1. Introduction
Vascular calcification, characterized by the active
deposition of calcium phosphate in the form of hydroxyapatite
crystals in the intima, media and adventitia layers of blood
vessels, is highly prevalent in chronic kidney disease, diabetes
mellitus, aging and atherosclerosis (1). When present, vascular calcification
increases the risk of adverse cardiovascular events and is highly
associated with cardiovascular mortality in the high-risk
population with diabetes mellitus and chronic kidney disease
(2). However, the cellular and
molecular mechanisms underlying vascular calcification are complex
and there are limited treatment options for the prevention and
treatment of vascular calcification in these diseases. Therefore,
much effort has been devoted to investigating the pathogenesis of
vascular calcification and developing more effective approaches to
its prevention in the last few decades, and great advances have
been made. In this context, the anti-aging protein klotho encoded
by the klotho gene reportedly has a protective role against
vascular calcification.
The klotho gene was firstly discovered in 1997 and
determined to be highly expressed in various tissue types,
including the kidney and brain. Loss-of-function mutation of klotho
in mice leads to a syndrome resembling human premature aging,
including hypoactivity, sterility, skin thinning, muscle atrophy,
osteoporosis, atherosclerosis and vascular calcification (3,4).
Conversely, klotho overexpression reversed the klotho-deficient
phenotypes. These results suggest that the anti-aging protein
klotho may exert an inhibitory role in vascular calcification. It
is therefore important to evaluate the precise effects of klotho on
vascular calcification and the underlying mechanisms, to identify
targets for novel therapies. Although vascular calcification is
thought to primarily originate from vascular smooth muscle cells
(VSMCs) (5), other cell types such
as stem cells have been reported to have a key role in the
development of vascular calcification. Of note, klotho deficiency
accelerates stem cell aging (6) and
exogenous administration of klotho attenuates osteogenic
differentiation of stem cells (7),
suggesting a key role of klotho in regulating stem cell-mediated
vascular calcification. Therefore, the present review will focus on
the potential role of dysregulation of stem cells in mediating
vascular calcification and provide novel insight into the cellular
and molecular mechanisms of how the anti-aging protein klotho
affects stem cells and vascular calcification. The anti-aging
protein klotho is proposed as a potential candidate therapeutic
agent to prevent vascular calcification.
2. Potential role of dysregulation of stem
cells in mediating vascular calcification
Potential role of stem cells in
mediating vascular calcification
Stem cells are a cluster of precursor cells with a
self-renewal ability and multi-directional differentiation
potential, which may differentiate into osteoblasts, chondrocytes,
cardiomyocytes or vascular cells in response to certain growth
factors (8-10).
Under normal conditions, these stem cells are able to differentiate
into vascular endothelial cells and VSMCs to mediate the
angiogenesis and maintenance of vascular homeostasis. During
vascular disease, including vascular calcification, these stem
cells are involved in the vascular repair or remodeling in response
to different microenvironmental factors (11-13),
including growth factors, reactive oxygen species and inflammatory
cytokines.
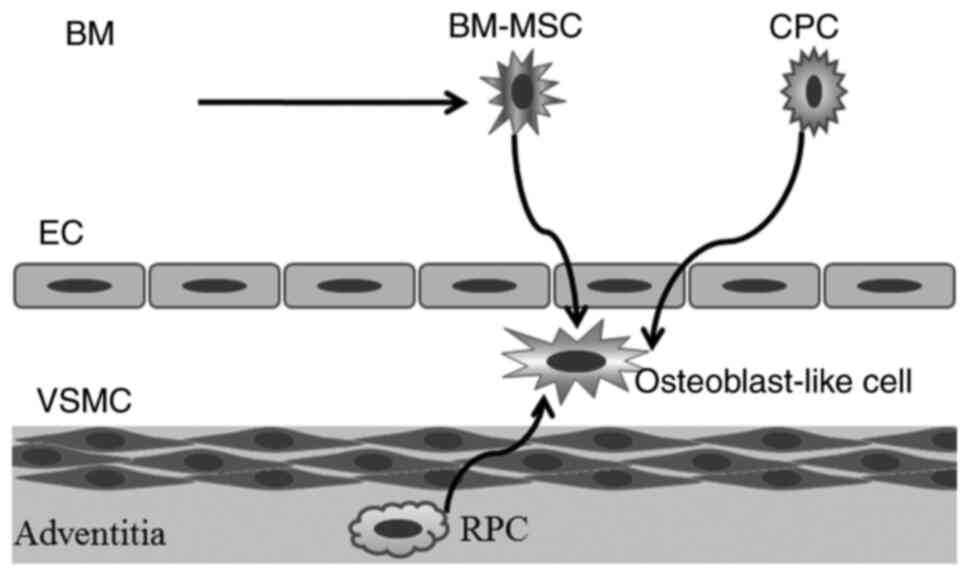
Vascular calcification is regarded as an active and
cell-mediated process and multiple types of stem cells, including
resident perivascular pericytes, circulating progenitor cells and
mesenchymal stem cells are involved in this process (Fig. 1) (14,15).
Although these stem cells are able to differentiate into vascular
endothelial cells and smooth muscle cells under normal conditions,
the differentiation potential of stem cells is largely influenced
by various diseases. On one hand, chronic kidney disease, diabetes
and aging have been reported to attenuate the vascular
differentiation potential of mesenchymal stem cells (16-18).
On the other hand, circulating progenitor cells that differentiate
into vascular cells undergo a phenotypic drift toward a procalcific
phenotype in diabetic patients (19) and hemodialysis patients with chronic
kidney disease (20), suggesting
that stem cells may function as possible players in vascular
calcification. In apolipoprotein E (-/-) mice with chronic kidney
disease, GLI family zinc finger 1+ mesenchymal stem
cell-like cells resident in the vascular wall have been suggested
to be a major source of osteoblast-like cells during calcification
in the media and intima (21).
Heterotopically implanted mesenchymal stem cells reportedly undergo
osteogenic differentiation in rat models of chronic kidney
disease-induced vascular calcification (22). Therefore, the above-mentioned
results provide crucial support for the view that stem cells have a
key role in mediating vascular calcification (Fig. 2).
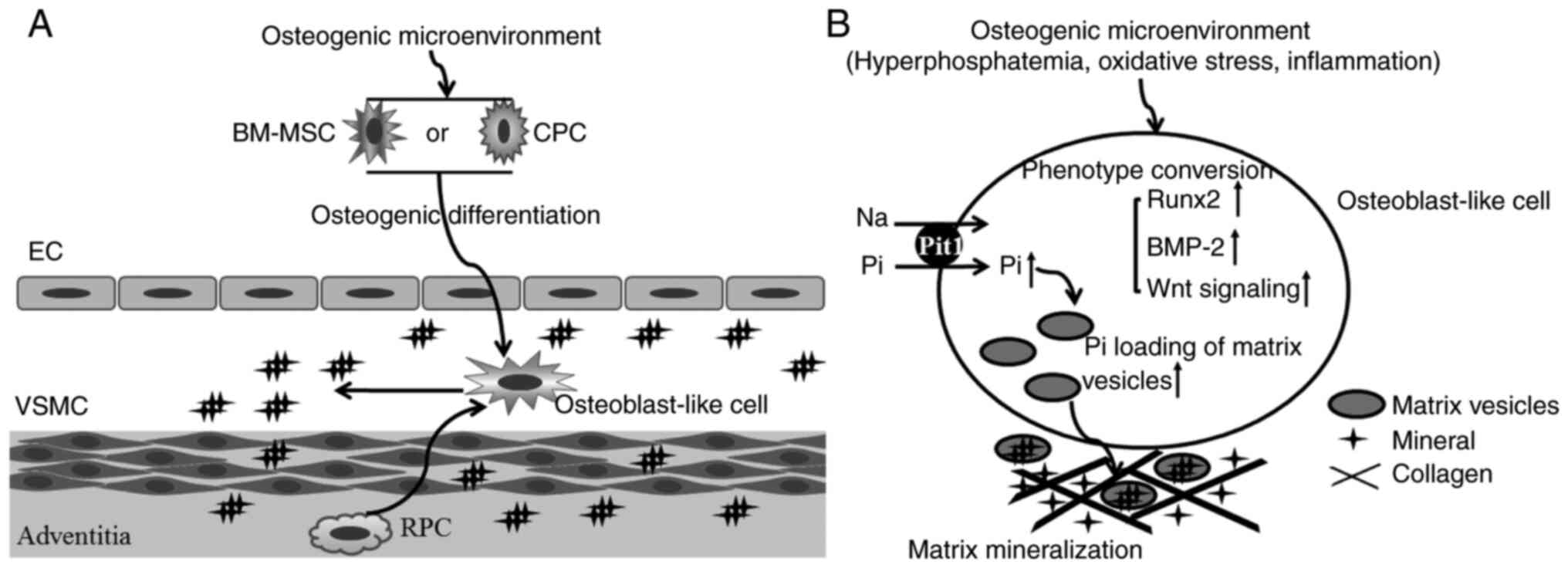 | Figure 2Involvement of stem/progenitor cells
in the calcification process. (A) In the calcification
microenvironment, stem/progenitor cells are induced to
differentiate into osteoblast-like cells. (B) These osteoblast-like
cells synthesize and release the osteogenesis matrix vesicles. EC,
endothelial cell; VSMC, vascular smooth muscle cell; BM-MSC, bone
marrow-derived mesenchymal stem cell; CPC, circulating progenitor
cell; RPC, resident pericytes; Pi, phosphate; Runx, Runt-related
transcription factor 2; BMP-2, bone morphogenetic protein 2; Pit1,
type III sodium-dependent phosphate transporter. |
Molecular mechanisms of stem
cell-mediated vascular calcification
Accumulating evidence suggests that the mechanisms
of vascular calcification are complex and involve multiple
microenvironmental factors, including hyperphosphatemia, oxidative
stress and inflammation.
Hyperphosphatemia is a common complication of
chronic kidney disease and functions as a key pathological factor
to promote the formation of arterial medial calcification in
chronic kidney disease (23).
Multiple types of stem cells, including circulating endothelial
progenitor cells and Gli1+-mesenchymal stem cells of the adventitia
are involved in the calcification process (23). The addition of high phosphate to
cultured mesenchymal stem cells reportedly results in high
expression of bone morphogenetic protein (BMP)-2(24), a key factor that promotes osteogenic
differentiation (25). BMP-2
promotes osteogenic differentiation of human bone marrow-derived
mesenchymal stem cells via the Smad/Runx family transcription
factor 2 (Runx2) pathway (26).
Thus, hyperphosphatemia may contribute to stem cell-mediated
vascular calcification through regulating the osteogenic
BMP-2/Runx2 pathway.
Oxidative stress is common in chronic kidney
disease, diabetes mellitus, atherosclerosis and aging. It is also
observed in association with vascular calcification in the diseases
mentioned above (27). In vascular
diseases, elevated production of hydrogen peroxide by residential
vascular cells increases oxidative stress in vascular lesions
(27) and promotes osteogenic
differentiation of VSMCs via the Akt signaling pathway (28). In embryonic stem cells treated with
glucose oxidase (GO), the GO-stimulated oxidative stress promotes
osteogenic differentiation and Runx expression through activating
the nuclear factor (erythroid-derived 2)-like 2/heme oxygenase-1
signaling and an extracellular signal-regulated kinase
(ERK)-mediated pathway (29). Thus,
oxidative stress may also act as an osteogenic factor to promote
stem cell-mediated vascular calcification.
The vascular inflammatory response involves the
recruitment of inflammatory cells (macrophages, neutrophils and
monocytes) and the release of inflammatory cytokines (TNF-α and
ILs) and is associated with vascular disease, including vascular
calcification (30,31). TNF-α has been reported to activate
osteogenic programming in VSMCs via the BMP-2, msh homeobox 2 and
Wnt signaling cascades (31). IL-1β
induces senescence of VSMCs to promote vascular calcification
through activating the NF-κB/p53/p21 signaling pathway (32). In mesenchymal stem cells from the
healthy aortas, treatment with TNF-α or IL-1β promoted the
transformation of those cells into an osteogenic phenotype
(33). Pharmacological targeting of
pro-inflammatory cytokines TNF-α and IL-1β ameliorated calcifying
phenotype conversion of vascular progenitors under uremic
conditions in vitro (34).
Thus, inflammation may also have a role in mediating stem
cell-mediated vascular calcification.
Although these different osteogenic stimuli promote
vascular calcification through regulating different signaling
pathways, the conversion of vascular cells into an osteogenic
phenotype is a key step in the development of vascular
calcification in response to various osteogenic stimuli. When
vascular cells acquire osteogenic differentiation potential, they
begin to produce mineralization-competent matrix vesicles and
release them into the extracellular fluid. Activated type III
sodium-dependent phosphate transporter (Pit1) in matrix vesicles
promotes phosphate loading of matrix vesicles and initiates
mineralization within the extracellular matrix (Fig. 2). Pit1 silencing was reported to
attenuate the osteogenic differentiation and calcification of
aortic smooth muscle cells (35).
3. Possible roles of klotho in regulating
vascular calcification
Effects of klotho deficiency on stem
cells and vascular calcification
A loss-of-function mutation of the klotho gene in
mice led to premature aging syndrome, including osteoporosis,
atherosclerosis and vascular calcification (4), suggesting a possible role of a lack of
klotho in mediating vascular calcification. The deficiency of the
klotho gene has been reported to upregulate the osteogenic
transcription factor Runx2 in aortic valves through the adenosine
monophosphate kinase-α pathway (36). Oxidative stress, a common risk
factor for chronic kidney disease, diabetes mellitus,
atherosclerosis and aging, may downregulate the expression of
endogenous klotho protein to promote vascular calcification
(37). Thus, the above-mentioned
results support a possible role of klotho deficiency in regulating
vascular calcification.
A possible mechanism underlying the role of klotho
in vascular calcification is that klotho deficiency alters the
functional features of stem cells. Klotho deficiency has been
reported to lead to impaired differentiation potential, cellular
senescence and apoptosis in stem cells during aging (6,38,39),
suggesting an important role of endogenous klotho in regulating
stem cell fate and function. Furthermore, klotho deficiency may
lead to the activation of calcification-associated signaling
pathways in stem cells, including the TGF-β1(6) and Wnt (40) pathways, both of which are reportedly
associated with the osteogenic differentiation potential of
vascular cells (41-43).
A recent study by Zhang et al (7) suggested that klotho significantly
attenuated the osteogenic differentiation potential of human bone
marrow-derived mesenchymal stem cells cultured in osteogenic
medium. Therefore, it is tempting to speculate from these
observations that klotho deficiency may contribute to vascular
calcification through regulating the osteogenic differentiation
potential of vascular stem cells. In the future, research efforts
should be made to investigate the effects of endogenous klotho
deficiency on the differentiation potential of stem cells during
vascular calcification.
Exogenous klotho as a therapeutic
agent
Various in vivo and in vitro studies
support an important protective role of exogenous klotho
administration in vascular calcification. First, genetic
overexpression of the klotho gene is able to reverse vascular
calcification (44,45). Furthermore, administration of
soluble klotho attenuates vascular calcification in vivo
(46) and in vitro (47). In addition, klotho mediates drug
treatment-induced suppression of vascular calcification (48,49).
However, the mechanisms of action remain elusive.
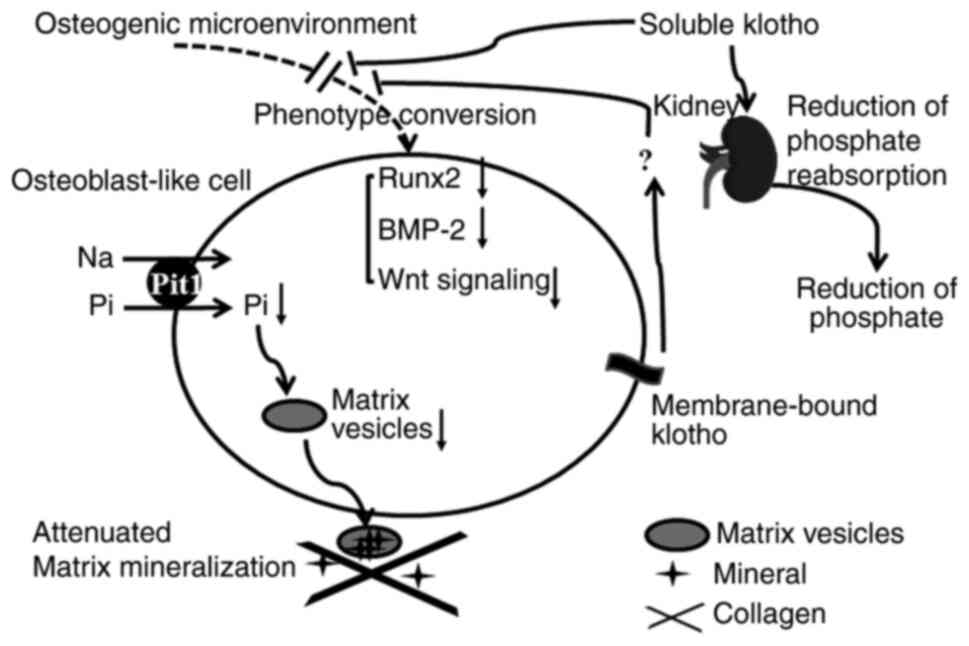
The precise mechanisms of klotho's actions are
complex and dependent on the reduction of phosphate and other
alternative mechanisms. As for reduction of phosphate, klotho
inhibits the reabsorption of phosphate in the kidney to decrease
serum phosphate levels, thus contributing to the improvement of
high phosphate-mediated vascular calcification. Additionally,
klotho directly inhibits the osteogenic phenotype conversion of
vascular progenitors through regulating Runx2, BMP-2 or Wnt
signaling pathways to reduce the release of matrix vesicles and
attenuate matrix mineralization (Fig.
3). On one hand, the delivery of soluble klotho attenuated
hyperphosphatemia and vascular calcification in a mouse model of
chronic kidney disease-mineral bone disorder (46). On the other hand, genetic
overexpression of klotho prevented chronic kidney disease-induced
medial calcification in a high-phosphate-independent manner
(50), suggesting a direct effect
of klotho on the vasculature. A possible explanation for the
contradictory results is that the two studies adopted different
animal models to investigate the role of klotho protein in
regulating vascular calcification.
To date, two forms of klotho protein have been
identified: Membrane-bound klotho and soluble klotho. In the study
by Hum et al (46), the
anti-calcification activity of soluble klotho was evaluated in a
transgenic mouse strain with loss-of-function mutation of the
klotho gene that exhibited phenotypes resembling human
premature-aging syndromes, including vascular calcification. The
study indicated that delivery of soluble klotho remedied
hyperphosphatemia and prevented vascular calcification in a
fibroblast growth factor receptor 1 (FGFR1)-dependent manner.
Conversely, in the study by Hu et al (50), klotho-attenuated vascular
calcification in chronic kidney disease was investigated in
transgenic mice with overexpression of membrane-bound klotho. The
study indicated that klotho overexpression ameliorated vascular
calcification by enhancing phosphaturia, preserving glomerular
filtration and directly inhibiting phosphate uptake by VSMCs.
Furthermore, Chen et al (47) reported that soluble klotho
ameliorated the calcification and osteogenic transition of VSMCs
through inhibiting the Wnt/β-catenin signaling pathway. Zhang et
al (7) indicated that soluble
α-klotho attenuated high-phosphate-induced calcification of human
bone marrow-derived mesenchymal stem cells via inactivation of the
FGFR1/ERK signaling pathway. In a study by Chen et al
(44), overexpression of
membrane-bound klotho attenuated high-phosphate-induced VSMC
calcification through inhibiting the Wnt7b/β-catenin pathway.
Another study by Chang et al (49) suggested that
intermedin1-53 increased the levels of membrane-bound
klotho protein in calcified VSMCs and klotho knockdown blocked the
inhibitory effect of intermedin1-53 on VSMC
calcification and their transformation into osteoblast-like cells.
Therefore, both soluble and membrane-bound klotho protein may have
therapeutic potential for vascular calcification.
4. Summary and perspectives
Since the klotho gene was first identified in 1997,
the understanding of its role as an endogenous inhibitor of
vascular diseases, including vascular calcification, has been
constantly growing. Klotho protein was determined to exert
pleiotropic protective effects against aging, chronic kidney
disease, diabetes mellitus, coronary artery disease,
atherosclerosis and cardiac hypertrophy (40,51-53).
However, the amount of currently available data about the effects
of klotho on vascular stem/progenitor cells during vascular
remodeling and diseases is limited. A better understanding of the
effects of klotho on stem cells during vascular calcification not
only provides novel insight into the precise role of stem cells in
the pathogenesis of calcification-associated vascular diseases but
also novel therapeutic targets for these diseases.
Patient and animal studies have indicated that
aging, chronic kidney disease and diabetes mellitus are able to
downregulate the expression of klotho (40,54).
Although the mechanisms underlying the downregulation of klotho
during calcification-associated diseases require to be fully
elucidated, overexpression of the klotho gene or administration of
exogenous klotho protein may be regarded as a potential therapeutic
approach for treating vascular calcification and
calcification-associated vascular diseases.
As mentioned above, previous studies have indicated
the possible role of klotho deficiency in regulating cell fate and
the function of stem cells in aging, and treatment with klotho
prevents the osteogenic differentiation of stem cells and induces
preservation of aging stem cells. Aging, diabetes mellitus and
chronic kidney disease may impair the vascular differentiation
potential of stem/progenitor cells and induce a phenotype drift of
stem cells toward a procalcific phenotype. Therefore, if soluble
klotho is able to protect stem cells or reverse the adverse
osteogenic differentiation of stem cells, klotho may be applied as
a promising therapeutic strategy for vascular repair and
calcification.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81641154), the
Special Fund of Hubei Science & Technology University for Hubei
Province Key Laboratory on Diabetes Mellitus (grant no.
2019-20XZ02) and the Key Clinical Specialty Discipline Construction
Program (grant no. LCZX201514).
Availability of data and materials
Not applicable.
Authors' contributions
LY performed the analysis of the current published
data. LY and ML contributed to drafting the original manuscript and
revising it critically for important intellectual content. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nicoll R and Henein M: Arterial
calcification: A new perspective? Int J Cardiol. 228:11–22.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rennenberg RJ, Kessels AG, Schurgers LJ,
van Engelshoven JM, de Leeuw PW and Kroon AA: Vascular
calcifications as a marker of increased cardiovascular risk: A
meta-analysis. Vasc Health Risk Manag. 5:185–197. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kuro-o M: Klotho in health and disease.
Curr Opin Nephrol Hypertens. 21:362–368. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kuro-o M: Klotho and the aging process.
Korean J Intern Med. 26:113–122. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Leopold JA: Vascular calcification:
Mechanisms of vascular smooth muscle cell calcification. Trends
Cardiovasc Med. 25:267–274. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ullah M and Sun Z: Klotho deficiency
accelerates stem cells aging by impairing telomerase activity. J
Gerontol A Biol Sci Med Sci. 74:1396–1407. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang W, Xue D, Hu D, Xie T, Tao Y, Zhu T,
Chen E and Pan Z: Secreted klotho protein attenuates osteogenic
differentiation of human bone marrow mesenchymal stem cells in
vitro via inactivation of the FGFR1/ERK signaling pathway. Growth
Factors. 33:356–365. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang L and Xu Q: Stem/Progenitor cells in
vascular regeneration. Arterioscler Thromb Vasc Biol. 34:1114–1119.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Campagnolo P, Wong MM and Xu Q: Progenitor
cells in arteriosclerosis: Good or bad guys? Antioxid Redox Signal.
15:1013–1027. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xie C, Ouyang L, Chen J, Zhang H, Luo P,
Wang J and Huang H: The emerging role of mesenchymal stem cells in
vascular calcification. Stem Cells Int.
2019(2875189)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zou S, Ren P, Zhang L, Azares AR, Zhang S,
Coselli JS, Shen YH and LeMaire SA: Activation of bone
marrow-derived cells and resident aortic cells during aortic
injury. J Surg Res. 245:1–12. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen Q, Yang M, Wu H, Zhou J, Wang W,
Zhang H, Zhao L, Zhu J, Zhou B, Xu Q and Zhang L: Genetic lineage
tracing analysis of c-kit+ stem/progenitor cells
revealed a contribution to vascular injury-induced neointimal
lesions. J Mol Cell Cardiol. 121:277–286. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Burtenshaw D, Hakimjavadi R, Redmond EM
and Cahill PA: Nox, reactive oxygen species and regulation of
vascular cell fate. Antioxidants (Basel). 14(90)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bostrom KI: Where do we stand on vascular
calcification? Vascul Pharmacol 2016:. 84:8–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Leszczynska A and Murphy JM: Vascular
calcification: Is it rather a stem/progenitor cells driven
phenomenon? Front Bioeng Biotechnol. 6(10)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dzhoyashvili NA, Efimenko AY, Kochegura
TN, Kalinina NI, Koptelova NV, Sukhareva OY, Shestakova MV,
Akchurin RS, Tkachuk VA and Parfyonova YV: Disturbed angiogenic
activity of adipose-derived stromal cells obtained from patients
with coronary artery disease and diabetes mellitus type 2. J Transl
Med. 12(337)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ikeda Y, Kumagai H, Motozawa Y, Suzuki J,
Akazawa H and Komuro I: Understanding vascular diseases: Lessons
from premature aging syndromes. Can J Cardiol. 32:650–658.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Khodayari S, Khodayari H, Amiri AZ, Eslami
M, Farhud D, Hescheler J and Nayernia K: Inflammatory
microenvironment of acute myocardial infarction prevents
regeneration of heart with stem cells therapy. Cell Physiol
Biochem. 53:887–909. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fadini GP, Albiero M, Menegazzo L, Boscaro
E, Agostini C, de Kreutzenberg SV, Rattazzi M and Avogaro A:
Procalcific phenotypic drift of circulating progenitor cells in
type 2 diabetes with coronary artery disease. Exp Diabetes Res.
2012(921685)2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cianciolo G, Capelli I, Cappuccilli M,
Scrivo A, Donadei C, Marchetti A, Rucci P and La Manna G: Is
chronic kidney disease-mineral and bone disorder associated with
the presence of endothelial progenitor cells with a calcifying
phenotype? Clin Kidney J. 10:389–396. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kramann R, Goettsch C, Wongboonsin J,
Iwata H, Schneider RK, Kuppe C, Kaesler N, Chang-Panesso M, Machado
FG, Gratwohl S, et al: Adventitial MSC-like cells are progenitors
of vascular smooth muscle cells and drive vascular calcification in
chronic kidney disease. Cell Stem Cell. 19:628–642. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kramann R, Kunter U, Brandenburg VM,
Leisten I, Ehling J, Klinkhammer BM, Knüchel R, Floege J and
Schneider RK: Osteogenesis of heterotopically transplanted
mesenchymal stromal cells in rat models of chronic kidney disease.
J Bone Miner Res. 28:2523–2534. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zununi Vahed S, Mostafavi S, Hosseiniyan
Khatibi SM, Shoja MM and Ardalan M: Vascular calcification: An
important understanding in nephrology. Vasc Health Risk Manag.
16:167–180. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guerrero F, Herencia C, Almaden Y,
Martinez-Moreno JM, Montes de Oca A, Rodriguez-Ortiz ME,
Diaz-Tocados JM, Canalejo A, Florio M, López I, et al: TGF-β
prevents phosphate-induced osteogenesis through inhibition of BMP
and Wnt/β-catenin pathways. PLoS One. 9(e89179)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Evrard S, Delanaye P, Kamel S, Cristol JP
and Cavalier E: SFBC/SN joined working group on vascular
calcifications. Vascular calcification: From pathophysiology to
biomarkers. Clin Chim Acta. 438:401–414. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang CL, Xiao F, Wang CD, Zhu JF, Shen C,
Zuo B, Wang H, Li D, Wang XY, Feng WJ, et al: Gremlin2 suppression
increases the BMP-2-induced osteogenesis of human bone
marrow-derived mesenchymal stem cells via the BMP-2/Smad/Runx2
signaling pathway. J Cell Biochem. 118:286–297. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen Y, Zhao X and Wu H: Arterial
stiffness: A focus on vascular calcification and its link to bone
mineralization. Arterioscler Thromb Vasc Biol. 40:1078–1093.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Byon CH, Javed A, Dai Q, Kappes JC,
Clemens TL, Darley-Usmar VM, McDonald JM and Chen Y: Oxidative
stress induces vascular calcification through modulation of the
osteogenic transcription factor Runx2 by AKT signaling. J Biol
Chem. 283:15319–15327. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sim HJ, Kim JH, Kook SH, Lee SY and Lee
JC: Glucose oxidase facilitates osteogenic differentiation and
mineralization of embryonic stem cells through the activation of
Nrf2 and ERK signal transduction pathways. Mol Cell Biochem.
419:157–163. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sprague AH and Khalil RA: Inflammatory
cytokines in vascular dysfunction and vascular disease. Biochem
Pharmacol. 78:539–552. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shobeiri N and Bendeck MP: Interleukin-1β
Is a key biomarker and mediator of inflammatory vascular
calcification. Arterioscler Thromb Vasc Biol. 37:179–180.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Han L, Zhang Y, Zhang M, Guo L, Wang J,
Zeng F, Xu D, Yin Z, Xu Y, Wang D and Zhou H:
Interleukin-1β-induced senescence promotes osteoblastic transition
of vascular smooth muscle cells. Kidney Blood Press Res.
45:314–330. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ciavarella C, Gallitto E, Ricci F, Buzzi
M, Stella A and Pasquinelli G: The crosstalk between vascular MSCs
and inflammatory mediators determines the pro-calcific remodelling
of human atherosclerotic aneurysm. Stem Cell Res Ther.
8(99)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hegner B, Schaub T, Janke D, Zickler D,
Lange C, Girndt M, Jankowski J, Schindler R and Dragun D: Targeting
proinflammatory cytokines ameliorates calcifying phenotype
conversion of vascular progenitors under uremic conditions in
vitro. Sci Rep. 8(12087)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Voelkl J, Alesutan I, Leibrock CB,
Quintanilla-Martinez L, Kuhn V, Feger M, Mia S, Ahmed MS,
Rosenblatt KP, Kuro-O M and Lang F: Spironolactone ameliorates
PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice.
J Clin Invest. 123:812–822. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Chen J, Lin Y and Sun Z: Deficiency in the
anti-aging gene Klotho promotes aortic valve fibrosis through
AMPKalpha-mediated activation of RUNX2. Aging Cell. 15:853–860.
2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sun H, Zhang F, Xu Y, Sun S, Wang H, Du Q,
Gu C, Black SM, Han Y and Tang H: Salusin-β promotes vascular
calcification via nicotinamide adenine dinucleotide
phosphate/reactive oxygen species-mediated klotho downregulation.
Antioxid Redox Signal. 31:1352–1370. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fan J and Sun Z: The antiaging gene klotho
regulates proliferation and differentiation of adipose-derived stem
cells. Stem Cells. 34:1615–1625. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Vadakke Madathil S, Coe LM, Casu C and
Sitara D: Klotho deficiency disrupts hematopoietic stem cell
development and erythropoiesis. Am J Pathol. 184:827–841.
2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bian A, Neyra JA, Zhan M and Hu MC:
Klotho, stem cells, and aging. Clin Interv Aging. 10:1233–1243.
2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bartoli-Leonard F, Wilkinson FL,
Langford-Smith AWW, Alexander MY and Weston R: The interplay of
SIRT1 and Wnt signaling in vascular calcification. Front Cardiovasc
Med. 5(183)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cai T, Sun D, Duan Y, Wen P, Dai C, Yang J
and He W: WNT/β-catenin signaling promotes VSMCs to osteogenic
transdifferentiation and calcification through directly modulating
Runx2 gene expression. Exp Cell Res. 345:206–217. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang W, Li C, Pang L, Shi C, Guo F, Chen
A, Cao X and Wan M: Mesenchymal stem cells recruited by active TGFβ
contribute to osteogenic vascular calcification. Stem Cells Dev.
23:1392–1404. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen YX, Huang C, Duan ZB, Xu CY and Chen
Y: Klotho/FGF23 axis mediates high phosphate-induced vascular
calcification in vascular smooth muscle cells via Wnt7b/β-catenin
pathway. Kaohsiung J Med Sci. 35:393–400. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi
H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et
al: Mutation of the mouse klotho gene leads to a syndrome
resembling ageing. Nature. 390:45–51. 1997.PubMed/NCBI View
Article : Google Scholar
|
|
46
|
Hum JM, O'Bryan LM, Tatiparthi AK, Cass
TA, Clinkenbeard EL, Cramer MS, Bhaskaran M, Johnson RL, Wilson JM,
Smith RC and White KE: Chronic hyperphosphatemia and vascular
calcification are reduced by stable delivery of soluble klotho. J
Am Soc Nephrol. 28:1162–1174. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chen T, Mao H, Chen C, Wu L, Wang N, Zhao
X, Qian J and Xing C: The role and mechanism of α-Klotho in the
calcification of rat aortic vascular smooth muscle cells. Biomed
Res Int. 2015(194362)2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Liu L, Liu Y, Zhang Y, Bi X, Nie L, Liu C,
Xiong J, He T, Xu X, Yu Y, et al: High phosphate-induced
downregulation of PPARγ contributes to CKD-associated vascular
calcification. J Mol Cell Cardiol. 114:264–275. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chang JR, Guo J, Wang Y, Hou YL, Lu WW,
Zhang JS, Yu YR, Xu MJ, Liu XY, Wang XJ, et al: Intermedin1-53
attenuates vascular calcification in rats with chronic kidney
disease by upregulation of α-Klotho. Kidney Int. 89:586–600.
2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hu MC, Shi M, Zhang J, Quinones H,
Griffith C, Kuro-o M and Moe OW: Klotho deficiency causes vascular
calcification in chronic kidney disease. J Am Soc Nephrol.
22:124–136. 2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Golembiewska E, Stepniewska J,
Kabat-Koperska J, Kedzierska K, Domanski M and Ciechanowski K: The
role of klotho protein in chronic kidney disease: Studies in
animals and humans. Curr Protein Pept Sci. 17:821–826.
2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kuro OM: The Klotho proteins in health and
disease. Nat Rev Nephrol. 15:27–44. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Olejnik A, Franczak A, Krzywonos-Zawadzka
A, Kaluzna-Oleksy M and Bil-Lula I: The biological role of klotho
protein in the development of cardiovascular diseases. Biomed Res
Int. 2018(5171945)2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Henaut L, Chillon JM, Kamel S and Massy
ZA: Updates on the mechanisms and the care of cardiovascular
calcification in chronic kidney disease. Semin Nephrol. 38:233–250.
2018.PubMed/NCBI View Article : Google Scholar
|