Introduction
Pollution with particulate matter of ≤2.5 µm in
diameter (PM2.5) is known to have significant deleterious effects
on human health (1-3).
The respiratory system exchanges gases with the ambient gas
environment directly; therefore, PM2.5-containing air can cause
airway lesions (4). The increase of
inflammatory cytokines has been revealed to be positively
correlated with the exposure dose and duration (5), and even a low dose of PM2.5 can cause
inflammation and pulmonary injury (6). Moreover, PM2.5 has a cumulative effect
on the respiratory system. For instance, the longer the exposure
time, the stronger the adverse influence of PM2.5 to the
respiratory system (7). Previous
studies have reported that PM2.5 can activate the NF-κB pathway, as
well as upregulate the transcription and secretion of
proinflammatory cytokines, including IL-1β, TNF-α, IL-6 and IL-8,
ultimately inducing widespread pulmonary inflammatory lesions
(8,9). In addition, it has been observed that
upregulation of AMP-activated protein kinase (AMPK) suppresses the
inflammatory response via inhibition of inflammatory cytokines and
NF-κB (10,11). Therefore, downregulation of the
NF-κB signaling pathway or activation of AMPK may be a valid method
for controlling PM2.5-induced respiratory inflammation and
preventing or limiting lung injury.
Ophiopogon japonicus (O. japonicus;
commonly known as Maidong in China) was first recorded in Shen
Nong's Materia Medica written during the Han dynasty and has been
widely applied in traditional Chinese medicine (12). Currently, O. japonicus is
often used in compound prescriptions as the main medicinal
ingredient, such as in YiQiFuMai injection, Sheng Mai Yin and
Xuanmai granule (13). According to
the Chinese Pharmacopoeia, O. japonicus has been applied for
1,000s of years for the treatment of inflammatory diseases, such as
pharyngitis, bronchitis, pneumonia and cough (14). Ophiopogonin D (OP-D) is a vital
bioactive steroidal glycoside extracted from the root of O.
japonicas (15). Accumulating
evidence has indicated that OP-D possesses a broad range of
pharmacological properties, including anti-inflammatory,
antioxidative and antitussive effects, as well as inhibition of
venous thrombosis (16-18).
However, it remains unknown whether OP-D is able to protect
alveolar epithelial cells from PM2.5-induced toxicity via its
anti-inflammatory effects. Therefore, it was hypothesized that OP-D
may be potentially useful for preventing PM2.5-induced pulmonary
inflammation.
The aim of the present study was to investigate the
protective effects of OP-D on PM2.5-induced pulmonary inflammation.
In addition, the molecular mechanisms of the anti-inflammatory
effects of OP-D were evaluated.
Materials and methods
Materials
OP-D, extracted from O. japonicus, was
obtained from Beijing Biotop Biotechnology Development (cat. no.
41753-55-3), dissolved in DMSO (Sigma-Aldrich; Merck KGaA), and
diluted with basal DMEM (Beijing Transgen Biotech) (19). The final concentration of DMSO in
the culture medium was 0.1% (v/v). The use of 0.1% DMSO alone in
DMEM was used as a negative control in the corresponding
experiments.
The AMPK-specific inhibitor Compound C (CC) was
purchased from Selleck Chemicals (cat. no. S7306). Streptomycin,
penicillin, FBS and trypsin were obtained from Gibco (Thermo Fisher
Scientific, Inc.). A BCA Protein assay kit (cat. no. 23250) and an
Enhanced Chemiluminescence western blotting Detection reagents kit
were obtained from Thermo Fisher Scientific. The CelLytic™ NuCLEAR™
Extraction kit was purchased from Sigma-Aldrich; Merck KGaA (cat.
no. NXTRACT-1KT). ELISA kits for IL-1β (cat. no. BPE20083), IL-6
(cat. no. BPE20012), IL-8 (cat. no. BPE20459) and TNF-α (cat. no.
BPE20220) were obtained from Shanghai Lengton Bioscience Co., Ltd.
The Cell Counting Kit-8 (CCK-8) was from Beijing Transgen Biotech
(cat. no. FC101-04).
Antibodies against AMPK (1:1,000; cat. no. A1229),
phosphorylated (p)-AMPKβ1-S108 (1:800; cat. no. AP0597), p-NF-κBp65
(1:1,000; cat. no. AP0417), NF-κBp65 (1:1,000; cat. no. A14754),
lamin B (1:2,000; cat. no. A1910) and GAPDH (1:2,000; cat. no.
AC033), as well as HRP goat anti-rabbit antibody (1:5,000; cat. no.
AS014), HRP goat anti-mouse antibody (1:5,000; cat. no. AS003) and
FITC goat anti-rabbit antibody (1:80; cat. no. AS011), were
obtained from ABclonal Biotech. All other reagents were obtained
from Sigma-Aldrich; Merck KGaA.
Cell culture
Mouse lung epithelial cells (MLE-12; The Cell Bank
of Type Culture Collection of the Chinese Academy of Sciences) were
cultured in complete DMEM containing 10% (v/v) FBS, 100 U/ml
penicillin, 100 g/ml streptomycin and 50 g/ml amphotericin B at
37˚C in an incubator with 5% atmospheric CO2. The media
was replaced every 2-3 days, and the cells were subcultured weekly
(1,500-3,000 cells/cm2).
PM2.5 collection and preparation
PM2.5 was collected between November 2016 and March
2017 in an urban area of Changchun, Jilin. The collection and
preparation procedures of PM2.5 were the same as those reported
previously (20). Briefly,
concentrated PM2.5 was gathered using a multistage particle counter
(100 l/min; total suspended particulate/PM10/PM5/PM2.5; Laoshan
Electronic Instrument Co., Ltd.). Daily PM2.5 samples were
collected on Teflon filters (90 mm; Whatman plc; Cytiva). The
PM-loaded filters were put into a 50-ml centrifuge tube, followed
by probe-sonication (700 W; 40 kHz; 25˚C) for 1 h in 40 ml Milli-Q
water. The suspension was freeze-dried in a vacuum for 12 h to
obtain PM2.5 powder, which was then weighed and stored at -80˚C.
Before the experiments, the PM2.5 powder was dissolved in PBS and
sonicated (700 W; 40 kHz; 25˚C) for 30 min to avoid aggregation of
particles.
Cell viability assay
Cell viability was assessed using a CCK-8 assay,
according to the manufacturer's protocol. A total of
4x103 MLE-12 cells per well in 100 µl media were
cultivated in 96-well plates and grown at 37˚C for 24 h. The cells
were treated with OP-D (0-320 µM) for 1 h at 37˚C and then reacted
with or without 15 µg/cm2 PM2.5 at 37˚C in an incubator
for 24 h. CCK-8 solution (10 µl) was added into each well, and the
cells were incubated for another 4 h at 37˚C. Finally, the
absorbance of each well was measured at a wavelength of 450 nm
using a microplate reader. Each sample assay was repeated three
times.
Immunofluorescence staining
MLE-12 cells (1x105 cells/well) on a
glass coverslip cultured in 24-well plates were treated with 15
µg/cm2 PM2.5 and 80 µM OP-D, cells were then fixed with
4% paraformaldehyde, blocked with 5% BSA (cat. no. A1933-25G;
Sigma-Aldrich; Merck KGaA) for 30 min at room temperature (RT) and
incubated with anti-NF-κBp65 (1:200) overnight at 4˚C. After
washing with PBS, the coverslip was treated with secondary antibody
(1:80) for 1 h at RT. Following washing with PBS, the nuclei were
stained with DAPI (Invitrogen; Thermo Fisher Scientific, Inc.) for
20 min at RT. The stained cells were then observed under an
FV-1,000 fluorescence microscope (Olympus Corporation) under x400
magnification. Semi-quantitative analysis was performed using
ImageJ 1.51t software (National Institutes of Health).
ELISA
Based on the experimental groups, the MLE-12 cells
(7x105 cells/well) were cultivated in six-well plates,
pretreated with OP-D at 10, 20, 40 or 80 µM with 10 µM CC for 1 h,
and then stimulated with 15 µg/cm2 PM2.5 for 24 h at
37˚C. The PM2.5 group was stimulated with the corresponding dose of
PM2.5. The OP-D 80 group was only treated with 80 µM OP-D. After
incubation, the cultured media samples were collected to determine
the levels of IL-1β, IL-6, IL-8 and TNF-α, using Mouse Quantikine
ELISA kits, according to the manufacturer's instructions. The cells
were harvested for western blot analysis.
Western blotting
Cell lysates were extracted via homogenization with
RIPA buffer (Beyotime Institute of Biotechnology) containing
phenylmethanesulfonyl fluoride, protease inhibitors and phosphatase
inhibitors. The nuclear fraction was isolated using a Nuclear and
Cytoplasmic extraction kit (cat. no. NXTRACT-1KT; Sigma-Aldrich;
Merck KGaA). The concentration of each supernatant was determined
using a BCA protein assay kit. Aliquoted proteins (60 µg/well) were
loaded onto a 10% SDS-PAGE, separated by electrophoresis and then
transferred onto a PVDF membrane. After blocking with 5% skimmed
milk in Tris-buffered saline-Tween-20 (TBS-T, 0.1% Tween-20) for 1
h at RT, the membrane was treated with the corresponding primary
antibody (AMPK, p-AMPK, NF-κBp65, p-NF-κBp65, GAPDH or lamin B) at
4˚C overnight, washed with TBST buffer three times and then treated
with horseradish peroxidase-conjugated secondary antibody for 1 h
at RT. After washing with TBST three times, the membranes were
visualized with enhanced chemiluminescence reagents. GAPDH and
lamin B were employed as cytosol and nuclear protein loading
controls, respectively. Semi-quantitative analysis was performed
using ImageJ 1.51t software.
Statistical analysis
Data are presented as the mean ± SD. Statistical
analysis was performed using SPSS 20.0 software (IBM Corp.).
Comparison of cell survival rate between the groups were performed
using two-way ANOVA, while other experimental groups were compared
using one-way ANOVA followed by Bonferroni's post hoc test.
Experiments were repeated three times. P<0.05 was considered to
indicate a statistically significant difference.
Results
OP-D prevents the PM2.5-induced
decrease of MLE-12 cell viability
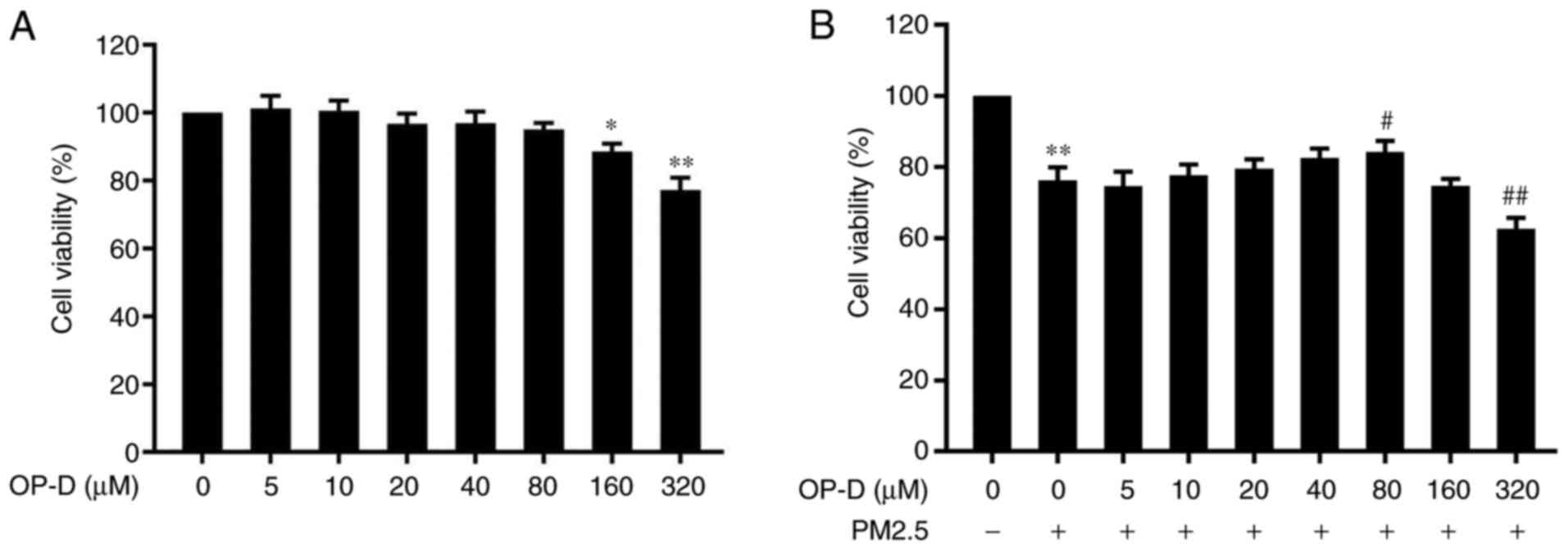
Cellular viability was assayed in the OP-D (0, 5,
10, 20, 40, 80, 160 and 320 µM)-treated MLE-12 cells. No cellular
cytotoxicity was observed at OP-D concentrations of 0-80 µM for 24
h in the presence or absence of PM2.5 (Fig. 1). However, higher concentrations
(160 and 320 µM) of OP-D caused significant cytotoxicity, thus
reducing the cellular viability (Fig.
1A). PM2.5 significantly decreased the MLE-12 cellular
viability, compared with the untreated cells (Fig. 1B). Moreover, pretreatment with 80 µM
OP-D for 1 h significantly attenuated the PM2.5-induced decrease of
cellular viability (Fig. 1B). Lower
concentrations (5-80 µM) of OP-D did not affect cellular viability,
however, 320 µM OP-D significantly further inhibited cellular
viability after PM2.5 treatment (Fig.
1B). Therefore, concentrations of 10, 20, 40 and 80 µM OP-D
were selected for the subsequent experiments.
OP-D attenuates the PM2.5-induced
levels of TNF-α, IL-6, IL-8 and IL-1β in MLE-12 cells
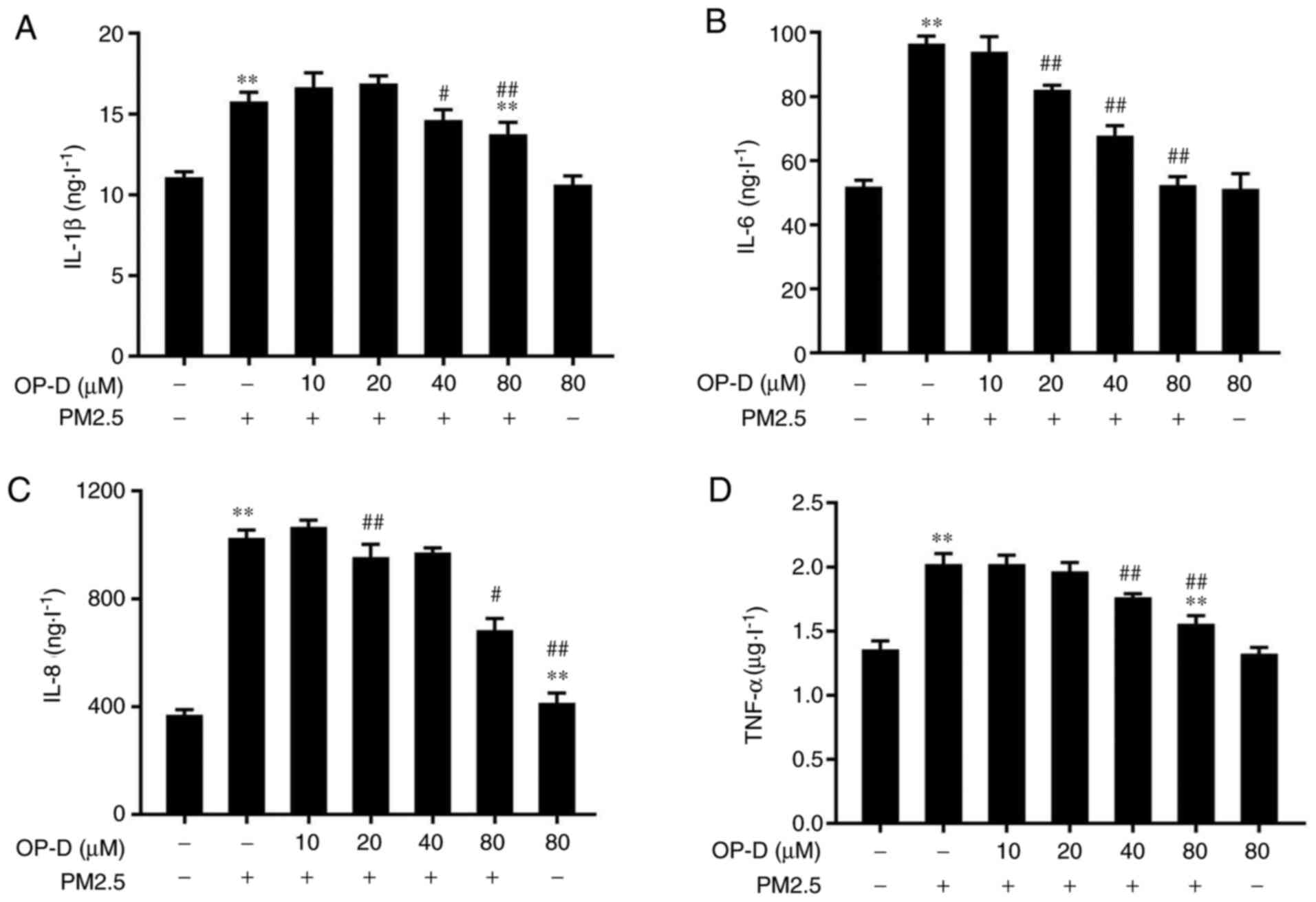
The anti-inflammatory activity of OP-D was
investigated in PM2.5-treated MLE-12 cells via ELISA to examine
levels of IL-1β, IL-6, IL-8 and TNF-α in the culture supernatant.
PM2.5 treatment significantly induced the expression of IL-1β
(Fig. 2A), IL-6 (Fig. 2B), IL-8 (Fig. 2C) and TNF-α (Fig. 2D) in the culture medium of MLE-12
cells, compared with the negative controls. Cells treated with OP-D
alone did not demonstrate any effect on the levels of
proinflammatory cytokines. However, pretreatment with OP-D at 20-80
µM significantly reduced the levels of the PM2.5-triggered
cytokines in a dose-dependent manner, compared with those of the
control PM2.5-treated cells (Fig.
2).
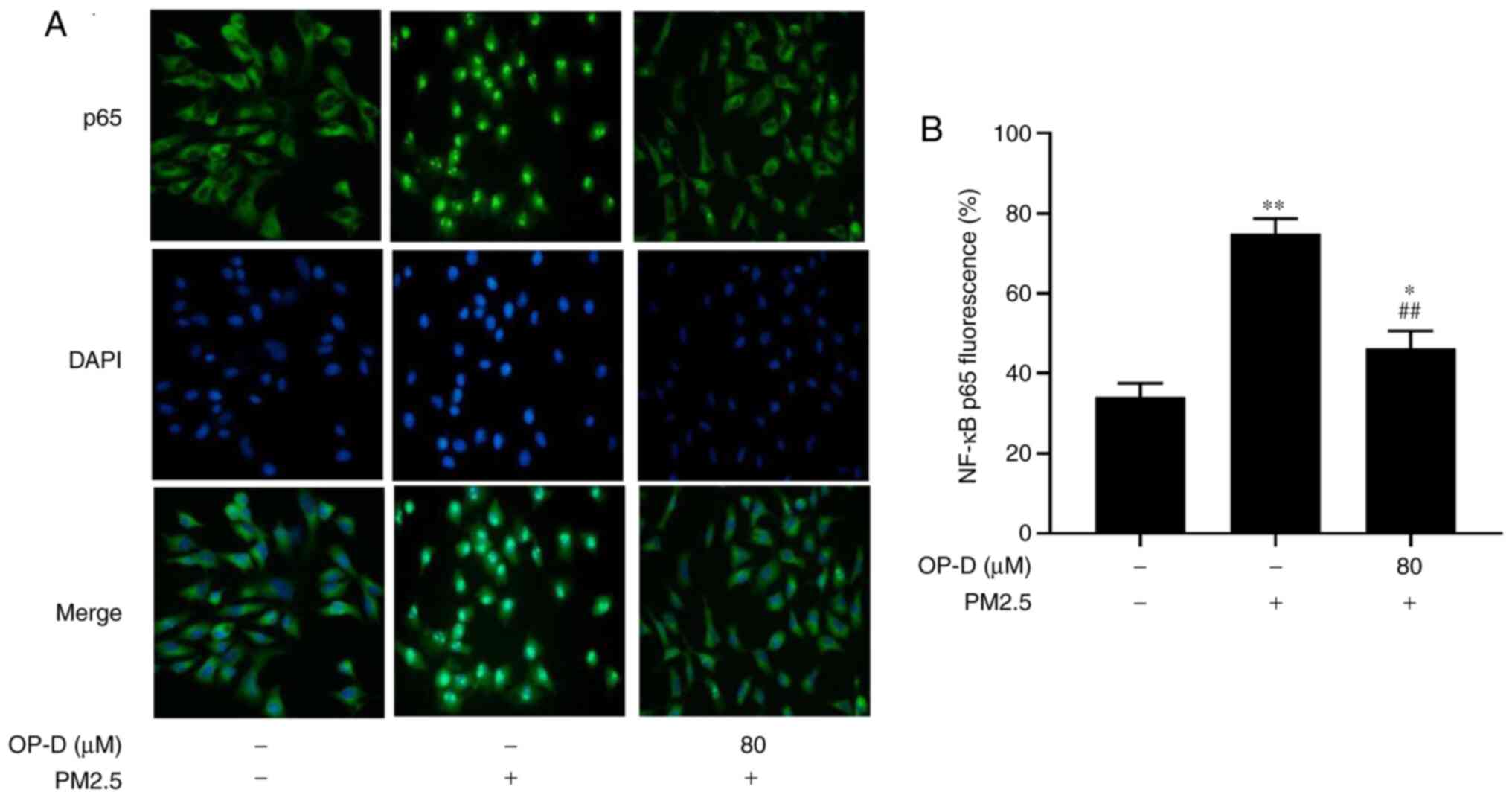
OP-D inhibits the NF-κB inflammatory
signaling induced by PM2.5 in MLE-12 cells
OP-D has been reported to reduce inflammation via
activation of the NF-κB signaling pathway (19), which can be suppressed by AMPK. To
further examine the anti-inflammatory activity of OP-D, the effects
of OP-D were detected on both NF-κB subcellular translocation and
the protein phosphorylation of AMPK and NF-κBp65 in MLE-12 cells
using immunofluorescence and western blot analysis.
PM2.5 treatment significantly induced NF-κB
translocation from the cytoplasm to the nucleus, compared with the
negative control in which most NF-κB expression still remained in
the cytoplasm, according to immunofluorescence staining (Fig. 3B). In contrast, the PM2.5-induced
nuclear translocation of NF-κBp65 was partly suppressed by
pretreatment with OP-D (Fig. 3).
Taken together, these findings suggest the potential
anti-inflammatory effect of OP-D in PM2.5-induced cellular
inflammation.
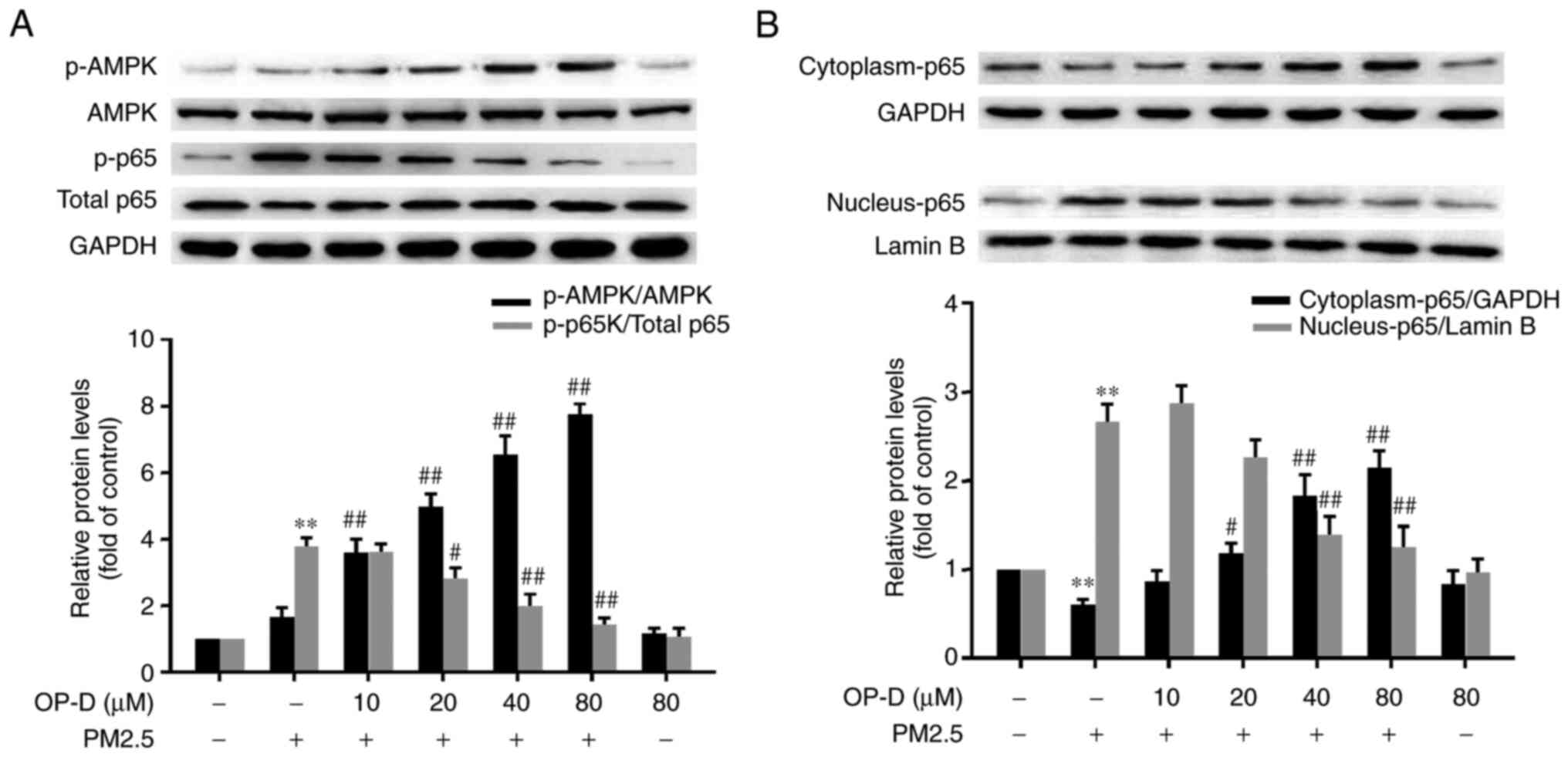
OP-D phosphorylates AMPK in
PM2.5-treated MLE-12 cells
Next, the activation of AMPK in MLE-12 cells was
investigated via western blot analysis. Exposure to PM2.5 did not
significantly alter the phosphorylation of AMPK; however, 10-80 µM
OP-D significantly increased the phosphorylation of AMPK in a
dose-dependent manner (Fig. 4A).
Moreover, there were no significant changes in the OP-D alone
group.
OP-D inhibits PM2.5-triggered NF-κB
activation
PM2.5 exposure significantly phosphorylated NF-κB,
and pretreatment with OP-D (20-80 µM) significantly attenuated this
activation in a dose-dependent manner (Fig. 4A) in MLE-12 cells. In addition,
similar to the immunofluorescence staining results (Fig. 3), PM2.5 treatment significantly
decreased NF-κB expression in the cytoplasm, while NF-κB expression
in the nuclei was significantly increased. These alterations were
significantly reversed by pretreatment with OP-D in a
dose-dependent manner (Fig.
4B).
CC inhibits the OP-D-induced
dephosphorylation of NF-κB in MLE-12 cells
To elucidate whether the anti-inflammatory effect of
OP-D is mediated via AMPK, all the OP-D-treated cells were
incubated with 10 µM CC simultaneously. The CC-treated cells did
not demonstrate any significant activation of AMPK (Fig. 5). In the PM2.5-exposed MLE-12 cells
cotreated with OP-D and CC, the 20-40 µM OP-D-mediated decrease of
NF-κB was significantly blocked; however, CC did not inhibit this
effect in the 80 µM OP-D-treated cells compared with the negative
control cells (Fig. 5). These
results indicated that AMPK mediated NF-κB activation during
PM2.5-induced inflammation.
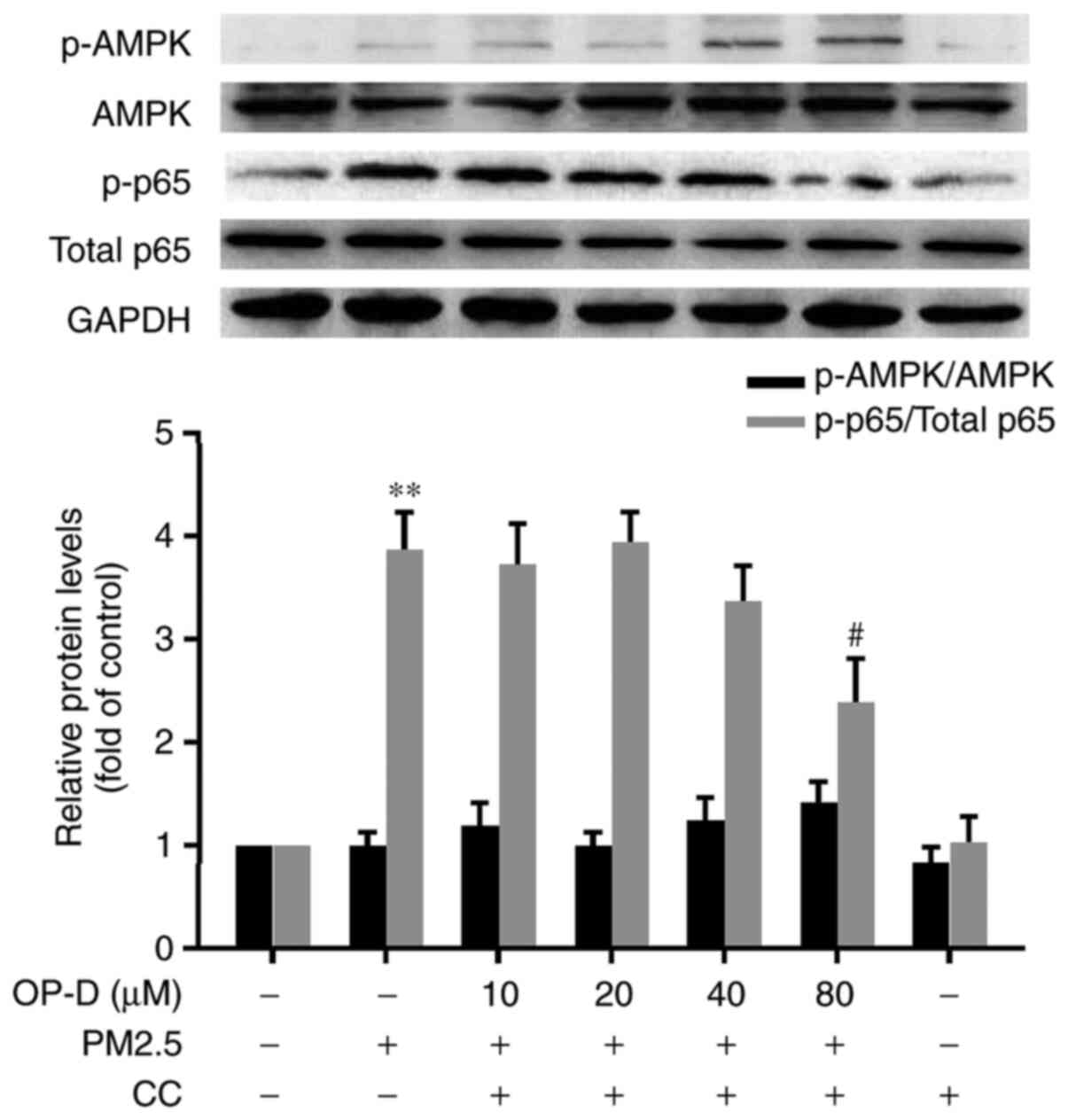 | Figure 5CC blocks the OP-D-mediated
inhibition of NF-κB activation in PM2.5-exposed MLE-12 cells.
MLE-12 cells were pretreated with different concentrations of OP-D
(10, 20, 40 or 80 µM) and 10 µM CC for 1 h, and then they were
exposed to 15 µg/cm2 PM2.5 for 24 h. The expression
levels of NF-κBp65, p-NF-κBp65, AMPK and p-AMPK were assessed using
western blotting and were semi-quantified. Data are presented as
the mean ± SD (n=3 in each group). **P<0.01 vs.
controls; #P<0.05 vs. PM2.5-treated cells. AMPK,
AMP-activated protein kinase; CC, Compound C; OP-D, Ophiopogonin D;
PM2.5, particulate matter of ≤2.5 µm in diameter; p-,
phosphorylated. |
CC upregulates the levels of TNF-α,
IL-6, IL-8 and IL-1β inhibited by OP-D in MLE-12 cells
The aforementioned results suggested that the
anti-inflammatory activity of OP-D is AMPK dependent. In order to
verify this finding, the downregulation of IL-1β (Fig. 6A), IL-6 (Fig. 6B), IL-8 (Fig. 6C) and TNF-α (Fig. 6D) by OP-D was measured in the
presence or absence of CC. CC alone did not demonstrate any notable
effect on the levels of the proinflammatory cytokines. Pretreatment
with 10 µM CC significantly reversed the downregulation of IL-1β,
IL-6, IL-8 and TNF-α induced by OP-D (80 µM), compared with the
group without CC treatment. Furthermore, the inhibition by OP-D did
not completely reverse to the control levels of TNF-α, IL-8 and
IL-1β, compared with the PM2.5-treated group. These results
indicated that downregulation of the inflammatory factors by OP-D
was partly via the AMPK pathway, which is consistent with the
western blotting results. Thus, the present results suggested that
OP-D attenuated the PM2.5-induced cell inflammation via activation
of AMPK and suppression of the NF-κB signaling pathway.
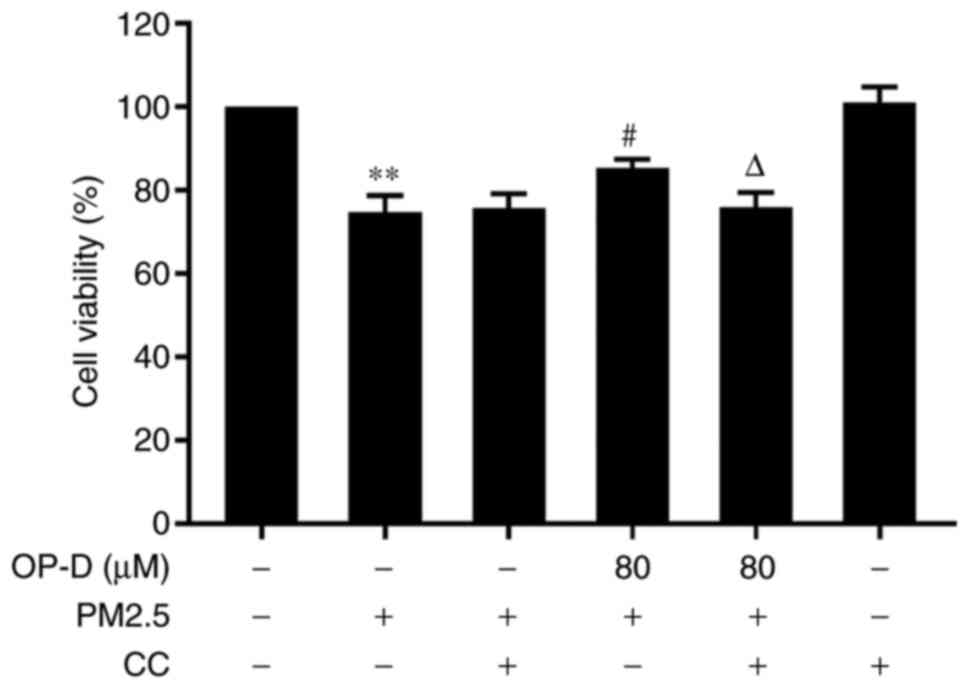
CC reduces the protective effect of
OP-D in MLE cells
CC was used to block the activation of AMPK in order
to observe whether the AMPK pathway is essential to protect
cellular viability. The present results demonstrated that blocking
the AMPK pathway with CC significantly reduced the protective
effect of OP-D on the PM2.5-induced cells (Fig. 7; P<0.05). These results indicated
that OP-D had a protective effect on MLE cells via activation of
the AMPK signaling pathway.
Discussion
To the best of our knowledge, the present study was
the first to demonstrate that OP-D has an anti-inflammatory effect
on PM2.5-injured alveolar epithelial cells. The present study
identified that PM2.5 activated the NF-κB signaling pathway and
then induced high expression levels of inflammatory factors, such
as IL-6, IL-8, IL-1β and TNF-α, leading to inflammatory stress in
the alveolar epithelial cells. In contrast, OP-D had an
anti-inflammatory role via activation of AMPK to inhibit the
PM2.5-activated NF-κB pathway. These findings suggested that the
anti-inflammatory activity of OP-D may be a valuable strategy for
protection against PM2.5- or other cause-induced pulmonary injury
progression.
Due to the use of heating systems during the long
winter season, air pollution in the city of Changchun is a serious
issue during this time period, which markedly increases the
concentrations of PM2.5. According to the environmental data
released by the Ministry of Ecological Environment of the P.R.
China, during the winter of 2017, the concentration of PM2.5 was
60-90 µg/m3 in Changchun, which reached 2-3 times that
of other seasons in the same area (21), he concentration, composition and
properties of the particles vary with the region and season. Our
previous study found that PM2.5 contained numerous chemical
components, including Al, Ca, Fe, K, Zn, Mg, polycyclic aromatic
hydrocarbons, organic carbon and elemental carbon, of which Al and
organic carbon were the most abundant in the PM2.5 samples in
Changchun, China, during the winter (22). In the present study, the samples of
PM2.5 were collected over five months in winter. Therefore, the
present data may be more reflective of what individuals may be
exposed to during the winter. Since PM2.5 is composed of extremely
complex components, the toxic effects of different batches may
reveal different results. Further work will be conducted to observe
the anti-inflammatory activity of OP-D on additional samples
collected from different cities.
As the first defense barrier between air and lung
tissue, the airway epithelium is extremely vulnerable to the
stimulation of harmful substances, such as PM2.5 or cigarette
smoke. For example, He et al (9) have established that PM2.5 can cause
inflammatory effects in MLE-12 cells, which are derived from a
female mouse. In addition, multiple studies in male mice have
reported that PM2.5 can activate inflammation and lead to lung
injury (23-25).
Altogether, these findings suggest that sex differences do not
affect the outcome of PM2.5 exposure; therefore, the MLE-12 cell
line was selected to perform this research.
Previous investigations have demonstrated that PM2.5
can lead to an inflammatory response, which is suggested to be the
basic pathogenesis of respiratory disorders of the respiratory
system (26) and can damage
pulmonary function (27), making
the lungs more susceptible to infection. For example, Jeong et
al (28) have revealed that the
epidermal growth factor receptor/mitogen-activated protein
kinase/NF-κB/IL-8 pathway may be a possible mechanism for
PM2.5-induced lung toxicity. In addition, Li et al (29) observed that PM2.5 may induce
inflammatory responses via the Toll-like receptor 4/p38/NF-κB
pathway. Notably, activated NF-κB serves a crucial role in
PM2.5-induced inflammatory diseases. To date, NF-κB is known to
consist of five family member protein monomers (p65/RelA, RelB,
cRel, p50 and p52) that form homodimers or heterodimers to bind DNA
differentially (30). Moreover,
p65/RelA is activated and translocated into the nucleus via the
formation of different heterodimers with p50 or p52 (31,32).
The present study only determined the translocation of p65, not of
the other forms, to investigate the activation of NF-κB. It has
previously been reported that PM2.5-induced p65 translocation
results in the release of certain inflammatory cytokines (20). After encountering inflammatory
irritants, p65, the most important subunit of NF-κB, undergoes
phosphorylation (activation) and translocates to the nucleus,
acting as a transcriptional activator to promote the expression of
various downstream proinflammatory mediators, including TNF-α, IL-6
and IL-1β (8). A previous study
revealed that PM2.5 exposure activates the NF-κB complex via
phosphorylation of nuclear p65 and cytoplasmic IκB kinase-α,
leading to nuclear p65/p50 DNA binding in human lung epithelial
cells in a time- and concentration-dependent manner (33). These findings are consistent with
the present results.
In the current study, immunofluorescence staining
demonstrated that there was a greater level of NF-κBp65 in the
nuclei of PM2.5-treated MLE-12 cells, compared with that of the
blank control group (Fig. 3).
Semi-quantitative analysis via western blotting indicated that the
nuclear translocation of NF-κBp65 and the phosphorylation level of
NF-κBp65 were ~3 (Fig. 4B) and four
(Fig. 4A) times greater, compared
with the blank control group. In addition, the cytoplasmic NF-κBp65
was reduced significantly only in the PM2.5-treated MLE-12 cells,
compared with that in the blank control group (Fig. 4B). It was also found that PM2.5
stimulation significantly induced the levels of IL-1β, IL-6, IL-8
and TNF-α in MLE-12 cells, compared with control cells (Fig. 2). However, the PM2.5-induced
inflammatory cytokine response may induce the death of pulmonary
epithelial cells, inhibit the junctional gap between these cells to
block intercellular communication and impair their function,
further leading to alveolar collapse (34,35).
OP-D significantly downregulated the expression of
the PM2.5-triggered cytokines in a dose-dependent manner (Fig. 2), indicating that OP-D may have
protective effects against PM2.5-induced inflammation.
PM2.5-induced nuclear translocation and phosphorylation of NF-κBp65
were partly suppressed by OP-D (Figs.
3 and 4), which suggested that
OP-D had a protective effect against PM2.5-induced inflammation via
the NF-κB signaling pathway.
AMPK is a crucial sensor that regulates the
intracellular ATP to AMP ratio in all eukaryotic cells (36). It has been reported that AMPK
activation attenuates cigarette smoke-induced inflammatory responses
in human lung epithelial cells to protect against the development
of emphysema (37). Moreover,
activated AMPK inhibits the inflammatory response via the
attenuation of NF-κB phosphorylation to block the production of
proinflammatory cytokines (38).
Based on these findings, it was hypothesized that the protective
mechanism of OP-D may be partly attributed to activation of AMPK
signaling in PM2.5-stimulated MLE-12 cells. Indeed, the present
results supported this hypothesis, as it was demonstrated that the
activity of OP-D inhibited the release of IL-6, IL-8, IL-1β and
TNF-α via activation of AMPK to block the NF-κB pathway in
PM2.5-stimulated MLE-12 cells (Figs.
5 and 6). Furthermore, the AMPK
signaling pathway was identified to have a significant protective
effect on cellular viability (Fig.
7). However, AMPK activation cannot completely inhibit the
activation of NF-κB and the production of cytokines, which suggests
that other signaling pathways may be involved in the
anti-inflammatory effect of OP-D in PM2.5-treated alveolar
epithelial cells. Therefore, the specific anti-inflammatory
mechanism of OP-D should be investigated in future studies. The
present study demonstrated the dose effects of OP-D in the cells;
however, whether the doses are equivalent in the in vivo
exposure has not been elucidated. Hence, in order to further
evaluate the pharmacological effect and value of OP-D, an
additional study on the function and dose correlation of OP-D is
underway in a mouse model of PM2.5-induced emphysema.
In conclusion, the present study demonstrated that
OP-D has anti-inflammatory effects against the PM2.5-induced damage
in alveolar epithelial cells. The mechanism of OP-D activity may be
via inhibiting the expression of inflammatory cytokines, such as
IL-6, IL-8, IL-1β and TNF-α, activating phosphorylated AMPK and
inhibiting activation of the NF-κB pathway. Therefore, OP-D could
potentially be an efficient and therapeutic drug for assisting in
the treatment of respiratory inflammation caused by PM2.5. The
present research also provides theoretical evidence for the
transformation of OP-D into clinical applications.
Acknowledgements
Not applicable.
Funding
This work was supported by the Project of Science
and Technology Agency of Jilin Province (Grant no. 2017J044) and
the 59th Chinese postdoctoral program of China (Grant No.
801161020425).
Availability of data and materials
The data used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HD and YW designed most of the investigation,
performed data analysis and wrote the manuscript. YW provided
experimental technical assistance, and DL and LS contributed to
interpretation of the data and analyses. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zheng Q, Liu H, Zhang J and Chen D: The
effect of ambient particle matters on hospital admissions for
cardiac arrhythmia: A multi-city case-crossover study in China.
Environ Health. 17(60)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liao J, Yu H, Xia W, Zhang B, Lu B, Cao Z,
Liang S, Hu K, Xu S and Li Y: Exposure to ambient fine particulate
matter during pregnancy and gestational weight gain. Environ Int.
119:407–412. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sram RJ, Veleminsky M Jr, Veleminsky M Sr
and Stejskalová J: The impact of air pollution to central nervous
system in children and adults. Neuro Endocrinol Lett. 38:389–396.
2017.PubMed/NCBI
|
|
4
|
Xing YF, Xu YH, Shi MH and Lian YX: The
impact of PM2.5 on the human respiratory system. J Thorac Dis.
8:E69–E74. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Happo MS, Salonen RO, Hälinen AI, Jalava
PI, Pennanen AS, Kosma VM, Sillanpää M, Hillamo R, Brunekreef B,
Katsouyanni K, et al: Dose and time dependency of inflammatory
responses in the mouse lung to urban air coarse, fine, and
ultrafine particles from six European cities. Inhal Toxicol.
19:227–246. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang G, Zhao J, Jiang R and Song W: Rat
lung response to ozone and fine particulate matter (PM2.5)
exposures. Environ Toxicol. 30:343–356. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
de F C Lichtenfels AJ, van der Plaat DA,
de Jong K, van Diemen CC, Postma DS, Nedeljkovic I, van Duijn CM,
Amin N, la Bastide-van Gemert S, de Vries M, et al: Long-term air
pollution exposure, genome-wide DNA methylation and lung function
in the lifelines cohort study. Environ Health Perspect.
126(027004)2018.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Zhang Y, Wang S, Zhu J, Li C, Zhang T, Liu
H, Xu Q, Ye X, Zhou L and Ye L: Effect of atmospheric PM2.5 on
expression levels of NF-κB genes and inflammatory cytokines
regulated by NF-κB in human macrophage. Inflammation. 41:784–794.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
He M, Ichinose T, Yoshida S, Ito T, He C,
Yoshida Y, Arashidani K, Takano H, Sun G and Shibamoto T:
PM2.5-induced lung inflammation in mice: Differences of
inflammatory response in macrophages and type II alveolar cells. J
Appl Toxicol. 37:1203–1218. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Peixoto CA, Oliveira WH, Araújo SMDR and
Nunes AKS: AMPK activation: Role in the signaling pathways of
neuroinflammation and neurodegeneration. Exp Neurol. 298:31–41.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Grahame Hardie D: AMP-activated protein
kinase: A key regulator of energy balance with many roles in human
disease. J Intern Med. 276:543–559. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang J, Hu Y, Wang D, Qin T, Liu C, Liu
X, Sheng X, Chang S, Fan Y, Guo LW and Nguyen TL: The optimization
of sulfation modification conditions for ophiopogonpolysaccharide
based on antiviral activity. Int J Biol Macromol. 51:657–662.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liang H, Xing Y, Chen J, Zhang D, Guo S
and Wang C: Antimicrobial activities of endophytic fungi isolated
from Ophiopogon japonicus (Liliaceae). BMC Complement Altern
Med. 12(238)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kou J, Sun Y, Lin Y, Cheng Z, Zheng W, Yu
B and Xu Q: Anti-inflammatory activities of aqueous extract from
Radix Ophiopogon japonicus and its two constituents. Biol
Pharm Bull. 28:1234–1238. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chang JM, Shen CC, Huang YL, Chien MY, Ou
JC, Shieh BJ and Chen CC: Five new homoisoflavonoids from the tuber
of Ophiopogon japonicus. J Nat Prod. 65:1731–1733.
2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kou J, Tian Y, Tang Y, Yan J and Yu B:
Antithrombotic activities of aqueous extract from Radix
Ophiopogon japonicus and its two constituents. Biol Pharm
Bull. 29:1267–1270. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Takahama K and Miyata T: Cough-diversity
and the peripheral mechanisms of production. Nihon Yakurigaku
Zasshi. 105:41–52. 1995.(In Japanese). PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qian J, Jiang F, Wang B, Yu Y, Zhang X,
Yin Z and Liu C: Ophiopogonin D prevents H2O2-induced injury in
primary human umbilical vein endothelial cells. J Ethnopharmacol.
128:438–445. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang YY, Meng C, Zhang XM, Yuan CH, Wen
MD, Chen Z, Dong DC, Gao YH, Liu C and Zhang Z: Ophiopogonin D
attenuates doxorubicin-induced autophagic cell death by relieving
mitochondrial damage in vitro and in vivo. J Pharmacol Exp Ther.
352:166–174. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Song L, Li D, Li X, Ma L, Bai X, Wen Z,
Zhang X, Chen D and Peng L: Exposure to PM2.5 induces aberrant
activation of NF-κB in human airway epithelial cells by
downregulating miR-331 expression. Environ Toxicol Pharmacol.
50:192–199. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Monthly report on Urban air quality from
the Ministry of ecology and environment of the People's Republic of
China. http://www.mee.gov.cn/hjzl/dqhj/cskqzlzkyb/index_2.shtml.
Accessed August 11, 2020.
|
|
22
|
Song L, Li D, Gu Y, Li X and Peng L:
Let-7a modulates particulate matter (≤2.5 µm)-induced oxidative
stress and injury in human airway epithelial cells by targeting
arginase 2. J Appl Toxicol. 36:1302–1310. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Ogino K, Nagaoka K, Ito T, Takemoto 1,
Okuda T, Nakayama SF, Ogino N, Seki Y, Hamada H, Takashiba S and
Fujikura Y: Involvement of PM2.5-bound protein and metals in
PM2.5-induced allergic airway inflammation in mice. Inhal Toxicol.
30:498–508. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ogino K, Nagaoka K, Okuda T, Oka A, Kubo
M, Eguchi E and Fujikura Y: PM2.5-induced airway inflammation and
hyperresponsiveness in NC/Nga mice. Environ Toxicol. 32:1047–1054.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yue W, Tong L, Liu X, Weng X, Chen X, Wang
D, Dudley SC, Weir EK, Ding W, Lu Z, et al: Short term Pm2.5
exposure caused a robust lung inflammation, vascular remodeling,
and exacerbated transition from left ventricular failure to right
ventricular hypertrophy. Redox Biol. 22(101161)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li R, Zhou R and Zhang J: Function of
PM2.5 in the pathogenesis of lung cancer and chronic airway
inflammatory diseases. Oncol Lett. 15:7506–7514. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Akther T, Ahmed M, Shohel M, Ferdousi FK
and Salam A: Particulate matters and gaseous pollutants in indoor
environment and association of ultra-fine particulate matters
(PM1) with lung function. Environ Sci Pollut Res Int.
26:5475–5484. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jeong SC, Cho Y, Song MK, Lee E and Ryu
JC: Epidermal growth factor receptor (EGFR)-MAPK-nuclear
factor(NF)-κB-IL8: A possible mechanism of particulate matter(PM)
2.5-induced lung toxicity. Environ Toxicol. 32:1628–1636.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li R, Zhao L, Tong J, Yan Y and Xu C: Fine
particulate matter and sulfur dioxide coexposures induce rat lung
pathological injury and inflammatory responses Via TLR4/p38/NF-κB
pathway. Int J Toxicol. 36:165–173. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tilborghs S, Corthouts J, Verhoeven Y,
Arias D, Rolfo C, Trinh XB and Dam PA: The role of nuclear
factor-kappa B signaling in human cervical cancer. Crit Rev Oncol
Hematol. 120:141–150. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Leung TH, Hoffmann A and Baltimore D: One
nucleotide in a kappaB site can determine cofactor specificity for
NF-kappaB dimers. Cell. 118:453–464. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mukherjee SP, Behar M, Birnbaum HA,
Hoffmann A, Wright PE and Ghosh G: Analysis of the RelA: CBP/p300
interaction reveals its involvement in NF-κB-driven transcription.
PLoS Biol. 11(e1001647)2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dagher Z, Garcon G, Billet S, Verdin A,
Ledoux F, Courcot D, Aboukais A and Shirali P: Role of nuclear
factor-kappa B activation in the adverse effects induced by air
pollution particulate matter (PM2.5) in human epithelial lung cells
(L132) in culture. J Appl Toxicol. 27:284–290. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Jacquemin B, Lanki T, Yli-Tuomi T, Vallius
M, Hoek G, Heinrich J, Timonen K and Pekkanen J: Source
category-specific PM2.5 and urinary levels of Clara cell protein
CC16. The ULTRA study. Inhal Toxicol. 21:1068–1076. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Longhin E, Holme JA, Gutzkow KB, Arlt VM,
Kucab JE, Camatini M and Gualtieri M: Cell cycle alterations
induced by urban PM2.5 in bronchial epithelial cells:
Characterization of the process and possible mechanisms involved.
Part Fibre Toxicol. 10(63)2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ke R, Xu Q, Li C, Luo L and Huang D:
Mechanisms of AMPK in the maintenance of ATP balance during energy
metabolism. Cell Biol Int. 42:384–392. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cheng XY, Li YY, Huang C, Li J and Yao HW:
AMP-activated protein kinase reduces inflammatory responses and
cellular senescence in pulmonary emphysema. Oncotarget.
8:22513–22523. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang J, Zhang Y, Xiao F, Liu Y, Wang J,
Gao H, Rong S, Yao Y, Li J and Xu G: The peroxisome
proliferator-activated receptor γ agonist pioglitazone prevents
NF-κB activation in cisplatin nephrotoxicity through the reduction
of p65 acetylation via the AMPK-SIRT1/p300 pathway. Biochem
Pharmacol. 101:100–111. 2016.PubMed/NCBI View Article : Google Scholar
|