Introduction
Ketamine, also known as K powder, is a substance
that has been widely abused in recent years (1). Chronic ketamine abusers frequently
suffer from severe lower urinary tract syndrome (LUTS), which is
characterized by the excessive frequency of urination, nocturia,
suprapubic discomfort and occasional hematuria (2). Pathologically, it is similar to
interstitial cystitis, also known as ketamine-induced cystitis
(KIC) (3). However, the
pathophysiology of bladder dysfunction in patients with KIC remains
to be elucidated.
Accumulating evidence has indicated that oxidative
stress-mediated injury serves an important role in KIC (4,5).
During oxidative stress, ATP production from the mitochondrial
respiratory chain triggers bladder hyperactivity. Ketamine
injections increase the production of intracellular reactive oxygen
species (ROS), which stimulates electron leakage from the
mitochondrial respiratory chain complexes (4). ROS can be removed from the body by
enzymes (for example, SOD and GSH-px) and non-enzymatic (for
example GSH, Vit C and Vit E) antioxidants, which serves as the
main defense system (6). During
oxidative stress, high ROS levels induce lipid peroxidation and the
formation of malondialdehyde (MDA), which is an end-product of
oxidation that influences the mitochondrial respiratory chain
complex and the activities of key enzymes (For example, SOD and
GSH) (7). Superoxide dismutase
(SOD) and glutathione-sulfhydryl (GSH) are the main antioxidant
enzymes that degrade and scavenge free radicals in vivo
(8). A previous study revealed that
chronic ketamine treatment increased MDA levels in the rat bladder
and reduced the expression of antioxidant enzymes SOD and GSH (Mi
et al, 2016, unpublished data). This indicated that ketamine
can target the bladder urothelium to release ROS and induce
inflammation in the bladder. The release of ROS in bladder
urothelium is mediated by the cytochrome C oxidase pathway, which
induces detrusor hyperactivity. Furthermore, oxidative stress
triggers the production of large quantities of proinflammatory
mediators nitric oxide (NO) and prostaglandin E2 from macrophages.
These products are generated by inducible nitric oxide synthase
(iNOS) and cyclooxygenase-2 (COX-2), respectively (9). These inflammatory mediators increase
vascular permeability in the urothelium, resulting in ulceration,
erythrocyte accumulation (hemorrhage), monocyte infiltration and
increased interstitial fibrosis between the detrusor smooth muscle
tracts in the rat bladder injured by ketamine treatment (10).
Aldehyde dehydrogenase 2 (aldh2) is a mitochondrial
enzyme that regulates aldehyde metabolism by eliminating cytotoxic
aldehydes, thereby reducing oxidative stress and inhibiting the
production of ROS-related toxic products (11). In particular, ~40% of the population
in Asia has a defective aldh2 gene compared with that in
Europe and Africa, where the prevalence is <5% (12). Individuals with the aldh2
allele deficiency are highly susceptible to adverse reactions to
ethanol and other stimulatory factors (For example,
4-hydroxy-2-nonenal and hypoxia) due to the accumulation of
aldehydes caused by the lack of aldh2 enzymes. Aldh2 has been
previously identified as a potential prognostic marker for bladder
urothelial carcinoma (13). In a
follow-up study performed in the USA, aldh2 variants were found to
be associated with a shorter time to first recurrence of bladder
cancer (14). This finding was
supported by another previous study by Ferreira-Teixeira et
al (15), who revealed that
aldh2 has the potential to predict the progression and metastasis
of invasive bladder cancer. Although a number of studies have
demonstrated the anti-oxidant effect of aldh2 in bladder tumors,
its role in cystitis remains poorly understood.
It has been previously reported that ketamine
treatment induces the translocation of the NF-κB subunit p65 into
the nucleus, activates COX-2 expression and production of
prostaglandin E2 in bladder tissues (16). As an important anti-oxidative stress
protein in the body, aldh2 has been found to suppress the
production of ROS and the related toxic aldehyde product MDA, in
turn inhibiting apoptosis by suppressing the NF-κB signaling
pathway in human pulmonary artery smooth muscle cells (5). Based on these aforementioned findings,
the present study hypothesized that aldh2 may serve a role in the
development of KIC.
Materials and methods
Animal models
A total of 45 aldh2 knock-out (aldh2KO) and
60 wild-type (WT) male Institute of Cancer Research (ICR) mice
(weight, 25±5 g; age, 8 weeks) were used for the present study. The
aldh2KO mice were obtained from the Genomics Center Laboratory of
Guangxi Medical University (Nanning, China; Fig. S1). The genotype of the mice was
verified by PCR using tail tissues cut from the aldh2 KO mice
(17). The aldh2 specific primer
(DNA) was designed on the BLAST website (https://blast.ncbi.nlm.nih.gov/Blast.cgi): Forward,
5'-GCTGGGCTGACAAGTACCAT-3' and reverse, 5'-TTGATCAAGTTGGCCACGTA-3'
(Takara Bio Technology Co., Ltd.). The aldh2 genotype was verified
by Takara TaqTM Version 2.0 (Code No. R004Q; Takara
Biotechnology Co., Ltd.) according to the manufacturer's
instructions. The thermocycling conditions were as follows: Initial
denaturation at 98˚C for 10 sec, followed by 30 cycles of 55˚C for
30 sec and 72˚C for 1 min. Agarose gel (1.5%) and ethidium bromide
(0.5 µg/ml) were used in the experiment. WT mice were purchased
from Hunan Slack Jingda Experimental Animal Co., Ltd. All mice were
kept at 23±1˚C and 50±5% humidity, with 12 h light/dark cycles and
had free access to food and water. The present study was approved
by the Ethics Committee of Guangxi Medical University (approval no.
20180129).
Firstly, 15 WT mice were randomly divided into the
ketamine group, which received 30 mg/kg for 4 and 8 weeks to
produce the ICR mouse model or the saline group, which received
normal saline (saline group) for 4 and 8 weeks, according to the
ICR mouse model previously established by Yeung et al
(18). Four groups were used with
3-4 WT mice within each group. Ketamine hydrochloride injections
(100 mg/2 ml) were purchased from China Fujian Gutian
Pharmaceutical Co., Ltd. Injections were administered by an
intraperitoneal injection every day at 9 am, where an injection
volume of 1 ml liquid volume (saline or ketamine solution) was used
to mimic chronic ketamine abuse. All mice were weighed weekly to
adjust the doses of ketamine given (30 mg/kg). Mice were sacrificed
by cervical dislocation following the 4- and 8-week treatments
after an anesthetic (40 mg/kg intravenous pentobarbital;
Sigma-Aldrich; Merck KGaA) was administered. Bladder tissues were
subsequently obtained and the expression levels of aldh2 was
quantified using quantitative PCR (qPCR), as aformentioned.
In addition, 45 WT and 45 KO mice were randomly
divided into the following groups: i) WT normal saline control
(WNS); ii) KO normal saline control (KNS); iii) WT low-dose
ketamine (WLK; 30 mg/kg); iv) KO low-dose ketamine (KLK; 30 mg/kg);
v) WT high-dose ketamine (WHK; 6 mg/kg); and vi) KO high-dose
ketamine (KHK; 60 mg/kg). Each group was then further divided into
3 subgroups (4, 8 and 12 weeks, 5 mice in each). Following ketamine
administration, mice bladder tissues were obtained from each mouse
following euthanasia at 4, 8 and 12 weeks as aforementioned.
Micturition behavior
Micturition frequency was monitored as previously
described by Gu et al (19).
Briefly, the short-term micturition frequency of freely moving mice
was observed at the end of weeks 4, 8 and 12 following ketamine
treatment. At these time-points, mice were placed in a metabolic
cage containing a mesh filter pad, where the short-term urination
frequency was recorded. A filter paper was soaked with a saturated
copper sulfate solution (CuSO4.5H2O) which
was dehydrated at 200˚C for 1 h prior to use. When the urine come
to contact with the filter paper, the anhydrous CuSO4
was rehydrated and turned blue. After allowing the mice to move
freely for 2 h, the filter paper was removed from the cage and the
number of urination events was determined by counting the number of
blue dots on the filter paper. Overlapping urination points with
distinctly different edges were considered separate urination
events and urine points >0.2 cm in diameter were counted
(Fig. S2).
Determination of ketamine metabolites
in serum and urine
A total of 1 ml of blood was obtained from the
mouse's tail. Then the blood was separated by centrifugation at
2,000 x g for 10 min at 4°C. The concentration of
ketamine and norketamine in the serum and urine (1 ml) was
determined using high-performance liquid chromatography. Briefly,
samples were collected on the day before the mice were euthanized.
The final mobile phase was prepared via mixing ammonium bicarbonate
solution (5 mM) adjusted with concentrated ammonia to a pH of 11.3
and acetonitrile in a ratio of 70:30. A Phenomenex LUX®
AMP (Phenomenex) 3 µm, 150x4.6 mm column served as the stationary
phase. All chemicals were of analytical grade. Chiral separation
experiments were carried out with an Agilent 1260 Series Liquid
Chromatograph (Agilent Technologies Inc.), equipped with an
autosampler and a diode array detector. Each analysis was performed
at ambient column temperature or a column temperature of
40°C. The measurements were performed under isocratic
conditions with a flow rate of 0.5 ml/min and an injection volume
of 1 µl. Ultraviolet detection was performed at 200 nm. Data
evaluation was performed via a ChemStation for LC 3D Systems Rev.
C. 01.07SR2 software (Agilent Technologies GmbH).
Measurement of oxidative stress
parameters
Bladder tissues were quickly excised from mice and
washed thoroughly with ice-cold normal PBS (pH 7.2). Tissues were
sectioned into small pieces by the shear with liquid nitrogen and
then homogenized using a glass homogenizer in ice-cold PBS. Next,
the solution was centrifuged at 20,000 rpm (41,800 x g) for 10 min
at 4°C. The supernatant was used to estimate the levels
of oxidative stress indicators SOD, GSH and MDA using their
respective ELISA kits (SOD, cat. no. 706002; GSH, cat. no. 703002;
MDA, grant no. 700870; Cayman Chemical Company), according to the
manufacturer's protocols.
Histopathology and immunohistochemical
examinations
Bladder tissues were fixed in 4% phosphate-buffered
paraformaldehyde for one day at room temperature, dehydrated in an
ascending ethanol gradient, cleared in xylene and embedded in
paraffin. The paraffinized tissues were then cut into 5-µm sections
and stained with hematoxylin and eosin (H&E) and Masson's
trichrome for 5 min at room temperature. Sections were then
examined under a light microscope (magnification, x100, x200, x400;
Olympus Corporation).
Immunohistochemical examination was performed using
the Zhongshan Jinqiao Detection kit (OriGene Technologies, Inc.).
Briefly, the tissue sections were deparaffinized and immersed in 3%
H2O2 for 30 min to quench endogenous
peroxidase activity. After being blocked with 2% BSA at room
temperature for 30 min, the sections were incubated with iNOS
antibody (1:400; cat. no. AF0199; Affinity Biosciences), COX-2
antibody (1:500; cat. no. 12282; Cell Signaling Technology, Inc.)
overnight at 4°C. Following incubation with the primary
antibodies, the tissue sections were incubated with the appropriate
biotinylated secondary antibody (1:5,00; cat. no. TA130016; OriGene
Technologies, Inc.) for 30 min at room temperature; after which,
they were incubated with 3,3'-diaminobenzidine for 2 min and
lightly counterstained with hematoxylin for 1 min at room
temperature. Lastly, sections were then examined under a light
microscope (magnification, x200; Olympus Corporation).
Western blotting
Harvested bladder tissues (10 mg) were homogenized
in liquid nitrogen and re-suspended. The lysates were centrifuged
at 14,500 x g for 15 min at 4˚C. Protein concentrations were
quantified using a bicinchoninic acid Protein Assay kit (Pierce;
Thermo Fisher Scientific, Inc.) and 5X protein loading buffer
(Beyotime Institute of Biotechnology) was add to the supernatant
(specifically 1:4). The samples (25 µg total protein/lane) were
separated using 10% SDS-PAGE gel electrophoresis and then
transferred onto PVDF membranes. After that, membranes were blocked
with 5% BSA at room temperature for 30 min. The membranes were
incubated with primary antibodies at 4˚C overnight and then washed
with TBS-supplemented with 0.1% Tween-20. The primary antibodies
used were as follows: iNOS (1:1,000; cat. no. 131205; Cell
Signaling technology, Inc.), Aldh2 (1:1,000; cat. no. 108306;
Abcam), COX-2 (1:1,000; cat. no. 12282; Cell Signaling technology,
Inc.), NF-κB (1:1,500; cat. no. 8242; Cell Signaling technology,
Inc.), α-smooth muscle actin (α-SMA; 1:1,000; cat. no. 68463; Cell
Signaling technology, Inc.), transforming growth factor-β (TGF-β;
1:1,000; cat. no. 3711; Cell Signaling technology, Inc.),
fibronectin (1:1,000; cat. no. AF5335; Affinity Biosciences) and
β-actin (1:5,000; cat. no. 12262; Cell Signaling technology, Inc.).
This was followed by incubation with the horseradish peroxidase
(HRP)-conjugated secondary antibodies (1:1,000; cat. no. A0208;
Beyotime Institute of Biotechnology) at 37˚C for 1 h. The membranes
were next incubated with Novex ECL HRP chemiluminescent substrate
reagent kits (Invitrogen; Thermo Fisher Scientific, Inc.) for 30
sec at room temperature and exposed to X-ray film. Protein signals
were quantified by scanning densitometry using a FluorChem Q system
(Alpha Innotech Corporation). The results of western blotting were
quantified using Quantity One Version 4.4.0 software (Bio-Rad
Laboratories, Inc.).
Reverse transcription (RT)-qPCR
(RT-qPCR) Total RNA extraction and RT
Minced bladder tissues were treated with
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol, which were fully lysed
and mixed. A total of 50% by volume of chloroform was added and the
upper aqueous phase was collected following centrifugation
(4°C; 15,000 x g). Equal volumes of isopropanol were
added and RNA was precipitated by centrifugation (4°C;
15,000 x g). RNA was washed with 70% alcohol, dried and dissolved
in RNA-free enzyme water. Subsequently, the purity and
concentration of the isolated RNA were detected using an
ultra-micronucleic acid detector AIGS (SuiZhen Biotechnology Co.,
Ltd.). RNA was reverse-transcribed to cDNA using a reverse
transcription kit MonAmp™ Mix (cat. no. MR05201; Monad Biotech Co.,
Ltd.), according to the manufacturer's protocol. Obtained cDNA was
stored at -20˚C until use.
qPCR and data analysis
The qPCR reaction system consisted of 5 µl SYBR
Green Premix Taq (cat. no. RN04006M; Monad Biotech Co., Ltd.), 1 µl
cDNA, 0.3 µl forward primer (10 µM), 0.3 µl reverse primer (10 µM)
and 3.4 µl H2O. The thermocycling conditions were as
follows: Initial denaturation at 95˚C for 30 sec, followed by 40
cycles of 95˚C for 5 sec, 60˚C for 30 sec and 72˚C for 15 sec.
Dissolution curve was produced using the standard dissolution curve
program (Agilent Aria Software; version 1.5; Agilent Technologies,
Inc.). The PCR reaction was carried out on an ABI 7500 quantitative
PCR instrument (Thermo Fisher Scientific, Inc.). The relative mRNA
expression data were calculated using the 2-ΔΔCq method
(20). The relative expression
value of a gene was normalized against the expression of Actb from
control group (WNS) mRNA. The Data was analyzed using the SPSS 22.0
software (IBM Corp). Primer sequences are presented in Table I.
 | Table IOligonucleotide sequences of the
primer pairs used for reverse transcription-quantitative PCR. |
Table I
Oligonucleotide sequences of the
primer pairs used for reverse transcription-quantitative PCR.
| Primer | Forward sequence
(5'-3') | Reverse sequence
(5'-3') |
|---|
| β-actin |
CAGCCTTCCTTCTTGGGTAT |
TGGCATAGAGGTCTTTACGG |
| COX-2 |
CAGATGACTGCCCAACTCCC |
TGAACCCAGGTCCTCGCTTA |
| iNOS |
TGGAGCGAGTTGTGGATTGT |
GTGAGGGCTTGGCTGAGTGA |
| NF-κB |
ACACGAGGCTACAACTCTGC |
GGTACCCCCAGAGACCTCAT |
| α-SMA |
GTACCCAGGCATTGCTGACA |
GCTGGAAGGTAGACAGCGAA |
| TGF-β |
AGGGCTACCATGCCAACTTC |
CCACGTAGTAGACGATGGGC |
| Fibronectin |
ATGAGAAGCCTGGATCCCCT |
GGAAGGGTAACCAGTTGGGG |
| Aldh2 |
TTCGGGGACGTAAAAGACGG |
GGTGTCCTTCTCCGGCATAG |
Statistical analysis
In the aldh2 gene expression experiment, 4 mice were
used. While in the rest of the experiments, 5 mice were analysed.
All data are presented as mean ± standard error of the mean.
Statistical analyses were performed using the Graph Pad Prism
software (version 7.0; GraphPad Software, Inc.). Mean differences
were compared with one-way ANOVA followed by multiple comparison by
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Ketamine increases aldh2 mRNA
expression in WT mice
The mRNA expression levels of aldh2 in bladder
tissues from WT mice were measured after ketamine treatment. At 4
weeks, the relative aldh2 mRNA levels were found to be 1.06±0.37 in
the saline group and 1.38±0.15 in the ketamine group (Fig. 1A). There was no significant
difference between these two groups (P=0.078). At 8 weeks, the
relative mRNA levels of aldh2 in the WT mice in the ketamine
treatment group was 1.84±0.24, which was significantly higher
compared with 1.02±0.15 in the saline group (P<0.01; Fig. 1A). These results suggest that
long-term treatment with ketamine increased the expression of aldh2
mRNA in the bladder tissues of WT mice.
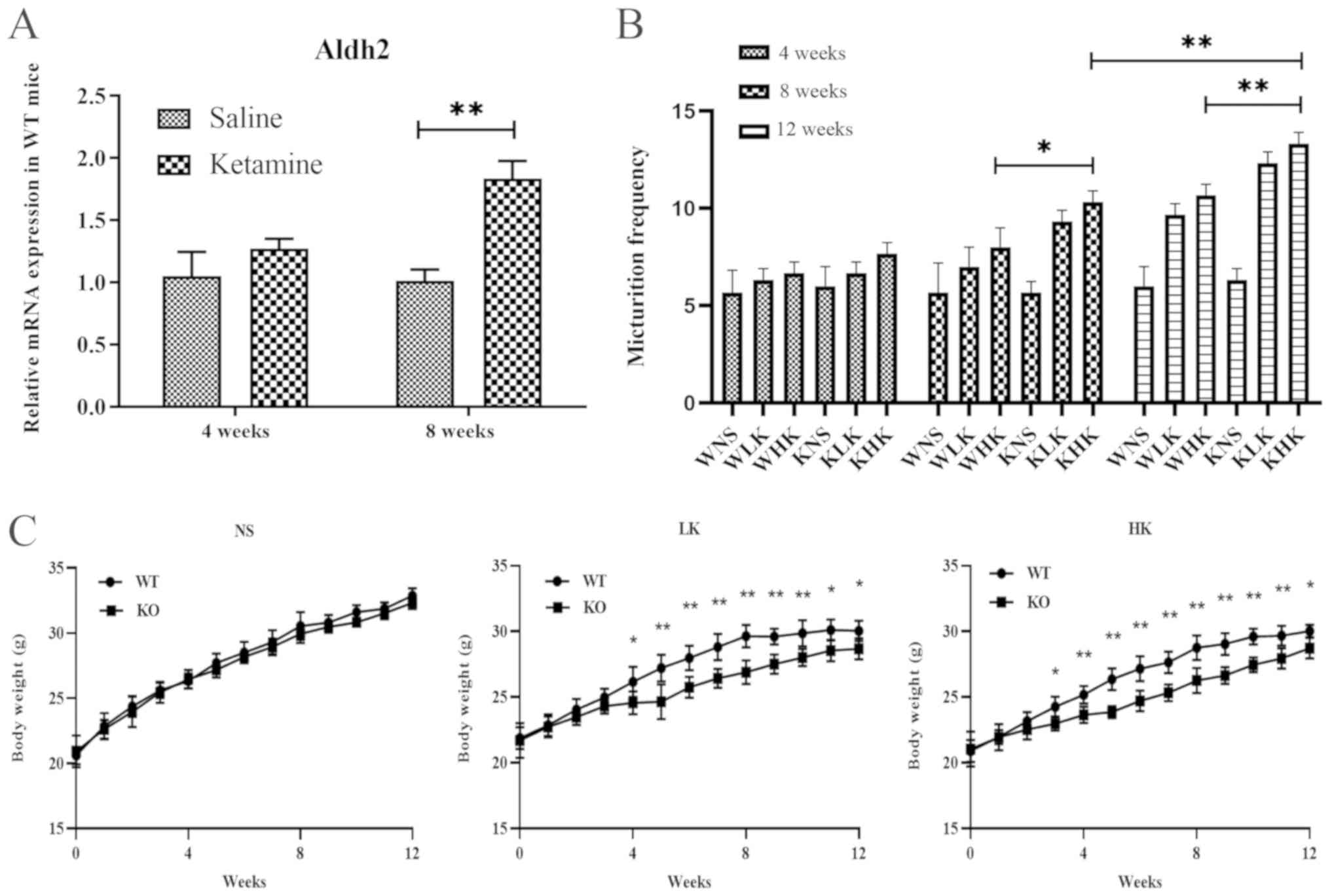 | Figure 1Ketamine treatment increases aldh2
mRNA expression in WT mice and increases urination frequency and
reduces body weight in KO mice. (A) Reverse
transcription-quantitative PCR was performed to measure the mRNA
expression of aldh2 in WT mice at 4 and 8 weeks following saline
and ketamine treatment. (B) Changes in urination frequency of KO
and WT mice during 4-, 8- and 12-week ketamine treatments. (C)
Weekly weight changes of KO and WT mice (each week, group WT vs.
group KO). Data were presented as the mean ± standard error of the
mean from ≥3 experimental repeats. *P<0.05 and
**P<0.01. Aldh2, aldehyde dehydrogenase 2; WT,
wild-type; KO, knock-out; WNS, wild-type normal saline control
group; WLK, wild-type low-dose ketamine group; WHK, wild-type
high-dose ketamine group; KNS, knock-out normal saline control
group; KLK, knock-out low-dose ketamine group; KHK, knock-out
high-dose ketamine group; NS, normal saline; LK, low-dose ketamine;
HK, high-dose ketamine; w, week. |
Ketamine treatment increases the
frequency of urination and suppresses weight gain in aldh2 KO
mice
Results of Aldh2 KO and WT mice were
compared, which revealed that prolonged ketamine treatment caused
urinary dysfunction (Fig. 1B) and
suppressed weight gain (Fig. 1C) in
KO mice. At week 8, the frequency of urination in the KHK group was
10.33±0.47, which was significantly higher compared with 8.00±0.82
in the WHK group (P<0.05; Fig.
1B). Similar trends were recorded at week 12 (KHK vs. WHK;
13.33±0.47 vs. 10.67±0.47; P<0.01; Fig. 1B). In terms of body weight, the
weight of mice in the HK group at week 12 was 28.76±0.93 g in WT
mice and 27.67±1.12 g in KO mice (P=0.032), where the average
weight gain from the beginning of the experiment to week 12 was
9.12±1.03 g in WT mice and 7.68±0.69 g in KO mice (P=0.042) in the
HK group (Fig. 1C). Similar results
were observed in the LK group (Fig.
1C). There were no significant differences in urination
frequency or body weight changes between WT and KO mice in the NS
group. These results suggest that ketamine treatment significantly
hindered weight gain whilst increasing urination frequency in KO
mice compared with that in WT mice.
Ketamine metabolism is unaffected by
aldh2 KO
The potential effects of aldh2 on ketamine
metabolism in WT and aldh2 KO mice was next investigated.
According to the serum and urine measurements of ketamine and
norketamine, the levels in the Aldh2 KO group did not differ
significantly compared with those in the WT group (Table II). These observations suggest that
ketamine and its metabolites induced similar toxic effects in
bladder tissues in WT and KO mice.
 | Table IIBiochemical indicators of ketamine in
blood and urine of mice in the different experimental groups. |
Table II
Biochemical indicators of ketamine in
blood and urine of mice in the different experimental groups.
| A, 4 weeks |
|---|
| | C (NS) | LK (30 mg/kg) | HK (60 mg/kg) |
|---|
| Indicator |
Aldh2-(n=15) |
Aldh2+(n=15) |
Aldh2-(n=15) |
Aldh2+(n=15) |
Aldh2-(n=15) |
Aldh2+(n=15) |
|---|
| Serum Ket
(ng/ml) | ND | ND | ND | ND | ND | ND |
| Serum Nket
(ng/ml) | ND | ND | ND | ND | ND | ND |
| Urine Ket
(ng/ml) | ND | ND | 868±95.9 | 896±94.3 | 1068±98.6 | 1,123±95.3 |
| Urine Nket
(ng/ml) | ND | ND | 2,2681±734.2 | 2,0248±870.4 | 23,425±726.9 | 24,786±102.3 |
| B, 8 weeks |
| | C (NS) | LK (30 mg/kg) | HK (60 mg/kg) |
| Indicator |
Aldh2-(n=15) |
Aldh2+(n=15) |
Aldh2-(n=15) |
Aldh2+(n=15) |
Aldh2-(n=15) |
Aldh2+(n=15) |
| Serum Nket
(ng/ml) | ND | ND | ND | ND | ND | ND |
| Urine Ket
(ng/ml) | ND | ND | 1,076±103.2 | 998±87.3 | 1,324±112.3 | 1,238±109.3 |
| Urine Nket
(ng/ml) | ND | ND | 24,564±924.3 | 23,652±898.3 | 27,347±923.4 | 25,863±954.2 |
| C, 12 weeks |
| | C (NS) | LK (30 mg/kg) | HK (60 mg/kg) |
| Indicator |
Aldh2-(n=15) |
Aldh2+(n=15) |
Aldh2-(n=15) |
Aldh2+(n=15) |
Aldh2-(n=15) |
Aldh2+(n=15) |
| Serum Nket
(ng/ml) | ND | ND | 1.2±0.32 | 2.3±0.26 | 4.6±0.67 | 3.2±0.34 |
| Urine Ket
(ng/ml) | ND | ND | 1,545±162.7 | 1,432±93.3 | 1,636±156.8 | 1,523±196.2 |
| Urine Nket
(ng/ml) | ND | ND | 23,691±1,423.3 | 26,538±1,397.2 | 28,877±1,452.3 | 29,376±1,254.8 |
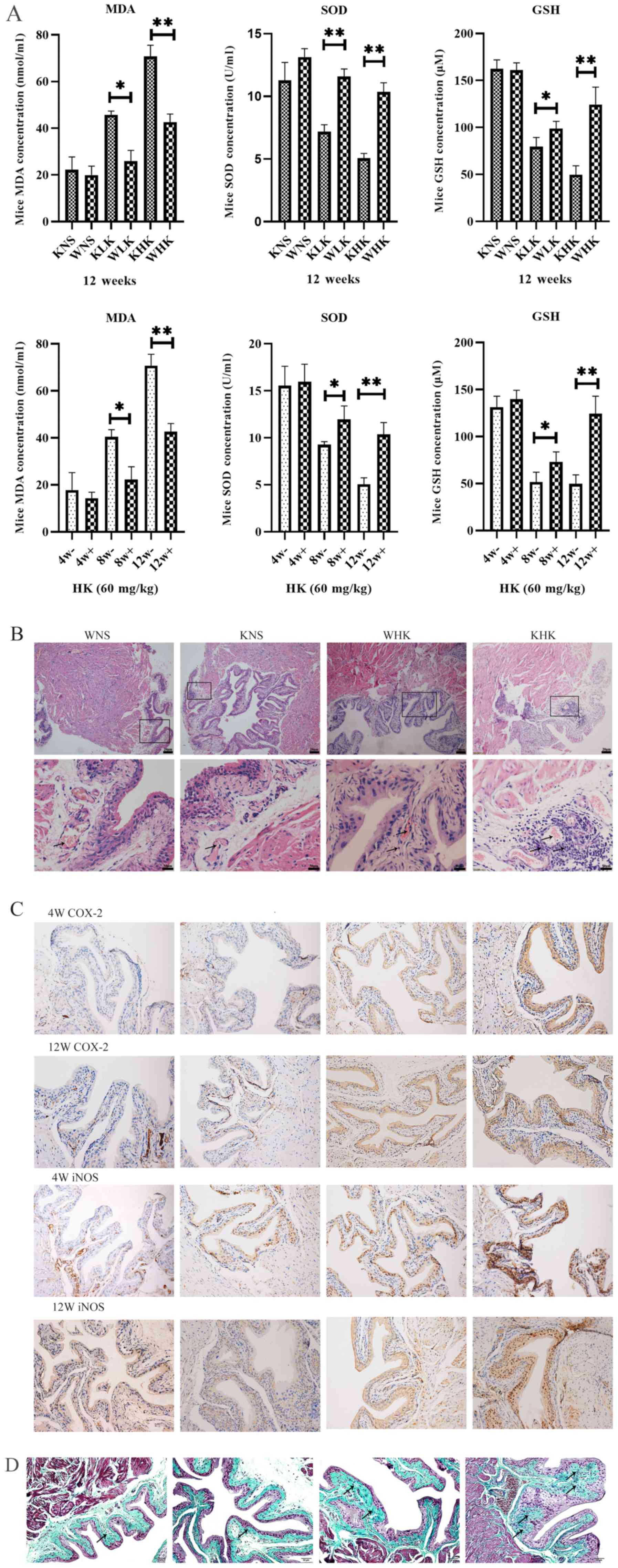
Ketamine increases oxidative stress
parameters in aldh2 KO mice
ELISA were performed to quantify the levels of
oxidative stress factors in bladder tissues. Results in Fig. 2A demonstrated the effects of
different treatment periods and doses of ketamine on oxidative
stress in the mouse bladder tissues at 12 weeks in all groups and
at 4, 8 and 12 weeks in the HK group. These assays reported that
levels of anti-oxidation factors SOD (P<0.01) and GSH
(P<0.01) were significantly decreased, whilst levels of the
lipid peroxidation indicator MDA (P<0.01) was significantly
increased in aldh2 KO mice compared with those in the WT
mice at week 12 in the HK group. At week 12, the KHK group
exhibited a significant decline in the level of SOD (5.05±0.56
U/ml) and GSH (49.75±13.45 µM) and a significant increase in MDA
(70.79±8.28 nmol/ml) in bladder tissues compared with WHK (SOD,
10.35±1.04 U/ml; P<0.01; GSH, 124.49±26.02 µM; P<0.05; MDA,
41.97±5.01 nmol/ml; P<0.01).
 | Figure 2Ketamine increases the level of
oxidative stress in aldh2 KO mice and aggravates
pathological damage. (A) Effects of ketamine on parameters of
oxidative stress in WT and KO mice at 4, 8 and 12 weeks as detected
by ELISA. Negative control mice were treated with NS.
*P<0.05 and **P<0.01. KO, knock-out;
NS, normal saline; KHK, knock-out high-dose ketamine group; SOD,
superoxide dismutase; GSH, glutathione-sulfhydryl; MDA,
malondialdehyde; WHK, wild-type high-dose ketamine group; COX-2,
cyclooxygenase 2; iNOS, inducible nitric oxide synthase; WT,
wild-type; KNS, knock-out normal saline control group; WNS,
wild-type normal saline control group; KLK, knock-out low-dose
ketamine group; WLK, wild-type low-dose ketamine group; HK,
high-dose ketamine; W, week; -, knock-out; +, wild-type. Ketamine
increases the level of oxidative stress in aldh2 KO mice and
aggravates pathological damage. (B) Representative hematoxylin and
eosin staining images of bladder tissues from KO and WT mice in
week 12. Magnification, x100 for the upper images; x400 for the
lower images. (C) Representative immunohistochemical staining
images of COX-2 and iNOS proteins in the bladder tissues of KO and
WT mice in weeks 4 and 12. The cytoplasm and cell membranes
exhibiting brown-yellow colors were considered as positive
expression of the target protein, which were mainly confined to the
bladder epithelium Magnification, x200. (D) Representative Masson
trichrome staining images of bladder tissues of KO and WT mice in
week 12. Collagen fibers stained green, muscle fibers stained red,
and nucleus stained blue-brown. Magnification, x200. Data are
presented as the mean ± standard error of the mean from ≥3
experimental repeats. *P<0.05 and
**P<0.01. KO, knock-out; NS, normal saline; KHK,
knock-out high-dose ketamine group; SOD, superoxide dismutase; GSH,
glutathione-sulfhydryl; MDA, malondialdehyde; WHK, wild-type
high-dose ketamine group; COX-2, cyclooxygenase 2; iNOS, inducible
nitric oxide synthase; WT, wild-type; KNS, knock-out normal saline
control group; WNS, wild-type normal saline control group; KLK,
knock-out low-dose ketamine group; WLK, wild-type low-dose ketamine
group; HK, high-dose ketamine; W, week; -, knock-out; +,
wild-type. |
Ketamine induces more severe
pathological damage in KO mice
At week 12, modest inflammatory cell infiltration
and massive intravascular congestion were observed via H&E
staining in the submucosal layer of the bladder tissues of mice in
the WHK group (Fig. 2B). In tissues
from mice in the KHK group, this appeared to be more severe, where
the mucosal barrier had disintegrated with numerous inflammatory
cells accumulated in the submucosa and extensive edema in the
bladder mucosa (Fig. 2B). No edema
could be observed in the bladder walls of tissues from the WNS and
KNS control groups, where the bladder mucosal barrier was intact
(Fig. 2B). All these qualitative
descriptions were pointed out by using arrows. Immunohistochemical
analysis revealed that COX-2 and iNOS protein expression in KO mice
was enhanced beneath the bladder mucosa compared with WT mice
(Fig. 2C). In week 4, COX-2 protein
expression in KHK group increased more than WHK and iNOS protein
expression in KHK and WHK group showed little difference. When in
week 12, the staining densities of COX-2 and iNOS in the KHK group
were markedly higher compared with those in the WHK group (Fig. 2C). Masson trichome staining of the
bladder tissues revealed that the bladder submucosa and muscular
layers were not notably affected in the WNS and KNS groups at week
12 (Fig. 2D). By contrast, the
lamina propria and submucosa structure had disintegrated in the WHK
and KHK groups, where the tissues exhibited diffuse interstitial
fibrosis. Furthermore, the area infiltrated by collagen fibers was
larger and the thickening of the knot tissues was clearer in the
KHK group compared with that in the WHK group. All these
qualitative descriptions are presented by arrows.
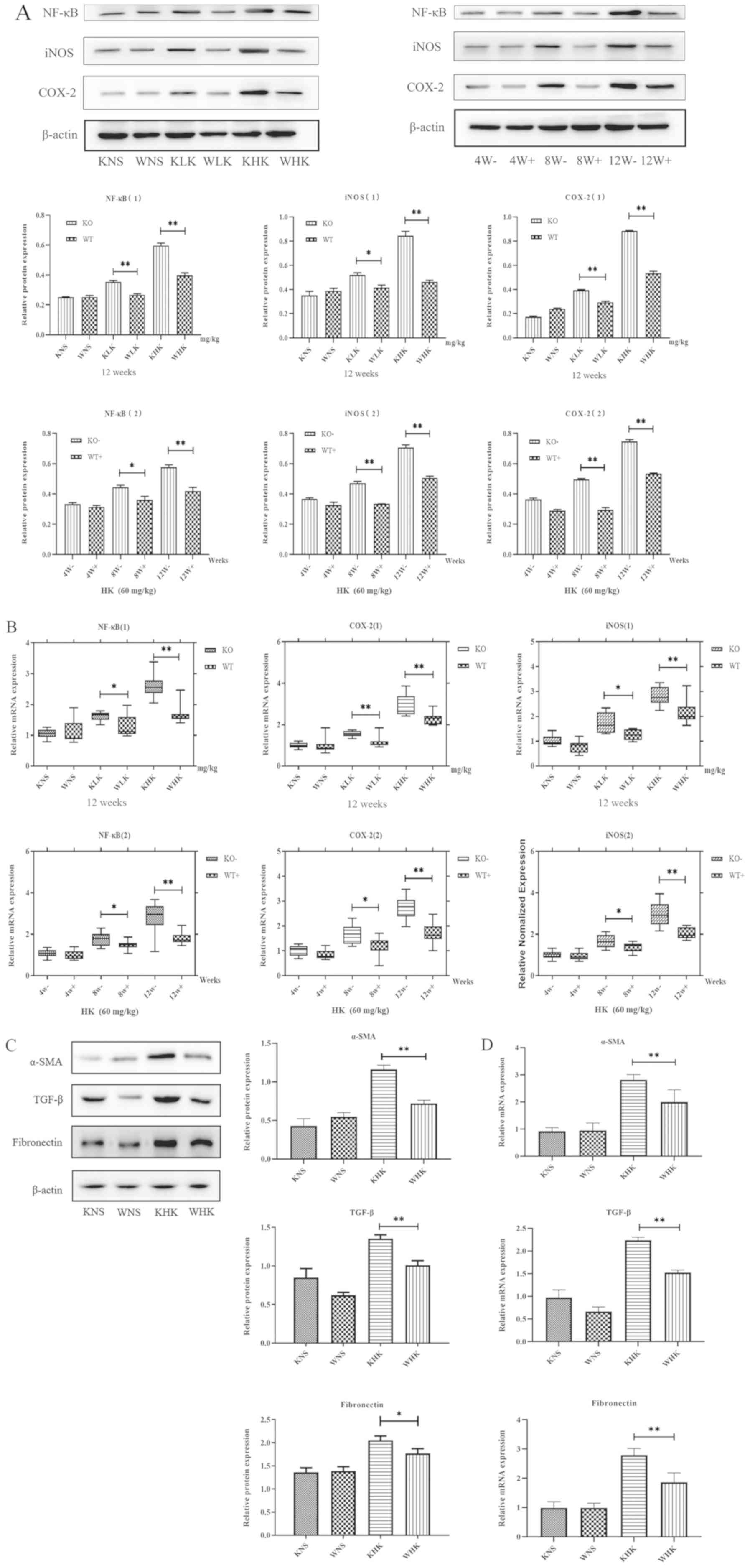
Inflammation of ketamine-induced
cystitis is more severe in KO mice
Furthermore, the expression of NF-κB, iNOS and
COX-2, indicators associated with inflammation, was verified by
measuring their protein and mRNA levels. The results demonstrated
that in the KHK group, the protein expression levels of COX-2, iNOS
and NF-κB were increased by 1.63 (P<0.01), 1.41 (P<0.01) and
1.51 (P<0.01) fold, respectively, compared with those in the WHK
group in week 12, in a concentration-dependent manner (Fig. 3A). In addition, when comparing the
LK groups (week 12) or week 8 (HK groups), the results also
demonstrated that NF-κB (P<0.05), iNOS (P<0.05) and COX-2
(P<0.01) protein expression was enhanced in KO mice compared
with those in WT mice. At 12 weeks in the HK group, the mRNA levels
of NF-κB, COX-2 and iNOS were significantly higher (all P<0.01)
in the KO group (2.620±0.350, 2.903±0.188 and 3.307±0.242,
respectively) compared with those in the WT group (1.884±0.311,
1.787±0.116 and 2.083±0.286, respectively; Fig. 3B). Furthermore, when comparing LK
groups (week 12) or week 8 (HK groups), the results also
demonstrated that NF-κB (P<0.05), iNOS (P<0.05) and COX-2
(P<0.05) protein expressions were enhanced in KO mice compared
with those in WT mice.
 | Figure 3Ketamine treatment induces
inflammation and fibrosis in KO mice. (A) Effects of ketamine on
inflammation protein COX-2, iNOS and NF-κB levels in mice.
*P<0.05 and **P<0.01. Data are
presented as the mean ± standard error of the mean from ≥3
experimental repeats. KO, knock-out; KHK, KO high-dose ketamine
group; WHK, wild-type high-dose ketamine group; COX-2,
cyclooxygenase 2; iNOS, inducible nitric oxide synthase; WT,
wild-type; α-SMA, α-smooth muscle actin; TGF-β, transforming growth
factor β; KNS, knock-out normal saline control group; WNS,
wild-type normal saline control group; KLK, knock-out low-dose
ketamine group; WLK, wild-type low-dose ketamine group; W, weeks;
-, knock-out; +, wild-type; HK, high-dose ketamine. Ketamine
treatment induces inflammation and fibrosis in KO mice. (B) mRNA
expression levels of inflammatory proteins COX-2, iNOS and NF-κB in
the mouse bladder tissues were detected by reverse
transcription-quantitative PCR. (C) Western blotting and (D)
reverse transcription-quantitative PCR were performed to measure
the expression of α-SMA, TGF-β and fibronectin in mouse bladder
tissues at week 12. *P<0.05 and
**P<0.01. Data are presented as the mean ± standard
error of the mean from ≥3 experimental repeats. KO, knock-out; KHK,
KO high-dose ketamine group; WHK, wild-type high-dose ketamine
group; COX-2, cyclooxygenase 2; iNOS, inducible nitric oxide
synthase; WT, wild-type; α-SMA, α-smooth muscle actin; TGF-β,
transforming growth factor β; KNS, knock-out normal saline control
group; WNS, wild-type normal saline control group; KLK, knock-out
low-dose ketamine group; WLK, wild-type low-dose ketamine group; W,
weeks; -, knock-out; +, wild-type; HK, high-dose ketamine. |
Fibrosis levels in ketamine-induced
cystitis is more severe in KO mice
Additionally, the protein expression levels of
fibrotic markers α-SMA (P<0.01), TGF-β (P<0.01) and
fibronectin (P<0.05) were found to be significantly higher in
mice in the KHK group compared with those in mice in the WHK group
(Fig. 3C), consistent with changes
in the expression of inflammatory proteins in KO and WT mice.
Subsequently, RT-qPCR analysis revealed that the mRNA expression
levels of α-SMA, TGF-β and fibronectin in KHK vs. WHK mice were
2.814±0.342 vs. 1.967±0.536, 2.237±0.118 vs. 1.524±0.102 and
2.791±0.395 vs. 1.866±0.551, respectively (all P<0.01) in week
12.
Discussion
To the best of our knowledge, the present study is
the first to investigate the impact of the aldh2 gene on
ketamine-induced cystitis. Aldh2 is a metabolic enzyme of certain
aldehydes, including 4-hydroxynonenal and MDA, which has a number
of enzymatic functions, including dehydrogenase and esterase. In
addition, aldh2 oxidizes 4-hydroxynonenal, MDA and other oxidation
products and converts them into non-toxic acids, thereby reducing
the degree of inflammation and inhibiting apoptosis. Using this
function, aldh2 has been previously reported to protect the
kidneys, liver, heart and other organs from damage (21). Hu et al (22) demonstrated that inhibiting aldh2
expression aggravated the inflammatory reaction in rats with
sepsis, increasing kidney damage. In another study, Wimborne et
al (23) previously revealed
that activation of aldh2 reduced the hepatotoxic effects of ethanol
and acetaminophen. Additionally, Xu et al (24) reported that aldh2 prevented
myocardial damage associated with pulmonary hypertension. These
previous studies demonstrated that that aldh2 served important
functions in regulating inflammation leading up to organ damage.
The low expression of aldh2 was found to associate closely with
renal fibrosis, myocardial fibrosis and urinary tract fibrosis
caused by urothelial tumors (25-27).
Tang et al (25) revealed
that aldh2 can be used as a common potential genetic target for
prognosis of various renal fibrosis diseases (renal fibrosis caused
by unilateral ureteral obstruction, ischemia-reperfusion injury or
cisplatin-induced). Mali et al (26) previously discovered that heart
damage and myocardial fibrosis as a result of chronic hyperglycemia
are associated with reduced aldh2 activity. A whole genome analysis
conducted by Wu et al (27)
demonstrated aldh2 is a potential prognostic marker for urothelial
carcinoma, where aldh2 expression associated with that of
urothelial fibrosis genes. However, the mechanism of aldh2 in the
development of KIC remains unclear. Due to its significant
anti-inflammatory and antifibrotic effects, the present study
hypothesized that aldh2 has a high probability of being a novel KIC
prevention target.
The results of the present study demonstrated that
following long-term treatment of ketamine, the expression of aldh2
in mice was significantly upregulated. Previous studies have
reported that the pathogenesis of KIC is associated with
inflammation and fibrosis (28,29).
Therefore, the present study hypothesized that following long-term
treatment of ketamine, the aldh2 gene may be upregulated to
protect against adverse factors, including oxidative stress,
inflammation and fibrosis in vivo. To investigate this, the
long-term effects of ketamine on aldh2 KO mice were compared
with those in WT mice.
KIC is a dynamic and complex process that is closely
associated with oxidative stress, which is in turn associated with
excessive ROS production (4). The
effects of ROS on micturition reflexes have been previously
demonstrated in several pathological states of the bladder,
including cyclophosphamide-induced hemorrhagic cystitis, ionizing
radiation cystitis and partial bladder outlet obstruction (30). High intracellular levels of ROS and
the imbalance between the oxidative and antioxidant systems
promotes lipid peroxidation and increases the formation of
aldehydes, including that of MDA, which is the end product in
kidney tissues. Additionally, GSH and SOD inactivate ROS production
in the mitochondria by enhancing the activity of catalase in
peroxisomes. Therefore, any aberrant changes in GSH and SOD can
cause serious damage to cellular DNA, lipids and proteins (5). The present study demonstrated that
under the same doses of ketamine, the levels of oxidative stress in
aldh2 KO mice were significantly higher compared with that
in WT mice. In addition, ketamine treatment significantly reduced
the expression of antioxidant enzymes whilst enhancing the
production of MDA in aldh2 KO mice.
During KIC, ROS stimulates NF-κB activation, leading
to downstream signaling pathways to induce tissue damage (31,32).
Generally, NF-κB is inactive under physiological conditions. Once
activated by phosphorylation, NF-κB dissociates from its inhibitory
unit, nuclear factor of κ light polypeptide gene enhancer in
B-cells inhibitor β, freeing the active p65-NF-κB. p65-NF-κB then
migrates to the nucleus, where it binds to its promoter sequence to
activate the expression of pro-inflammatory cytokines (33). In a previous study, hyaluronan
instillation treatment significantly inhibited the activation of
the NF-κB signaling pathway by suppressing oxidative stress
(34). The results of the present
study revealed that the expression of the active NF-κB unit in
aldh2 KO mice was higher compared with that in WT,
indicating a more stressful condition. Treating aldh2 KO
mice with ketamine activated the NF-κB pathway, which may be
associated with the absence of aldh2 in its anti-oxidative stress
effects. COX-2 and iNOS are sensitive markers of inflammation
caused by oxidative stress (35).
Previous studies have demonstrated that COX-2 regulated the
inflammatory response in rats with KIC via the NF-κB pathway
(15,31), such that COX-2 overexpression can
serve an important role in altering the urinary pattern during KIC
(36). Numerous previous studies on
cyclophosphamide-induced cystitis (37,38)
revealed the role of COX-2 in bladder overactivity, which is
associated with inflammation or hypertrophy. Additionally,
treatment with COX-2 inhibitors has been demonstrated to improve
ifosfamide-induced bladder injury and alter urinary overactivity
(39). iNOS is usually expressed in
macrophages. When activated by pathogens or cytokines, such as ROS,
it synthesizes nitric oxide (NO) to promote cell apoptosis
(40). A previous study reported
that iNOS was upregulated in cyclophosphamide-induced cystitis
(38). Other studies have
previously revealed that ketamine or its urinary metabolites
exerted direct toxic effects on bladder epithelial cells due to the
activation of iNOS in the mitochondria and the resulting high
levels of NO in urine (19,36). In the present study, COX-2 and iNOS
were found to be highly expressed in aldh2 KO mice.
Numerous animal studies have demontrated that
long-term ketamine treatment can lead to bladder fibrosis, which is
an important cause of bladder abnormalities, including decreased
capacity, lower compliance and impaired detrusor function (41,42).
Song et al (41)
demonstrated that the inflammatory mediators in KIC increased the
expression of collagen type-I (COL-I) and α-SMA, which leads to
thickening of the bladder basement membrane. Furthermore, Shen
et al (42) conducted whole
genome analysis and reported that the expression of fibrotic genes,
including fibronectin, TGF-β1 and COL-I, were upregulated in
bladder tissues with KIC. This enriched expression was considered
to be a sensitive marker of active fibrosis development. The
present study revealed that compared with WT mice, the severity of
ketamine-induced fibrosis in aldh2 KO mice was considerably
higher and the expression of α-SMA, TGF-β1 and fibronectin were
significantly higher. These results indicated that the expression
of aldh2 alleviates bladder fibrosis induced by KIC. Due to the
effects of inflammation and fibrosis in vivo, aldh2
KO mice gained weight slower and urinated more frequently compared
with that in WT mice. This may be due to the chronic physiological
stress caused by inflammation and fibrosis, which were increased in
aldh2 KO mice.
The results of the present study revealed that aldh2
inhibited the NF-κB signaling pathway, thereby preventing fibrosis
by suppressing lipid peroxidation in KIC and reducing COX-2 and
iNOS expression. Data from the present study advises that Asian
people should refrain from ingesting ketamine, since they are at a
higher risk of developing mutations in the aldh2 gene, which
can result in the development of severe LUTS and bladder
contracture. Future studies should include cellular tests and crowd
verification experiments to validate the results of the present
study. Future studies are also required to clarify whether
ketamine-induced cystitis is race-specific.
Supplementary Material
Generation of aldh2 KO and WT
mice. (A) Western blot analysis of aldh2 expression in ICR mouse
liver. The targeting mouse liver mitochondrial fractions were
subjected to immunoblot analysis with anti-Aldh2 antibody. Lane 1
is recombinant aldh2 protein. Lanes 2-4 are Aldh2+/+, +/- and -/-,
respectively. (B) PCR analysis of aldh2 DNA extracted from ICR
mouse tails. Both lanes 1 and 3 indicate aldh2+/-. Lanes 2 and 4
indicate aldh2-/- and +/+, respectively. Lane 5 indicates markers.
The WT (+/+, +/-) mouse showed a 208 bp fragment, and in KO (-/-)
primer reaction system, a 280 bp fragment appeared only. (C) The
mechanism of oxidative stress in ketamine-induced cystitis. Aldh2,
aldehyde dehydrogenase 2; KO, knock-out; WT, wild-type; ICR,
Institute of Cancer Research; KIC, ketamine-induced cystitis; ROS,
reactive oxygen species; RNS, reactive nitrogen species; NF-κB,
nuclear factor-κ-light-chain-enhancer of B cells; iNOS, inducible
nitric oxide synthase; COX-2, cyclooxygenase 2.
Measurement of the frequency of
micturition based on the number of micturition points in
aldh2 KO and WT mice. aldh2, aldehyde dehydrogenase 2; WT,
wild-type; KO, knock-out; WNS, wild-type normal saline control
group; WLK, wild-type low-dose ketamine group; WHK, wild-type
high-dose ketamine group; KNS, knock-out normal saline control
group; KLK, knock-out low-dose ketamine group; KHK, knock-out
high-dose ketamine group.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81860142) and the
Natural Science Foundation of Guangxi Zhuang Autonomous Region
(grant no. 2017GXNSFAA198279).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
XJX and HM designed this study. XJX and SHC
performed the experiments. XJX, SHC and HM performed the
statistical analysis. XJX and HM drafted and revised the
manuscript. HM contributed reagents, materials and experimental
platforms. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Guangxi Medical University (approval no.
20180129).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang JW, Kivovich V and Gordon L: Ketamine
abuse syndrome: Hepatobiliary and urinary pathology among
adolescents in Flushing, NY. Pediatr Emerg Care. 33:e24–e26.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Persson J: Wherefore ketamine? Curr Opin
Anaesthesiol. 23:455–60. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Parkin MC, Turfus SC, Smith NW, Halket JM,
Braithwaite RA, Elliott SP, Osselton MD, Cowan DA and Kicman AT:
Detection of ketamine and its metabolites in urine by ultra high
pressure liquid chromatography-tandem mass spectrometry. J
Chromatogr B Analyt Technol Biomed Life Sci. 876:137–142.
2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu KM, Chuang SM, Long CY, Lee YL, Wang
CC, Lu MC, Lin RJ, Lu JH, Jang MY, Wu WJ, et al: Ketamine-induced
ulcerative cystitis and bladder apoptosis involve oxidative stress
mediated by mitochondria and the endoplasmic reticulum. Am J
Physiol Renal Physiol. 309:F318–F331. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xu T, Liu S, Ma T, Jia Z, Zhang Z and Wang
A: Aldehyde dehydrogenase 2 protects against oxidative stress
associated with pulmonary arterial hypertension. Redox Biol.
11:286–296. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bondeva T and Wolf G: Reactive oxygen
species in diabetic nephropathy: Friend or foe? Nephrol Dial
Transplant. 29:1998–2003. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim MJ and Lim Y: Protective effect of
short-term genistein supplementation on the early stage in
diabetes-induced renal damage. Mediators Inflamm.
2013(510212)2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jomova K and Valko M: Advances in
metal-induced oxidative stress and human disease. Toxicology.
283:65–87. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Choi YJ, Kim HS, Lee J, Chung J, Lee JS,
Choi JS, Yoon TR, Kim HK and Chung HY: Down-regulation of oxidative
stress and COX-2 and iNOS expressions by dimethyl lithospermate in
aged rat kidney. Arch Pharm Res. 37:1032–1038. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lin HC, Lee HS, Chiueh TS, Lin YC, Lin HA,
Lin YC, Cha TL and Meng E: Histopathological assessment of
inflammation and expression of inflammatory markers in patients
with ketamine-induced cystitis. Mol Med Rep. 11:2421–2428.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ge W, Guo R and Ren J: AMP-dependent
kinase and autophagic flux are involved in aldehyde
dehydrogenase-2-induced protection against cardiac toxicity of
ethanol. Free Radic Biol Med. 51:1736–48. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Clarke TK, Adams MJ, Davies G, Howard DM,
Hall LS, Padmanabhan S, Murray AD, Smith BH, Campbell A, Hayward C,
et al: Genome-wide association study of alcohol consumption and
genetic overlap with other health-related traits in UK Biobank
(N=112 117). Mol Psychiatry. 22:1376–1384. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu S, Chen J, Dong P, Zhang S, He Y, Sun
L, Zhu J, Cheng Y, Li X, Tang A, et al: Global gene expression
profiling identifies ALDH2, CCNE1 and SMAD3 as potential prognostic
markers in upper tract urothelial carcinoma. BMC Cancer.
14(836)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Andrew AS, Gui J, Hu T, Wyszynski A,
Marsit CJ, Kelsey KT, Schned AR, Tanyos SA, Pendleton EM, Ekstrom
RM, et al: Genetic polymorphisms modify bladder cancer recurrence
and survival in a USA population-based prognostic study. BJU Int.
115:238–247. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ferreira-Teixeira M, Parada B,
Rodrigues-Santos P, Alves V, Ramalho JS, Caramelo F, Sousa V, Reis
F and Gomes CM: Functional and molecular characterization of cancer
stem-like cells in bladder cancer: A potential signature for
muscle-invasive tumors. Oncotarget. 6:36185–201. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chuang SM, Lu JH, Lin KL, Long CY, Lee YC,
Hsiao HP, Tsai CC, Wu WJ, Yang HJ and Juan YS: Epigenetic
regulation of COX2 expression by DNA hypomethylation via NFkappaB
activation in ketamineinduced ulcerative cystitis. Int J Mol Med.
44:797–812. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jin S, Chen J, Chen L, Histen G, Lin Z,
Gross S, Hixon J, Chen Y, Kung C, Chen Y, et al: ALDH2(E487K)
mutation increases protein turnover and promotes murine
hepatocarcinogenesis. Proc Natl Acad Sci USA. 112:9088–9093.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yeung LY, Rudd JA, Lam WP, Mak YT and Yew
DT: Mice are prone to kidney pathology after prolonged ketamine
addiction. Toxicol Lett. 191:275–278. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gu D, Huang J, Yin Y, Shan Z, Zheng S and
Wu P: Long-term ketamine abuse induces cystitis in rats by
impairing the bladder epithelial barrier. Mol Biol Rep. 41:7313–22.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Choi JW, Kim JH, Cho SC, Ha MK, Song KY,
Youn HD and Park SC: Malondialdehyde inhibits an AMPK-mediated
nuclear translocation and repression activity of ALDH2 in
transcription. Biochem Biophys Res Commun. 404:400–406.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hu JF, Wang HX, Li HH, Hu J, Yu Y and Gao
QQ: Inhibition of ALDH2 expression aggravates renal injury in a rat
sepsis syndrome model. Exp Ther Med. 14:2249–2254. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wimborne HJ, Hu J, Takemoto K, Nguyen N,
Jaeschke H, Lemasters JJ and Zhong Z: Aldehyde dehydrogenase-2
activation decreases acetaminophen hepatotoxicity by prevention of
mitochondrial depolarization. Toxicol Appl Pharmacol.
396(114982)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xu T, Liu SY, Ma TT, Jia ZY, Zhang ZF and
Wang AM: Aldehyde dehydrogenase 2 protects against oxidative stress
associated with pulmonary arterial hypertension. Redox Biol.
11:286–296. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tang S, Huang T, Jing H, Huang Z, Chen H,
Fan Y, Zhong J and Zhou J: Aldehyde dehydrogenase-2 acts as a
potential genetic target for renal fibrosis. Life Sci.
239(117015)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mali VR, Pan G, Deshpande M, Thandavarayan
RA, Xu J, Yang XP and Palaniyandi SS: Cardiac mitochondrial
respiratory dysfunction and tissue damage in chronic hyperglycemia
correlate with reduced aldehyde Dehydrogenase-2 activity. PLoS One.
11(e0163158)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu S, Chen JH, Dong P, Zhang SQ, He YY,
Sun L, Zhu JL, Cheng YB, Li XX, Tang AF, et al: Global gene
expression profiling identifies ALDH2, CCNE1 and SMAD3 as potential
prognostic markers in upper tract urothelial carcinoma. BMC Cancer.
14(836)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Q, Wu Q, Wang J, Chen Y, Zhang G,
Chen J, Zhao J and Wu P: Ketamine analog methoxetamine induced
inflammation and dysfunction of bladder in rats. Int J Mol Sci.
18(117)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim A, Yu HY, Heo J, Song M, Shin JH, Lim
J, Yoon SJ, Kim Y, Lee S, Kim SW, et al: Mesenchymal stem cells
protect against the tissue fibrosis of ketamine-induced cystitis in
rat bladder. Sci Rep. 6(30881)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chiba K, Yamaguchi K, Ando M, Miyake H and
Fujisawa M: Expression pattern of testicular claudin-11 in
infertile men. Urology. 80:1161.e13–e17. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Juan YS, Lee YL, Long CY, Wong JH, Jang
MY, Lu JH, Wu WJ, Huang YS, Chang WC and Chuang SM: Translocation
of NF-κB and expression of cyclooxygenase-2 are enhanced by
ketamine-induced ulcerative cystitis in rat bladder. Am J Pathol.
185:2269–2285. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xi XJ, Zeng JJ, Lu Y, Chen SH, Jiang ZW,
He PJ and Mi H: Extracellular vesicles enhance oxidative stress
through P38/NF-kB pathway in ketamine-induced ulcerative cystitis.
J Cell Mol Med. 24:7609–7624. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang Q, Lenardo MJ and Baltimore D: 30
years of NF-κB: A blossoming of relevance to human pathobiology.
Cell. 168:37–57. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee YL, Lin KL, Chuang SM, Lee YC, Lu MC,
Wu BN, Wu WJ, Yuan SF, Ho WT and Juan YS: Elucidating mechanisms of
bladder repair after hyaluronan instillation in ketamine-induced
ulcerative cystitis in animal model. Am J Pathol. 187:1945–1959.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Arena A, Zimmer TS, van Scheppingen J,
Korotkov A, Anink JJ, Mühlebner A, Jansen FE, van Hecke W, Spliet
WG, van Rijen PC, et al: Oxidative stress and inflammation in a
spectrum of epileptogenic cortical malformations: Molecular
insights into their interdependence. Brain Pathol. 29:351–365.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chuang SM, Liu KM, Li YL, Jang MY, Lee HH,
Wu WJ, Chang WC, Levin RM and Juan YS: Dual involvements of
cyclooxygenase and nitric oxide synthase expressions in
ketamine-induced ulcerative cystitis in rat bladder. Neurourol
Urodyn. 32:1137–1143. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hu VY, Malley S, Dattilio A, Folsom JB,
Zvara P and Vizzard MA: COX-2 and prostanoid expression in
micturition pathways after cyclophosphamide-induced cystitis in the
rat. Am J Physiol Regul Integr Comp Physiol. 284:R574–R585.
2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Oter S, Korkmaz A, Oztas E, Yildirim I,
Topal T and Bilgic H: Inducible nitric oxide synthase inhibition in
cyclophosphamide induced hemorrhagic cystitis in rats. Urol Res.
32:185–189. 2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Macedo FY, Mour ão LT, Palheta RC Jr, Juca
DM, Lima RC Jr, Neto Jde S, Magalhaes PJ, Santos AA, Souza MH,
Brito GA and Ribeiro RA: Cyclooxygenase-2 contributes to functional
changes seen on experimental hemorrhagic cystitis induced by
ifosfamide in rat urinary bladder. Cancer Chemother Pharmacol.
67:935–943. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kumar A, Singh KP, Bali P, Anwar S, Kaul
A, Singh OP, Gupta BK, Kumari N, Noor Alam M, Raziuddin M, et al:
iNOS polymorphism modulates iNOS/NO expression via impaired
antioxidant and ROS content in P. vivax and P. falciparum
infection. Redox Biol. 15:192–206. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Song M, Yu HY, Chun JY, Shin DM, Song SH,
Choo MS and Song YS: The fibrosis of ketamine, a noncompetitive
N-methyl-d-aspartic acid receptor antagonist dose-dependent change
in a ketamine-induced cystitis rat model. Drug Chem Toxicol.
39:206–212. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Shen CH, Wang SC, Wang ST, Lin SM, Wu JD,
Lin CT and Liu YW: Evaluation of urinary bladder fibrogenesis in a
mouse model of long-term ketamine injection. Mol Med Rep.
14:1880–1890. 2016.PubMed/NCBI View Article : Google Scholar
|

















