs group; AGE + 0.05
mg/ml, AGE-induced HUVECs + 0.05 mg/ml PNS group; AGE + 0.5 mg/ml,
AGE-induced HUVECs + 0.5 mg/ml PNS group; AGE + 1 mg/ml,
AGE-induced HUVECs + 1 mg/ml PNS group. HUVECs, human umbilical
vein endothelial cells; AGE, advanced glycation end products; PNS,
Panax notoginseng saponins; MCP-1, monocyte chemoattractant
protein-1; MDA, malondialdehyde; SOD, superoxide dismutase. |
PNS promotes the protein expression
levels of SIRT1 in AGE-induced HUVECs
To investigate the mechanism by which AGE-induced
HUVECs are regulated when treated with PNS, the protein expression
levels of SIRT1 protein were investigated in HUVECs. AGEs
significantly reduced the protein expression levels of SIRT1 in
AGE-induced HUVECs compared with the control group (P<0.01:
Fig. 5). However, 0.5 or 1 mg/ml
PNS treatment significantly increased the protein expression levels
of SIRT1 in AGE-induced HUVECs compared with the AGE-induced HUVECs
model (P<0.01; Fig. 5).
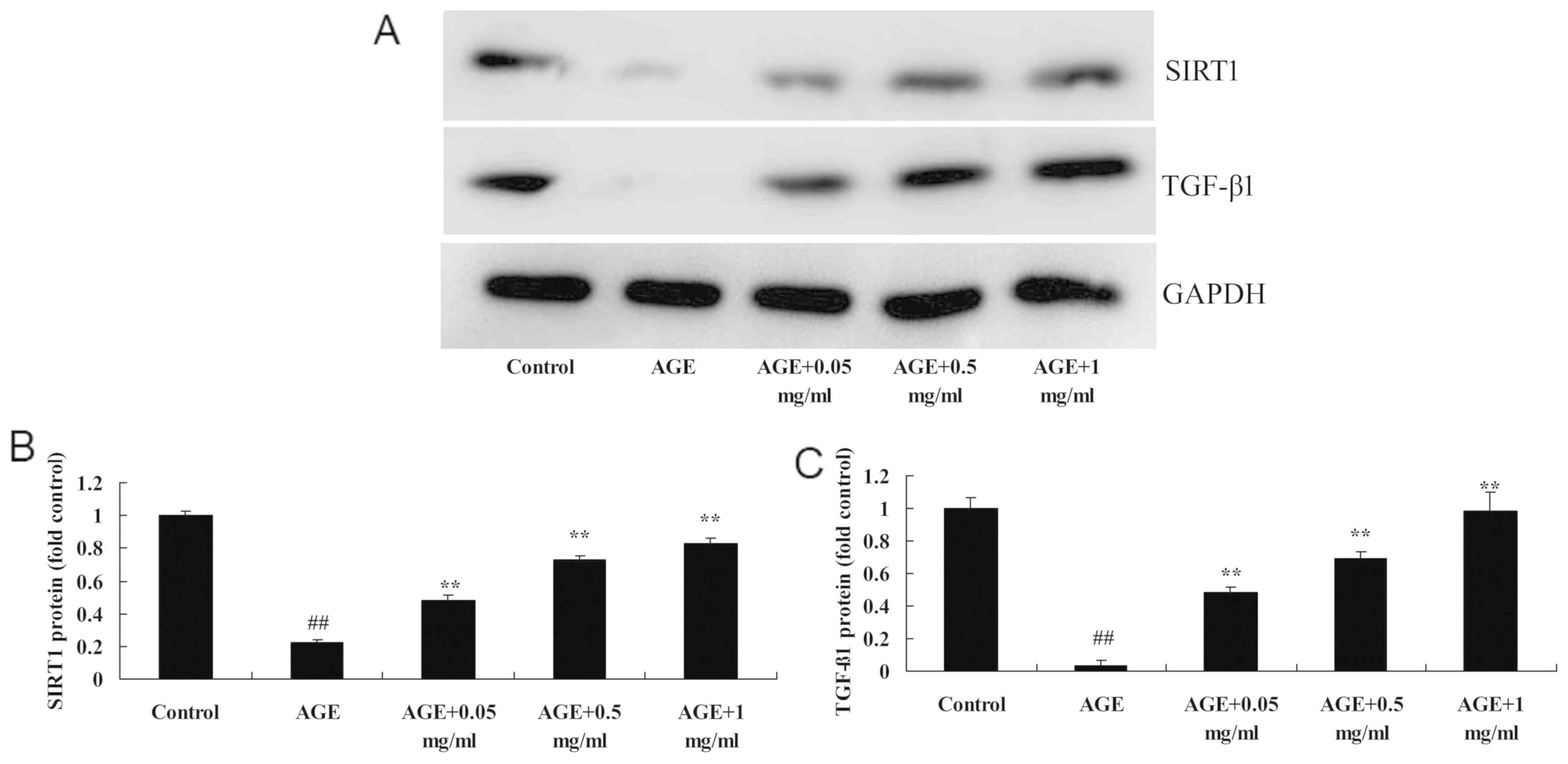 | Figure 5PNS activates SIRT1 and TGF-β1
protein expression of AGE-induced HUVECs. (A) Western blotting and
(B and C) statistical analysis was performed and indicated that PNS
suppressed SIRT1 and TGF-β1 protein expression levels of
AGE-induced HUVECs. ##P<0.01 vs. Control group;
**P<0.01 vs. AGE group. Control, HUVECs without AGE
and PNS (Control group); AGE, AGE-induced HUVECs group; AGE + 0.05
mg/ml, AGE-induced HUVECs + 0.05 mg/ml PNS group; AGE + 0.5 mg/ml,
AGE-induced HUVECs + 0.5 mg/ml PNS group; AGE + 1 mg/ml,
AGE-induced HUVECs + 1 mg/ml PNS group. HUVECs, human umbilical
vein endothelial cells; AGE, advanced glycation end products; PNS,
Panax notoginseng saponins; SIRT1, silent information
regulator 1; TGF-β1, transforming growth factor-β1. |
PNS promotes the protein expression
levels of TGF-β1 in AGE-induced HUVECs
To investigate whether TGF-β1 regulates AGE-induced
HUVECs by PNS, the present study analyzed the protein expression
levels of TGF-β1 in AGE-induced HUVECs. As revealed in Fig. 6, the protein expression levels of
TGF-β1 were significantly reduced compared with the control group
(P<0.01). TGF-β1 protein expression levels were significantly
increased by 0.5 or 1 mg/ml PNS treatment compared with the
AGE-induced HUVECs model (P<0.01; Fig. 5).
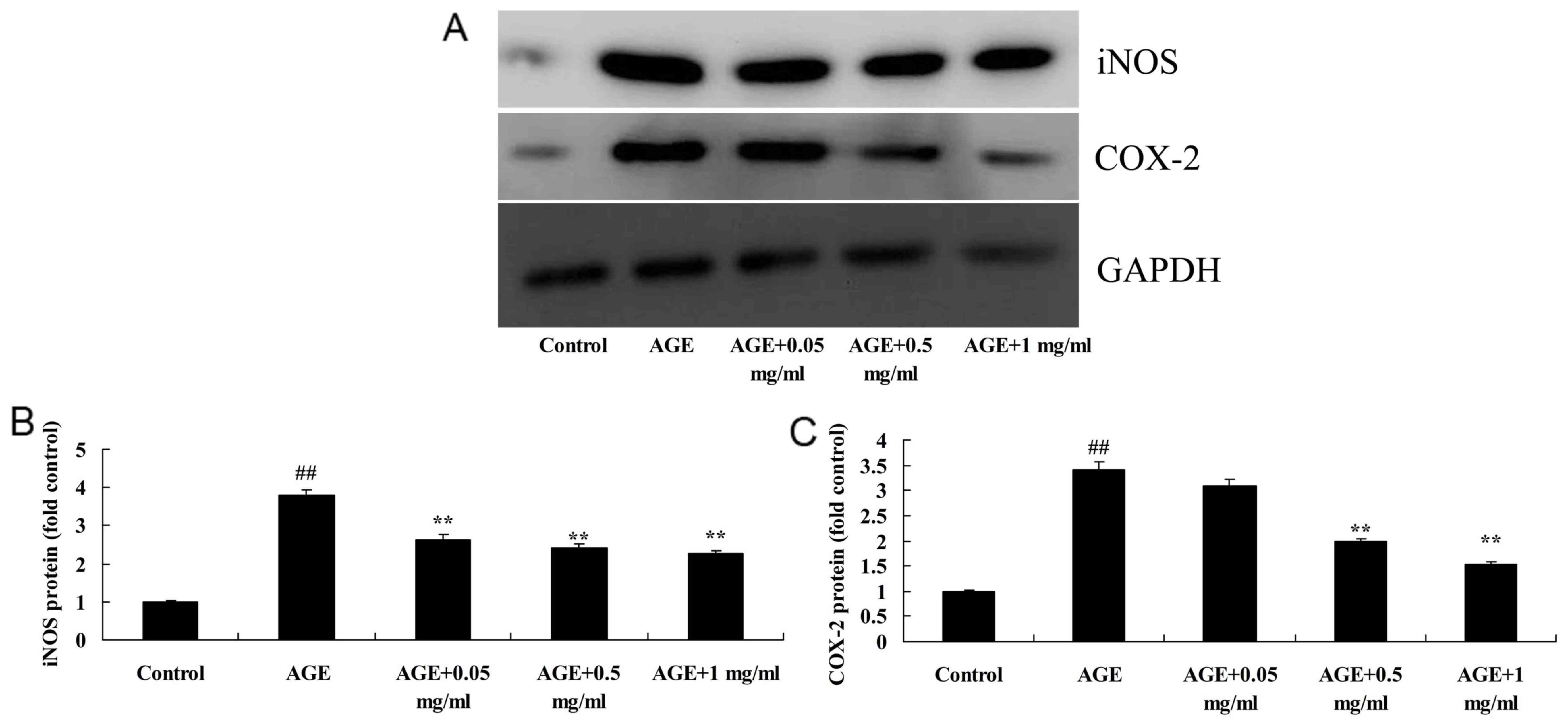 | Figure 6PNS suppresses iNOS and COX-2 protein
expression levels in AGE-induced HUVECs. (A) Western blotting and
(B and C) statistical analysis indicated that PNS suppressed iNOS
and COX-2 protein expression levels in AGE-induced HUVECs.
##P<0.01 vs. Control group; **P<0.01
vs. AGE group. Control, HUVECs without AGE and PNS (Control group);
AGE, AGE-induced HUVECs group; AGE + 0.05 mg/ml, AGE-induced HUVECs
+ 0.05 mg/ml PNS group; AGE + 0.5 mg/ml, AGE-induced HUVECs + 0.5
mg/ml PNS group; AGE + 1 mg/ml, AGE-induced HUVECs + 1 mg/ml PNS
group. HUVECs, human umbilical vein endothelial cells; AGE,
advanced glycation end products; PNS, Panax notoginseng
saponins; iNOS, inducible nitric oxide synthase; COX-2,
cyclooxyggenase-2. |
PNS suppresses the protein expression
levels of iNOS in AGE-induced HUVECs
The present study observed that the iNOS protein
expression levels of AGE-induced HUVECs were significantly
increased compared with the control group (P<0.01; Fig. 6). However, 0.5 or 1 mg/ml PNS
treatment significantly reduced AGE-induced iNOS protein expression
levels in HUVECs compared with the AGE-induced HUVECs model
(P<0.01; Fig. 6).
PNS suppresses the protein expression
levels of COX-2 in AGE-induced HUVECs
To further explore the effect of PNS in AGE-induced
HUVECs, the protein expression levels of COX-2 in AGE-induced
HUVECs were examined. As indicated in Fig. 6, COX-2 protein expression levels in
AGE-induced HUVECs were significantly increased compared with the
control group (P<0.01). Treatment with 0.5 and 1 mg/ml PNS
significantly inhibited AGE-induced COX-2 protein expression levels
in HUVECs compared with the AGE-induced HUVECs model (P<0.01;
Fig. 6).
Discussion
It is largely acknowledged that oxidative stress is
a dangerous factor of coronary heart disease (17). Changes in vascular endothelial
function and structure in diabetes is one of predominant causes of
disability and mortality (18). An
animal study indicated that, during the progression of disease in
rats, AGEs in tissues are increased and impact the normal functions
of tissues (18). For
cardiovascular protection, candesartan is typically administered in
clinic settings, which has been shown to downregulate the
expression of AGE receptors in diabetic rats and reduce the
generation of AGEs (5). A previous
study has demonstrated that long-term hyperglycemia reduces
mitochondrial functions and generates superfluous superoxide.
Tissue proteins and nucleic acids undergo glycation and generate
multiple AGEs (19). AGEs can coat
mitochondrial proteins, restrain mitochondrial proteins and promote
the generation of super-oxygen ions (20). During the progression of
hyperglycemia, damage to the cell accumulates (20). It has been demonstrated that even if
hyperglycemia is corrected, the damage provoked by AGEs is
irreversible (21). SOD levels and
suppressed AGE-induced iNOS and COX-2 expression in AGE-induced
HUVECs. Peng et al (21)
demonstrated that P. notoginseng flower saponins (PNFS)
significantly downregulated iNOS gene expression in RAW264.7
macrophages. Ding et al (16) demonstrated that PNFS reduced acute
ethanol-induced liver injury through reducing ethanol-mediated
oxidative stress.
TGF-β1 has multiple roles in regulating
cardiovascular physiology and disease (22). One important role of TGF-β1 is to
regulate endothelial cell function in the blood vascular system
(23). Unbalanced TGF-β1 signaling
results in abnormalities in embryo vascular development (24). Furthermore, TGF-β1 has an important
role in the formation of new blood capillaries (24). Furthermore, a previous study
indicated that TGF-β1 regulated proliferation, apoptosis, changes
of permeability and morphogenesis of endothelial cells (24). It has been demonstrated that, in
patients with typical precursors of cardiovascular disease, such as
obesity or diabetes, TGF-β1 plasma concentration is increased
(23). The present findings
revealed that PNS treatment significantly induced TGF-β1 protein
expression in AGE-induced HUVECs. Hu et al (25) revealed that PNS significantly
inhibited the expression of TGF-β1 in rats with peritoneal
fibrosis.
Aging is the response of body to multiple factors
within the internal and external environment (26). Conditions such as radiation, anoxia,
peroxidation, high glucose or hyperlipidaemia promote aging;
however, aging is not determined by a single factor. In the
literature, various aging markers have been indicated, such as
telomerase, β-galactosidase and SIRT1 gene (26). SIRT1 has been implicated in gene
silencing, resisting stress and prolonging lifespan (27). Furthermore, multiple studies have
indicated that the SIRT1 gene has an important role on aging
(26,27). Previous results demonstrated that
knockout of SIRT1 may promote animal aging (26). Furthermore, a previous study has
suggested that SIRT1 is a key factor for resisting outside
stimulation, oxidative stress, inflammation and autophagy (7). Reports have demonstrated that SIRT1
may prevent oxidative stress by inducing premature-aging of HUVECs
and inhibiting premature senility of oxidative stress, while
increased SIRT1 expression plays an important role in the aging
phenotype of HUVECs (28,29). Results from the present study
demonstrated that PNS treatment significantly increased SIRT1
protein expression in AGE-induced HUVECs and suggested that the
SIRT1 pathway has an important role in the sensitization effect of
PNS to vasculopathy. Du et al (30) reported that PNS protects the kidneys
of rats from diabetes by upregulating SIRT1 and antioxidant
effects.
In conclusion, the present study revealed that PNS
significantly promoted the cell viability, inhibited the
AGE-induced apoptotic rate, inhibited MCP-1 and MDA levels,
increased SOD levels and suppressed AGE-induced iNOS and COX-2
protein expression levels in HUVECs, potentially through
upregulating SIRT1 and TGF-β1. The present study indicated the
SIRT1 and TGF-β1 are likely involved in PNS-induced protection of
AGE-induced cardiovascular injury.
Acknowledgements
Not applicable.
Funding
This work was partly supported by the National
Natural Science Foundation of China (grant no. 81570272; Bo Yang),
the Beijing Natural Science Foundation (grant no. 7132227; Bo
Yang), the Nova Programme from Beijing Municipal Science and
Technology Commission (grant no. Z141107001814113-XXHZ- 201401; Bo
Yang) and Discovery Foundation from The Chinese Medical Doctor
Association (grant no. DFCMDA201311; Bo Yang).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YB designed the experiment. YB, ZJ, SZJ, WQ, ZH, LCW
and CYK performed the experiments. YB analyzed the data. YB wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Writing Committee for the Diabetic
Retinopathy Clinical Research Network. Gross JG, Glassman AR,
Jampol LM, Inusah S, Aiello LP, Antoszyk AN, Baker CW, Berger BB,
Bressler NM, et alPanretinal photocoagulation vs intravitreous
ranibizumab for proliferative diabetic retinopathy: A randomized
clinical trial. JAMA. 314:2137–2146. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
de Franciscis S, Gallelli L, Battaglia L,
Molinari V, Montemurro R, Stillitano DM, Buffone G and Serra R:
Cilostazol prevents foot ulcers in diabetic patients with
peripheral vascular disease. Int Wound J. 12:250–253.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Davis KE, Prasad C, Vijayagopal P, Juma S,
Adams-Huet B and Imrhan V: Contribution of dietary advanced
glycation end products (AGE) to circulating AGE: Role of dietary
fat. Br J Nutr. 114:1797–1806. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Komosinska-Vassev K, Olczyk P,
Winsz-Szczotka K, Klimek K and Olczyk K: Plasma biomarkers of
oxidative and AGE-mediated damage of proteins and
glycosaminoglycans during healthy ageing: A possible association
with ECM metabolism. Mech Ageing Dev. 133:538–548. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Calfee CS, Ware LB, Eisner MD, Parsons PE,
Thompson BT, Wickersham N and Matthay MA: NHLBI ARDS Network.
Plasma receptor for advanced glycation end products and clinical
outcomes in acute lung injury. Thorax. 63:1083–1089.
2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hipps D, Ausania F, Manas DM, Rose JD and
French JJ: Selective interarterial radiation therapy (SIRT) in
colorectal liver metastases: How do we monitor response? HPB Surg.
2013(570808)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mortuza R, Chen S, Feng B, Sen S and
Chakrabarti S: High glucose induced alteration of SIRTs in
endothelial cells causes rapid aging in a p300 and FOXO regulated
pathway. PLoS One. 8(e54514)2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Donato AJ, Morgan RG, Walker AE and
Lesniewski LA: Cellular and molecular biology of aging endothelial
cells. J Mol Cell Cardiol. 89:122–135. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Roy A, Zhang M, Saad Y and Kolattukudy PE:
Antidicer RNAse activity of monocyte chemotactic protein-induced
protein-1 is critical for inducing angiogenesis. Am J Physiol Cell
Physiol. 305:C1021–C1032. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Marampon F, Gravina GL, Scarsella L,
Festuccia C, Lovat F, Ciccarelli C, Zani BM, Polidoro L, Grassi D,
Desideri G, et al: Angiotensin-converting-enzyme inhibition
counteracts angiotensin II-mediated endothelial cell dysfunction by
modulating the p38/SirT1 axis. J Hypertens. 31:1972–1983.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Madani Z, Malaisse WJ and Ait-Yahia D: A
comparison between the impact of two types of dietary protein on
brain glucose concentrations and oxidative stress in high
fructose-induced metabolic syndrome rats. Biomed Rep. 3:731–735.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mooradian AD, Onstead-Haas L and Haas MJ:
Asymmetrical cross-talk between the endoplasmic reticulum stress
and oxidative stress caused by dextrose. Life Sci. 144:37–48.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bone DB, Antic M, Vilas G and Hammond JR:
Oxidative stress modulates nucleobase transport in microvascular
endothelial cells. Microvasc Res. 95:68–75. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nagar H, Jung SB, Kwon SK, Park JB, Shong
M, Song HJ, Jeon BH, Irani K and Kim CS: CRIF1 deficiency induces
p66shc-mediated oxidative stress and endothelial activation. PLoS
One. 9(e98670)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Roy A and Kolattukudy PE: Monocyte
chemotactic protein-induced protein (MCPIP) promotes inflammatory
angiogenesis via sequential induction of oxidative stress,
endoplasmic reticulum stress and autophagy. Cell Signal.
24:2123–2131. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ding RB, Tian K, Cao YW, Bao JL, Wang M,
He C, Hu Y, Su H and Wan JB: Protective effect of panax
notoginseng saponins on acute ethanol-induced liver injury is
associated with ameliorating hepatic lipid accumulation and
reducing ethanol-mediated oxidative stress. J Agric Food Chem.
63:2413–2422. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lopes-Virella MF, Baker NL, Hunt KJ, Lyons
TJ, Jenkins AJ and Virella G: DCCT/EDIC Study Group. High
concentrations of AGE-LDL and oxidized LDL in circulating immune
complexes are associated with progression of retinopathy in type 1
diabetes. Diabetes Care. 35:1333–1340. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ng ZX, Chua KH, Iqbal T and Kuppusamy UR:
Soluble receptor for advanced glycation end-product
(sRAGE)/pentosidine ratio: A potential risk factor determinant for
type 2 diabetic retinopathy. Int J Mol Sci. 14:7480–7491.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Stróżecki P, Kurowski R, Flisiński M,
Stefańska A, Odrowąż-Sypniewska G and Manitius J: Advanced
glycation end products and arterial stiffness in patients with
diabetic nephropathy and patients with chronic kidney disease
without diabetes. Pol Arch Med Wewn. 123:609–616. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
You WH, Wang P, Li MQ, Zhang Y, Peng YL
and Zhang FL: Therapeutic effects of modified Danggui Sini
Decoction on plasma level of advanced glycation end products in
patients with Wagner grade 0 diabetic foot: A randomized controlled
trial. Zhong Xi Yi Jie He Xue Bao. 7:622–628. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Peng XX, Zhang SH, Wang XL, Ye TJ, Li H,
Yan XF, Wei L, Wu ZP, Hu J, Zou CP, et al: Panax notoginseng
flower saponins (PNFS) inhibit LPS-stimulated NO overproduction and
iNOS gene overexpression via the suppression of TLR4-mediated
MAPK/NF-kappa B signaling pathways in RAW264.7 macrophages. Chin
Med. 10(15)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Eiselein L, Nyunt T, Lamé MW, Ng KF,
Wilson DW, Rutledge JC and Aung HH: TGRL lipolysis products induce
stress protein ATF3 via the TGF-β receptor pathway in human aortic
endothelial cells. PLoS One. 10(e0145523)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Star GP, Giovinazzo M and Langleben D:
Effects of bone morphogenic proteins and transforming growth
factor-beta on In-vitro production of endothelin-1 by human
pulmonary microvascular endothelial cells. Vascul Pharmacol.
50:45–50. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ferrari G, Terushkin V, Wolff MJ, Zhang X,
Valacca C, Poggio P, Pintucci G and Mignatti P: TGF-β1 induces
endothelial cell apoptosis by shifting VEGF activation of p38(MAPK)
from the prosurvival p38β to proapoptotic p38α. Mol Cancer Res.
10:605–614. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hu W, Zhang Y and Sigdel KR: The effects
of Panax notoginseng saponins on the cytokines and
peritoneal function in rats with peritoneal fibrosis. Ren Fail.
37:1507–1513. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gao R, Wang Y, Pan Q, Huang G, Li N, Mou J
and Wang D: Fuzhisan, a chinese herbal medicine, suppresses
beta-secretase gene transcription via upregulation of SIRT1
expression in N2a-APP695 cells. Int J Clin Exp Med. 8:7231–7240.
2015.PubMed/NCBI
|
|
27
|
Takizawa Y, Kosuge Y, Awaji H, Tamura E,
Takai A, Yanai T, Yamamoto R, Kokame K, Miyata T, Nakata R and
Inoue H: Up-regulation of endothelial nitric oxide synthase (eNOS),
silent mating type information regulation 2 homologue 1 (SIRT1) and
autophagy-related genes by repeated treatments with resveratrol in
human umbilical vein endothelial cells. Br J Nutr. 110:2150–2155.
2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ota H, Eto M, Kano MR, Kahyo T, Setou M,
Ogawa S, Iijima K, Akishita M and Ouchi Y: Induction of endothelial
nitric oxide synthase, SIRT1, and catalase by statins inhibits
endothelial senescence through the Akt pathway. Arterioscler Thromb
Vasc Biol. 30:2205–2211. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Suo R, Zhao ZZ, Tang ZH, Ren Z, Liu X, Liu
LS, Wang Z, Tang CK, Wei DH and Jiang ZS: Hydrogen sulfide prevents
H2O2-induced senescence in human umbilical
vein endothelial cells through SIRT1 activation. Mol Med Rep.
7:1865–1870. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Du YG, Wang LP, Qian JW, Zhang KN and Chai
KF: Panax notoginseng saponins protect kidney from diabetes
by up-regulating silent information regulator 1 and activating
antioxidant proteins in rats. Chin J Integr Med. 22:910–917.
2016.PubMed/NCBI View Article : Google Scholar
|