Introduction
In heart failure (HF), aberrant intracellular
calcium (Ca2+) homeostasis is associated with systolic
and diastolic dysfunction and arrhythmogenesis (1). Although a number of factors are
involved in Ca2+-handling abnormalities, alterations in
sarco/endoplasmic reticulum Ca2+-dependent ATPase 2a
(SERCA2a) appear to serve the most important function (2). SERCA2a is expressed in the cardiac
sarcoplasmic reticulum (SR), which serves a central role in
intracellular Ca2+ handling by pumping Ca2+
from the cytosol into the SR lumen (3). This enzyme reduces the cytosolic
calcium concentration and ensures that an adequate store of calcium
is available within the SR for release during the ensuing systole.
The expression and activity of SERCA2a significantly decrease
during HF onset, which impairs the uptake of Ca2+ into
the SR, reduces the subsequent release of Ca2+ from the
SR, in turn causing heart dysfunction. Therefore, impaired
Ca2+ reuptake resulting from the decreased abundance and
reduced activity of SERCA2a is a characteristic of HF (4). SERCA2a-knockout mice have been
previously demonstrated to develop systolic and diastolic
dysfunction, further supporting the important role of SERCA2a in HF
(5). Reduced SERCA2a mRNA
expression has been detected in the failing myocardium of humans in
addition to animal models (6,7),
making the SERCA2a pathway an attractive therapeutic target.
One of the most common methods for investigating the
role of a specific protein in the normal heart environment is to
transfer small hairpin (sh)RNA or a coding DNA sequence into
cultured cardiomyocytes via a plasmid or a viral vector (8-11). However, this
approach is accompanied with difficulties that mimic stress factors
during the process of myocardial remodeling in vitro
(12). In addition, neonatal
cardiomyocytes, which are widely used with this method due to their
ease of transfection, exhibit physiological and pathological
properties that differ from those of adult cells (13). Other approaches, such as
intravascular (via the caudal vein) or intraperitoneal systemic
injections (14-16), are relatively
costly and time consuming, which usually result in low transfection
rates and efficacy due to accumulation of the transgene in the
bloodstream and nontargeted organs (17-19).
Based on information provided by previous studies
(8-11,14-16), the present study attempted to modify the approach
for gene delivery to the heart. Specifically, SERCA2a-knockdown
lentivirus was directly injected into the myocardium of adult rats
under ultrasound guidance. A detailed study was then performed to
test the effectiveness and feasibility of this method and to
confirm the role of SERCA2a expression in cardiac dysfunction.
Materials and methods
Animals
A total of 65 Sprague-Dawley male rats (7-8 weeks
old, ~250 g; Shanghai Kayon Biological Technology Co., Ltd.) were
used. They were kept on a 12-h light/dark cycle in a
temperature-controlled room (22˚C; relative humidity, 45%) with
ad libitum access to food and water. The specific criteria
used to determine when animals should be euthanized was the end of
the experiment or when the ultrasound echocardiography (UCG) and
electrocardiograms (ECGs) were finished. The duration of the
experiment was 5 days. Animal health and behavior were monitored
twice a day, every morning and afternoon. The total number of rats
used was 65, of which 61 were euthanized at the end of the
experiment, with 4 rats being found lifeless. The potential causes
of mortality for the 4 deceased rats may be toxicity of the
lentiviral vector or other infections caused by the experimental
procedure. In a previous study by Saliba et al (20), it was suggested that significant
transfection occurred in the liver, lungs or other organs, which
may be the cause of vector toxicity; in addition, other causes,
such as an increase in total collagen in the left ventricle of the
heart where the adenoviral vector was injected, have been proposed.
However, that previous study used an adenoviral vector, whilst the
present study used lentiviral vector. Prendiville et al
(21) also reported animal
mortality due to this technique and possibly due to complications
caused by this experimental procedure.
The present study was approved by the Affiliated
Suzhou Hospital of Nanjing Medical University Animal Care and Use
Committee (Suzhou, China). Isoflurane was used for anesthetizing
the rats. All animal welfare considerations were taken into
account, including efforts to minimize suffering and distress, use
of anesthetics and special housing conditions. Before the
procedure, all animals were intubated and anaesthetized with
mechanical ventilation by inhalation of 1-1.5% isoflurane in 100%
oxygen continuously. Body temperature was maintained by a heating
pad. To reduce the possibility of perioperative infection, the
procedures were performed in a sterile laminar flow hood, where the
virus was delivered aseptically. When the experiment was finished,
rats were euthanized intraperitoneally with pentobarbital (200
mg/kg). The death of rats in the present study was verified by
identifying the cessation of breathing and heartbeat by
ultrasound.
Ultrasound-guided virus injection
The rats were randomized into four groups as
follows: i) Two groups received cardiac injections of either a
lentiviral vector for the shRNA-mediated knockdown of SERCA2a
(n=20) or the viral vector alone (n=15); ii) one group received the
SERCA2a shRNA-knockdown virus via a single-bolus tail-vein
injection (n=20); and iii) the untreated control group, which did
not receive any virus (n=10). The SERCA2a shRNA construct had the
following sequence: 5'AAATTCTAACTAGTAAGTCTTTTTTGGAATTAAT-3'. Third
generation lentiviral systems were used to generate the
pGLV1/U6/GFP vectors (Shanghai GeneChem Co., Ltd.). The anterior
wall of the left ventricle (LV) of the rat heart was transfected
with the virus, where each rat received two injections (100
µl/site). The sites of the two injections were located in the
middle segment of the anterior wall, which divided the whole
anterior wall into basal, middle and apical segments. The viral
constructs were diluted with 0.9% saline to a final concentration
of 1x109 viral particles in 200 µl prior to delivery.
Under ultrasound guidance, the virus solution was then injected
into the anterior myocardium of the LV as follows.
First, the animals were subjected to anesthesia with
isoflurane. The chest hair was removed, following which the rats
were placed in a supine position on a platform. Electrode pads were
then placed on the rats for continuous ECG monitoring throughout
the procedure.
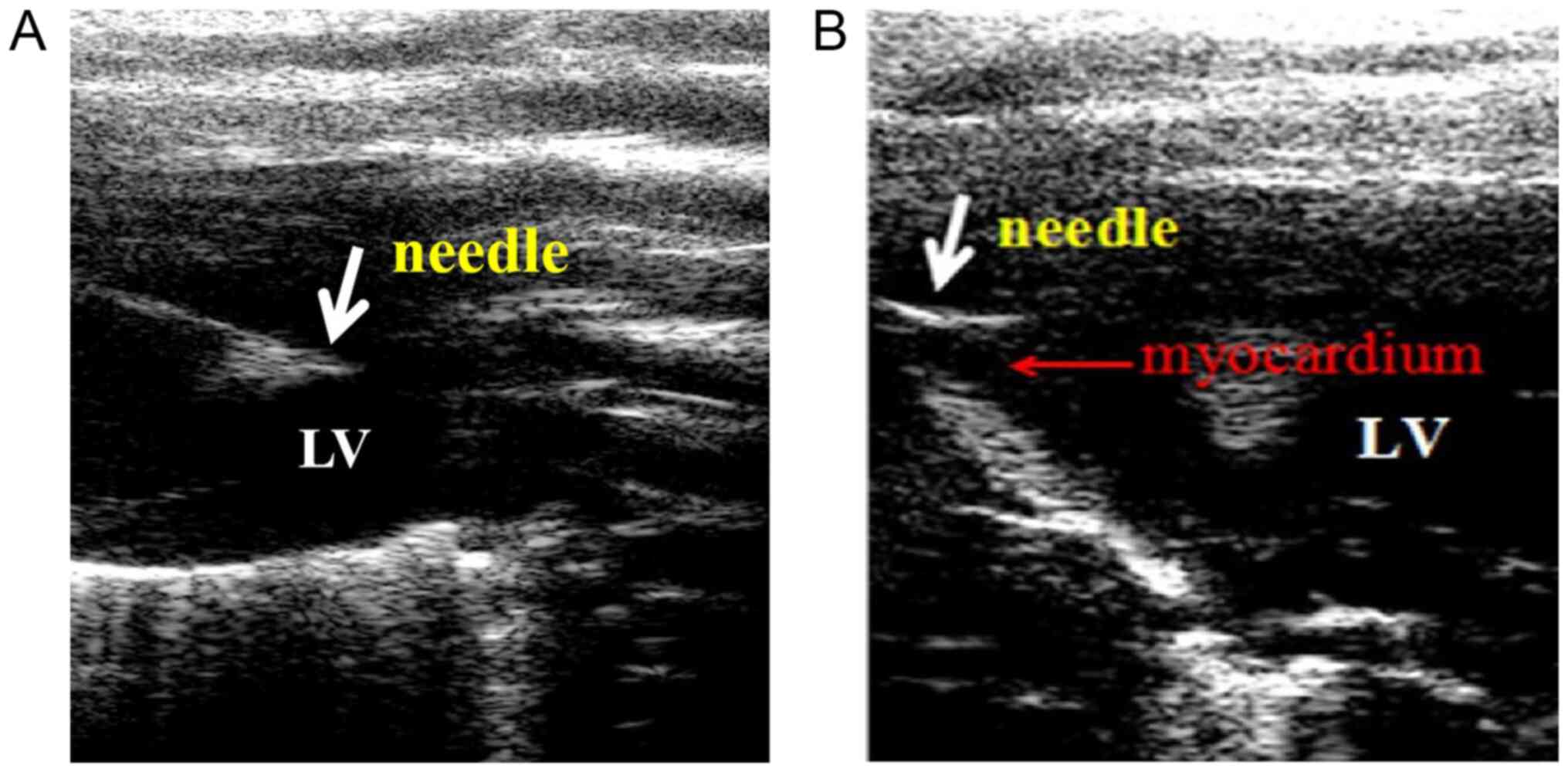
A high-resolution ultrasound system (Vevo 770;
VisualSonics, Inc.) coupled with a high-frequency linear array
transducer (12-38 MHz) was used. Specifically, it was necessary for
the needle and injection area of the myocardium to be shown in the
same sonographic view during the operation to ensure that the
entire virus delivery process was clearly visualized. The gain,
depth and focus of the two-dimensional ultrasound image were
adjusted for optimal visualization. Under ultrasound guidance, the
needle was introduced intercostally through the chest wall to the
cavity of the LV and then withdrawn until its tip reached the
target position (one of two sites along the anterior myocardium) in
the LV anterior wall over a short period of time (~20 sec). The
cardiac injections were visualized on the ultrasound monitor, where
all images were documented for analysis.
Determination of transfection
efficiency
Vector-GFP-transfected ventricles in the cardiac
injection group (n=4) and the tail-vein injection group (n=4) were
subjected to examination of GFP labeling. GFP expressed at the site
of injection in the anterior myocardium was detected and examined
using a Zeiss Axioplan fluorescent microscope (Zeiss AG). GFP
expression was detected on day 5 after viral infection. The
anterior wall of the heart was sectioned, with slices being taken
at a similar position in both groups. Tissues were dehydrated using
30% sucrose-PBS and embedded with optimal cutting temperature
compound, frozen at -80˚C and sliced into 15-µm sections. Three
sections were obtained from each animal and observed under a
confocal microscope at a magnification of x200. Six randomly
selected fields of view were analyzed for each section. The average
percentage of GFP-positive cells within the total cell population
was calculated from the sections obtained from each animal. The
number of GFP-positive cells relative to the total number of cells
was defined as the gene transfection efficiency.
Analysis of LV function by UCG and
ECG
M-mode UCG of the rats were acquired using the same
ultrasound system 5 days after the cardiac injections. M-mode
echocardiogram was performed on the parasternal long-axis section
of the LV to record the end-systolic and the corresponding
end-diastolic LV anterior wall thickness (LVAWs and LVAWd) and LV
inner diastolic and systolic dimensions (LVIDd and LVIDs). The LV
ejection fraction (EF) and fraction shortening (FS) were calculated
using Visual Sonics analysis software (Vevo770; Visualsonics Inc.).
ECG was performed on day 5. A 3-min ECG sample was recorded from
each animal for analysis. The ST-T segment and the wide portion of
the QRS segment were calculated from the mean curve from the ECG
usingLabChart7software (AD Instruments).
Histology
The hearts were harvested 5 days after the UCG and
ECG procedures, fixed in 10% polyformaldehyde for 24 h at room
temperature, embedded in paraffin, then cut into 10-µm sections.
Samples were then placed in xylene for 5-10 min, dehydrated in a
descending alcohol series (100, 90, 80 and 70%) and rinsed with
distilled water. Sections were then stained with hematoxylin for 5
min and eosin for 1-3 min at room temperature. The area of damage
was calculated as a percentage of the total area. Three fields of
view were selected for one section, with a total of three sections
obtained per animal. A mean score was calculated from analysis of
animals per group (n=3). All histopathological changes were
evaluated in a blinded manner by two investigators (YX and LP). The
intercellular space, heart tissue edema and inflammatory
infiltration were all assessed under a light microscope
(magnification, x400).
Western blotting
Protein was extracted from samples using 1% PMSF and
1% protease inhibitor cocktail in RIPA (Santa Cruz Biotechnology,
Inc.). The Pierce BCA assay kit (Thermo Fisher Scientific, Inc.)
was used to determine protein concentration and 20 µg protein was
loaded onto an 8% SDS-PAGE gel. After transferal to PVDF membranes,
5% non-fat milk with TBS and 0.1% Tween-20 was applied at room
temperature for 1 h. The following primary antibodies were added to
samples and incubated overnight at 4˚C: Rabbit anti-SERCA2a (cat.
no. PA5-78836; 1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.)
and mouse anti-GAPDH (cat. no. sc-32233; 1:1,500; Santa Cruz
Biotechnology, Inc.). Subsequently, membranes were treated with
donkey anti-rabbit DyLight™594-conjugated secondary antibodies
(cat. no. SA5-10040; 1:2,000) and goat anti-mouse
DyLight™488-conjugated secondary antibodies (cat. no. A32723;
1:5,000; each, Invitrogen; Thermo Fisher Scientific, Inc.) and
incubated for 1 h at room temperature. Samples were then washed
with TBS and 0.1% Tween-20 and visualized with the Super Signal
West Pico/Dura chemiluminescent substrate (Pierce; Thermo Fisher
Scientific, Inc.).
Statistical analysis
All the experiments were repeated at least three
times, and the data are presented as the mean ± standard deviation.
The differences between groups were assessed by post hoc Tukey's
test for multiple comparisons. P<0.05 was considered to indicate
a statistically significant difference.
Results
Ultrasound-guided application improved
myocardium-specific transfection
The ultrasound-guided cardiac injections resulted in
substantial myocardial gene transfection. Fig. 1 illustrates the process used for the
injection of SERCA2a-knockdown lentivirus into the heart of the
rats using the ultrasound-guided technique. As shown in Fig. S1, enhanced GFP signals for the
vector-GFP-transfected ventricles were observed on day 5,
indicating that the viral infection was successful. The number of
GFP-positive cells in the cardiac injection group (86%) was
significantly higher compared with that in the tail-vein injection
group (48%; P<0.01). This finding visually demonstrated that the
ultrasound-guided approach is superior compared with intravenous
injections. Additionally, the inter-animal variation in the
transfection efficiency was found to be low, as shown by the small
standard deviation observed in Fig.
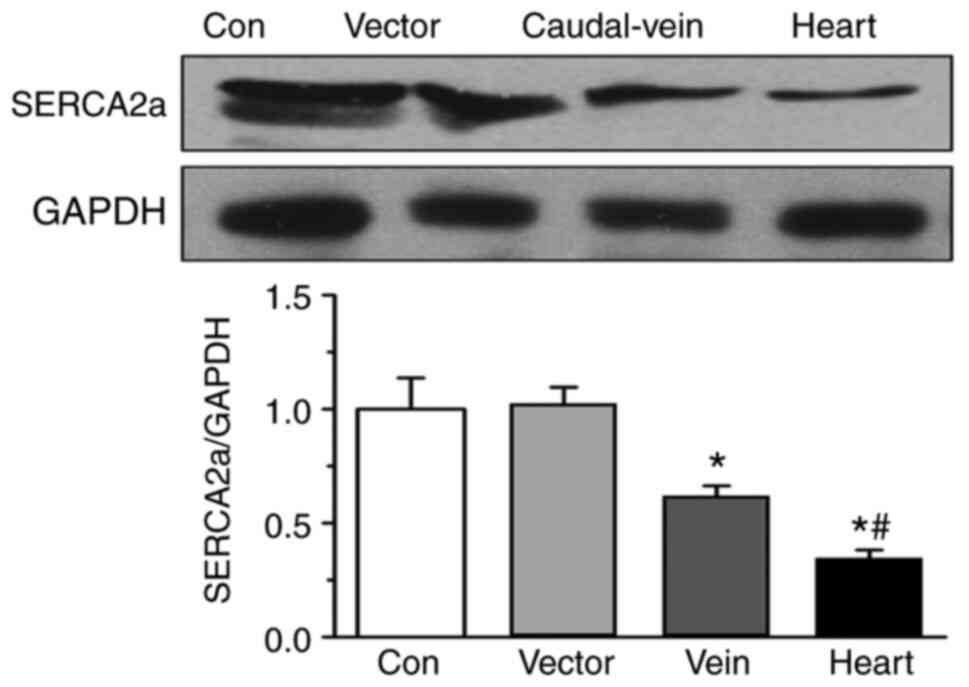
S1. Western blotting data demonstrated that SERCA2a expression
on day 5 was significantly lower in the cardiac injection group
compared with that in the tail-vein injection, vector-only and
control groups (Fig. 2). This
observation suggests that ultrasound-guided application improved
myocardium-specific transfection.
SERCA2a knockdown induces
cardiomyocyte injury
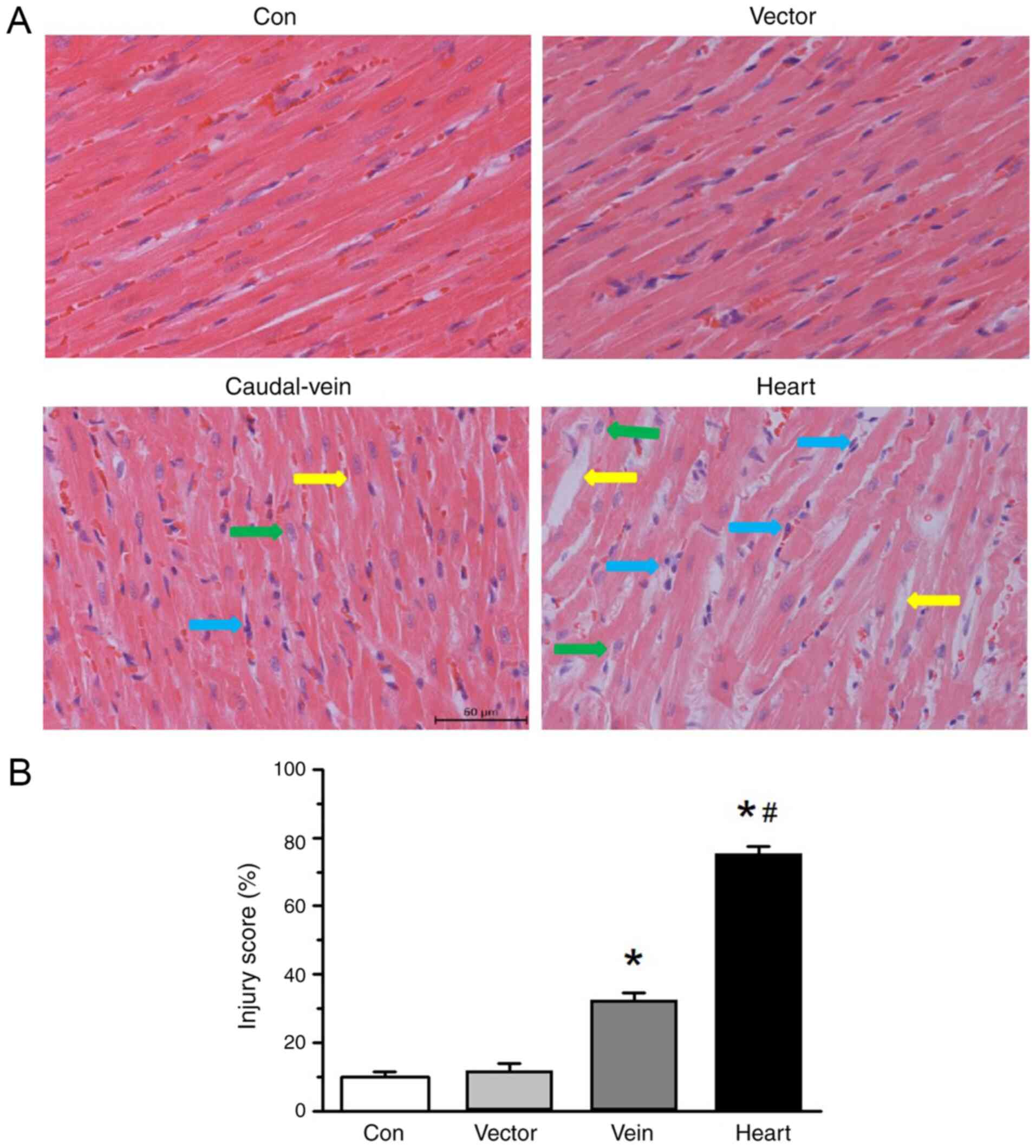
Hematoxylin and eosin staining results demonstrated
a mixture of pathological changes in the mice administered with
cardiac injections of the SERCA2a-knockdown virus, including
cardiomyocyte hypertrophy, myocardial disarray, intercellular space
alterations, heart tissue edema and inflammatory cell infiltration.
To a limited extent, similar changes were also evident in the group
of mice that received SERCA2a-knockdown virus injections via the
tail vein (Fig. 3A). Neither the
group that received the injection with the vector alone nor the
control group exhibited these types of histopathological changes.
The injury score was observed in >30% of the myocardium in the
hearts of the mice that received injections of the
SERCA2a-knockdown virus via the tail vein, whereas >70% of the
myocardium in the hearts of the mice belonging to the cardiac
injection group was injured (Fig.
3B). These data suggest that SERCA2a knockdown induces
cardiomyocyte injury.
SERCA2a knockdown induces
cardiomyocyte dysfuncton
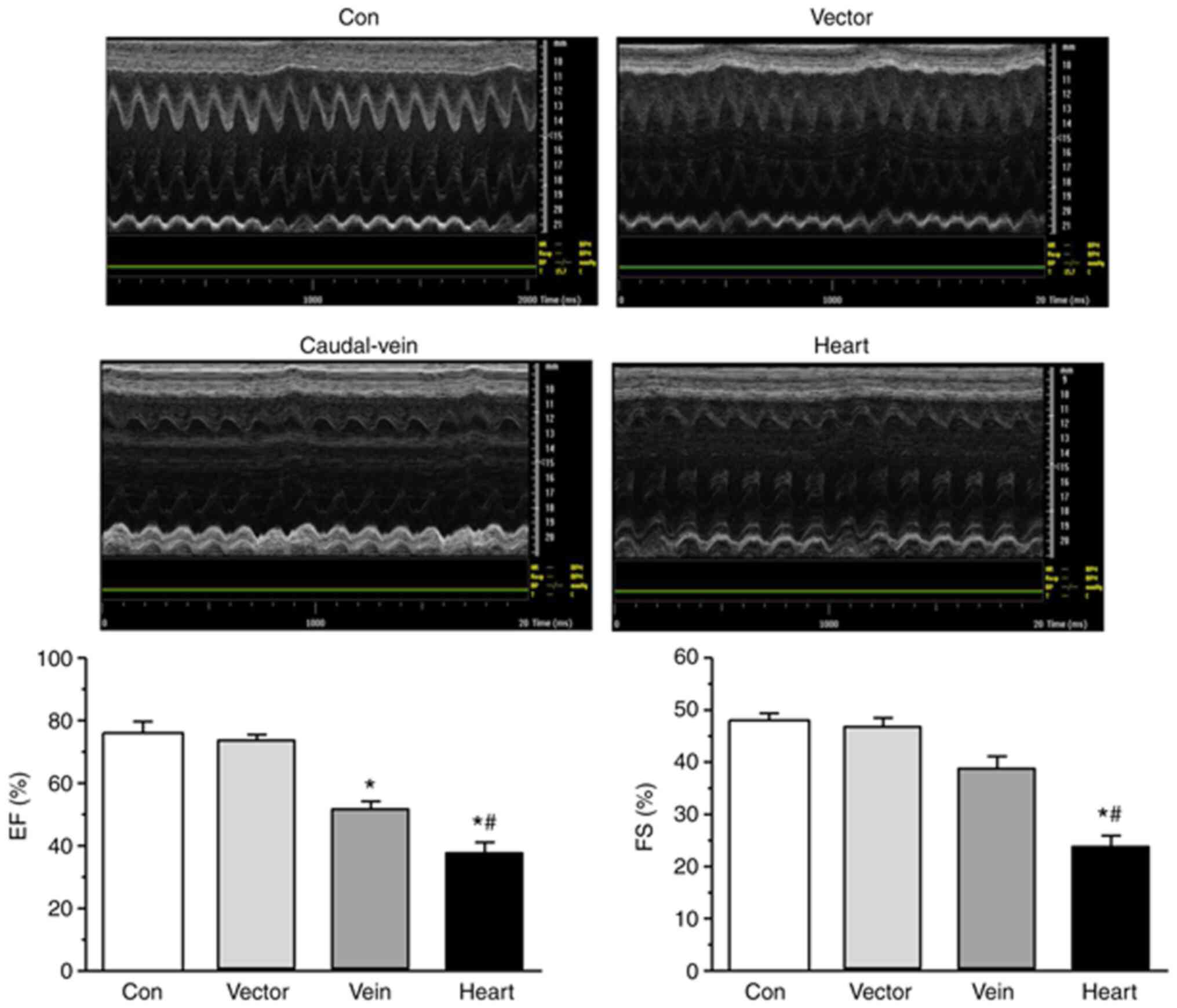
To confirm the role of SERCA2a in cardiomyocyte
dysfunction in vivo, the effects of knocking down SERCA2a levels
via the injection of SERCA2a-knockdown virus into the rat heart on
the EF and FS of the LV were calculated using UCG. EF and FS were
found to be significantly lower in the myocardium transfected with
the SERCA2a-knockdown lentivirus compared with those in the
myocardium of the rats of the vector and tail-vein injection
groups, suggesting that the cardiac injection group exhibited
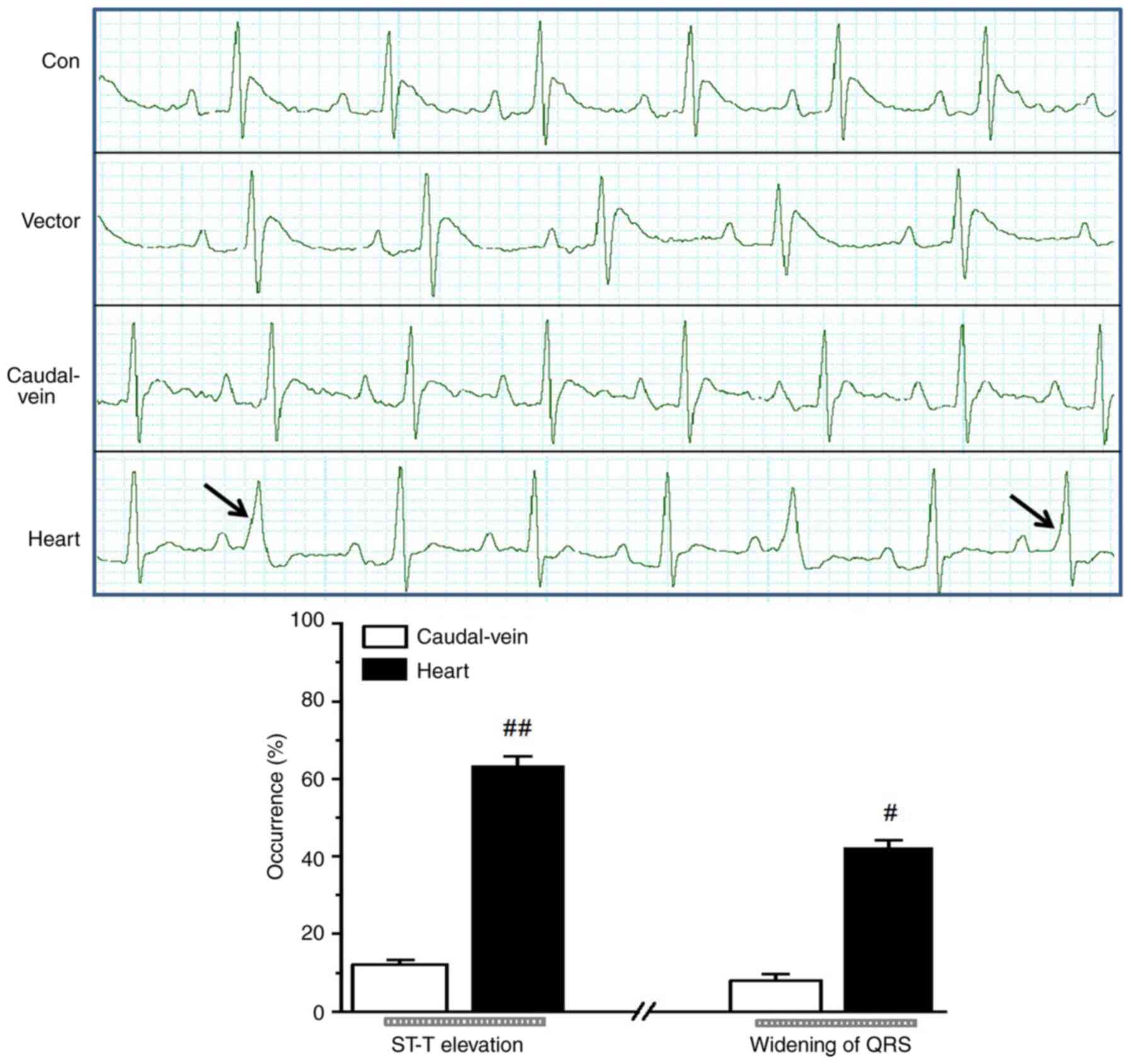
marked deterioration in cardiac function (Fig. 4). In addition, ST-segment elevation,
widening of the QRS complex and premature beats were observed in
the ECG recordings obtained from the rats belonging to the
injection groups, which suggested that reduced SERCA2a expression
altered the ventricular conduction properties. Additionally, higher
occurrences of ST-T elevation and greater QRS widening were
observed in the cardiac injection group compared with those in the
tail-vein injection group (Fig.
5).
Discussion
The results of the present study demonstrated that
ultrasound-guided cardiac viral injection efficiently induced gene
transfer into the heart of the rats, as shown by the observed
reduction in SERCA2a expression in cardiomyocytes and greater
cardiac dysfunction detected in the rats that received cardiac
SERCA2a-shRNA injections compared with the rats that received
identical injections via the tail-vein (22,23).
Previous studies have associated cardiac dysfunction with reduced
SERCA2a expression in humans and animals (24,25).
Results found in the present study therefore confirmed the pivotal
role of SERCA2a in the physiological function of the rat heart.
Ultrasound-guided cardiac injection has a number of
benefits for gene transfection. Compared with the caudal vein
injection approach, this technique resulted in enhanced,
cost-effective, tissue-specific transfection that required lower
viral titers. The majority of studies involving the transfection of
a gene into the heart have used systemic injection, which is
associated with a number of shortcomings, including dilution of the
transgene in the circulation and the nonspecific uptake of the
transgene by surrounding cells, reducing the expression levels in
the heart (26,27). In addition, the number of cells that
have been successfully transfected remains difficult to ascertain.
The present study found that the efficiency of systemic
transfection was markedly lower compared with that by local
myocardial-specific transfection via direct cardiac injection.
However, immunogenicity and toxicity are important aspects of the
technique used in the present study that require investigation in
subsequent studies.
Another major advantage of ultrasound-guided cardiac
injection is the drastically reduced invasiveness. Saliba et
al (20) achieved viral
delivery to the heart via open-chest injection. Compared with the
approach of Saliba et al (20), ultrasound-guided injection did not
induce any significant changes in ventricular function or the ECG
findings when comparing control and empty vector groups, where it
was possible to perform the real-time monitoring of cardiac
function at any time following gene delivery. Therefore, the key
processes and order of procedures in the approach presented in the
present study differed from those of this previously described
technique. The present study describes a feasible and semi-invasive
method that avoids open-chest surgery, which is associated with
morbidity and mortality (28),
thereby allowing the animals to recover more rapidly with minimal
inflammatory sequelae. Prendiville et al (21) previously introduced a technique
involving ultrasound-guided cardiac injection in mice and
demonstrated its advantages. Based on that study, the present study
delivered SERCA2a-knockdown lentivirus into rats via
ultrasound-guided cardiac injection, the results of which were then
with those that received the same lentivirus via tail-vein
injection. The resulting SERCA2a protein expression was
subsequently assessed, which demonstrated further the function of
SERCA2a in the heart.
A potential limitation of the present study is the
potential use of a lentiviral vector in patient treatment. The
majority of studies into this are still in the early stages, where
further basic and clinical research is necessary to verify the
long-term safety and efficacy of the application of lentiviral
vectors on patients for therapeutic purposes.
In conclusion, the present study improved our
previous method for myocardial gene delivery by administering
ultrasound-guided minimally-invasive injections directly into the
rat heart. This technique provided a safe method for conducting
protein functional studies. The present study also investigated and
confirmed the critical physiological role of SERCA2a in the
myocardium. The simplicity and directness of the proposed approach
suggest broader applicability to the study of other proteins.
Supplementary Material
Figure S1. The GFP expression levels
in the cardiac injection (n=4) and tail.vein injection groups (n=4)
were detected by fluorescence microscopy. GFP expression was
detected on day 5 after viral injection. The number of GFP-positive
cells is presented as a percentage of the total cell population.
**P<0.01 vs. vein.
Acknowledgements
Not applicable.
Funding
The present study was supported by a Suzhou Basic
Research in Medical and Health Application Grant (grant no.
SYSD2016109) and Special Diagnosis Techniques for Clinical Key
Diseases of Suzhou Municipal Health and Family Planning Commission
(grant no. LCZX201610).
Availability of data and materials
The datasets used and/or analyzed during the
currenty study are available from the corresponding author on
reasonable request.
Authors' contributions
ZG and XY performed the experiments and analyzed the
data. ZG performed injections and design the experiments. HJ
performed H&E staining. KS planned and performed western
blotting. PL and QZ determined the UCG and ECG. XD and XY
contributed to the writing the manuscript and analyzing the data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Affiliated
Suzhou Hospital of Nanjing Medical University Animal Care and Use
Committee (Suzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Val-Blasco A, Piedras MJGM, Ruiz-Hurtado
G, Suarez N, Prieto P, Gonzalez-Ramos S, Gómez-Hurtado N, Delgado
C, Pereira L, Benito G, et al: Role of NOD1 in heart failure
progression via regulation of Ca2+ handling. J Am Coll
Cardiol. 69:423–433. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hamilton S and Terentyev D: Altered
intracellular calcium homeostasis and arrhythmogenesis in the aged
heart. Int J Mol Sci. 20(E2386)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Meyer M, Schillinger W, Pieske B,
Holubarsch C, Heilmann C, Posival H, Kuwajima G, Mikoshiba K, Just
H and Hasenfuss G: Alterations of sarcoplasmic reticulum proteins
in failing human dilated cardiomyopathy. Circulation. 92:778–784.
1995.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Marks AR: Calcium cycling proteins and
heart failure: Mechanisms and therapeutics. J Clin Invest.
123:46–52. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Andersson KB, Birkeland JA, Finsen AV,
Louch WE, Sjaastad I, Wang Y, Chen J, Molkentin JD, Chien KR,
Sejersted OM, et al: Moderate heart dysfunction in mice with
inducible cardiomyocyte-specific excision of the Serca2 gene. J Mol
Cell Cardiol. 47:180–187. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chaanine AH, Nonnenmacher M, Kohlbrenner
E, Jin D, Kovacic JC, Akar FG, Hajjar RJ and Weber T: Effect of
bortezomib on the efficacy of AAV9.SERCA2a treatment to preserve
cardiac function in a rat pressure-overload model of heart failure.
Gene Ther. 21:379–386. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kho C, Lee A, Jeong D, Oh JG, Gorski PA,
Fish K, Sanchez R, DeVita RJ, Christensen G, Dahl R, et al:
Small-molecule activation of SERCA2a SUMOylation for the treatment
of heart failure. Nat Commun. 6(7229)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Doroudgar S, Quijada P, Konstandin M,
Ilves K, Broughton K, Khalafalla FG, Casillas A, Nguyen K, Gude N,
Toko H, et al: S100A4 protects the myocardium against ischemic
stress. J Mol Cell Cardiol. 100:54–63. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Isner JM: Myocardial gene therapy. Nature.
415:234–239. 2002.PubMed/NCBI
|
|
10
|
Jiang J, Burgon PG, Wakimoto H, Onoue K,
Gorham JM, O'Meara CC, Fomovsky G, McConnell BK, Lee RT, Seidman
JG, et al: Cardiac myosin binding protein C regulates postnatal
myocyte cytokinesis. Proc Natl Acad Sci USA. 112:9046–9051.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bühring HJ, Lang F, Sorg RV, Langer H and
Gawaz M: Activated platelets interfere with recruitment of
mesenchymal stem cells to apoptotic cardiac cells via high mobility
group box 1/Toll-like receptor 4-mediated Down-regulation of
hepatocyte growth factor receptor MET. J BiolChem. 289:11068–11082.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pironti G, Bersellini-Farinotti A, Agalave
NM, Sandor K, Fernandez-Zafra T, Jurczak A, Lund LH, Svensson CI
and Andersson DC: Cardiomyopathy, oxidative stress and impaired
contractility in a rheumatoid arthritis mouse model. Heart.
104:2026–2034. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bardot ES and Dubois NC: A watershed
finding for heart regeneration. Cell. 176:947–949. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fletcher ML, Ogg MC, Lu L, Ogg RJ and
Boughter JD Jr: Overlapping representation of primary tastes in a
defined region of the gustatory cortex. J Neurosci. 37:7595–7605.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hardee CL, Arévalo-Soliz LM, Hornstein BD
and Zechiedrich L: Advances in Non-Viral DNA Vectors for Gene
Therapy. Genes (Basel). 8(65)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Roostalu U, Aldeiri B, Albertini A,
Humphreys N, Simonsen-Jackson M, Wong JKF and Cossu G: Distinct
cellular mechanisms underlie smooth muscle turnover in vascular
development and repair. Circ Res. 122:267–281. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bevan AK, Duque S, Foust KD, Morales PR,
Braun L, Schmelzer L, Chan CM, McCrate M, Chicoine LG, Coley BD, et
al: Systemic gene delivery in large species for targeting spinal
cord, brain, and peripheral tissues for pediatric disorders. Mol
Ther. 19:1971–1980. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bruegmann T, van Bremen T, Vogt CC, Send
T, Fleischmann BK and Sasse P: Optogenetic control of contractile
function in skeletal muscle. Nat Commun. 6(7153)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
von Burstin J, Diersch S, Schneider G,
Reichert M, Rustgi AK and Schmid RM: Detection of tumor suppressor
genes in cancer development by a novel shRNA-based method. Mol
Cancer Res. 13:863–869. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Saliba Y, Mougenot N, Jacquet A, Atassi F,
Hatem S, Farès N and Lompré AM: A new method of ultrasonic nonviral
gene delivery to the adult myocardium. J Mol Cell Cardiol.
53:801–808. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Prendiville TW, Ma Q, Lin Z, Zhou P, He A
and Pu WT: Ultrasound-guided transthoracic intramyocardial
injection in mice. J Vis Exp. 90(e51566)2014.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Hadri L, Kratlian RG, Benard L, Maron BA,
Dorfmüller P, Ladage D, Guignabert C, Ishikawa K, Aguero J, Ibanez
B, et al: Therapeutic efficacy of AAV1.SERCA2a in
monocrotaline-induced pulmonary arterial hypertension. Circulation.
128:512–523. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Verbist KC, Field MB and Klonowski KD:
Cutting edge: IL-15-independent maintenance of mucosally generated
memory CD8 T cells. J Immunol. 186:6667–6671. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kronenbitter A, Funk F, Hackert K,
Gorreßen S, Glaser D, Boknik P, Poschmann G, Stühler K, Isić M,
Krüger M, et al: Impaired Ca2+ cycling of nonischemic
myocytes contributes to sarcomere dysfunction early after
myocardial infarction. J Mol Cell Cardiol. 119:28–39.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zsebo K, Yaroshinsky A, Rudy JJ, Wagner K,
Greenberg B, Jessup M and Hajjar RJ: Long-term effects of
AAV1/SERCA2a gene transfer in patients with severe heart failure:
Analysis of recurrent cardiovascular events and mortality. Circ
Res. 114:101–108. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mays LE, Wang L, Lin J, Bell P, Crawford
A, Wherry EJ and Wilson JM: AAV8 induces tolerance in murine muscle
as a result of poor APC transduction, T cell exhaustion, and
minimal MHCI upregulation on target cells. Mol Ther. 22:28–41.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shao CH, Capek HL, Patel KP, Wang M, Tang
K, DeSouza C, Nagai R, Mayhan W, Periasamy M and Bidasee KR:
Carbonylation contributes to SERCA2a activity loss and diastolic
dysfunction in a rat model of type 1 diabetes. Diabetes.
60:947–959. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Parsa CJ, Reed RC, Walton GB, Pascal LS,
Thompson RB, Petrofski JA, Emani SM, Folgar F, Riel RU, Nicchitta
CV, et al: Catheter-mediated subselective intracoronary gene
delivery to the rabbit heart: Introduction of a novel method. J
Gene Med. 7:595–603. 2005.PubMed/NCBI View
Article : Google Scholar
|