Introduction
Osteonecrosis of the femoral head (ONFH) is
characterized by the collapse of the femoral head and by joint
damage, and is caused by osteocyte death as a result of
insufficient blood supply (1). ONFH
is a destructive long-term condition with a poorly understood
pathogenic mechanism (2,3). A number of factors have been
demonstrated to increase the risk of osteonecrotic lesions,
including corticosteroid use, alcohol consumption, trauma and
abnormalities in coagulation (4).
In particular, the risk of steroid-induced osteonecrosis is
dependent on drug dosage, the route of drug delivery and underlying
disease states (5). At present,
total hip replacement is the only invasive therapy for advanced
osteonecrosis (6). Although
low-intensity pulsed ultrasound (LIPUS) has been proposed as an
alternative noninvasive therapeutic option for ONFH (7), it remains necessary to explore other
effective strategies to minimize treatment time. The use of bone
morphogenetic proteins (BMPs) and LIPUS have been studied
intensively as a prospective augmentation approach for
tendon-to-bone healing, where the delivery of BMPs into the bone
defect area combined with LIPUS has garnered attention for its
potential to prevent steroid-induced ONFH (8).
Biomaterials, including polymer scaffolds, possess
physical, mechanical and chemical properties that can be designed
to carry integrin signals, growth factors and cytokines for bone
regeneration and repair (9).
Bioactive small molecules can be encapsulated or entrapped into
scaffolds in such a way that the final constructs are able release
bioactive compounds into the area of interest in a controlled
manner (10). Of note, the BMP
family of proteins, including BMP-2, BMP-4 and BMP-7, have the
potential to induce endochondral bone formation when implanted into
mammals (11). BMP-2 has been
previously demonstrated to be an osteoconductive growth factor
beneficial for ONFH by promoting cartilage repair and inducing
osteoblast proliferation or differentiation (12). Recombinant human BMP-2 (rhBMP-2) may
improve the clinical efficacy of impacted bone graft surgery by
enhancing bone repair in ONFH (13). Several types of biodegradable
poly-α-hydroxy acid polymers, including polylactic acid (PLA),
polyglycolic acid (PGA) and their copolymer polylactic-co-glycolic
acid (PLGA), are popular options for the construction of scaffolds
in delivering rhBMP (14). rhBMP-2
tethered to biomaterials has been revealed to enhance the stability
and retention of biomaterials in the location of regeneration
(15). In recent decades,
poly-L-lactic acid (PLLA), and poly-ε-caprolactone (PCL) have
emerged as potentially viable biodegradable polymers for
therapeutic tissue engineering (16,17).
Therefore, the present study aimed to investigate the ability of a
novel composite PLLA/PLGA/PCL scaffold for BMP-2 delivery and to
assess the therapeutic action of this system in conjunction with
LIPUS in terms of bone formation, angiogenesis and differentiation
in a rat model of steroid-induced ONFH.
Materials and methods
Ethics statement
All animal experiments were performed in accordance
with the principles and procedures of the Guide for the Care and
Use of Laboratory Animals (18).
And approved by the Animal Ethics Committee of The Second
Affiliated Hospital of Zhejiang University School of Medicine.
Encapsulation of BMP-2-loaded PLGA
microspheres
PLGA microspheres were manufactured by double
emulsion. Briefly, 0.25 g polyvinyl alcohol (PVA) hydrogel was
diluted in 250 ml deionized water in a beaker. The diluted PVA was
subsequently stirred at 60˚C until the PVA was dissolved and cooled
to room temperature. PLGA (0.3 g) was dissolved in 3 ml
dichloromethane. The configured PVA and PLGA solutions were cooled
separately in an ice bath. The PLGA solution was then mixed with
500 µl PBS or PBS supplemented with BMP-2 (Recombinant Human Bone
Morphogenetic Protein 2, 1 µg/ml, Beijing BioLab Technology Co.,
Ltd.) or PBS supplemented with BSA (1 µg/ml, Beijing BioLab
Technology Co., Ltd.), ultrasonically dispersed using a sonicator
(4˚C, 200 W, 20 kHz, 10 sec) until the solution turned milky white
and added dropwise into the PVA solution. The PVA-PLGA mixture was
then ultrasonically dispersed until it turned milky white (4˚C, 200
W, 20 kHz, 2 min), following which the mixture was stirred using a
magnetic stirrer for 4-5 h at room temperature to allow the
evaporation of dichloromethane. After the microspheres were formed,
the mixture was centrifuged at 1,776 x g for 5 min at room
temperature, where the resulting supernatant was discarded, the
pellet was washed three times using deionized water. The pellet was
then freeze-dried to obtain empty or BMP-2 loaded PLGA
microspheres.
Determination of loading and
encapsulation efficiency
In total, 10 mg BMP-2-loaded microspheres were mixed
with 0.9 ml of NaOH solution (1 M) and 0.1 ml PBS. After the
mixture was stirred at room temperature for 2 h, 1 ml 0.9 M HCl was
added to neutralize the system. Protein concentration was then
determined in 20 µl of this sample using a bicinchoninic acid
protein assay kit (BCA; Beyotime Institute of Biotechnology). BMP-2
protein content was then calculated in 2 ml sample solution, which
was equated as the mass of BMP-2 in the microspheres. The loading
and encapsulation efficiency of BMP-2 were calculated according to
the following formulas: Loading efficiency (%)=(mass of BMP-2
protein in microspheres)/(total mass of microspheres) x100%;
encapsulation efficiency (%)=(mass of BMP-2 protein in
microspheres)/(mass of BMP-2 protein used in the initial batch)
x100%.
Packaging of BMP-2-loaded PLGA
microspheres into the scaffold
The mixture of PLLA, PLGA and PCL were dissolved in
tetrahydrofuran at a mass ratio of 3:4:3 and stirred at 60˚C until
fully dissolved. The solution was then transferred to a 2.5 ml
syringe barrel and stored at -80˚C overnight. The frozen sample was
then cut into ~1 mm slices, placed in an ice-water mixture and
incubated at 4˚C for 3 days. Deionized water was renewed three
times every day to completely displace the tetrahydrofuran. After
three days of incubation, the PLLA/PLGA/PCL composite scaffold was
removed and freeze-dried.
To obtain BMP-2-loaded microspheres packaged with
the PLGA-PLLA/PLGA/PCL composite scaffold, a total of 10 mg
nanostructured BMP-2-loaded microspheres were first uniformly
dissolved in 1 ml n-hexane, where 500 µl BMP-2-loaded
microspheres/n-hexane mixture was dropped onto one side of the
dried PLLA/PLGA/PCL nanofiber scaffold. After the n-hexane was
volatilized at room temperature, the other side was packed with
another 500 µl BMP-2-loaded microspheres. Both sides of the BMP-2
microsphere-containing scaffold were immersed in a
n-hexane-tetrahydrofuran mixture (9:1) to physically bind the
microspheres to the PLLA/PLGA/PCL nano-scaffold, which was
subsequently removed by vacuum drying in a dry box at 30˚C for
three days.
Scanning electron microscopy
(SEM)
The microspheres were first dissolved in ethanol
after they were made, where an ultrasonic wave was applied in the
ethanol solution containing the microspheres for dispersion
purposes. The dispersed liquid was aspirated using a pipette gun
and dropped onto the aluminum foil. After the anhydrous ethanol
evaporated at room temperature, the aluminum foil was pasted onto
the electron microscope carrier platform using a carbon conductive
tape (Hitachi, Ltd.). For the scaffold samples, the conductive
adhesive was directly pasted onto the carrier platform. The samples
were then sprayed with gold sputter coating and observed by
scanning electron microscopy (SEM) under an accelerating voltage
(10 kV) at x1,000 magnification. Using Image pro plus 6.0 software
(Media cybernetics, Inc.) to measure the particle size of PLGA
microspheres in SEM photos, the average particle size was
estimated.
In vitro release of BSA from the
PLGA-PLLA/PLGA/PCL composite scaffold
This assay was performed in accordance with a
previously described protocol (19). In brief, the BSA-PLGA-PLLA/PLGA/PCL
composite scaffold containing 10 mg BSA (1 µg/ml, Beijing BioLab
Technology Co., Ltd.) microspheres was added into a 5 ml centrifuge
tube containing 2 ml PBS (pH 7.4). The centrifuge tube was then
placed on a shaking table at 37˚C and 1,118 x g. Upon reaching the
configured time points (days 1-7, 14, 15, 17, 21, 22, 28, 30, 40,
50 and 60), 1 ml solution was replaced with 1 ml fresh PBS. The
concentration of BSA released into the obtained solution was
determined at each time point using a BCA kit.
Experimental rat model of ONFH
In total, 40 male Sprague-Dawley rats (age, 10
weeks; weight, 300-320 g; license number, SCXK 2009-0004; Hunan SJA
Laboratory Animal Co., Ltd.) were raised for 1 week at the
Laboratory Animal Center, The Second Affiliated hospital of
Zhejiang University School of Medicine (Zhejiang, China) according
to standard feeding conditions (temperature, 18-26˚C; humidity,
40-70%; light-dark cycle, 12:12 h; access to water and food, ad
libitum) with 5 rats per cage. The rats were randomly divided
into four groups (n=10): Normal group (sham-operated rats); Model
group (rats with osteonecrosis); LIPUS group (rats treated with
LIPUS); and BMP-2 + LIPUS group (rats implanted with the
BMP-2-loaded PLGA-PLLA/PLGA/PCL composite scaffold treated with
LIPUS). All rats were provided with a standardized diet and were
allowed unrestricted activities.
ONFH establishment
The osteonecrosis model was established in a total
of 30 randomly selected rats (all rats apart from rats in the
Normal group). In brief, the rats were intraperitoneally injected
with lipopolysaccharide (20 µg/kg) on day 0 and 1, once per day at
an interval of 24 h. Furthermore, both gluteal muscles were
alternately injected with methylprednisolone (20 mg/kg) on days 2,
3 and 4 once a day at an interval of 24 h. At present, controversy
remains regarding the use of hormones alone or in combination with
adjuvants, including methylprednisolone and endotoxin (20). To ensure successful establishment of
the model, methylprednisolone was administered in conjunction with
lipopolysaccharide (20 µg/kg) to induce hormone-related
osteonecrosis (21). In
sham-operated rats, the abdominal cavity and gluteal muscles were
injected with a 0.9% sodium chloride solution (normal saline) at
the same time points as the model rats as aforementioned.
LIPUS and BMP-2 intervention
Rats in the LIPUS and the BMP-2 + LIPUS groups were
subjected to bilateral femoral head LIPUS intervention (30 mW/cmz;
Cosmogamma® US13; AC International) two weeks after
model establishment, for 20 min per day for 12 weeks until
sacrifice. For the rats in the BMP-2 + LIPUS group, the scaffolds
were inserted into the rats 24 h prior to LIPUS treatment. The rats
were first anesthetized with pentobarbital sodium (40 mg/kg). The
femoral head area was first sterilized, where an incision was made
to expose the femoral head. A hole 3 mm in diameter was then made
on the posteromedial surface of the femoral neck using a drill. The
composite scaffold was made into a cylinder with a diameter of ~3
mm and a thickness of 3 mm, which were then implanted into the
defect of rats prior to suture. Before irradiation, all rats were
anaesthetized by an intraperitoneal injection of pentobarbital
sodium (40 mg/kg). In detail, the hip joint was located to
accurately determine the irradiation points by shaking the
bilateral hind thighs. Following fur removal, the probe was smeared
with a coupling agent to ensure close skin adherence. The dietary,
mental and physical changes of all rats were monitored weekly.
Healthy mental status was defined as actively inquisitive but
without biting, mental depression, drowsiness or laziness. The
fasting body weights of all the rats were measured using an
electronic scale before modeling every week. The rats were treated
for 12 weeks. Throughout the study, if any rats were adjudged to be
suffering in accordance with the items of ‘endpoints in animal
study proposals’ in the ‘animal care and use procedures’ section of
the National Institutes of Health guidelines (22), they would be immediately sacrificed
by an intraperitoneal injection of pentobarbital (150 mg/kg).
Micro-CT scan
All rats were euthanized by an intraperitoneal
injection of pentobarbital (150 mg/kg) 12 weeks after ONFH
establishment. The femoral head of the rats was extracted and fixed
with 10% paraformaldehyde at 4˚C for 4 h before the bone
architecture was observed using a micro-CT scanner (scanning
resolution, 45 µm; GE eXplore 120 model; GE Healthcare). The
central region of the femoral head was set as the region of
interest. The parameters measured were as follows: Bone mineral
density (BMD), bone volume/total volume (BV/TV), trabecular number
(Tb.N), trabecular thickness (Tb.Th) and trabecular separation
(Tb.Sp).
Monitoring of biomechanical
variables
The proximal femoral specimen was mounted on an
experimental platform of a universal mechanical tester (ZwickRoell
GmBH). The femur was kept moist during the experiment. A steel
needle was placed directly above the femoral head and horizontal to
the proximal femoral force line. A three-point bending test was
performed on a three-point bending device to determine the maximum
bending load and displacement of the femoral head by applying the
following parameters: Maximum loading weight, 20 kg; span at both
ends, 20 m; and loading speed, 5 mm/min.
Measurement of calcium (Ca) and
phosphorus (P) content
The biomechanically measured femur was dried at 95˚C
and ground into a powder. A total of 0.1 g accurately weighed femur
powder was placed in a digestion tube, soaked in 8 ml nitric acid
overnight at room temperature and detached according to the
following procedure: Stage I at 1,600 W, heated for 5 min and
maintained at 120˚C for 2 min; and stage II at 1,600 W, heated for
8 min and maintained at 185˚C for 25 min. The decomposition
solution obtained was then brought to 50 ml and diluted 50 times in
water. The Ca and P contents in the femur were determined (MARS 5
Digestion Microwave System; CEM Microwave Technology, Ltd.) under
the following operating conditions: Radio-frequency power, 1,150 W;
auxiliary gas flow rate, 0.5 l/min; flushing pumping rate, 50
r/min; analysis pumping rate, 50 r/min; stable pumping time, 5 sec;
and maximum integration time of the signal, 30 sec.
Angiogenesis assay
The femoral head tissues were fixed with 10%
formaldehyde at 4˚C overnight and decalcified with 10% EDTA at room
temperature until the tissue becomes soft. Paraffin-embedded
tissues were then sliced into 5-µm thick sections, which was then
incubated at 60˚C overnight. Prior to dewaxing, the tissue sections
were placed at room temperature for 60 min, following which they
were rehydrated in xylene for 20 min and then a descending gradient
of anhydrous ethanol (95% ethanol and 70% ethanol, 5 min
respectively). Endogenous catalase was then blocked using 3%
H2O2 for 15 min at room temperature. This is
followed by the antigen retrieval step, in which the tissue
sections were incubated in 0.1 M citric acid solution with pH 6.0,
at 100˚C for 20 min. The tissue sections were then blocked using
10% goat serum (cat. no. ab138478; Abcam) at 37˚C for 30 min,
following which they were incubated with anti-α-SMA antibody
(1:100; cat. no. BM0002; Wuhan Boster Biological Technology, Ltd.),
overnight at 4˚C. The next day, the sections were incubated with
anti IgG secondary antibody (1:500; cat. no. G-21040; Thermo Fisher
Scientific, Inc.) at 37˚C for 30 min, following which they were
treated with reagents in the DAB Kit according to manufacturer's
protocols (Wuhan Boster Biological Technology, Ltd.). The tissues
sections were then stained with hematoxylin for 30 sec at room
temperature and dehydrated using an ascending alcohol gradient
followed by xylene. After mounting the sections using neutral
resin, staining was then observed under a light microscope
(magnification, x200, Olympus CX41; Olympus Corporation). Five
sections were randomly selected for the assessment of vessel number
and diameter.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues in accordance
with the specifications of TRIzol® reagent (Thermo
Fisher Scientific, Inc.) and 15 ng the RNA was reverse transcribed
into cDNA using PrimeScript™ RT reagent Kit with gDNA Eraser
(Takara Biotechnology Co., Ltd.) according to manufacturer's
protocol (42˚C for 15 min and 85˚C for 5 sec). qPCR was
subsequently conducted in an ABI 7500 qPCR instrument using
SYBR® Premix Ex Taq™ II (Takara Bio, Inc.). The
thermocycling conditions were as follows: Initial denaturation at
95˚C 1 min; followed by 40 cycles of 95˚C 10 sec and 62˚C for 25
sec. The primer sequences used for qPCR were synthesized by The
Beijing Genomics Institute (Beijing, China) and are listed in
Table I. The expression of target
genes was calculated using the 2-ΔΔCq method (23) with GAPDH as the internal
reference.
 | Table IPrimers for reverse transcription
quantitative polymerase chain reaction. |
Table I
Primers for reverse transcription
quantitative polymerase chain reaction.
| Gene | Primer
sequence |
|---|
| BMP-2 | F:
5'-GGGACCCGCTGTCTTCTAGT-3' |
| | R:
5'-TCAACTCAAATTCGCTGAGGAC-3' |
| TGF-β1 | F:
5'-CCACCTGCAAGACCATCGAC-3' |
| | R:
5'-CTGGCGAGCCTTAGTTTGGAC-3' |
| RUNX2 | F:
5'-GCACAAACATGGCCAGATTCA-3' |
| | R:
5'-AAGCCATGGTGCCCGTTAG-3' |
| Col I | F:
5'-GACATGTTCAGCTTTGTGGACCTC-3' |
| | R:
5'-GGGACCCTTAGGCCATTGTGTA-3' |
| Osteocalcin | F:
5'-GCAATAAGGTAGTGAACAGACTCC-3' |
| | R:
5'-CCATAGATGCGTTTGTAGGCGG-3' |
| GAPDH | F:
5'-CATGAGAAGTATGACAACAGCCT-3' |
| | R:
5'-AGTCCTTCCACGATACCAAAGT-3' |
Western blot analysis
Total protein was extracted from the cell lysates or
tissues using 1% Triton X-100 solution, which was quantified using
a BCA kit (Sigma-Aldrich; Merck KGaA). The protein samples (50 µg
per lane) were separated by 10% SDS-PAGE was transferred onto
polyvinylidene fluoride membranes. The membrane was subsequently
blocked with 50 g/l skim milk powder at room temperature for 1 h
and washed three times with Tris-buffered saline supplemented with
0.1% Tween-20 (TBS-T). Afterwards, the membrane was incubated
overnight at 4˚C with the following primary rabbit anti-rat
antibodies (all from Abcam): BMP-2 (1:1,000; cat. no. ab6285),
transforming growth factor-β1 (TGF-β1; 1:100; cat. no. ab64715),
runt-related transcription factor 2 (RUNX2; 1:1,000; cat. no.
ab76956), Collagen I (Col I; 1:3,000; cat. no. ab6308), Osteocalcin
(OCN; 1:500; cat. no. ab93876) and β-actin (1:5,000; cat. no.
ab8227). The membranes were then washed a further three times with
TBS-T and incubated with a horseradish peroxidase-conjugated
secondary antibody against Immunoglobulin G (1:3,000; ab205718;
Abcam) for 1 h at room temperature, followed by further washing
with TBS-T. Protein signals were detected using an enhanced
chemiluminescence fluorescence detection kit (EMD Millipore) and
quantified using Image Quant software (version 5.2, GE Healthcare
Life Sciences).
Alizarin red staining
MC3T3-E1 cells (Type Culture Collection of the
Chinese Academy of Sciences), usually cultured in 90% α-MEM media
(cat. no. 11900024; Gibco; Thermo Fisher Scientific, Inc.)
containing 1.5 g/l NaHCO3, 43.2 mg/l inositol (Sinopharm
Chemical Reagent Co., Ltd.), 8.82 mg/l folic acid (Sinopharm
Chemical Reagent Co., Ltd.) and 7.8 mg/l β-mercaptoethanol
(Sigma-Aldrich; Merck KGaA) and 10% FBS at 37˚C and 5%
CO2. They were seeded into 12-well plates at a density
of 5x104 cells/well. Osteogenic induction medium (300
µl; cat. no. PH-B-002; Puhe Biotechnology Co., Ltd.; https://puhe.biomart.cn/) containing the PLLA/PLGA/PCL
nano-scaffolds, PLGA-PLLA/PLGA/PCL composite scaffolds and
BMP-2-PLGA-PLLA/PLGA/PCL composite scaffolds was then added to the
cells, followed by incubation for 14 days at 37˚C. The medium was
removed at the 14th day. The cells were washed twice with PBS,
fixed in 4% polyformaldehyde at 4˚C for 4 h, washed once with
deionized water and stained with 2% alizarin red (20 mg/ml,
pH=4.1-4.3) at room temperature for 20 min. Any extra alizarin red
was subsequently removed using deionized water followed by
fluorescent microscopy (magnification: x200; Model: IX71; Olympus
Corporation).
Statistical analysis
The experimental data were analyzed using SPSS 21.0
software (IBM Corp.). Measurement data are presented as the mean ±
standard deviation. Comparisons of two groups of data conforming to
normal distribution were conducted using Student's t-test. Data
between multiple groups were compared using one-way analysis of
variance followed by Tukey's post hoc test. P<0.05 was
considered indicate a statistically significant difference.
Results
Characterization of BMP-2-loaded PLGA
microspheres incorporated into PLLA/PLGA/PCL composite
scaffolds
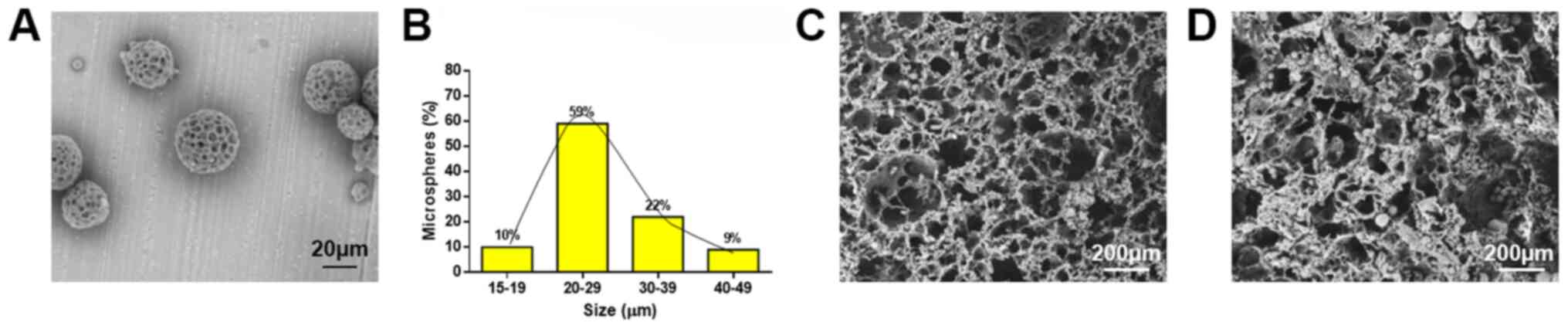
The surface morphology of the BMP-2-loaded PLGA
microspheres was observed using electron microscopy. The
BMP-2-loaded PLGA microspheres exhibited relatively spherical
shapes (Fig. 1A). The sizes of the
particles were relatively uniform, with 81% of the PLGA
microspheres presented with sizes between 20 and 39 µm (Fig. 1B). According to the formula, the
PLGA microspheres could load 0.77±0.62% of the BMP-2 protein with
an encapsulation efficiency of 74.87±0.58%, suggesting that the
obtained microspheres could encapsulate more protein. However, the
average protein loading rate was lower could be due to the large
quantity of microspheres prepared. Under electron microscopic
observation, the PLLA/PLGA/PCL scaffolds presented with a
microscopically porous structure with good connectivity between the
channels (Fig. 1C), which was
suitable for cell adhesion and growth. Therefore, the PLLA/PLGA/PCL
scaffold could be used as a biomimetic scaffold. After the
BMP-2-loaded PLGA microspheres were incorporated into the
PLLA/PLGA/PCL scaffold, it was observed that the PLGA microspheres
were dispersed onto the surface and into some channels of the
PLLA/PLGA/PCL scaffold structure (Fig.
1D).
PLLA/PLGA/PCL composite scaffold
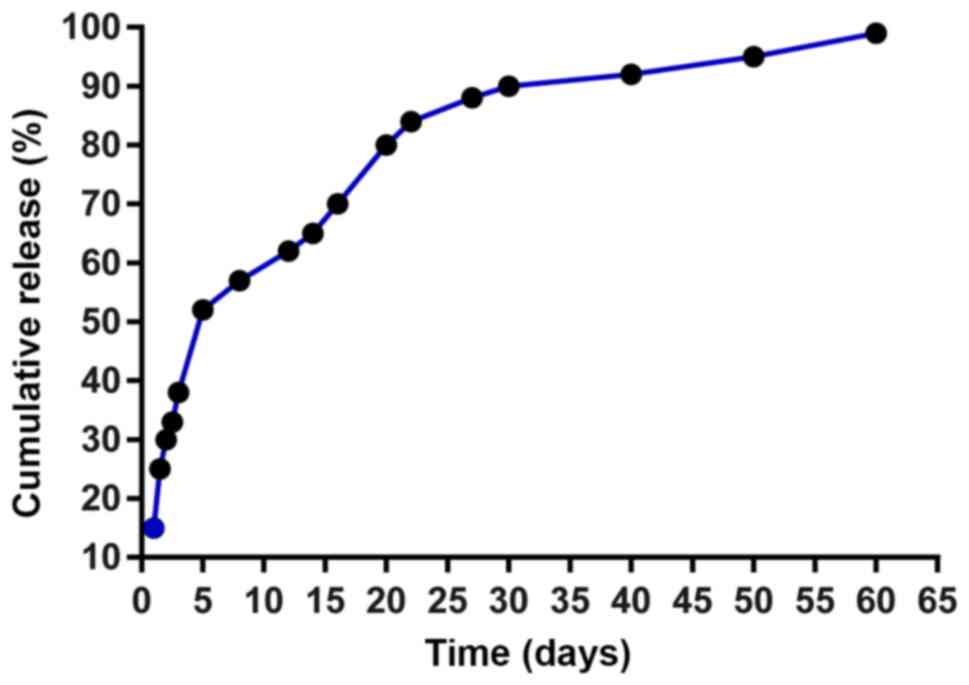
releases BSA in a sustained manner
The release of BSA from PLGA microspheres was rapid,
as >50% of BSA was already released within five days (Fig. 2). This sudden release of protein at
the initial stage could be attributed to the small amount of
protein adsorbed on the surface of microspheres. The release of BSA
became slower from day 5 onwards, and ~90% of the BSA were released
after 1 month. The reason for this observation was that the release
of the BSA loaded inside the PLGA microspheres required a
relatively long channel or a break in the matrix barrier. The
results indicated that PLGA microspheres served as a microcarrier
of protein.
Delivery of BMP-2 using the
PLGA-PLLA/PLGA/PCL composite scaffold combined with LIPUS relieves
steroid-induced femoral head necrosis
Following model construction, behavioral activities,
diet, feces, mental status and body weights of the rats were
observed regularly. The rats in the normal group exhibited good
mental states, movement, natural hair luster and no hair loss.
Before LIPUS or BMP-2 treatment, the rats in the model, LIPUS and
LIPUS + BMP-2 groups presented with thin and sloppy stool, mental
depression, anorexia and emaciation after 3 days of model
establishment. However, these symptoms disappeared after 2 weeks
and the rats recovered. No rats were found dead or required
immediate euthanasia during the experimental process. Micro-CT scan
results revealed that the BMD, BV/TV, Tb.N and Tb.Th of the rats in
the model group were significantly lower compared with those in the
normal group (P<0.05), whereas the rats in the LIPUS and LIPUS +
BMP-2 groups exhibited significantly higher BMD, BV/TV, Tb.N and
Tb.Th compared with those in the model group (P<0.05). The Tb.Sp
of rats in the model group was significantly higher compared with
those in the normal group (P<0.05), whereas the Tb.Sp of rats in
the LIPUS and LIPUS + BMP-2 groups were significantly lower
compared with those in the model group (P<0.05; Table II). These results indicated that
LIPUS partially alleviated steroid-induced ONFH and that the use of
BMP-2-loaded PLGA-PLLA/PLGA/PCL composite scaffolds combined with
LIPUS was also conducive to the recovery of this disease.
 | Table IIMicro-architectural parameters of
femoral head in normal mice or mice with osteonecrosis. |
Table II
Micro-architectural parameters of
femoral head in normal mice or mice with osteonecrosis.
| Parameter | Normal | Model | LIPUS | LIPUS±BMP-2 |
|---|
| BMD (mg/cc) | 648.94±39.25 |
564.91±17.91a |
619.94±21.25b |
628.94±27.25b |
| BV/TV | 59.29±1.37 |
37.93±1.04a |
49.29±1.13b |
53.29±1.18b |
| Tb.N (1/mm) | 2.95±0.14 |
2.29±0.21a |
2.65±0.10b |
2.75±0.11b |
| Tb.Sp (µm) | 174.36±53.30 |
262.29±81.50a |
201.36±58.70b |
185.36±55.40b |
| Tb.Th (µm) | 204.44±12.13 |
158.32±9.23a |
174.44±10.47b |
187.44±11.83b |
Delivery of BMP-2 by
PLGA-PLLA/PLGA/PCL composite scaffolds in addition to LIPUS
improves load-carrying capacity
By measuring the kinesiological parameters, it was
found that the maximum bending load and displacement of rats in the
model group were significantly lower compared with those in the
normal group (P<0.05). In contrast, the maximum bending load and
displacement of the femoral head were significantly higher in the
LIPUS + BMP-2 group compared with the model group (P<0.05;
Table III). Together, these data
suggested that the load-carrying capacity of the femoral head of
rats with steroid-induced ONFH was inferior compared with normal
rats, which can be alleviated by LIPUS treatment and additively by
the delivery of BMP-2 by PLGA-PLLA/PLGA/PCL composite
scaffolds.
 | Table IIIKinematical parameters of femoral
head in normal mice or mice with osteonecrosis. |
Table III
Kinematical parameters of femoral
head in normal mice or mice with osteonecrosis.
| Parameter | Normal | Model | LIPUS | LIPUS + BMP-2 |
|---|
| Maximum bending
load (N) | 145.2±22.0 |
124.6±8.0a | 134.1±13.7 |
141.0±11.2b |
| Maximum
displacement (cm) | 0.53±0.05 |
0.42±0.04a | 0.46±0.04 |
0.50±0.06b |
Delivery of BMP-2 by
PLGA-PLLA/PLGA/PCL composite scaffold combined with LIPUS
accelerates bone formation
During the bone formation process, calcium and
phosphorus in the blood are deposited in bone tissues, in a process
known as osteoid calcification, which is key to bone formation
(24). By measuring Ca and P
contents in rat femoral head samples it was found that those in the
model group were significantly lower compared with the normal group
(P<0.05). In comparison with the model group, the femoral Ca and
P contents of were significantly higher in the LIPUS + BMP-2 group
(P<0.05; Table IV). These
findings suggest that the implantation of BMP-2-loaded
PLGA-PLLA/PLGA/PCL composite scaffold plus LIPUS treatment
accelerated bone formation in rats with steroid-induced ONFH.
 | Table IVKinematical parameters of femoral
head in normal mice or mice with osteonecrosis. |
Table IV
Kinematical parameters of femoral
head in normal mice or mice with osteonecrosis.
| Cotent | Normal | Model | LIPUS | LIPUS + BMP-2 |
|---|
| Femoral Ca
(mg/kg) | 261.1±10.3 |
232.5±8.0a | 246.6±10.1 |
253.7±7.7b |
| Femoral P
(mg/kg) | 162.8±9.2 |
134.4±7.5a | 148.4±8.7 |
154.6±9.0b |
Delivery of BMP-2 by
PLGA-PLLA/PLGA/PCL composite scaffold in addition to LIPUS
accelerates bone angiogenesis
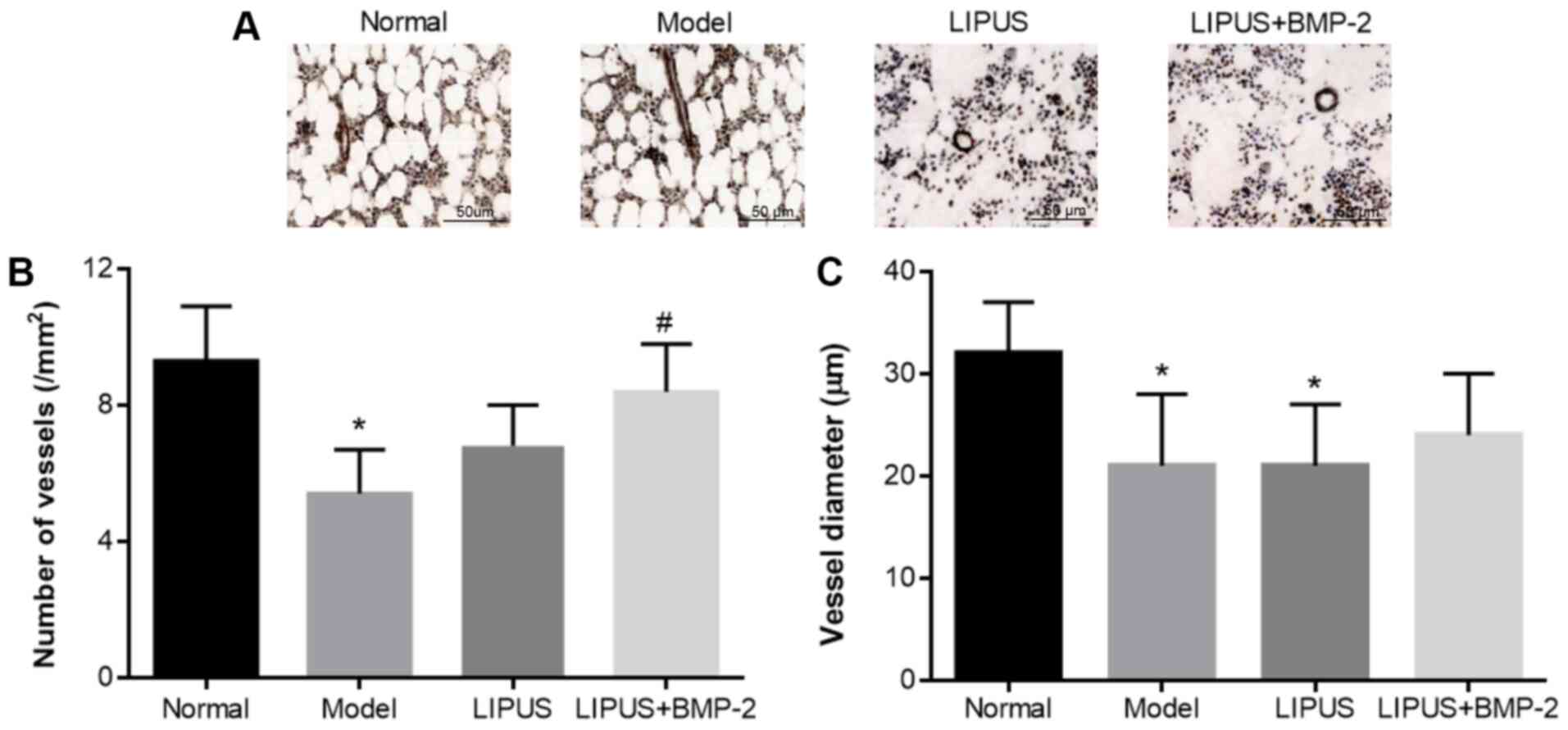
Blood vessels were visualized in femoral head tissue
sections stained with α-SMA. The number and diameter of blood
vessels were significantly reduced in the model group compared with
the normal group (P<0.05), whereas the number of blood vessels
was dramatically increased in the LIPUS + BMP-2 group compared with
the model group (P<0.05; Fig.
3). The diameter of the blood vessels was slightly increased in
the LIPUS + BMP-2 group compared with that in the model group but
without statistical significance (Fig.
3C). These findings suggest that angiogenesis was promoted by
the delivery of BMP-2 by PLGA-PLLA/PLGA/PCL composite scaffolds
combined with LIPUS treatment.
Delivery of BMP-2 by
PLGA-PLLA/PLGA/PCL composite scaffold in addition to LIPUS enhances
osteocyte differentiation
The mRNA and protein expression of BMP-2, TGF-β1,
RUNX2, Col I and OCN in tissues were subsequently determined using
RT-qPCR and western blot analysis. The expression of BMP-2, TGF-β1,
RUNX2, Col I and OCN was significantly lower in the model group
compared that in the normal group (P<0.05), which was
significantly reversed by either LIPUS alone or LIPUS + BMP-2
(P<0.05; Fig. 4A and B). The highest level of BMP-2 was observed
in the LIPUS + BMP-2 group, suggesting that BMP-2 was released
following composite scaffold implantation, which subsequently
induced bone formation. According to the results of alizarin red
staining (Fig. 4C) in MC3T3-E1
cells, calcium deposition was markedly higher in the
BMP-2-PLGA-PLLA/PLGA/PCL composite scaffolds group, compared with
those treated with PLLA/PLGA/PCL nano-scaffolds and
PLGA-PLLA/PLGA/PCL composite scaffolds. In conclusion, BMP-2
delivery through the PLGA-PLLA/PLGA/PCL composite scaffold in
addition to LIPUS treatment upregulated the expression of the
osteoblast-specific transcription factors RUNX2, Col I and OCN.
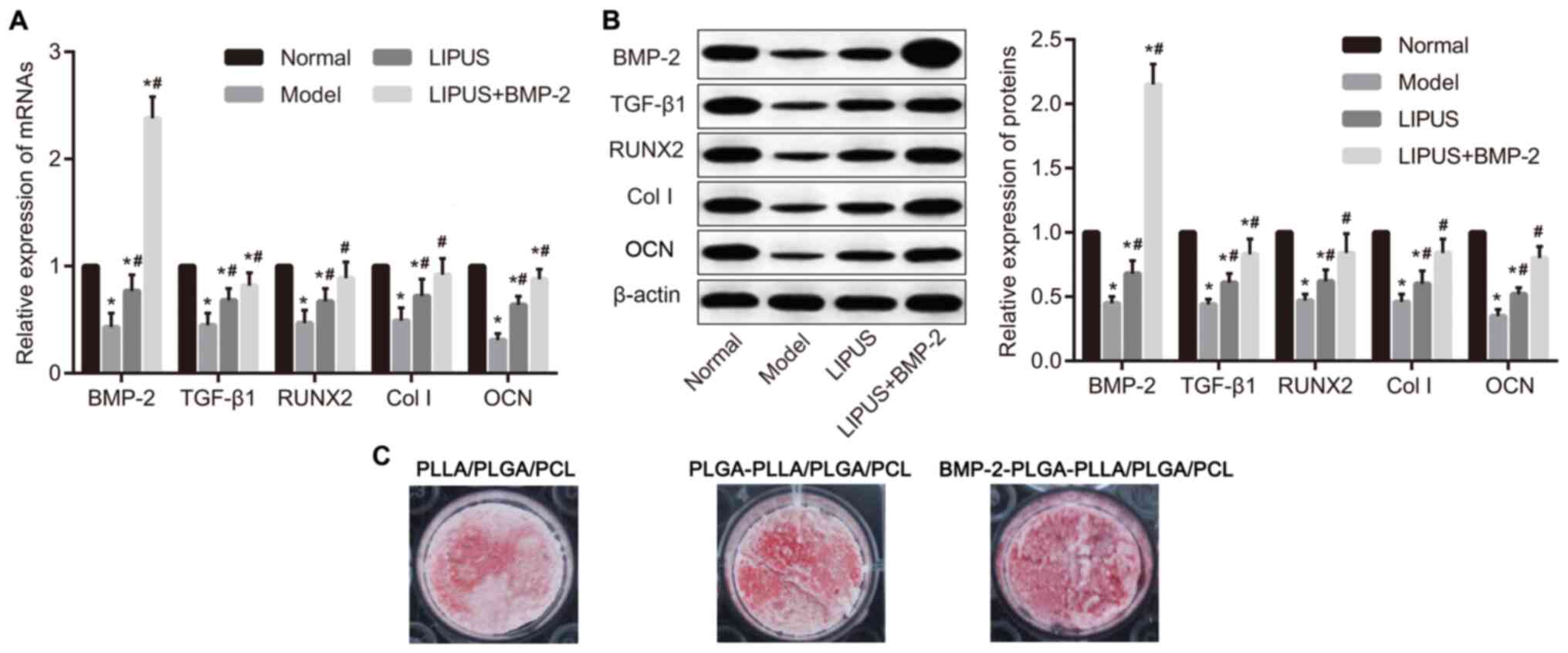 | Figure 4Delivery of BMP-2 by
PLGA-PLLA/PLGA/PCL composite scaffold increases TGF-β1, RUNX2, Col
I and OCN expression combined with LIPUS treatment. (A) Measurement
of BMP-2, TGF-β1, RUNX2 and Col I and OCN mRNA expression by
reverse transcription-quantitative PCR, performed using tissues.
(B) Measurement of BMP-2, TGF-β1, RUNX2, Col I and OCN protein
expression by western blotting, performed using tissues. (C)
Measurement of calcium deposition by alizarin red staining,
performed using MC3T3-E1 cell lines. *P<0.05 vs.
Normal and #P<0.05 vs. Model. BMP-2, bone
morphogenetic protein-2; PLGA, polylactic-co-glycolic acid; PLLA,
poly-L-lactic acid; PCL, poly-ε-caprolactone; TGF-β1, transforming
growth factor β1; RUNX2, runt-related transcription factor 2; Col
I, type I collagen; OCN, osteocalcin. |
Discussion
The sustained release of BMP-2 from nano-scaffolds
is capable of enhancing bone regeneration, resulting in superior
bone quality compared with the direct administration of
BMP-2(25). In the present study
BMP-2-loaded PLGA microspheres were successfully incorporated into
PLLA/PLGA/PCL composite scaffolds. The data suggested that BMP-2
delivered using PLLA/PLGA/PCL composite scaffolds promoted bone
formation and repair in rat ONFH models in combination with
LIPUS.
In the present study, it was determined that LIPUS
alone or both LIPUS + BMP-2-loaded scaffolds increased BMD, BV/TV,
Tb.N and Tb.Th but reduced Tb.Sp in rats with steroid-induced ONFH
and improved bone load-carrying capacity. In addition, it was
demonstrated that LIPUS partially alleviated steroid-induced
femoral head necrosis, the effect of which was potentiated
additively when combined with BMP-2 delivery using the
PLGA-PLLA/PLGA/PCL composite scaffold. In a previous study, a
porous PLGA/TCP composite scaffold incorporated with icaritin could
repair bone defects and prevent hip joint collapse in a
steroid-induced rabbit model of osteonecrosis (26), whilst 2.0% nanosilver particle-PLGA
composite grafts incorporated with BMP-2 contributed to the healing
of femoral defects as a result of infection within 12 weeks with
antibacterial activity in another study (27). Additionally, the implantation of PCL
scaffolds loaded with BMP-2/BMP-7 nanocapsules accelerated the
healing of iliac crest defects in rats (28), whereas BMP-2 conjugation into
three-dimensional PCL scaffolds has been previously revealed to be
a feasible approach in stimulating the osteoinductive capability of
bone marrow stromal cells (29).
PLLA nanosheets also had a good capacity for use as a
sustained-release vehicle of rhBMP-2, enhancing bone regeneration
(30). Considering the capability
of PLGA, PCL and PLLA as nanocapsules in BMP-2 delivery, the
present study first constructed a delivery system of BMP-2 using a
PLLA/PLGA/PCL composite scaffold and provided evidence that the
release of BMP-2 from the PLLA/PLGA/PCL composite scaffold
potentiated protective effects against steroid-induced ONFH.
In the present study, LIPUS combined with the
release of BMP-2 from the PLLA/PLGA/PCL composite scaffold was
observed to increase Ca and P contents and upregulate the
expression of the osteoblast-specific transcription factor RUNX2,
Col I and OCN, further demonstrating its inductive role in bone
formation and repair. LIPUS has been previously shown to enhance
bone repair through osteogenesis and neovascularization in
steroid-associated osteonecrosis (31). Mechanistically, LIPUS stimulates
bone regeneration by exerting pro-osteogenic effects by promoting
the expression of genes associated with osteogenesis in
osteoblasts, including RUNX2, alkaline phosphatase and osteorix
(32,33). Col I stimulates the proliferation
and osteogenesis of human mesenchymal stem cells (34). Increased expression of OCN is
indicative of the therapeutic effects of deferoxamine on
early-stage ONFH in a rabbit model (35), consistent with the present study. It
has been previously demonstrated that daily LIPUS can stimulate the
release of BMPs in the rat clonal cell line ROS 17/2.8 during
osteogenic differentiation processes (36), whilst another study revealed that
LIPUS enhanced rhBMP-2-induced bone formation (37). LIPUS induces angiogenesis through
the upregulation of vascular endothelial growth factor (VEGF)
expression, expediting osteoporotic fracture healing (38). Data from the present study suggest
that blood vessels were more numerous and blood vessel diameters
were greater following LIPUS treatment in combination with the
implantation of BMP-2-containing PLGA-PLLA/PLGA/PCL composite
scaffolds, implicating increased angiogenesis. As previously
reported, LIPUS enhances angiogenesis by increasing VEGF after
acute myocardial infarction (39).
Additionally, BMP-2 treatment stimulated angiogenesis in human
endothelial progenitor cells (40).
The incorporation of either BMP-2 or VEGF inside porous scaffolds
has been demonstrated to induce both angiogenesis and osteogenesis
in a previous study (41). After
osteonecrosis, the enlarged adipocytes caused blood vessel
compression, resulting in reduced local blood supply and blood
vessel diameter. Following LIPUS treatment and BMP-2 delivery,
angiogenesis was induced. Since the newly formed blood vessels were
small, the diameter of the blood vessels in this group was not
markedly different from that of the blood vessels in the model
group.
In the present study, TGF-β signaling was induced
following the implantation of the BMP-2-containing PLLA/PLGA/PCL
composite scaffold plus LIPUS treatment. The TGF-β signaling
pathway has also been demonstrated to be an osteoclast-specific
signaling pathway which can also induce bone formation (42). BMP and TGF-β are two important
factors associated with the proliferation and differentiation of
osteoblasts (43), such that the
interplay between TGF-β and BMP signaling has been highlighted in
osteoblast differentiation and bone formation (44,45).
Since TGF-β has been shown to enhance BMP-9-stimulated osteogenesis
of mesenchymal stem cells in a previous study (46), it can be speculated that TGF-β
signaling may participate in the mechanism underlying the
protection of the BMP-2 delivered from PLLA/PLGA/PCL composite
scaffolds against ONFH. However, further studies are necessary to
test this hypothesis.
It should be noted that the number of samples in the
present study was limited according to the Animal Ethics Committee
of The Second Affiliated Hospital of Zhejiang University School of
Medicine, suggesting the need for further research with a larger
sample size. The effects of bare scaffolds on the alleviation of
steroid-induced ONFH were acquiescently ruled out in the present
study and should be seriously considered in future
investigations.
Taken together, results in the present study
demonstrate that the PLLA/PLGA/PCL composite scaffold is able to
deliver BMP-2 into rats with steroid-induced ONFH, implicating
PLLA/PLGA/PCL composite scaffold to be a potential carrier for
BMP-2 delivery. In addition, the present study also demonstrated
the combined application of BMP-2 and LIPUS contributed to bone
formation and repair for steroid-induced ONFH. This strategy can be
potentially applied for future clinical applications.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Zhejiang
Provincial Natural Science Foundation of China (grant no.
LQ18H070002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HXZ and CHZ conceived, designed the current study,
and developed the methodology. XZC, ZLS and XBY acquired and
analyzed data. HXZ reviewed and revised the manuscript. The final
version of the manuscript was read and approved by all authors.
Ethical approval and consent to
participate
The present study was approved by The Second
Affiliated Hospital, Zhejiang University School of Medicine
(approval no. A2018014; Zhejiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tripathy SK, Goyal T and Sen RK:
Management of femoral head osteonecrosis: Current concepts. Indian
J Orthop. 49:28–45. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Issa K, Pivec R, Kapadia BH, Banerjee S
and Mont MA: Osteonecrosis of the femoral head: The total hip
replacement solution. Bone Joint J. 95-B (11 Suppl A):S46–S50.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mont MA, Cherian JJ, Sierra RJ, Jones LC
and Lieberman JR: Nontraumatic osteonecrosis of the femoral head:
Where do we stand today? A ten-year update. J Bone Joint Surg Am.
97:1604–1627. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zalavras CG and Lieberman JR:
Osteonecrosis of the femoral head: Evaluation and treatment. J Am
Acad Orthop Surg. 22:455–464. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Powell C, Chang C, Naguwa SM, Cheema G and
Gershwin ME: Steroid induced osteonecrosis: An analysis of steroid
dosing risk. Autoimmun Rev. 9:721–743. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Powell C, Chang C and Gershwin ME: Current
concepts on the pathogenesis and natural history of steroid-induced
osteonecrosis. Clin Rev Allergy Immunol. 41:102–113.
2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yan SG, Huang LY and Cai XZ: Low-intensity
pulsed ultrasound: A potential non-invasive therapy for femoral
head osteonecrosis. Med Hypotheses. 76:4–7. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Atesok K, Fu FH, Wolf MR, Ochi M, Jazrawi
LM, Doral MN, Lubowitz JH and Rodeo SA: Augmentation of
tendon-to-bone healing. J Bone Joint Surg Am. 96:513–521.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Agarwal R and Garcia AJ: Biomaterial
strategies for engineering implants for enhanced osseointegration
and bone repair. Adv Drug Deliv Rev. 94:53–62. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Balmayor ER: Targeted delivery as key for
the success of small osteoinductive molecules. Adv Drug Deliv Rev.
94:13–27. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Salazar VS, Gamer LW and Rosen V: BMP
signalling in skeletal development, disease and repair. Nat Rev
Endocrinol. 12:203–221. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang C, Zang H and Zhou D: Bone
morphogenetic protein-2 exhibits therapeutic benefits for
osteonecrosis of the femoral head through induction of cartilage
and bone cells. Exp Ther Med. 15:4298–4308. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun W, Li Z, Gao F, Shi Z, Zhang Q and Guo
W: Recombinant human bone morphogenetic protein-2 in debridement
and impacted bone graft for the treatment of femoral head
osteonecrosis. PLoS One. 9(e100424)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lo KW, Ulery BD, Ashe KM and Laurencin CT:
Studies of bone morphogenetic protein-based surgical repair. Adv
Drug Deliv Rev. 64:1277–1291. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
King WJ and Krebsbach PH: Growth factor
delivery: How surface interactions modulate release in vitro and in
vivo. Adv Drug Deliv Rev. 64:1239–1256. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fitzgerald R, Bass LM, Goldberg DJ,
Graivier MH and Lorenc ZP: Physiochemical characteristics of
poly-L-lactic acid (PLLA). Aesthet Surg J. 38 (Suppl 1):S13–S17.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Martins AF, Facchi SP, da Camara PCF,
Camargo SEA, Camargo CHR, Popat KC and Kipper MJ: Novel
poly(ε-caprolactone)/amino-functionalized tannin electrospun
membranes as scaffolds for tissue engineering. J Colloid Interface
Sci. 525:21–30. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Barthold SW, Bayne KA, Davis MA, Bayne K
and Davis M: Guide for the care and use of laboratory animals.
Publication no. 85-23(rev.). 327:963–965. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Mercado AE, Ma J, He X and Jabbari E:
Release characteristics and osteogenic activity of recombinant
human bone morphogenetic protein-2 grafted to novel self-assembled
poly(lactide-co-glycolide fumarate) nanoparticles. J Control
Release. 140:148–156. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Drescher W, Bünger MH, Weigert K, Bünger C
and Hansen ES: Methylprednisolone enhances contraction of porcine
femoral head epiphyseal arteries. Clin Orthop Relat Res. 112–117.
2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dong Y, Li Y, Huang C, Gao K and Weng X:
Systemic application of teriparatide for steroid induced
osteonecrosis in a rat model. BMC Musculoskelet Disord.
16(163)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mcpherson C: Regulation of animal care and
research? NIH's opinion. J Anim Sci. 51:492–496. 1980.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Florencio-Silva R, Sasso GR, Sasso-Cerri
E, Simões MJ and Cerri PS: Biology of bone tissue: Structure,
function, and factors that influence bone cells. Biomed Res Int.
2015(421746)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tian H, Du J, Wen J, Liu Y, Montgomery SR,
Scott TP, Aghdasi B, Xiong C, Suzuki A, Hayashi T, et al:
Growth-factor nanocapsules that enable tunable controlled release
for bone regeneration. ACS Nano. 10:7362–7369. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qin L, Yao D, Zheng L, Liu WC, Liu Z, Lei
M, Huang L, Xie X, Wang X, Chen Y, et al: Phytomolecule icaritin
incorporated PLGA/TCP scaffold for steroid-associated
osteonecrosis: Proof-of-concept for prevention of hip joint
collapse in bipedal emus and mechanistic study in quadrupedal
rabbits. Biomaterials. 59:125–143. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zheng Z, Yin W, Zara JN, Li W, Kwak J,
Mamidi R, Lee M, Siu RK, Ngo R, Wang J, et al: The use of BMP-2
coupled-nanosilver-PLGA composite grafts to induce bone repair in
grossly infected segmental defects. Biomaterials. 31:9293–9300.
2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yilgor P, Yilmaz G, Onal MB, Solmaz I,
Gundogdu S, Keskil S, Sousa RA, Reis RL, Hasirci N and Hasirci V:
An in vivo study on the effect of scaffold geometry and growth
factor release on the healing of bone defects. J Tissue Eng Regen
Med. 7:687–696. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang H, Migneco F, Lin CY and Hollister
SJ: Chemically-conjugated bone morphogenetic protein-2 on
three-dimensional polycaprolactone scaffolds stimulates osteogenic
activity in bone marrow stromal cells. Tissue Eng Part A.
16:3441–3448. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Huang KC, Yano F, Murahashi Y, Takano S,
Kitaura Y, Chang SH, Soma K, Ueng SWN, Tanaka S, Ishihara K, et al:
Sandwich-type PLLA-nanosheets loaded with BMP-2 induce bone
regeneration in critical-sized mouse calvarial defects. Acta
Biomater. 59:12–20. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhu H, Cai X, Lin T, Shi Z and Yan S:
Low-intensity pulsed ultrasound enhances bone repair in a rabbit
model of steroid-associated osteonecrosis. Clin Orthop Relat Res.
473:1830–1839. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gleizal A, Ferreira S, Lavandier B, Simon
B, Béziat JL and Béra JC: The impact of low intensity pulsed
ultrasound on mouse skull bone osteoblast cultures. Rev Stomatol
Chir Maxillofac. 111:280–285. 2010.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
33
|
Zhou XY, Wu SY, Zhang ZC, Wang F, Yang YL,
Li M and Wei XZ: Low-intensity pulsed ultrasound promotes
endothelial cell-mediated osteogenesis in a conditioned medium
coculture system with osteoblasts. Medicine (Baltimore).
96(e8397)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tsai KS, Kao SY, Wang CY, Wang YJ, Wang JP
and Hung SC: Type I collagen promotes proliferation and
osteogenesis of human mesenchymal stem cells via activation of ERK
and Akt pathways. J Biomed Mater Res A. 94:673–682. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li J, Fan L, Yu Z, Dang X and Wang K: The
effect of deferoxamine on angiogenesis and bone repair in
steroid-induced osteonecrosis of rabbit femoral heads. Exp Biol Med
(Maywood). 240:273–280. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Suzuki A, Takayama T, Suzuki N, Kojima T,
Ota N, Asano S and Ito K: Daily low-intensity pulsed ultrasound
stimulates production of bone morphogenetic protein in ROS 17/2.8
cells. J Oral Sci. 51:29–36. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wijdicks CA, Virdi AS, Sena K, Sumner DR
and Leven RM: Ultrasound enhances recombinant human BMP-2 induced
ectopic bone formation in a rat model. Ultrasound Med Biol.
35:1629–1637. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cheung WH, Chow SK, Sun MH, Qin L and
Leung KS: Low-intensity pulsed ultrasound accelerated callus
formation, angiogenesis and callus remodeling in osteoporotic
fracture healing. Ultrasound Med Biol. 37:231–238. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shindo T, Ito K, Ogata T, Hatanaka K,
Kurosawa R, Eguchi K, Kagaya Y, Hanawa K, Aizawa K, Shiroto T, et
al: Low-lntensity pulsed ultrasound enhances angiogenesis and
ameliorates left ventricular dysfunction in a mouse model of acute
myocardial infarction. Arterioscler Thromb Vasc Biol. 36:1220–1229.
2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen WC, Chung CH, Lu YC, Wu MH, Chou PH,
Yen JY, Lai YW, Wang GS, Liu SC, Cheng JK, et al: BMP-2 induces
angiogenesis by provoking integrin α6 expression in human
endothelial progenitor cells. Biochem Pharmacol. 150:256–266.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lv J, Xiu P, Tan J, Jia Z, Cai H and Liu
Z: Enhanced angiogenesis and osteogenesis in critical bone defects
by the controlled release of BMP-2 and VEGF: Implantation of
electron beam melting-fabricated porous Ti6Al4V scaffolds
incorporating growth factor-doped fibrin glue. Biomed Mater.
10(035013)2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Weivoda MM, Ruan M, Pederson L, Hachfeld
C, Davey RA, Zajac JD, Westendorf JJ, Khosla S and Oursler MJ:
Osteoclast TGF-β receptor signaling induces Wnt1 secretion and
couples bone resorption to bone formation. J Bone Miner Res.
31:76–85. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fujiwara M and Ozono K: Cytokines and
osteogenesis. Clin Calcium. 24:845–851. 2014.PubMed/NCBI(In Japanese).
|
|
44
|
Liu DD, Zhang JC, Zhang Q, Wang SX and
Yang MS: TGF-β/BMP signaling pathway is involved in cerium-promoted
osteogenic differentiation of mesenchymal stem cells. J Cell
Biochem. 114:1105–1114. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chen G, Deng C and Li YP: TGF-β and BMP
signaling in osteoblast differentiation and bone formation. Int J
Biol Sci. 8:272–288. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li XL, Liu YB, Ma EG, Shen WX, Li H and
Zhang YN: Synergistic effect of BMP9 and TGF-β in the proliferation
and differentiation of osteoblasts. Genet Mol Res. 14:7605–7615.
2015.PubMed/NCBI View Article : Google Scholar
|