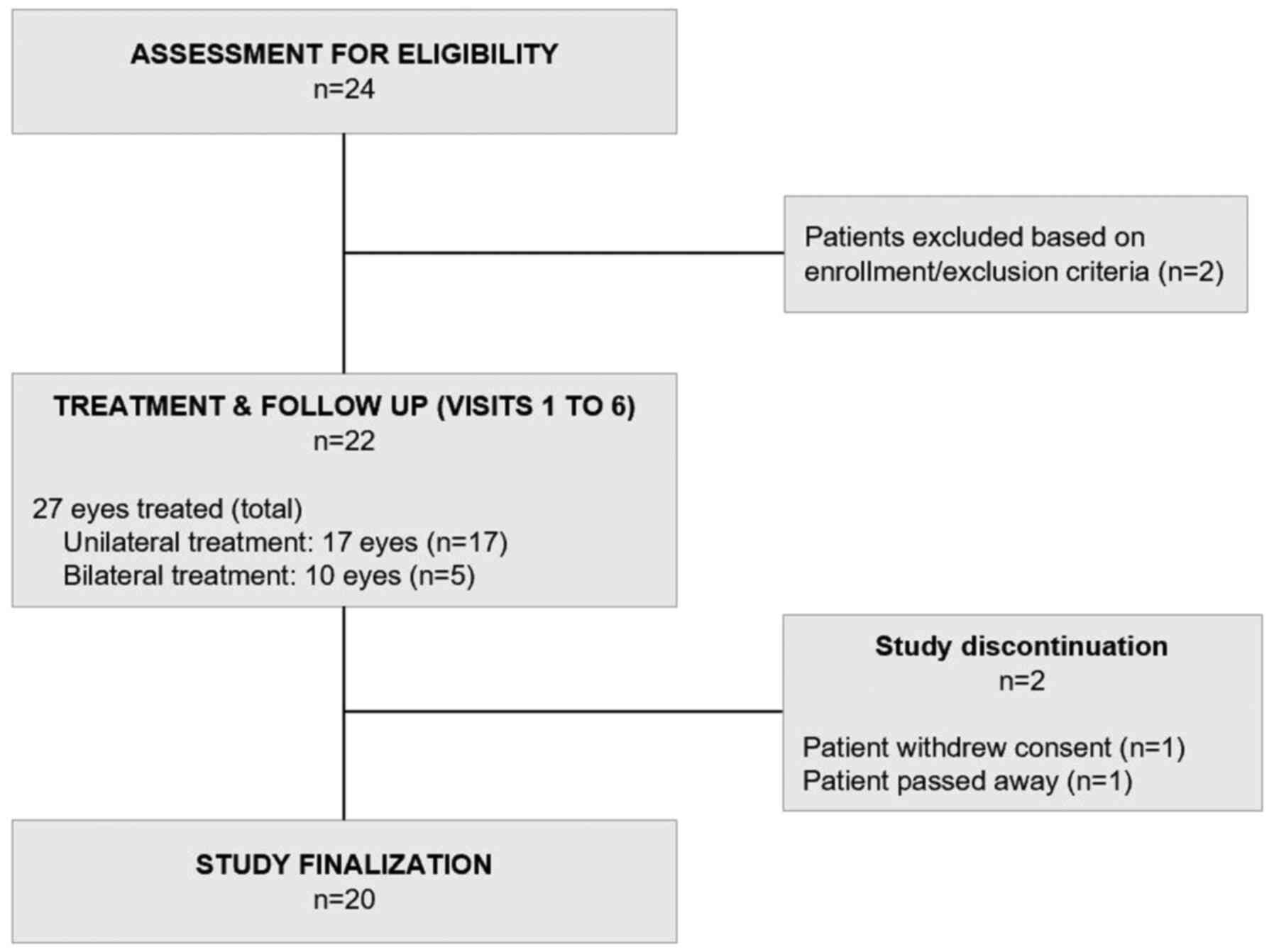
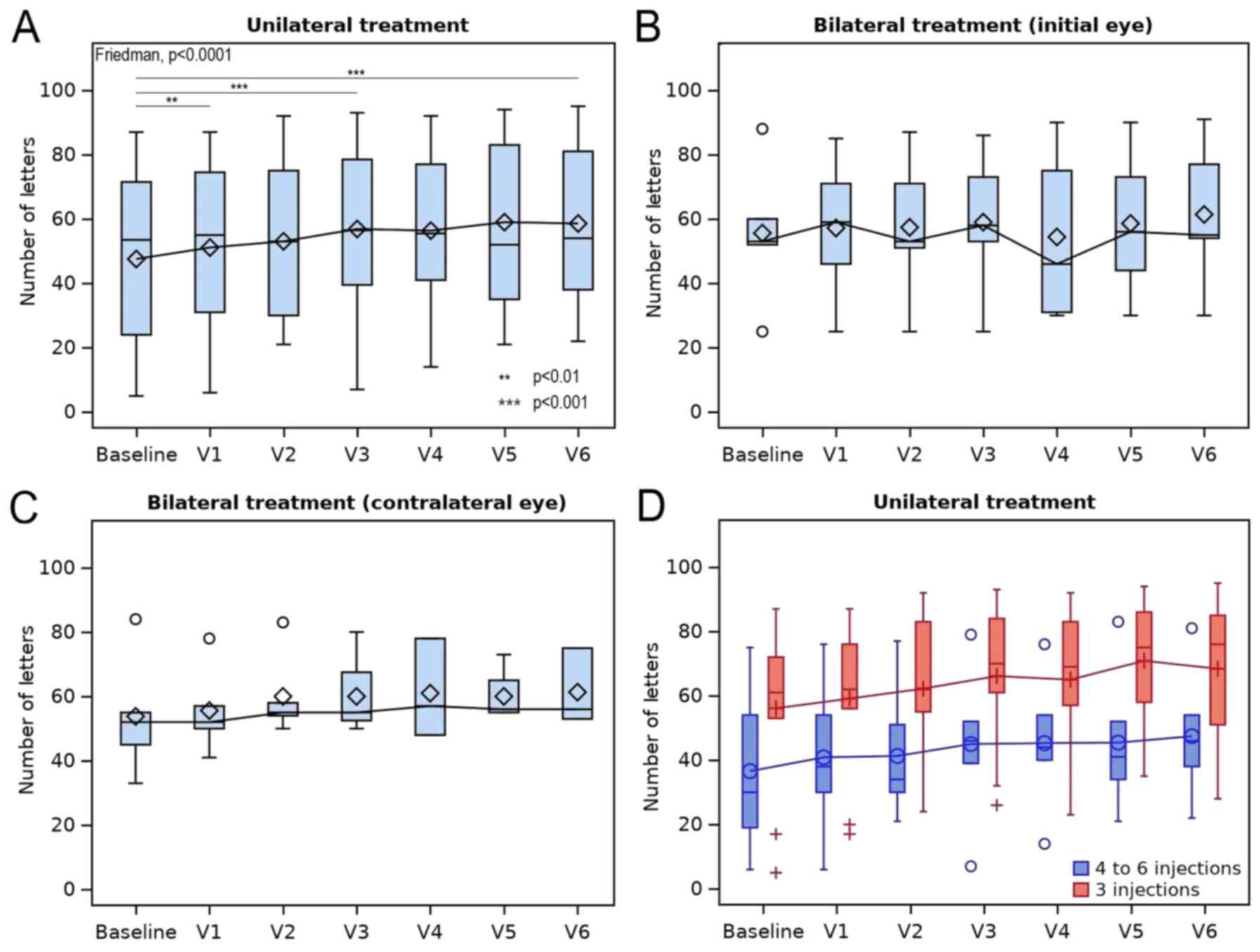
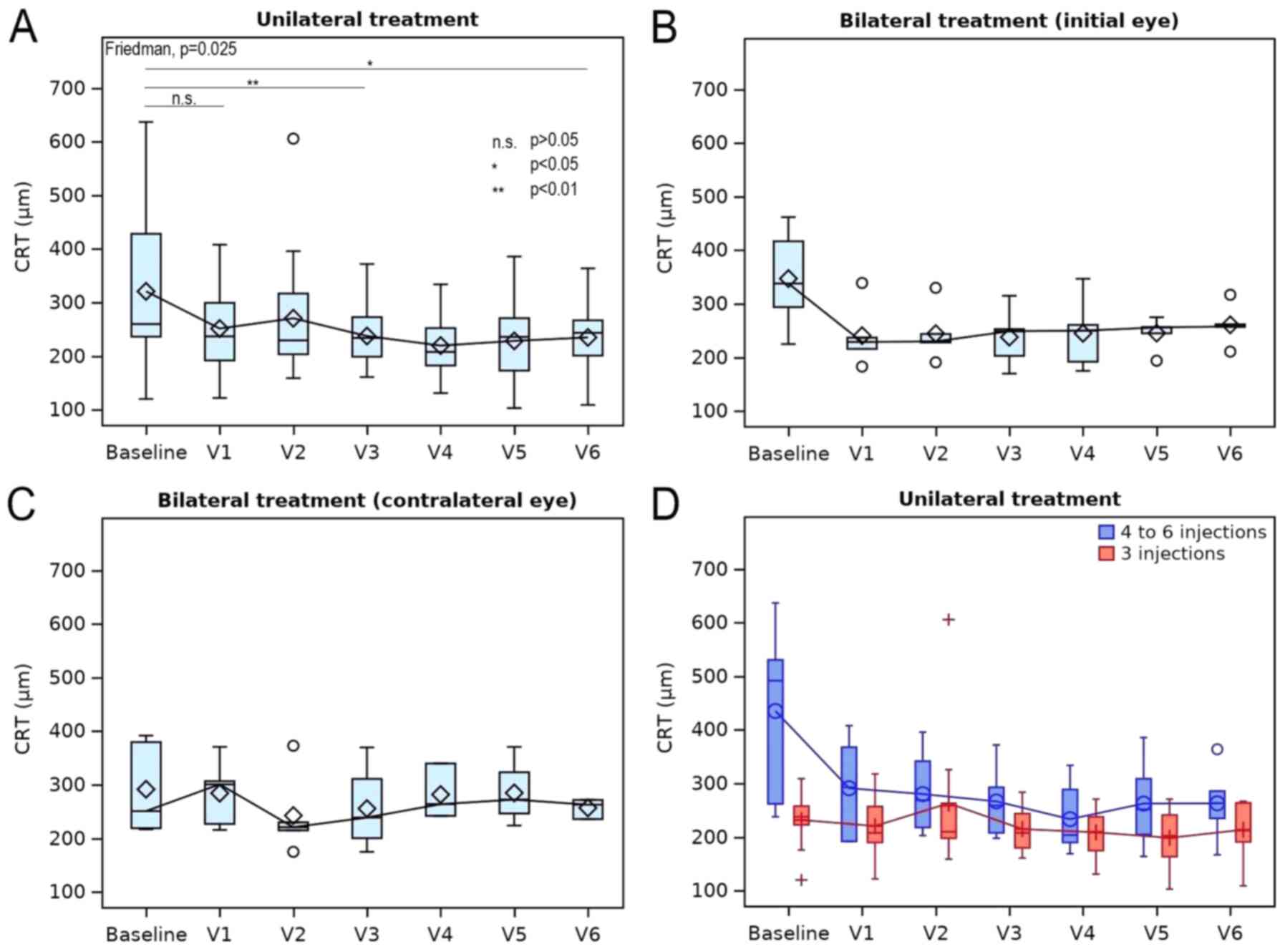
|
1
|
Friedman DS, O'Colmain BJ, Muñoz B, Tomany
SC, McCarty C, de Jong PT, Nemesure B, Mitchell P and Kempen J: Eye
Diseases Prevalence Research Group. Prevalence of age-related
macular degeneration in the United States. Arch Ophthalmol.
122:564–572. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Flaxel CJ, Adelman RA, Bailey ST, Fawzi A,
Lim JI, Vemulakonda GA and Ying Gs: Age-Related Macular
Degeneration Preferred Practice Pattern®, Ophthalmology
(2019), doi: https://doi.org/10.1016/j.ophtha.2019.09.024.
|
|
3
|
Ferris FL III, Fine SL and Hyman L:
Age-related macular degeneration and blindness due to neovascular
maculopathy. Arch Ophthalmol. 102:1640–1642. 1984.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zarbin MA: Age-related macular
degeneration: Review of pathogenesis. Eur J Ophthalmol. 8:199–206.
1998.PubMed/NCBI
|
|
5
|
Beatty S, Koh H, Phil M, Henson D and
Boulton M: The role of oxidative stress in the pathogenesis of
age-related macular degeneration. Surv Ophthalmol. 45:115–134.
2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Holz FG, Schmitz-Valckenberg S and
Fleckenstein M: Recent developments in the treatment of age-related
macular degeneration. J Clin Invest. 124:1430–1438. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Heinemann V and Hoff PM: Bevacizumab plus
irinotecan-based regimens in the treatment of metastatic colorectal
cancer. Oncology. 79:118–128. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Johnson DR, Leeper HE and Uhm JH:
Glioblastoma survival in the United States improved after food and
drug administration approval of bevacizumab: A population-based
analysis. Cancer. 119:3489–3495. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ferrara N, Hillan KJ, Gerber HP and
Novotny W: Discovery and development of bevacizumab, an anti-VEGF
antibody for treating cancer. Nat Rev Drug Discov. 3:391–400.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Rosenfeld PJ, Moshfeghi AA and Puliafito
CA: Optical coherence tomography findings after an intravitreal
injection of bevacizumab (avastin) for neovascular age-related
macular degeneration. Ophthalmic Surg Lasers Imaging. 36:331–335.
2005.PubMed/NCBI
|
|
11
|
Modi YS, Tanchon C and Ehlers JP:
Comparative safety and tolerability of anti-VEGF therapy in
age-related macular degeneration. Drug Saf. 38:279–293.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jan S, Nazim M, Karim S and Hussain Z:
Intravitreal bevacizumab: Indications and complications. J Ayub Med
Coll Abbottabad. 28:364–368. 2016.PubMed/NCBI
|
|
13
|
Bro T, Derebecka M, Jørstad ØK and
Grzybowski A: Off-label use of bevacizumab for wet age-related
macular degeneration in Europe. Graefes Arch Clin Exp Ophthalmol.
258:503–511. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Martin DF, Maguire MG, Ying GS, Grunwald
JE, Fine SL and Jaffe GJ: CATT Research Group. Ranibizumab and
bevacizumab for neovascular age-related macular degeneration. N
Engl J Med. 364:1897–1908. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chakravarthy U, Harding SP, Rogers CA,
Downes SM, Lotery AJ, Wordsworth S and Reeves BC: IVAN Study
Investigators. Ranibizumab versus bevacizumab to treat neovascular
age-related macular degeneration: one-year findings from the IVAN
randomized trial. Ophthalmology. 119:1399–1411. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schauwvlieghe AM, Dijkman G, Hooymans JM,
Verbraak FD, Hoyng CB, Dijkgraaf MG, Peto T, Vingerling JR and
Schlingemann RO: Comparing the effectiveness of bevacizumab to
ranibizumab in patients with exudative age-related macular
degeneration. The BRAMD study. PLoS One.
11(e0153052)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Krebs I, Schmetterer L, Boltz A, Told R,
Vécsei-Marlovits V, Egger S, Schönherr U, Haas A, Ansari-Shahrezaei
S and Susanne Binder: MANTA Research Group. A randomised
double-masked trial comparing the visual outcome after treatment
with ranibizumab or bevacizumab in patients with neovascular
age-related macular degeneration. Br J Ophthalmol. 97:266–271.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kodjikian L, Souied EH, Mimoun G,
Mauget-Faÿsse M, Behar-Cohen F, Decullier E, Huot L and Aulagner G:
GEFAL Study Group. Ranibizumab versus bevacizumab for neovascular
age-related macular degeneration: Results from the GEFAL
noninferiority randomized trial. Ophthalmology. 120:2300–2309.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Berg K, Pedersen TR, Sandvik L and
Bragadóttir R: Comparison of ranibizumab and bevacizumab for
neovascular age-related macular degeneration according to LUCAS
treat-and-extend protocol. Ophthalmology. 122:146–152.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Poku E, Rathbone J, Wong R, Everson-Hock
E, Essat M, Pandor A and Wailoo A: The safety of intravitreal
bevacizumab monotherapy in adult ophthalmic conditions: Systematic
review. BMJ Open. 4(e005244)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Solomon SD, Lindsley K, Vedula SS,
Krzystolik MG and Hawkins BS: Anti-vascular endothelial growth
factor for neovascular age-related macular degeneration. Cochrane
Database Syst Rev. 3(CD005139)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Moja L, Lucenteforte E, Kwag KH, Bertele
V, Campomori A, Chakravarthy U, D'Amico R, Dickersin K, Kodjikian
L, Lindsley K, et al: Systemic safety of bevacizumab versus
ranibizumab for neovascular age-related macular degeneration.
Cochrane Database Syst Rev. 2014(CD011230)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jain P, Sheth J, Anantharaman G and
Gopalakrishnan M: Real-world evidence of safety profile of
intravitreal bevacizumab (Avastin) in an Indian scenario. Indian J
Ophthalmol. 65:596–602. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Foss AJ, Childs M, Reeves BC, Empeslidis
T, Tesha P, Dhar-Munshi S, Mughal S, Culliford L, Rogers CA, Tan W
and Montgomery A: Comparing different dosing regimens of
bevacizumab in the treatment of neovascular macular degeneration:
Study protocol for a randomised controlled trial. Trials.
16(85)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ross EL, Hutton DW, Stein JD, Bressler NM,
Jampol LM and Glassman AR: Diabetic Retinopathy Clinical Research
Network. Cost-effectiveness of aflibercept, bevacizumab, and
ranibizumab for diabetic macular edema treatment: Analysis from the
diabetic retinopathy clinical research network comparative
effectiveness trial. JAMA Ophthalmol. 134:888–896. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Age-Related Eye Disease Study System for
Classifying Age-related Macular Degeneration From Stereoscopic
Color Fundus Photographs. AREDS Report No. 6. Am J Ophthalmol 132:
668-681, 2001.
|
|
27
|
International Conference on Harmonization
of Technical Requirements for Registration of Pharmaceuticals for
Human Use (ICH). MedDRA® the Medical Dictionary for
Regulatory Activities. A registered trademark of the International
Federation of Pharmaceutical Manufacturers and Associations
(IFPMA); Chantilly, VA: Northrop Grumman MSSO (distributors). March
13, 2019. http://www.meddra.org/.
|
|
28
|
Bakri SJ, Thorne JE, Ho AC, Ehlers JP,
Schoenberger SD, Yeh S and Kim SJ: Safety and efficacy of
anti-vascular endothelial growth factor therapies for neovascular
age-related macular degeneration: A report by the american academy
of ophthalmology. Ophthalmology. 126:55–63. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Etminan M, Maberley DA, Babiuk DW and
Carleton BC: Risk of myocardial infarction and stroke with single
or repeated doses of intravitreal bevacizumab in age-related
macular degeneration. Am J Ophthalmol. 166:53–58. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ng WY, Tan GS, Ong PG, Cheng CY, Cheung
CY, Wong DW, Mathur R, Chow KY, Wong TY and Cheung GC: Incidence of
myocardial infarction, stroke, and death in patients with
age-related macular degeneration treated with intravitreal
anti-vascular endothelial growth factor therapy. Am J Ophthalmol.
159:557–64.e1. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Beato J, Pedrosa AC, Pinheiro-Costa J,
Freitas-da-Costa P, Falcão MS, Melo A, Estrela-Silva S,
Falcão-Reism F and Carneiro ÂM: Long-term effect of anti-VEGF
agents on intraocular pressure in age-related macular degeneration.
Ophthalmic Res. 56:30–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Matsuyama K, Ogata N, Matsuoka M, Wada M,
Takahashi K and Nishimura T: Plasma levels of vascular endothelial
growth factor and pigment epithelium-derived factor before and
after intravitreal injection of bevacizumab. Br J Ophthalmol.
94:1215–1218. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Carneiro AM, Costa R, Falcão MS,
Barthelmes D, Mendonça LS, Fonseca SL, Gonçalves R, Gonçalves C,
Falcão-Reis FM and Soares R: Vascular endothelial growth factor
plasma levels before and after treatment of neovascular age-related
macular degeneration with bevacizumab or ranibizumab. Acta
Ophthalmol. 90:e25–e30. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zehetner C, Kirchmair R, Huber S,
Kralinger MT and Kieselbach GF: Plasma levels of vascular
endothelial growth factor before and after intravitreal injection
of bevacizumab, ranibizumab and pegaptanib in patients with
age-related macular degeneration, and in patients with diabetic
macular oedema. Br J Ophthalmol. 97:454–459. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kwon JW, Jee D and La TY: The association
between myocardial infarction and intravitreal bevacizumab
injection. Medicine (Baltimore). 97(e0198)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Enseleit F, Michels S, Sudano I, Stahel M,
Zweifel S, Schlager O, Becker M, Winnik S, Nägele M, Flammer AJ, et
al: SAVE-AMD: Safety of VEGF inhibitors in age-related macular
degeneration. Ophthalmologica. 238:205–216. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Domigan CK, Warren CM, Antanesian V,
Happel K, Ziyad S, Lee S, Krall A, Duan L, Torres-Collado AX,
Castellani LW, et al: Autocrine VEGF maintains endothelial survival
through regulation of metabolism and autophagy. J Cell Sci.
128:2236–2248. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zechariah A, ElAli A, Doeppner TR, Jin F,
Hasan MR, Helfrich I, Mies G and Hermann DM: Vascular endothelial
growth factor promotes pericyte coverage of brain capillaries,
improves cerebral blood flow during subsequent focal cerebral
ischemia, and preserves the metabolic penumbra. Stroke.
44:1690–1697. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen HX and Cleck JN: Adverse effects of
anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol.
6:465–477. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kiss S, Dugel PU, Khanani AM, Broder MS,
Chang E, Sun GH and Turpcu A: Endophthalmitis rates among patients
receiving intravitreal anti-VEGF injections: A USA claims analysis.
Clin Ophthalmol. 12:1625–1635. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Adelman RA, Zheng Q and Mayer HR:
Persistent ocular hypertension following intravitreal bevacizumab
and ranibizumab injections. J Ocul Pharmacol Ther. 26:105–110.
2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Choi DY, Ortube MC, McCannel CA, Sarraf D,
Hubschman JP, McCannel TA and Gorin MB: Sustained elevated
intraocular pressures after intravitreal injection of bevacizumab,
ranibizumab, and pegaptanib. Retina. 31:1028–1035. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Good TJ, Kimura AE, Mandava N and Kahook
MY: Sustained elevation of intraocular pressure after intravitreal
injections of anti-VEGF agents. Br J Ophthalmol. 95:1111–1114.
2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hoang QV, Tsuang AJ, Gelman R, Mendonca
LS, Della Torre KE, Jung JJ and Freund KB: Clinical predictors of
sustained intraocular pressure elevation due to intravitreal
anti-vascular endothelial growth factor therapy. Retina.
33:179–187. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mathalone N, Arodi-Golan A, Sar S, Wolfson
Y, Shalem M, Lavi I and Geyer O: Sustained elevation of intraocular
pressure after intravitreal injections of bevacizumab in eyes with
neovascular age-related macular degeneration. Graefes Arch Clin Exp
Ophthalmol. 250:1435–1440. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Segal O, Ferencz JR, Cohen P, Nemet AY and
Nesher R: Persistent elevation of intraocular pressure following
intravitreal injection of bevacizumab. Isr Med Assoc J. 15:352–355.
2013.PubMed/NCBI
|
|
47
|
Sniegowski M, Mandava N and Kahook MY:
Sustained intraocular pressure elevation after intravitreal
injection of bevacizumab and ranibizumab associated with
trabeculitis. Open Ophthalmol J. 4:28–29. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Dedania VS and Bakri SJ: Sustained
elevation of intraocular pressure after intravitreal anti-VEGF
agents: What is the evidence? Retina. 35:841–858. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wen JC, Reina-Torres E, Sherwood JM,
Challa P, Liu KC, Li G, Chang JY, Cousins SW, Schuman SG, Mettu PS,
et al: Intravitreal anti-VEGF injections reduce aqueous outflow
facility in patients with neovascular age-related macular
degeneration. Invest Ophthalmol Vis Sci. 58:1893–1898.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sengul A, Rasier R, Ciftci C, Artunay O,
Kockar A, Bahcecioglu H and Yuzbasioglu E: Short-term effects of
intravitreal ranibizumab and bevacizumab administration on 24-h
ambulatory blood pressure monitoring recordings in normotensive
patients with age-related macular degeneration. Eye (Lond).
31:677–683. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Sagiv O, Zloto O, Moroz I and Moisseiev J:
Different clinical courses on long-term follow-up of age-related
macular degeneration patients treated with intravitreal
anti-vascular endothelial growth factor injections.
Ophthalmologica. 238:217–225. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Tufail A, Patel PJ, Egan C, Hykin P, da
Cruz L, Gregor Z, Dowler J, Majid MA, Bailey C, Mohamed Q, et al:
Bevacizumab for neovascular age related macular degeneration (ABC
trial): Multicentre randomised double masked study. BMJ.
340(c2459)2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang W and Zhang X: Systemic adverse
events after intravitreal bevacizumab versus ranibizumab for
age-related macular degeneration: A meta-analysis. PLoS One.
9(e109744)2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Müller S, Ehlken C, Bauer-Steinhusen U,
Lechtenfeld W, Hasanbasic Z, Agostini H and Wilke T: Treatment of
age-related neovascular macular degeneration: The patient's
perspective. Graefes Arch Clin Exp Ophthalmol. 255:2237–2246.
2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Senra H, Ali Z, Balaskas K and Aslam T:
Psychological impact of anti-VEGF treatments for wet macular
degeneration-a review. Graefes Arch Clin Exp Ophthalmol.
254:1873–1880. 2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Gianniou C, Dirani A, Ferrini W,
Marchionno L, Decugis D, Deli A, Ambresin A and Mantel I: Two-year
outcome of an observe-and-plan regimen for neovascular age-related
macular degeneration: How to alleviate the clinical burden with
maintained functional results. Eye (Lond). 29:450–451.
2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Alexandru MR and Alexandra NM: Wet
age-related macular degeneration management and follow-up. Rom J
Ophthalmol. 60:9–13. 2016.PubMed/NCBI
|