Introduction
All species maintain a 24-h solar cycle of rest and
activity, and disrupting this cycle affects adaptation and
homeostasis (1). Similar to fish
that do not sense the water except if they are placed in a dry
environment, the quotidian normality of sleep is not perceived
until it is disrupted (1). For
example, 30% of the adult population complain of transient
insomnia, and 10% experience chronic insomnia that disrupts daytime
function (2). Patients with chronic
insomnia experience low work productivity and a high number of
absenteeism incidents, accidents and hospitalizations, resulting in
treatment costs of 60 billion dollars annually (3). Schisandra, a Traditional Chinese
Medicine, is the dried ripe fruit of Schisandra chinensis
(Turcz.) Baill. (Magnoliaceae), and has been used to treat insomnia
for hundreds of years (4,5). Schisandrin B (SchB) is a monomeric
compound of Schisandra lignans (Fig.
1). According to a previous study in rats, SchB exerts sedative
and hypnotic effects, which are associated with the upregulation of
the inhibitory neurotransmitter γ-aminobutyric acid (GABA) and both
the mRNA and protein expression of its two receptors,
GABAA receptor α1 (GABAA Rα1) and
GABAA receptor γ2 (GABAA Rγ2) (6). However, reports have demonstrated that
as well as GABA, 5-hydroxytryptamine (5-HT), another central
neurotransmitter, also plays an important role in sleep regulation
(7,8). The decarboxylation of
5-hydroxytryptophan, a 5-HT precursor, is a key step during the
synthesis of 5-HT, and the tryptophan hydrogenase enzyme is
essential for this step (9).
Parachlorophenylalanine (PCPA) can antagonize tryptophan
hydrogenase and inhibit the synthesis of 5-HT, resulting in
insomnia with less slow wave sleep and circadian rhythm
disturbances (10,11). Therefore, in the present study, a
PCPA-induced insomnia rat model was used to determine whether SchB
exerted its sedative and hypnotic effects by regulating 5-HT. The
study aimed to provide experimental evidence for further
development of SchB as a sleep-promoting supplement and
medicine.
Materials and methods
Chemicals and reagents
SchB (Chengdu Pukang Biotechnology Co., Ltd.), the
crude powder of Schisandra sphenanthera Rehd. et Wils, was
added to 10X the volume of ethanol and extracted three times for 1
h each. The combined filtrate was then concentrated to a small
volume, and the sample was mixed with diatomite and extracted three
times with n-hexane-ethyl acetate at a 10:1 ratio; the filtrate was
then concentrated once more. The sample was mixed with silica gel
for gradient elution with petroleum ether-ethyl acetate (1:0-5:1),
and the sample fraction containing SchB was collected and
concentrated until dry, and then dissolved in anhydrous ethanol at
4-10˚C until crystallization. After repeated recrystallization with
anhydrous ethanol (2-3 times), the purity of SchB reached >98%.
PCPA was purchased from Sigma-Aldrich (Merck KGaA), and rat GABA,
glutamate (Glu), glutamic acid decarboxylase (GAD), γ-aminobutyric
acid transaminase (GABA-T), 5-HT and 5-hydroxyindoleacetic acid
(5-HIAA) ELISA kits were purchased from Zhongsheng Beikong
Biotechnology Co., Ltd. (http://www.zhongsheng.com.cn/). GABAA Rα1
(1:1,000; cat. no. ab252430) and GABAA Rγ2 (1:1,000;
cat. no. ab87328) were acquired from Abcam. 5-hydroxytryptamine
receptor 1A (HTR1A; 1:1,000; cat. no. A2801), β-actin (1:100,000;
cat. no. AC026) and HRP goat anti-rabbit IgG (H+L) (1:5,000; cat.
no. AS014) were acquired from ABclonal Biotech Co., Ltd. All
antibodies were diluted with TBS with containing 0.1% Tween-20
(TBST).
Experimental animals
A total of 52 eight week-old male Wistar rats,
weighing 200±10 g, were provided by Changchun Yis Experimental
Animal Technology Co., Ltd., license no. SCXK (Jilin) 2016-0003.
The animals were maintained in separate cages in a quiet
environment at 18-22˚C and 50-60% humidity with a 12-h light/dark
cycle and under specific pathogen-free conditions, with free access
to food and water, and were acclimated to the laboratory
environment for 7 days before experimentation.
Animal grouping and treatment
For reproduction of the insomnia model, rats were
maintained for 1 week and then intraperitoneally administered with
PCPA (300 mg/kg) once a day for 3 days. The disappearance of the
circadian rhythm and continuous animal activity during the day
indicated successful model establishment.
For sleep behavioral experiments, 24 male Wistar
rats were randomly divided into the blank control (CON), model
(PCPA), SchB, and PCPA + SchB groups (6 rats per group). Rats in
the CON and PCPA groups were administered equal volumes of
distilled water, and those in the SchB and PCPA + SchB groups
received 3.5 mg/kg SchB once a day for 7 days. In our previous
research, we observed the sedative and hypnotic effects of SchB in
mice at an effective dose of 5 mg/kg (6). Based on this, we calculated the
equivalent dose of 3.5 mg/kg for rats, which was effective in the
preliminary experiment. So, 3.5 mg/kg SchB was selected as the
appropriate dose in the present study. Following the sleep
behavioral experiments, the rats were anesthetized with ether, as
it is easy to evaporate using a simple device, rarely causes animal
death due to overdose, and has no effects on the experimental data.
The status of anesthesia was judged by falling, relaxed limbs and
loss of the skin pain reflex. The presence of a heartbeat and
breathing confirmed that the rats were alive. The rats were
sacrificed by exsanguination from the abdominal aorta under ether
anesthesia for 10 min (12). The
animal experiments were approved by the Institutional Animal Care
and Use Committee of Beihua University (approval no. 20190301).
A total of 28 male Wistar rats (grouped and treated
as aforementioned; 7 rats per group) were used for the detection of
biomarkers associated with sleep regulation. The rats were
sacrificed by exsanguination from the abdominal aorta after 10 min
of ether anesthesia. The hypothalamus was then removed from the
brain tissue, homogenized, and separated to harvest the
supernatant. The levels of 5-HT, 5-HIAA, GABA and Glu, as well as
the activities of GAD and GABA-T in the rat hypothalamus were
determined by ELISA. Protein expression of HTR1A, GABAA
Rα1 and GABAA Rγ2 was detected by western blotting.
Sleep experiments for the synergistic
effects of SchB and pentobarbital sodium
On the 7th day, 40 min after the last administration
of SchB, the rats in all four groups were intraperitoneally treated
with a subthreshold dose of pentobarbital sodium (28 mg/kg), and
the number of sleeping rats was observed. After another two days,
the rats received a threshold dose of pentobarbital sodium (35
mg/kg) to distinguish sleep latency and duration. At 30 min
post-pentobarbital sodium administration, the number of sleeping
rats (determined by the disappearance of the righting reflex for
>1 min), the sleep latency (the time from the start of
administration to the start of sleep) and the sleep duration were
observed.
Detection of 5-HT, 5-HIAA, GABA and
Glu levels, and GAD and GABA-T activity in the rat
hypothalamus
On the 7th day, 40 min after the last administration
of SchB, the rats were anesthetized with ether and sacrificed by
exsanguination from the abdominal aorta. The hypothalamus was
rapidly isolated on ice, homogenized and separated to generate the
supernatant. 5-HT, 5-HIAA, GABA and Glu levels, as well as the
activities of GAD and GABA-T were detected by ELISA kits (5-HT,
cat. no. 201506; 5-HIAA, cat. no. 201506; GABA, cat. no. 201512;
Glu, cat. no. 201512; GAD, cat. no. 201512; and GABA-T, cat. no.
201512) from Zhongsheng Beikong Biotechnology Co., Ltd. (http://www.zhongsheng.com.cn/), according to the
manufacturer's instructions.
Western blot detection of HTR1A,
GABAA Rα1 and GABAA Rγ2 protein expression in
the rat hypothalamus
The hypothalamus of each rat was immersed in lysis
buffer (protein lysate; Beijing Solarbio Science & Technology
Co., Ltd.) on ice for 1 h, and the supernatant was separated by
centrifugation at 16,000 x g and 4˚C for 10 min. Protein
concentration was determined using the BCA method, and the target
proteins (60 µg per lane) were separated via SDS-PAGE on a 10% gel,
and subsequently transferred to PVDF membranes for 2 h. The
membranes were rinsed with Tris buffer for 5 min, and then blocked
with blocking buffer (TBST buffer containing 5% skimmed milk
powder) for 1 h at room temperature. The blocking buffer was
discarded, and the corresponding primary antibodies were added and
incubated at 4˚C overnight. The membranes were washed with TBST
five times for 5 min each time, and incubated for 2 h with the
corresponding secondary antibodies. The membranes were washed with
TBST five times (5 min each) and were visualized using the enhanced
electrochemiluminescence method. ImageJ (version 1.51j8; National
Institutes of Health) was used to perform the western blotting
densitometric analysis.
Statistical analysis
SPSS 20.0 software (IBM Corp.) was used for all
statistical analyses. All quantitative data are expressed as the
mean ± SD, the mean value is taken from each rat in each group
treated with or without SchB. The data between the two groups were
compared using one-way ANOVA (followed by Tukey's test). Each assay
was performed in three experimental repeats. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of SchB in combination with a
subthreshold dose of pentobarbital sodium on the number of sleeping
rats
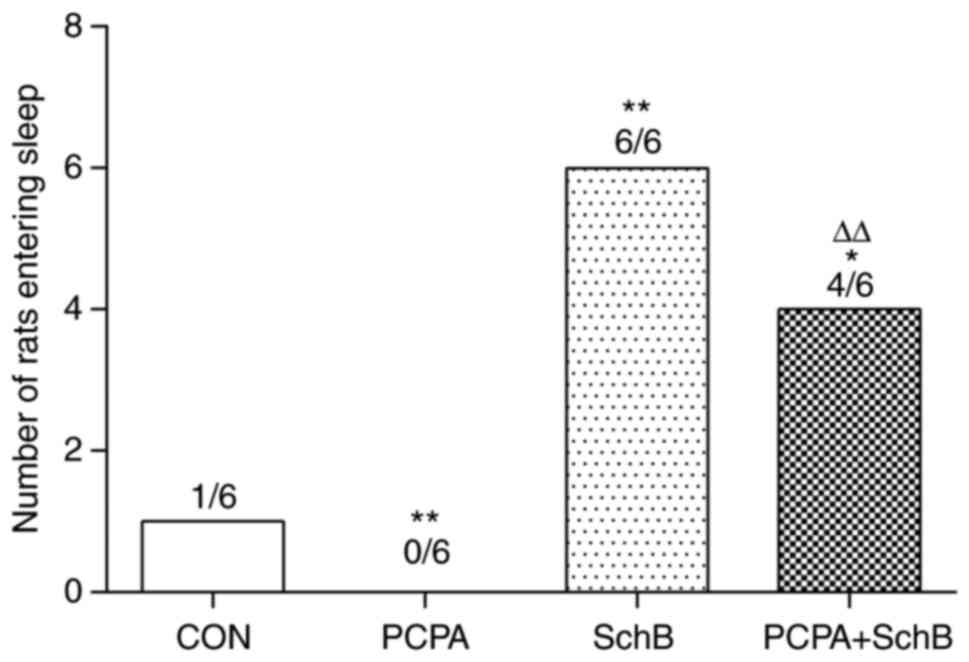
To evaluate the hypnotic effects of SchB, a sleep
experiment was performed on rats treated with a subthreshold dose
of pentobarbital sodium. As shown in Fig. 2, the number of sleeping rats in the
SchB group was significantly increased compared with that in the
CON group (P<0.01), indicating the hypnotic role of SchB.
Compared with the PCPA group, the number of sleeping rats in the
PCPA + SchB group was significantly higher (P<0.01), suggesting
that SchB and pentobarbital sodium exerted a synergistic effect to
induce sleep in rats.
Effects of SchB in combination with a
threshold dose of pentobarbital sodium on sleep regulation in
rats
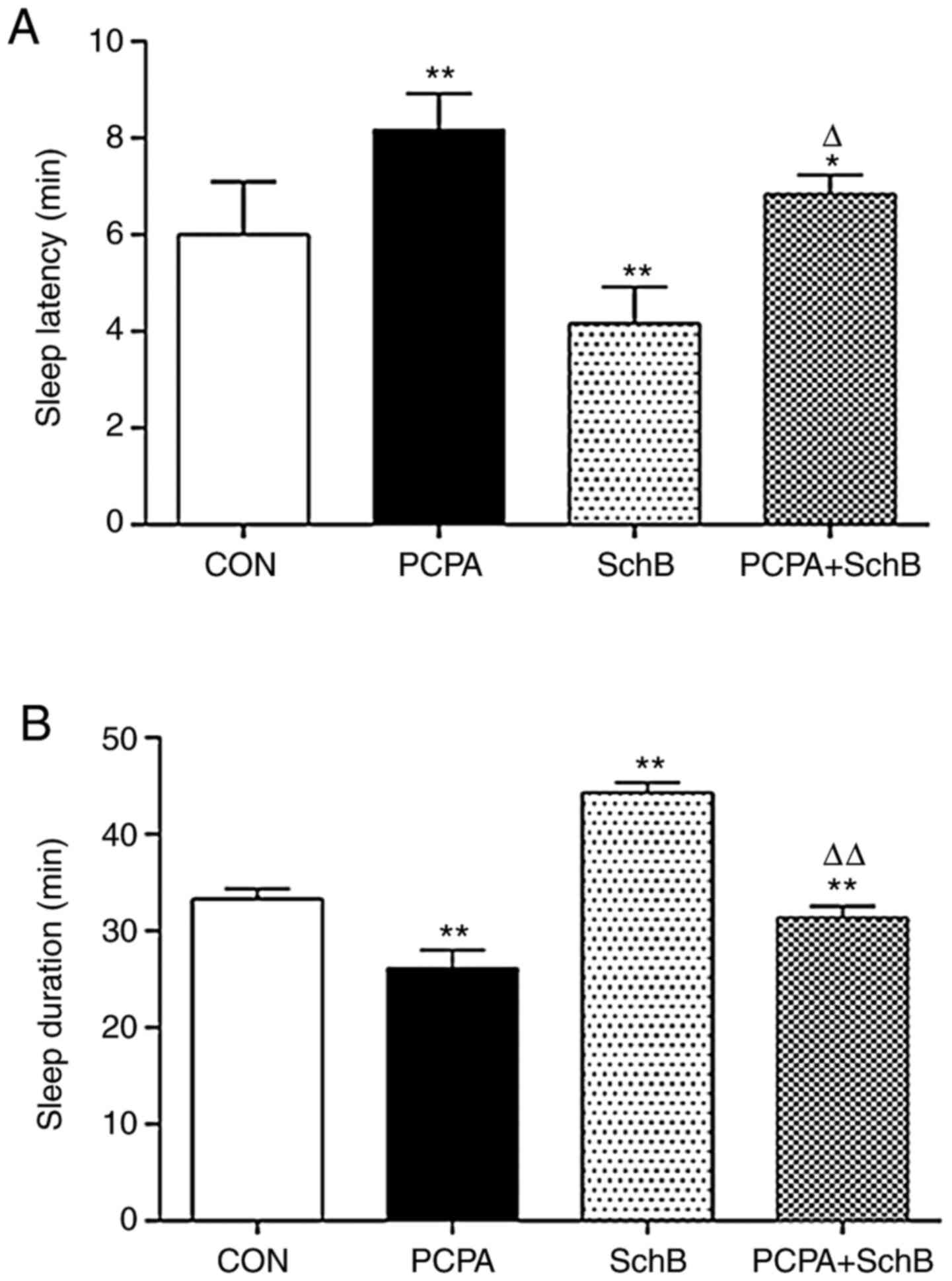
The effects of SchB in combination with a threshold
dose of pentobarbital sodium on sleep latency and duration were
observed in rats. The results indicate that compared with the CON
group, the sleep latency time of the SchB group was significantly
shorter, and the sleep duration was significantly prolonged
(P<0.01). Compared with the PCPA group, sleep latency was
reduced and sleep duration was significantly prolonged in the PCPA
+ SchB group (P<0.05 and P<0.01, respectively) (Fig. 3). In our previous study, SchB alone
could not produce significant hypnotic effects. However, following
the addition of a subthreshold (28 mg/kg) or threshold (35 mg/kg)
dose of pentobarbital sodium, SchB showed a significant synergistic
effect with pentobarbital sodium in comparison with control groups
(6).
Effects of SchB on the levels of 5-HT
and 5-HIAA in the hypothalamus
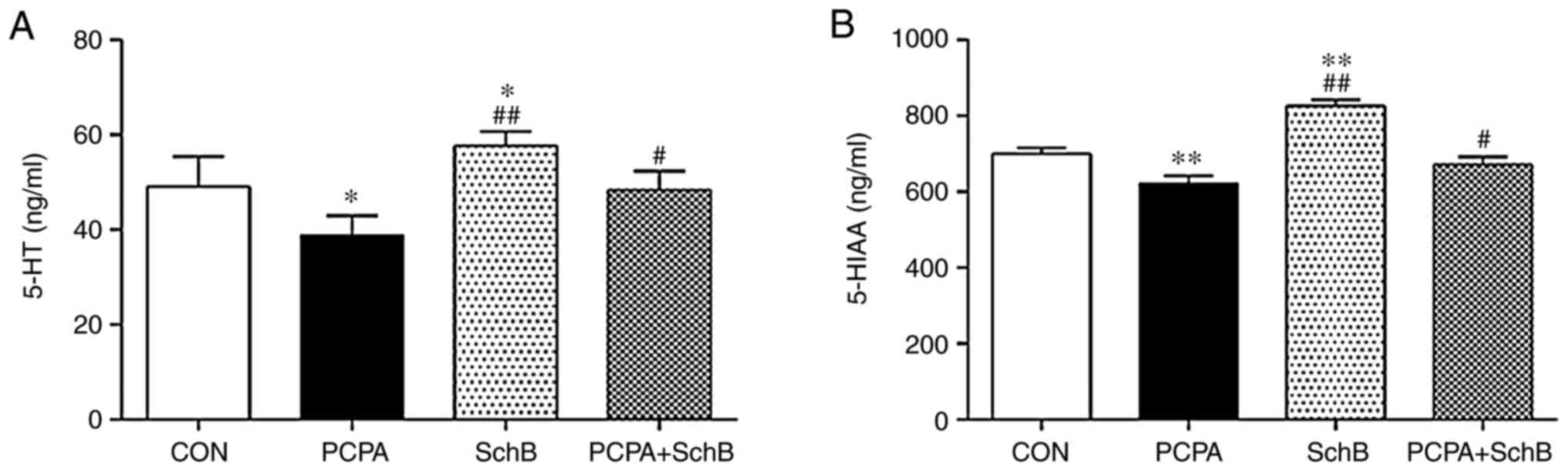
5-HT is an important central neurotransmitter in
sleep regulation, of which 5-HIAA is a primary metabolite (13). Compared with the CON group, the
levels of 5-HT and 5-HIAA in the hypothalami of rats in the SchB
group were significantly increased (P<0.05 and P<0.01,
respectively). Compared with the PCPA group, the levels of 5-HT and
5-HIAA in the hypothalami of rats in the PCPA + SchB group were
also significantly increased (P<0.05), suggesting that SchB
exerted a certain therapeutic effect on PCPA-induced insomnia
(Fig. 4).
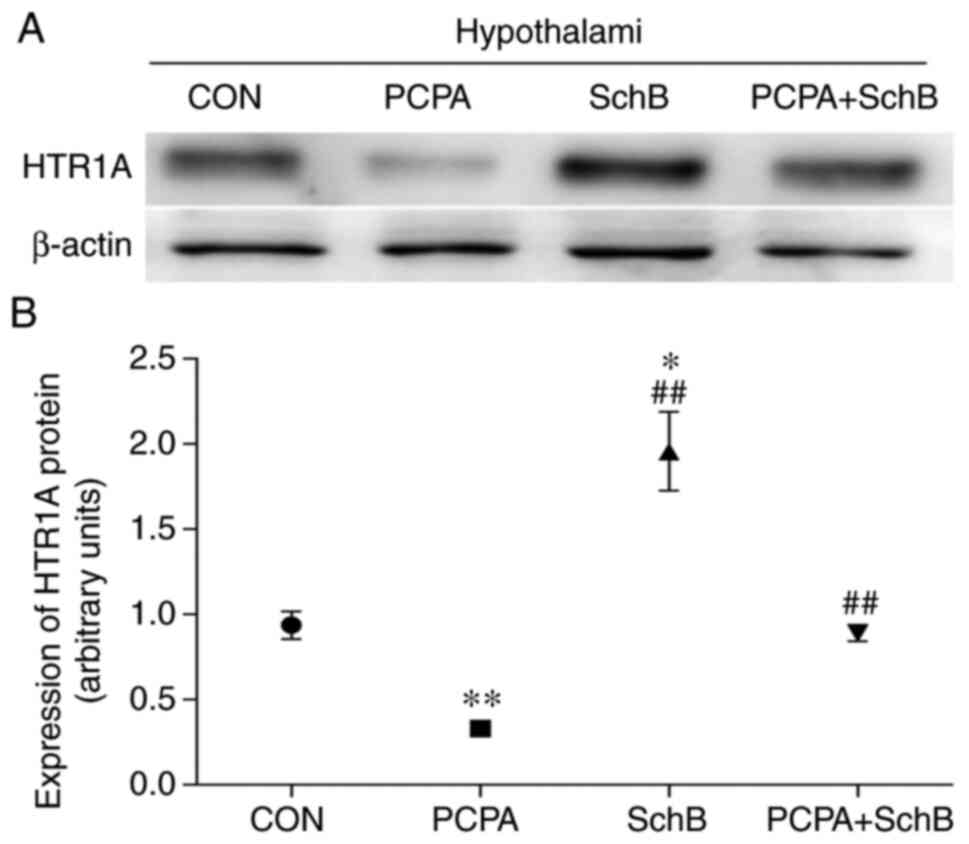
Effects of SchB on HTRlA protein
expression in the hypothalamus
As shown in Fig. 5,
HTRlA protein expression in the hypothalami of rats in the SchB
group was significantly higher than that in the CON group
(P<0.05), and the content of HTRlA in the hypothalami of rats in
the PCPA + SchB group was significantly higher than that in the
PCPA group (P<0.01). These findings suggested that SchB played a
sedative and hypnotic role by regulating the expression of HTRlA in
the rat hypothalamus.
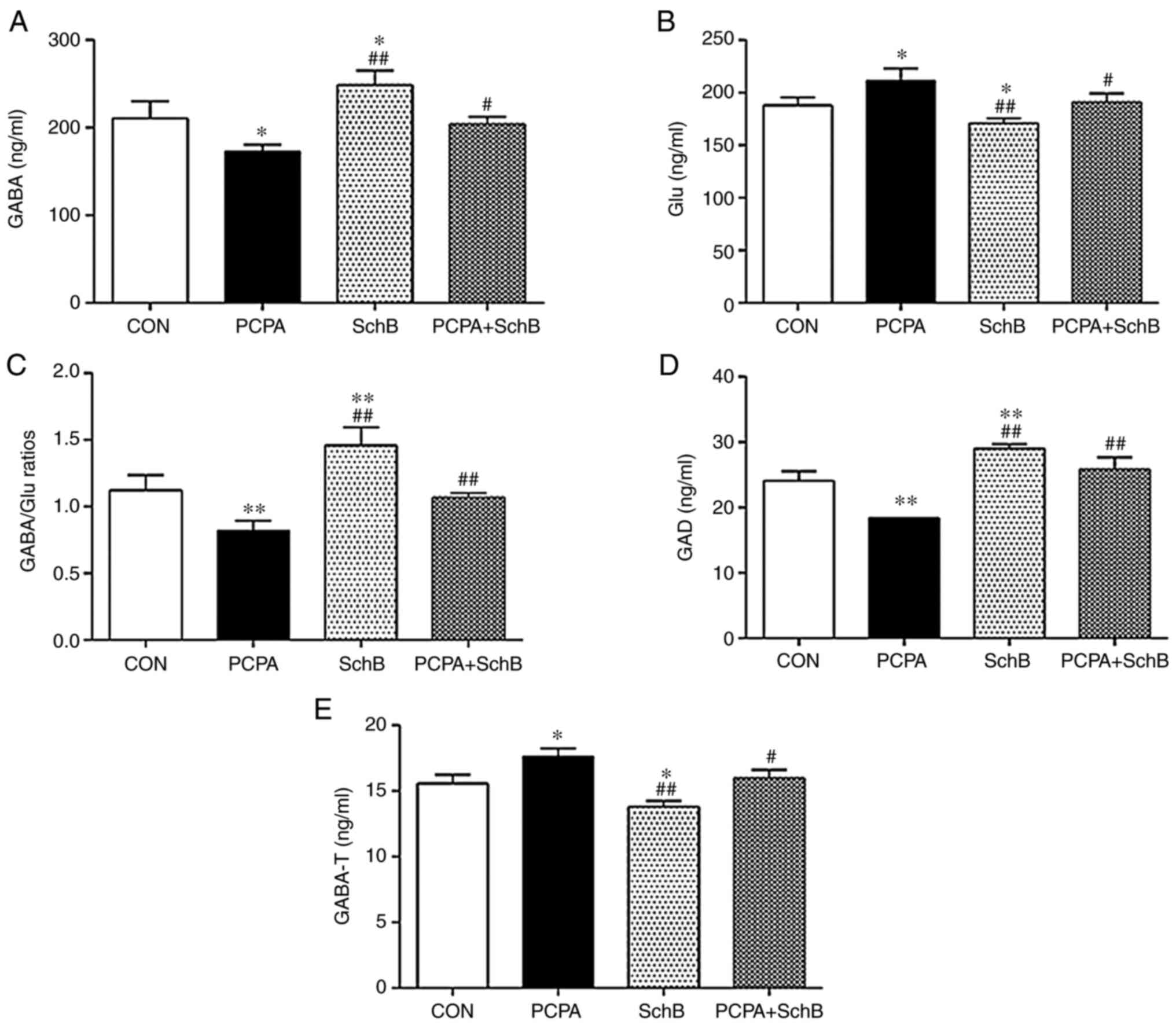
Effects of SchB on the levels of GABA
and Glu, and the activity of GAD and GABA-T in the rat
hypothalamus
GABA is the primary inhibitory neurotransmitter in
the central nervous system of mammals (14). GAD converts Glu to GABA (15) and GABA-T is the hydrolytic enzyme of
GABA (16). Therefore, the
activities of GAD and GABA-T were detected in the present study.
Compared with the CON group, the expression levels of GABA in the
hypothalami of rats in the SchB group were increased, the
expression of Glu was decreased, and the GABA/Glu ratio was
significantly increased. Furthermore, GAD activity was increased,
whereas the activity of GABA-T significantly decreased. Compared
with the PCPA group, the expression levels of GABA were increased
and those of Glu were decreased; the ratio of GABA/Glu was
increased, GAD activity was increased and GABA-T activity was
significantly decreased in the PCPA + SchB group (P<0.05 or
P<0.01) (Fig. 6).
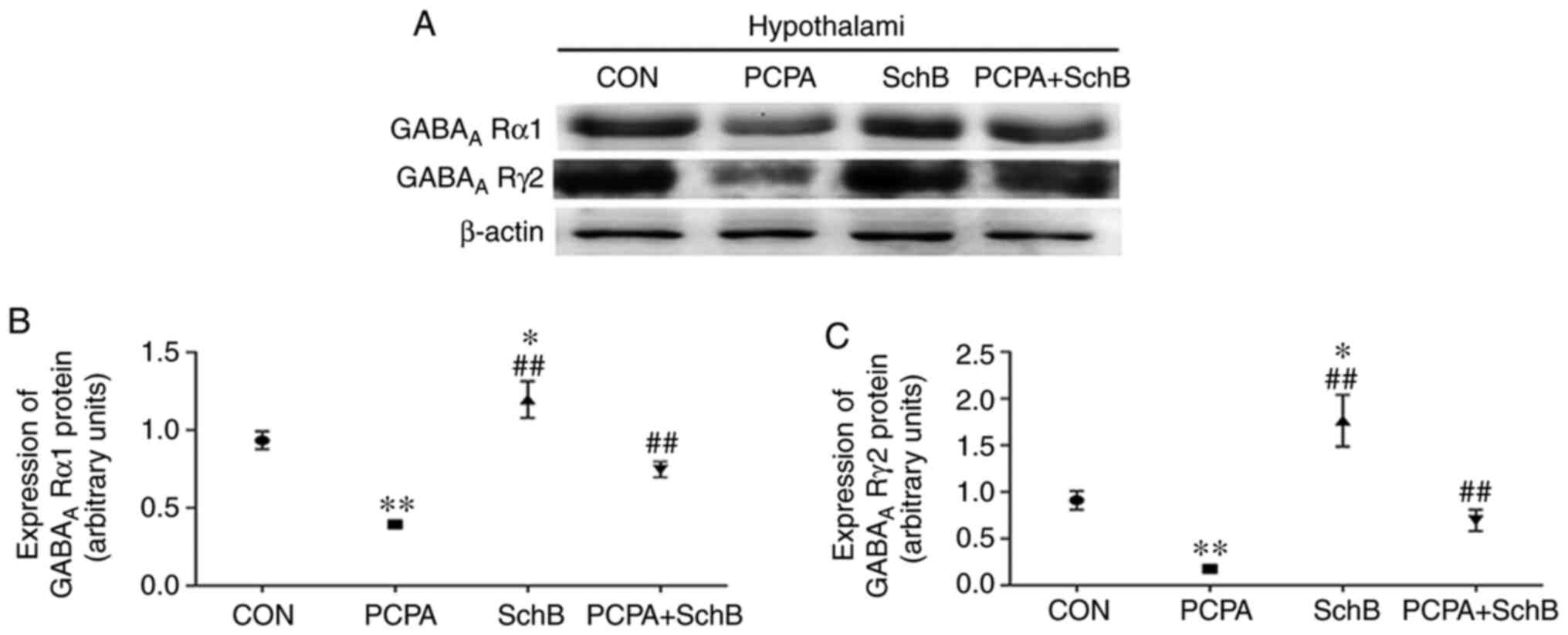
Effects of SchB on GABAA
Rα1 and GABAA Rγ2 protein expression in the rat
hypothalamus
The results demonstrated that the expression levels
of GABAA Rα1 and GABAA Rγ2 protein in the
hypothalami of rats in the SchB group were significantly higher
than those in the CON group, and that GABAA Rα1 and
GABAA Rγ2 protein expression in the hypothalami of the
PCPA + SchB group were significantly higher than in the PCPA group
(P<0.05 or P<0.01) (Fig. 7).
Collectively, these data indicated that SchB exerted its sedative
and hypnotic effects by regulating the expression of
GABAA Rα1 and GABAA Rγ2 in the rat
hypothalamus.
Discussion
As early as 1969, Jouvet (17) and Koella (18) proposed the theory that 5-HT induces
sleep. Later research indicated that low levels of 5-HT in the
brain resulted in insomnia, which could be reversed by the recovery
of 5-HT levels in the brain (19,20).
PCPA has the capacity to selectively inhibit tryptophan activity,
which blocks the synthesis of 5-HT (21,22).
However, once PCPA is metabolized, the synthesis of 5-HT can be
restored without substantial neuronal damage (23), and thus is recognized as a suitable
tool for establishing animal models of insomnia. In the present
study, PCPA predictably decreased the number of sleeping rats,
prolonged the latency of sleep and decreased sleep duration.
Conversely, SchB administration increased the number of sleeping
rats, reduced sleep latency and increased sleep duration in rats
treated with PCPA, indicating that SchB may improve insomnia
resulting from low 5-HT levels.
5-HT neurons affect the higher hypothalamic center
through ascending projections, and 5-HT and its receptors in the
hypothalamus are important components in the regulation of sleep
and arousal (24,25). 5-HIAA is a metabolite of 5-HT. 5-HT
is deaminated and converted into 5-hydroxyindole acetaldehyde by
monoamine oxidase, which is itself catalyzed by aldehyde
dehydrogenase into 5-HIAA; thus, the levels of 5-HIAA in the brain
reflect the activity of the 5-HT-nergic nervous system (13,26).
5-HIAA is associated with analgesia and sleep.
Previous studies have revealed that in animal models of insomnia or
sleep deprivation, 5-HIAA levels decrease with those of 5-HT
(27,28). The HTR1A gene encodes the 5-HT1A
receptor, the latter of which binds to the free form of 5-HT in the
synaptic space, thus mediating 5-HT function, increasing its
nervous system activity and promoting sleep (29). HTR1A is the most abundantly
expressed 5-HT receptor in the mammalian brain. Among various
biochemical reactions involving the 15 receptor subtypes of 5-HT
(and the associated receptor family), 5-HT dysfunction is the most
closely associated with HTR1A (30-32).
The results of the present study demonstrated that the expression
levels of 5-HT and 5-HIAA in the hypothalamus, and protein
expression in the hypothalami of rats with PCPA-induced insomnia,
were significantly decreased, but subsequently increased by SchB,
suggesting that the hypnotic effect of SchB may be associated with
5-HT.
GABA and Glu are the primary neurotransmitters in
the central nervous system of mammals (14,33,34).
When the levels of Glu are increased, neurons are activated,
promoting arousal and awakening of the body. When the level of GABA
is increased, neuronal excitation is inhibited and arousal is
inactivated, thus promoting sleep (35,36).
The GABA/Glu ratio maintains the balance between the inhibition and
excitation of nerve cells, and therefore, is often used to study
and evaluate the excitatory and inhibitory state of central nervous
system function. Specific binding of GABA and its receptor
instigates post-synaptic membrane chloride influx, resulting in
membrane hyperpolarization and the inhibition of neuronal activity.
This inhibition can be specifically blocked by GABA receptor
inhibitors (37). GABAA
Rα1 and GABAA Rγ2 are two important subunits of the GABA
receptor. In our previous study, SchB was found to increase the
levels of GABA, decrease the levels of Glu, increase the ratio of
GABA/Glu, and increase the expression of GABAA Rα1 and
GABAA Rγ2 in the hypothalami of rats (4). In the present study, similar changes
in these indicators were observed in the hypothalamic tissues of
rats treated with both SchB and PCPA, further confirming that the
hypnotic effect of SchB is associated with an increase in GABA and
a decrease of Glu.
GAD is a rate-limiting enzyme in the synthesis of
GABA from Glu (38), and under GAD
catalysis, the levels of GABA and Glu are increased and decreased,
respectively (15,39). GABA-T hydrolyses GABA. Mature GABA
is first deaminated by GABA-T to form succinic semialdehyde (SSA);
SSA is then converted to succinic acid, (catalyzed by succinate
semialdehyde dehydrogenase), and the end product (succinic acid)
then enters the tricarboxylic acid cycle to form Glu once again
(16,40-42).
It is therefore evident that the activities of GAD and GABA-T can
directly influence the levels of GABA and Glu. The present results
revealed that SchB significantly increased GAD activity and
decreased GABA-T activity in rats, with or without PCPA treatment,
suggesting that SchB altered the levels of GABA and Glu via GAD and
GABA-T.
In conclusion, the present study demonstrated that
SchB was able to improve PCPA-induced insomnia in rats, which may
be partly associated with its ability to elevate the levels of 5-HT
and GABA in the hypothalamus. The present study provided
experimental evidence showing the potential of Schisandra and SchB
to be further developed into sleep-promoting health supplements and
treatments for insomnia.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from Jilin
Provincial Education Department (grant no. JJKH20180376KJ); the
Jilin City Bureau of Science and Technology (grant no. 20163024);
the Jilin Provincial Department of science and Technology (grant
nos. 20170309006YY, 20200201521JC, 20200404053YY and
20200404022YY); the Jilin Science and technology innovation
development plan project (grant no. 20190601177); the Jilin
provincial health and Family Planning Commission (grant no.
2018J089); the Jilin Provincial Development and Reform Commission
(grant no. 2020C033-2); and the Jilin Administration of traditional
Chinese Medicine (grant no. 2020121).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JLL and JGC conceived and designed the study. MYW,
JHS, CMW, NL and SJ performed the animal experiments. MYW and HL
performed the data analysis. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the
Institutional Animal Care and Use Committee of Beihua University
(Jilin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davis EM, Ramani C and Quigg M: Neurologic
manifestations of systemic disease: Sleep disorders. Curr Treat
Options Neurol. 22(30)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Morin CM, LeBlanc M, Daley M, Gregoire JP
and Merette C: Epidemiology of insomnia: Prevalence, self-help
treatments, consultations, and determinants of help-seeking
behaviors. Sleep Med. 7:123–130. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chilcott LA and Shapiro CM: The
socioeconomic impact of insomnia. An overview. Pharmacoeconomics.
10 (Suppl 1):S1–S14. 1996.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang M, Xu L and Yang H: Schisandra
chinensis fructus and its active ingredients as promising
resources for the treatment of neurological diseases. Int J Mol
Sci. 19(1970)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhu H and Zhang L, Wang G, He Z, Zhao Y,
Xu Y, Gao Y and Zhang L: Sedative and hypnotic effects of
supercritical carbon dioxide fluid extraction from Schisandra
Chinensis in mice. J Food Drug Anal. 24:831–838.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li N, Liu J, Wang M, Yu Z, Zhu K, Gao J,
Wang C, Sun J, Chen J and Li H: Sedative and hypnotic effects of
Schisandrin B through increasing GABA/Glu ratio and upregulating
the expression of GABAA in mice and rats. Biomed
Pharmacother. 103:509–516. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chazalon M, Dumas S, Bernard JF, Sahly I,
Tronche F, de Kerchove d'Exaerde A, Hamon M, Adrien J, Fabre V and
Bonnavion P: The GABAergic Gudden's dorsal tegmental nucleus: A new
relay for serotonergic regulation of Sleep-wake behavior in the
mouse. Neuropharmacology. 138:135–330. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Murillo-Rodríguez E, Di Marzo V, Machado
S, Rocha NB, Veras AB, Neto GAM, Budde H, Arias-Carrión O and
Arankowsky-Sandoval G: Role of N-arachidonoyl-serotonin
(AA-5-HT) in sleep-wake cycle architecture, sleep homeostasis, and
neurotransmitters regulation. Front Mol Neurosci.
10(152)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sahu A, Gopalakrishnan L, Gaur N,
Chatterjee O, Mol P, Modi PK, Dagamajalu S, Advani J, Jain S and
Keshava Prasad TS: The 5-hydroxytryptamine signaling map: An
overview of serotonin-serotonin receptor mediated signaling
network. J Cell Commun Signal. 12:731–735. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang CY, Chang ET and Lai HL: Comparing
the effects of music and exercise with music for older adults with
insomnia. Appl Nurs Res. 32:104–110. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhu HY, Zhang D, Zhang Q, Zhao Y, He ZM,
Gao YG and Zhang LX: 4-Hydroxybenzyl alcohol derivatives and their
sedative-hypnotic activities. RSC Adv. 8:19539–19550. 2018.
|
|
12
|
Mitterhauser M, Wadsak W, Wabnegger L,
Sieghart W, Viernstein H, Kletter K and Dudczak R: In vivo and in
vitro evaluation of [18F]FETO with respect to the adrenocortical
and GABAergic system in rats. Eur J Nucl Med Mol Imaging.
30:1398–1401. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huang C, Li J, Lu L, Ren X, Li Y, Huang Q,
Lan Y and Wang Y: Interaction between serotonin transporter
gene-linked polymorphic region (5-HTTLPR) and Job-related stress in
insomnia: A cross-sectional study in Sichuan, China. Sleep Med.
15:1269–1275. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Malomouzh A, Ilyin V and Nikolsky E:
Components of the GABAergic signaling in the peripheral cholinergic
synapses of vertebrates: A review. Amino Acids. 51:1093–1102.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shi F, Luan M and Li Y: Ribosomal binding
site sequences and promoters for expressing glutamate decarboxylase
and producing γ-aminobutyrate in Corynebacterium glutamicum.
AMB Express. 8(61)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jalil SU, Ahmad I and Ansari MI:
Functional loss of GABA transaminase (GABA-T) expressed early leaf
senescence under various stress conditions in, Arabidopsis
thaliana. Curr Plant Biol. 9-10:11–22. 2017.
|
|
17
|
Jouvet M: Biogenic amines and the state of
sleep. Science. 163:32–41. 1969.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Koella WP: What is the functional role of
central nervous serotonin? Neurosci Res (N Y). 2:229–251.
1969.PubMed/NCBI
|
|
19
|
Si L, Wang Y, Wuyun G, Bao L and Agula B:
The effect of Mongolian medical acupuncture on cytokines and
neurotransmitters in the brain tissue of insomniac rats. Eur J
Integr Med. 7:492–498. 2015.
|
|
20
|
Monti JM, Perumal SRP, Spence DW and
Torterolo P: The involvement of 5-HT2A receptor in the
regulation of sleep and wakefulness, and the potential therapeutic
use of selective 5-HT2A Receptor antagonists
and inverse agonists for the treatment of an insomnia disorder.
5-HT2A Receptors Central Nervous System. 32:311–337. 2018.
|
|
21
|
Compan V, Dusticier N, Nieoullon A and
Daszuta A: Opposite changes in striatal neuropeptide Y
immunoreactivity after partial and complete serotonergic depletion
in the rat. Synapse. 24:87–96. 1996.
|
|
22
|
Gong H, Ni CX, Liu YZ, Zhang Y, Su WJ,
Lian YJ, Peng W and Jiang CL: Mindfulness meditation for insomnia:
A meta-analysis of randomized controlled trials. J Psychosom Res.
89:1–6. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nakazato T: Dual-mode dopamine increases
mediated by 5-HT1B and 5-HT2C receptors
inhibition, inducing impulsive behavior in trained rats. Exp Brain
Res. 237:2573–2584. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Thorpy MJ: Recently approved and upcoming
treatments for narcolepsy. CNS Drugs. 34:9–27. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun Y, Zhang N, Qu Y, Cao Y, Li J, Yang Y,
Yang T and Sun Y: Shuangxia decoction alleviates
p-chlorophenylalanine induced insomnia through the modification of
serotonergic and immune system. Metab Brain Dis. 35:315–325.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fredj Z, Ali M, Singh B and Dempsey E:
Simultaneous voltammetric detection of 5-hydroxyindole-3-acetic
acid and 5-hydroxytryptamine using a glassy carbon electrode
modified with conducting polymer and platinised carbon nanofibers.
Mikrochim Acta. 185(412)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen ML, Gao J, He XR and Chen Q:
Involvement of the cerebral monoamine neurotransmitters system in
antidepressant-like effects of a Chinese herbal decoction, baihe
dihuang tang, in mice model. Evid Based Complement Alternat Med.
2012(419257)2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shi R, Han Y, Yan Y, Qiao HY, He J, Lian
WW, Xia CY, Li TL, Zhang WK and Xu JK: Loganin exerts sedative and
hypnotic effects via modulation of the serotonergic system and
GABAergic neurons. Front Pharmacol. 10(409)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nakamura Y, Ishii J and Kondo A:
Applications of yeast-based signaling sensor for characterization
of antagonist and analysis of Site-directed mutants of the human
serotonin 1A receptor. Biotechnol Bioeng. 112:1906–1915.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Philippe TJ, Vahid-Ansari F, Donaldson ZR,
Le François B, Zahrai A, Turcotte-Cardin V, Daigle M, James J, Hen
R, Merali Z and Albert PR: Loss of MeCP2 in adult 5-HT neurons
induces 5-HT1A autoreceptors, with opposite sex-dependent anxiety
and depression phenotypes. Sci Rep. 8(5788)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cho SM, Shimizu M, Lee CJ, Han DS, Jung
CK, Jo JH and Kim YM: Hypnotic effects and binding studies for
GABA(A) and 5-HT(2C) receptors of traditional medicinal plants used
in Asia for insomnia. J Ethnopharmacol. 132:225–232.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yücel Y, Coşkun S, Cengiz B, Özdemir HH,
Uzar E, Çim A, Camkurt MA and Aluclu MU: Association of
polymorphisms within the serotonin receptor genes 5-HTR1A, 5-HTR1B,
5-HTR2A and 5-HTR2C and migraine susceptibility in a Turkish
Population. Clin Psychopharmacol Neurosci. 14:250–255.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang XT, Li LN, Wang Q, Chang T, Wen YJ,
Yuan Rui, Du XM, Tang SN and Gao L: Effect of Xylazine anesthesia
on Glu and Gaba amino acid neurotransmitters in rat. J Northeast
Agricultural Univ (English Ed). 26:46–52. 2019.
|
|
34
|
Lee DW, Chung S, Yoo HJ, Kim SJ, Woo CW,
Kim ST, Lee DH, Kim KW, Kim JK, Lee JS, et al: Neurochemical
changes associated with stress-induced sleep disturbance in rats:
In vivo and in vitro measurements. PLoS One.
11(e0153346)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xie F, Li X, Bao M, Shi R, Yue Y, Guan Y
and Wang Y: Anesthetic propofol normalized the increased release of
glutamate and γ-amino butyric acid in hippocampus after paradoxical
sleep deprivation in rats. Neurol Res. 37:1102–1107.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fan X, Qu F, Wang JJ, Du X and Liu WC:
Decreased γ-aminobutyric acid levels in the brainstem in patients
with possible sleep bruxism: A pilot study. J Oral Rehabil.
44:934–940. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Khwanchai P, Chinprahast N, Pichyangkura R
and Chaiwanichsiri S: Gamma-aminobutyric acid and glutamic acid
contents, and the GAD activity in germinated brown rice (Oryza
sativa L.): Effect of rice cultivars. Food Sci Biotechnol.
23:373–379. 2014.
|
|
38
|
Sutter JM, Tästensen JB, Johnsen U, Soppa
J and Schönheit P: Key enzymes of the semiphosphorylative
Entner-doudoroff pathway in the haloarchaeon haloferax volcanii:
Characterization of glucose dehydrogenase, gluconate dehydratase,
and 2-keto-3-deoxy-6-phosphogluconate aldolase. J Bacteriol.
198:2251–2262. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fernandez-Mendoza J, Baker JH, Vgontzas
AN, Gaines J, Liao D and Bixler EO: Insomnia symptoms with
objective short sleep duration are associad with systemic
inflammation in adolescents. Brain Behav Immun. 61:110–116.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Huang YJ, Hu L, Li H, Huang Y, Li Y, Yang
J, Gu J and Xu H: PKA-mediated phosphorylation of CREB and NMDA
receptor 2B in the hippocampus of offspring rats is involved in
transmission of mental disorders across a generation. Psychiatry
Res. 280(112497)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ravasz D, Kacso G, Fodor V, Horvath K,
Adam-Vizi V and Chinopoulos C: Catabolism of GABA, succinic
semialdehyde or gamma-hydroxybutyrate through the GABA shunt impair
mitochondrial substrate-level phosphorylation. Neurochem Int.
109:41–53. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Boostan N and Yazdanparast R: Augmentation
of endogenous GABA pool size induced by Magainin II peptide.
Biochem Biophys Res Commun. 506:891–894. 2018.PubMed/NCBI View Article : Google Scholar
|