Introduction
Age-related macular degeneration (AMD) is the major
cause of irreversible vision loss in individuals aged >50 years
(1) and is classified as atrophic
(or dry) or neovascular (wet or exudative). The neovascular
variant, despite accounting for only 10% of cases, is responsible
for 90% of cases of severe vision loss, defined as a visual acuity
(VA) of 20/200 or worse (2,3).
It is thought that oxidative stress in the retinal
pigment epithelium (RPE) leads to poor performance of its cells
with the consequent formation of extracellular debris, affecting
the permeability of Bruch’s membrane, eventually triggering atrophy
of the RPE or choroidal neovascularization. If inadequately
treated, neovascular AMD (nAMD) leads to vision loss or blindness
caused by leakage, hemorrhage, RPE detachments and scar formation
(4,5).
Medical research has identified the vascular
endothelial growth factor (VEGF) as a key pathophysiological factor
in the development of nAMD, with an essential role in angiogenesis,
vascular permeability and inflammatory response. Hence, intraocular
inhibition of this angiogenic factor is a natural therapeutic
target (6). The most used anti-VEGF
drugs for nAMD are bevacizumab, ranibizumab and aflibercept.
Bevacizumab is a monoclonal antibody that inhibits
all isoforms of VEGF-A and its systemic use was approved for the
treatment of colorectal cancer and glioblastoma (7,8).
Systemic adverse reactions associated with intravenous
administration in oncology indications include thromboembolic
events (e.g., myocardial infarction, cerebrovascular accident,
central nervous system hemorrhage), hemoptysis, gastrointestinal
perforation and wound healing complications (9). However, the intravitreal dose of
bevacizumab is 200-400 times lower than the intravenous dose
(10).
Since the introduction of intravitreal bevacizumab
therapy in 2005, this medication has been frequently used by retina
specialists as an off-label treatment for nAMD (11,12),
although a recent survey among ophthalmologists from 20 European
countries revealed a broad disparity in the off-label use of
bevacizumab between countries, from non-existent (0%) to very high
(97%), as well as diverging opinions expressed by governmental
institutions and national ophthalmological societies (13).
Bevacizumab exhibited comparable efficacy to other
anti-VEGF therapies (e.g., ranibizumab) in numerous trials
conducted worldwide [CATT (14),
IVAN (15), BRAMD (16), MANTA (17), GEFAL (18) and LUCAS (19) trials] with thousands of patients,
and it was indicated that intravitreal bevacizumab
(Avastin®) was not inferior to ranibizumab
(Lucentis®) in terms of efficacy and safety (14-20).
These conclusions were reaffirmed by two meta-analyses involving
5,496 and 3,665 patients with nAMD, where both anti-VEGF agents
demonstrated comparable effects in terms of vision improvement and
anatomical changes, as well as safety (21,22).
Evidence obtained from the landmark trials along with the preferred
standard practice of retina specialists supports the widespread use
of bevacizumab for nAMD.
In response to the requirement for a proper
adaptation, a sterile-dosage form (vial) containing 5 mg of
bevacizumab in 0.2 ml injectable solution for single-dose
administration has been developed. This pharmaceutical form is
intended to assure intravitreal injection with minimum risk of
contamination and adverse consequences, including endophthalmitis
and blindness, associated with reutilization or repackaging of
bevacizumab vials for oncological use. Bevacizumab also has the
advantage of reducing cost of therapy when compared with other
anti-VEGF alternatives, thus helping reduce the financial burden
over multiple injections (23-25).
The objective of the present study was to assess the
safety and clinical effectiveness of a single-dose form of
bevacizumab administered via the intravitreal route in a sample of
patients with nAMD naïve to anti-VEGF therapy.
Materials and methods
Study design
The present study was an open-label, interventional,
single-arm, uncontrolled, prospective and multicenter clinical
study to assess the safety and clinical effectiveness of
intravitreal injection of bevacizumab in a sample of 22 patients
with advanced nAMD (category 4 according to the Age-Related Eye
Disease Study classification) (26). The study protocol was approved by
the Argentinian National Administration of Drugs, Foods and Medical
Technology (ANMAT; approval no. 0829/2017) and by an independent
ethics committee (Comité Independiente de Ética-FEFyM, Pte. J.E.
Uriburu 774 1 Piso, Ciudad Autónoma de Buenos Aires, C1027AAP,
Argentina) and was performed between February 2017 and May 2018 at
the three specialized research centers participating in this study
(Consultorios Oftalmológicos Benisek-Ascarza, Consultorio
Oftalmológico Julio Manzitti and Instituto Scorsetti). The present
study was performed in accordance with Good Clinical Practices and
ethical principles laid out in the Declaration of Helsinki. The
study protocol was registered at clinicaltrials.gov (ref. no. NCT03668054).
The eligibility of patients was assessed using the
following inclusion criteria: i) Aged 50 years or above; ii)
diagnosed with nAMD and iii) indication for antiangiogenic therapy.
The exclusion criteria wereas follows: i) Patients with any
contraindication for bevacizumab therapy; ii) prior intravitreal
and/or systemic antiangiogenic therapy; iii) nAMD in the healing
period or disciform scar; iv) pregnant, breastfeeding or
childbearing-aged females; v) any person with choroidal
neovascularization not associated with nAMD; vi) history of retinal
or intraocular surgery in the affected eye in the last 3 months;
vii) vitrectomy in the affected eye; viii) any significant ocular
infection having compromised or able to compromise the affected
eye; ix) ocular inflammatory disease; x) myopia exceeding-8
diopters; xi) extensive subfoveal subretinal hemorrhage >2
papillary diameters; xii) coexistence of other severe ocular
diseases (uncontrolled ocular hypertension, terminal glaucoma,
diabetic retinopathy, retinal vein thrombosis, optic atrophy);
xiii) history of stroke or myocardial infarction in the last 6
months; xiv) history of coagulopathy and xv) patients physically or
mentally incompetent to perform relevant visual tests.
After obtainment of written informed consent,
patients meeting the eligibility criteria underwent baseline
assessments, which included VA measurement, intraocular pressure
(IOP) measurement by tonometry (Goldmann Applanation Tonometer),
slit-lamp biomicroscopy (Topcon SL-3E) and optical coherence
tomography (SOCT Copernicus/OPTOPOL Technology). Safety follow-up
was performed at day 1 post-injection [recording of adverse events
(AE), vital signs, biomicroscopy and tonometry] and overall safety
and therapy response were assessed at day 28 post-injection through
a full ophthalmologic examination that included imaging by OCT.
Data collected from patient visits were entered by the
investigators in a study-specific electronic case report form.
Administration of study treatment
Bevacizumab (Lumiere®) is supplied as a
sterile vial containing 5 mg drug in 0.2 ml of injecTable solution
intended for single use. After baseline assessments, patients
received the first intravitreal injection of bevacizumab at a
unique dose of 1.25 mg/0.05 ml and under aseptic conditions. Prior
to injection, adequate anesthesia and a broad-spectrum topical
microbicide were applied. Bevacizumab injections were repeated in
the affected eye until 3 doses were reached. Continuation of the
therapy (up to 6 injections) was decided by the investigator based
on safety, tolerability and response. The time interval between
injections was not less than 4 weeks. All patients, regardless of
the quantity of doses received (3 doses or between 4 and 6 doses)
were controlled monthly by the investigator until the finalization
of the study.
Safety assessments
Drug safety was assessed in the study population
that received at least one intravitreal injection of bevacizumab by
monitoring the local and systemic AE pattern and severity,
evolution of IOP and vital signs (systolic and diastolic blood
pressure and heart rate). The primary outcome was the number of
patients developing treatment-associated AEs and safety results
were compared with the published literature on the use of
bevacizumab for the treatment of nAMD. All AEs were coded using the
Medical Dictionary for Regulatory Activities (MedDRA) (27).
Evaluation of clinical
effectiveness
Therapy response was analyzed in the study
population who received at least 3 doses of bevacizumab in the
affected eye by determining the VA and anatomical changes on OCT.
The secondary outcomes were the number of patients developing
changes in central retinal thickness (CRT) and VA, determined from
the number of letters read, from baseline to months 1, 3 and 6
after therapy onset. CRT (in µm) was measured using scanned OCT
images and VA was assessed using retro-illuminated, standardized
Early Treatment Diabetic Retinopathy Study charts.
Statistical analysis
Descriptive statistics were used to summarize the
data. Continuous variables were expressed as the median and
interquartile range (IQR) or mean and standard deviation (SD) were
determined, whereas categorical variables were expressed as n (%).
Safety assessment included the estimation of AE rates per 100
injections of bevacizumab (at overall level and by MedDRA
term/seriousness), whereas response to therapy was assessed by the
change in the median value of CRT and number of letters over the
study period. A non-parametric method (Friedman's test) was used to
detect the overall significance, and post-hoc analyses with
Bonferroni adjustment were performed to assess changes between the
baseline and visits 1, 3 and 6. All tests were two-tailed and
P<0.05 was considered to indicate statistical significance. All
analyses were performed using SAS® version 9.4 (SAS
Institute).
Results
Patients
A total of 22 patients with a median age of 77 years
(range, 61-90 years) and a female-to-male ratio of 15:7 were
enrolled and received the study drug. The medical history included
ophthalmic, metabolic and/or cardiovascular comorbidities
frequently associated with the age group of patients presenting
with nAMD (Table I).
 | Table ISummary of demographics and baseline
characteristics of the study population (n=22). |
Table I
Summary of demographics and baseline
characteristics of the study population (n=22).
| Characteristic | Value |
|---|
| Age (years) | 77 (61-90) |
| Females | 15 (68.2) |
| Tobacco
usea | |
|
Never used
tobacco | 8 (36.4) |
|
Ever used
tobacco | 2 (9.1) |
|
Current
tobacco user | 3 (13.6) |
| Medical
history | |
|
Cataract | 20 (90.9) |
|
Arterial
hypertension | 18 (81.8) |
|
Insomnia | 6 (27.3) |
|
Hypercholesterolemia | 5 (22.7) |
|
Prostatic
benign hyperplasia | 5 (22.7) |
|
Hypothyroidism | 5 (22.7) |
|
Arrhythmia | 4 (18.2) |
|
Diabetes
mellitus | 4 (18.2) |
|
Right bundle
branch block | 3 (13.6) |
|
Depression | 3 (13.6) |
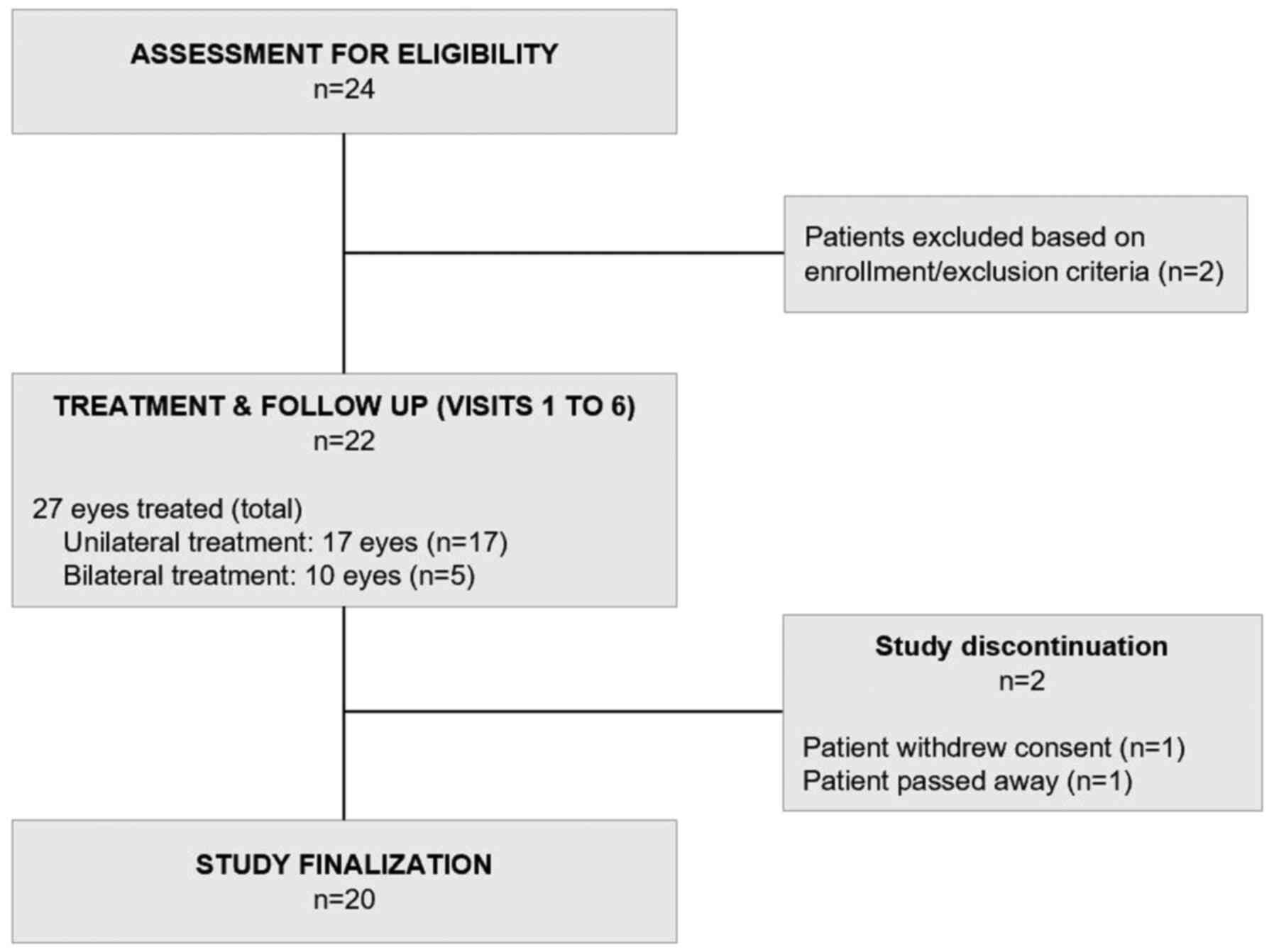
A total of 110 bevacizumab injections were
administered to 27 eyes treated (Fig.
1 and Table II). With the
exception of one patient who withdrew consent after the first
injection, the remaining patients (n=21 patients and 26 eyes
treated) received the minimum scheme of 3 doses in the affected
eye, as per the study protocol. In a subgroup of these patients
(n=5 patients, 10 eyes treated), therapy was given in both eyes due
to bilateral disease, with a median interval for therapy onset
between eyes of 2 days, albeit with a broad dispersion (range,
1-132 days).
 | Table IISummary of treatment schemes. |
Table II
Summary of treatment schemes.
| Characteristic | Unilateral
treatment (17 eyes) | Bilateral treatment
(10 eyes) |
|---|
| Total bevacizumab
injections received | | |
|
n | 68 | 42 |
|
Mean ±
SD | 4.0±1.6 | 8.4±1.3 |
| Received 3 loading
doses (only) in affected eye | 9 (52.9) | 5 (50.0) |
| Received between
4-6 doses in affected eye | 7 (41.2) | 5 (50.0) |
No protocol violations or major deviations were
identified and no patients were lost to follow-up.
Safety
A total of 87 notifications of AEs were received
from the patients during the 6-month follow-up period and the
cumulative AE rate was 79 AE notifications for every 100
intravitreal injections (Table
III). The median time to the first occurrence of an AE since
therapy onset was 16 days. Most of the AEs were not serious (n=84;
96.6%), listed (i.e., AEs whose nature and severity was consistent
with the product information; n=57; 51.8%) and occurred after the
third injection (n=49; 44.5%). The development of conjunctival
hemorrhage, the most frequently reported AE in both sexes (29.9% of
patients), resulted in full recovery in all cases and its
occurrence was attributed to the injection procedure. In addition,
most of the AEs reported whose nature and severity were not
consistent with the product information were considered as not
related to the study drug. Therapy with bevacizumab was not
suspended due to safety reasons in any case. Non-ocular hemorrhagic
events included one episode of mild hematuria (non-serious AE),
which lasted for one day in a patient who had a previous history of
such episodes, and was considered as not being associated with the
study drug. No other non-ocular hemorrhages were reported. There
were no reports of endophthalmitis (sterile or infectious),
uveitis, retinal detachment, vitreous hemorrhage, anaphylactic
reactions or arteriothrombotic events.
 | Table IIISummary of cumulative AE reporting
rates. |
Table III
Summary of cumulative AE reporting
rates.
| Outcome | Frequency | AE rate (per 100
injections) |
|---|
| Total | 87 | 79.1 |
| Per seriousness
category | | |
|
Not
serious | 84 | 76.4 |
|
Serious | 3 | 2.7 |
| Per
listednessa | | |
|
Listed | 57 | 51.8 |
|
Unlisted | 30 | 27.3 |
| Per time of
occurrence | | |
|
Up to the
third injection | 38 | 34.5 |
|
After the
third injection | 49 | 44.5 |
| Per AE term (MedDRA
PT)b | | |
|
Ophthalmologic
reactions | | |
|
Conjunctival
hemorrhagec | 26 | 23.6 |
|
Conjunctival
hyperemiac | 5 | 4.5 |
|
Eye
painc | 4 | 3.6 |
|
Neovascular
AMDd,e | 4 | 3.6 |
|
Conjunctivitis/viral
conjunctivitise | 3 | 2.7 |
|
Cataract/nuclear
cataractc | 3 | 2.7 |
|
Ocular
hypertensionc | 2 | 1.8 |
|
Systemic
reactions | | |
|
Hypertensionc | 3 | 2.7 |
|
Headachef | 2 | 1.8 |
IOP variation from baseline values was assessed at
all visits and no clinically relevant changes in this parameter
were identified since the onset of bevacizumab therapy in the
affected eye, regardless of the number of doses received (Table IV). No increase in IOP was
evidenced during or immediately after the injection procedure.
Likewise, non-clinically relevant IOP changes were observed in the
untreated eyes. A total of 3 occurrences of IOP increment
(non-serious AEs) were reported with complete recovery in all
cases; two of them were considered by the investigator as not
related to bevacizumab therapy and the other one as possibly
related.
 | Table IVMeasurements of intraocular pressure
(mmHg). |
Table IV
Measurements of intraocular pressure
(mmHg).
| | Difference from
baseline |
|---|
| Item | Baseline | 1 month (V1) | 3 months (V3) | 6 months (V6) |
|---|
| All eyes treated
(overall) | 12.6±2.6 | -0.6±1.7 | -0.2±3.1 | -0.9±2.6 |
| By treatment
strategy | | | | |
|
Unilateral | 12.6±2.9 | -0.4±0.9 | -0.1±3.4 | -0.4±2.8 |
|
Bilateral
(in eye initially treated) | 12.4±1.5 | -0.2±1.8 | +0.8±1.3 | -0.8±2.2 |
|
Bilateral
(in eye contralaterally treated) | 13.0±2.4 | 1.0±3.4 | -2.0±4.2 | n.d. |
| Received 3 loading
doses (only) | | | | |
|
All eyes
treated (overall) | 13.1±2.2 | -0.9±1.9 | -0.9±1.9 | -1.5±1.8 |
|
Unilateral
therapy | 13.1±2.3 | -0.6±1.0 | -6.1±6.1 | -7.4±5.8 |
|
Bilateral
therapya | 13.2±2.2) | -1.6±3.0 | -1.3±2.6 | -2.3±2.4 |
| Received between 4
and 6 doses | | | | |
|
All eyes
treated (overall) | 10.9±4.5 | -0.2±1.3 | +0.6±3.0 | +0.6±2.2 |
|
Unilateral
therapy | 11.9±3.8 | -0.4±0.5 | +0.4±4.0 | +1.2±2.6 |
|
Bilateral
therapya | 9.6±5.5 | +0.2±1.9 | +0.8±0.8 | -0.3±1.3 |
Only minor changes of no clinical significance in
blood pressure and heart rate were observed during follow-up.
Furthermore, two occurrences of arterial hypertension and one
occurrence of bradycardia were notified; all of them were
considered by the investigator as not serious and not related to
bevacizumab therapy. In addition, no drug interactions were
identified.
Clinical effectiveness
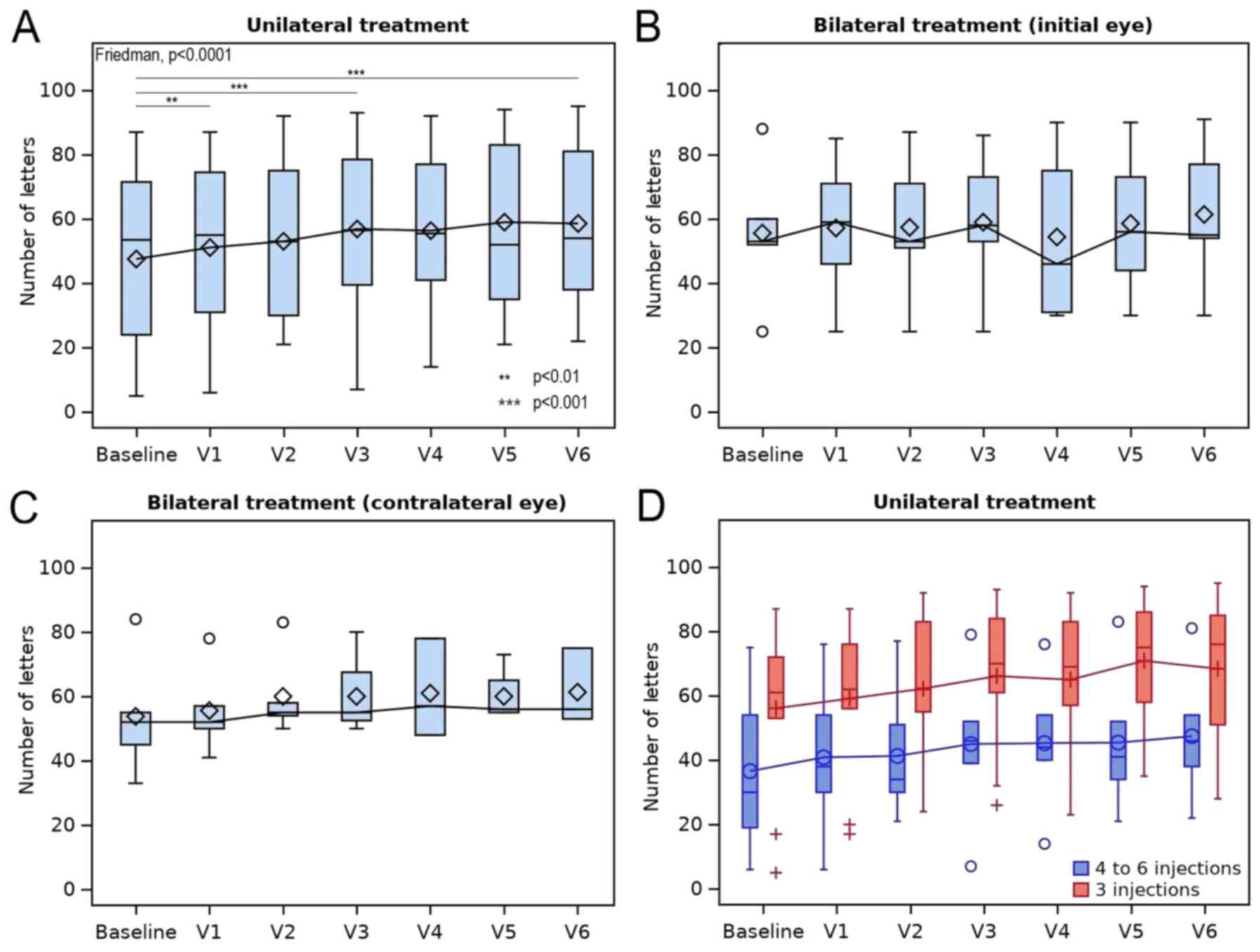
A sustained overall response was observed at 1, 3
and 6 months after therapy onset. A statistically significant
improvement in VA was observed for unilateral therapy, as evidenced
at the sixth month by a median (IQR) change of +8 (+6.5, +12.5)
letters (mean ± SD, +8.2±8.8 letters; range, -18 to +19 letters;
P<0.0001). Pairwise comparisons revealed significant differences
from baseline at 1 month (P=0.009), as well as at 3 and 6 months
(P<0.0001; Table V and Fig. 2A). Furthermore, a small gain in VA
[median (IQR), +2 (-0.5, +5) letters; mean ± SD, +1.7±5.7 letters;
range, -9 to +11] was observed in the contralateral (untreated) eye
at 6 months, which was not significant and of low clinical
relevance (results not shown).
 | Table VMeasurements of visual acuity (in
number of letters). |
Table V
Measurements of visual acuity (in
number of letters).
| | Change from
baselinec |
|---|
| Item | Eyes (n) | Baseline | 1 month (V1) | 3 months (V3) | 6 months (V6) |
|---|
| All eyes treated
(overall) | 26 | 53 (30.7,
68.5) | +1 (0, 5) | +6 (0, 11) | +7.0 (3, 11) |
| By treatment
strategy | | | | | |
|
Unilateral | 16 | 53.5 (26.5,
71.2) | +1.5 (0, 5.5) | +8.5 (5.5,
13.5) | +8 (6.5, 12.5) |
|
Bilateral
(in eye initially treated) | 5 | 53 (52, 60) | -1 (-3, 0) | 0 (-2, 6) | +3 (3, 5) |
|
Bilateral
(in eye contralaterally treated) | 5 | 52 (45, 55) | +5 (-3, 5) | -1 (-2.5, 2.5) | -2 (-5.5,
1)a |
| Received 3 loading
doses (only) | | | | | |
|
All eyes
treated (overall) | 14 | 60.5 (53, 78) | +1.5 (0, 45) | +6 (4, 13) | +7.5 (0.8, 11) |
|
Unilateral
therapy | 9 | 61 (53, 72) | +2 (1, 3) | +8 (6, 13) | +8 (6.7, 11) |
|
Bilateral
therapyb | 5 | 60 (53, 84) | -1 (-3, 8) | -3 (-4.7, 3.5) | -1.5 (-6.7,
8.2) |
| Received between 4
and 6 doses | | | | | |
|
All eyes
treated (overall) | 12 | 44 (28, 52.5) | +0.5 (0, 5.5) | +5 (0, 10) | +6 (3.5, 13) |
|
Unilateral
therapy | 7 | 30 (24, 48.5) | +1 (0, 8) | +9 (2.5, 13.5) | +10 (7, 16.5) |
|
Bilateral
therapyb | 5 | 52 (45, 52) | 0 (-3, 5) | 0 (0, 6) | +3.5 (1.7,
4.2) |
No significant change in VA was observed in the
group receiving bilateral therapy (P=0.924), with a median (IQR)
gain of +3 (3, 5) letters (mean ± SD, +5.8±11 letters; range, -6 to
+24 letters) at the sixth month in the eyes treated first and no
response was observed in the contralateral eye [median (IQR), -2
(-5.5, 1) letters; mean ± SD, -2.3±6.5; range, -9 to +4; Table V and Fig. 2B and C].
Regarding the number of injections, the response to
therapy observed after six months for unilateral therapy was
greater in eyes that received between 4 and 6 injections [median
(IQR), +10 (7, 16,5) letters; mean ± SD, +10.8±6.8 letters; range,
0 to +19 letters] when compared to eyes that received 3 injections
[median (IQR), +8 (6.7, 11) letters; mean ± SD, +5.87±10 letters;
range, -18 to +14 letters; Fig.
2D]. Likewise, a similar overall response was observed for
bilateral therapy [4-6 injections group: Median (IQR), +3.5 (1.7,
4.2) letters; mean ± SD, +2.50±3.1 letters; range, -2 to +5
letters; 3 injections group: Median (IQR), -1.5 (-6.7, 8.2)
letters; mean ± SD, +3.00±14.9 letters; range, -9 to +24 letters;
Fig. 2D]. Continuation of the
therapy after the third dose was usually decided in patients
exhibiting lower values of baseline VA (Table V).
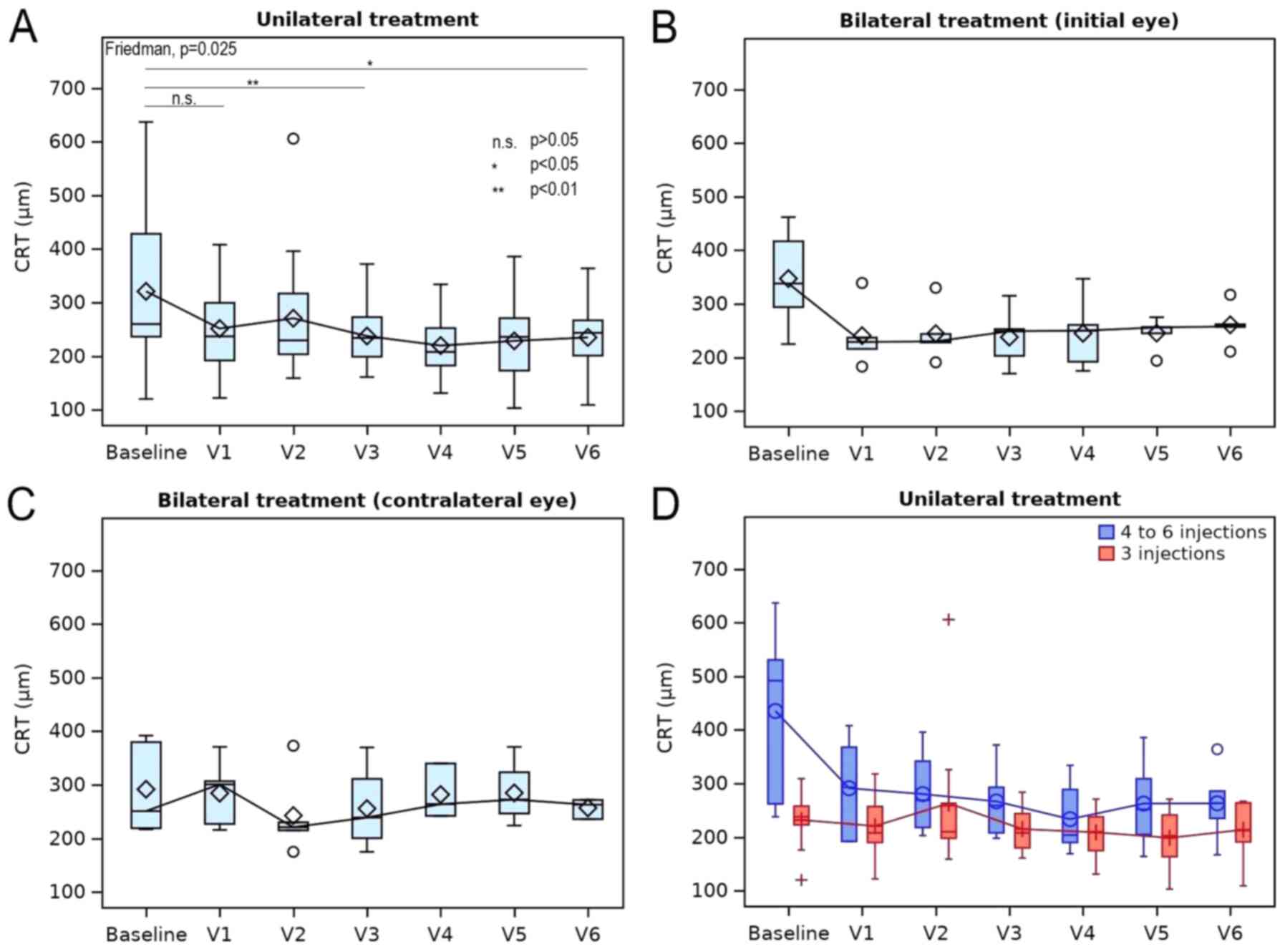
A favorable response in OCT results was observed for
patients receiving unilateral therapy after six months, as
evidenced by a statistically significant change in the CRT
(P=0.025). A median (IQR) reduction in the CRT of -24 (-84.2, -3.2)
µm [mean ± SD: -75.5±120.3 µm; range, -385 to +30 µm] was
determined for unilateral therapy (Fig.
3A and Table VI). Pairwise
comparisons revealed significant differences from the baseline at 3
months (P=0.007) and 6 months (P=0.027). Comparison between the
baseline and 1 month indicated no significant change in CRT
(P=0.071).
 | Table VIMeasurements of CRT [in µm]. |
Table VI
Measurements of CRT [in µm].
| | Change from
baselinec |
|---|
| Item | Eyes (n) | Baseline | 1 month (V1) | 3 months (V3) | 6 months (V6) |
|---|
| All eyes treated
(overall) | 26 | 266.5 (235.7,
389) | -32 (-119.2,
2.7) | -64 (-120, -9) | -25.5 (-140,
-1.5) |
| By treatment
strategy | | | | | |
|
Unilateral | 16 | 260 (237.2,
396.7) | -32 (-83.2,
1.2) | -57 (-93.7,
-7.7) | -24 (-84.2,
-3.2) |
|
Bilateral
(in eye initially treated) | 5 | 338 (294, 417) | -111 (-122,
-78) | -102 (-124,
-85) | -81 (-155,
-14) |
|
Bilateral
(in eye contralaterally treated) | 5 | 251 (219, 380) | +10 (-9, 56) | -4.5 (-61.7,
3) | +12 (-72,
33.5)a |
| Received 3 loading
doses (only) | | | | | |
|
All eyes
treated (overall) | 14 | 236.5 (220,
267.7) | -6.5 (-40.5,
9.7) | -22 (-77, 9) | -7.5 (-39.2,
9.5) |
|
Unilateral
therapy | 9 | 238 (223, 258) | -14 (-18, 2) | -9 (-50, 9) | -7.5 (-39.2,
1.7) |
|
Bilateral
therapyb | 5 | 225 (219, 294) | +10 (-111, 12) | -73 (-146.2,
-14.2) | +4.5 (-61.5,
31) |
| Received between 4
and 6 doses | | | | | |
|
All eyes
treated (overall) | 12 | 386 (319,
499.7) | -100 (-185,
-36.7) | -93.5 (-220.2,
-55.5) | -125 (-201.7,
-22.5) |
|
Unilateral
therapy | 7 | 492 (313.5,
527) | -123 (-221,
-58) | -120 (-256,
-70.5) | -156 (-232,
-26) |
|
Bilateral
therapyb | 5 | 380 (338, 392) | -78 (-122, -9) | -85 (-102,
-10) | -118 (-155.2,
-57.7) |
For bilateral therapy, no significant change in CRT
was obtained (P=0.218). In the eyes treated first, a reduction in
the median (IQR) CRT of -81 (-155, -14) µm (mean ± SD, -86.2±94.6;
range, -204 to +23) was observed at six months, while no response
was observed in the contralateral eye [median (IQR), +12 (-72,
33.5) µm; mean ± SD, -29.6±111.5 µm; range, -156 to +55 µm;
Table VI and Fig. 3B and C].
The favorable response in OCT results was also
greater in eyes receiving between 4 and 6 injections for unilateral
therapy [median (IQR): -156 (-232, -26) µm; mean ± SD, -156.3±150.9
µm; range, -385 to -1 µm] when compared to the eyes that received 3
injections [median (IQR), -7.5 (-39.2, 1.7) µm; mean ± SD, -14.8±28
µm; range, -52 to +30 µm; Fig. 3D;
Table VI] and similar results were
observed for bilateral therapy in eyes receiving 4-6 injections
[median (IQR), -118 (-155.2, -57.7) µm; mean ± SD, -95±79.5 µm;
range, -204 to +23 µm] vs. 3 injections [median (IQR), +4.5 (-61.5,
31) µm; mean ± SD, -35.0±116.1 µm; range, -14 to +55 µm]. In
addition, in eyes treated unilaterally, a small reduction in the
CRT [median (IQR), -1 (-8, 3.7) µm; mean ± SD, -3.1±13.4 µm; range,
-39 to +12 µm] was observed in the contralateral (untreated) eye at
the sixth month, but this was not significant and of low clinical
significance (results not shown).
In most patients (n=16 patients and 17 eyes
treated), the ophthalmological assessment by slit-lamp
biomicroscopy showed an improvement in the AMD throughout the
treatment course with bevacizumab. Improvements encompassed both
the decrease in the disease intensity and/or its change from
exudative to dry AMD.
No clinically significant abnormalities were
reported outside the expected alterations in patients with AMD.
Discussion
In the present study, the safety and clinical
effectiveness of bevacizumab (Lumiere®) administered by
the intravitreal route for the treatment of nAMD were assessed. The
study was designed to represent the patient population usually
requiring treatment for nAMD (inclusion/exclusion criteria). Safety
assessment was the primary objective of the present study. In this
regard, and based on data collected from the study population, no
noteworthy safety risks for patients receiving a minimum of 3 doses
during the 6-month period (as per the protocol) were identified.
These results were consistent with those of previous studies
(11,14-19,23,28-31),
further confirming that bevacizumab has a favorable safety profile
for its use in nAMD. None of the serious (or severe) systemic AEs
that may be associated with the parenteral administration of
anti-VEGF drugs were observed (14-19).
Although anti-VEGF drugs are injected into the eye in small
quantities, several authors have raised concerns regarding
potential AE(s) resulting from the systemic suppression of VEGF
(32-36).
VEGF is necessary for the normal functioning of the endothelium,
where it promotes vascular integrity and endothelial cell survival
(37,38). Clinical experience in oncology
indicated that blocking of VEGF is associated with AEs in the
systemic circulation (39) and as
patients with nAMD are usually of advanced age and have an
increased risk of cardiovascular events, the requirement to assess
cardiovascular safety is warranted. These systemic AEs include
cardiovascular and arterial thromboembolic effects (e.g., stroke
and myocardial infarction), but also renal and gastrointestinal
effects, as well as wound healing complications. Due to this,
patients with a recent history of stroke/myocardial infarction were
not included in the present study and caution should be taken in
patients with a history of recent cardiovascular disease or stroke,
as they may have a greater risk for systemic AEs.
Another major safety concern associated with
intravitreal administration is the development of severe
ophthalmologic infections (e.g., infectious endophthalmitis), which
were reported in previous studies (21,40).
Besides a single case of bilateral non-serious viral
conjunctivitis, no other ocular infections were reported in the
present study. The factors towards such a favorable safety outcome
would include proper drug storage/handling at the research center,
drug preparation and administration under strict aseptic
conditions, use of periprocedural topical broad-spectrum
antibiotics (e.g., gatifloxacin) and proper post-procedural
guidance provided to the patient. Furthermore, the pharmaceutical
form of bevacizumab (Lumiere®) as a single-dose vial
avoids the possibility of drug reutilization.
Additional safety variables assessed throughout the
study included IOP and vital signs (blood pressure and heart rate).
Results from certain studies raised concern regarding the impact of
intravitreal anti-VEGF therapy in the IOP and reported a sustained
IOP elevation (i.e., IOP ≥21 or 22 mmHg and elevation ≥6 mmHg from
baseline and on, at least, two consecutive visits) in 3-11% of
patients who received repeated anti-VEGF injections (41-49).
In the present study, patients with severe ocular hypertension were
not included and the IOP was bilaterally monitored at days 1 and 28
post-injection. It was observed that the average baseline IOP
values kept stable up to the sixth month and only three occurrences
of non-serious AEs associated with elevated IOP were reported.
Furthermore, changes observed in blood pressure and heart rate were
minor and non-clinically relevant throughout the follow-up period,
which is consistent with the observations reported in another study
(50).
The assessment of clinical effectiveness (i.e.,
response to treatment) in patients receiving a minimum of 3 doses
was the secondary objective of the present study and was assessed
by analyzing variations over time in VA and CRT. Even though the
study design and sample size did not allow for inference with
estimations, treatment with intravitreal bevacizumab resulted in a
favorable effect in terms of VA improvement and CRT reduction over
the 6-month period. VA assessment was achieved at the sixth month
in a total of 23 eyes: 19 of these (82.6%) had either maintained
(no change from baseline) or improved VA at the 6-month follow-up.
These results are consistent with those of another clinical study
(51).
The subgroup of patients receiving only 3 doses had
less improvement in anatomical and functional outcomes when
compared to those who received between 4 and 6 injections in the
affected eye. The gain in VA obtained in the present study is
comparable with that of previous studies and is associated with the
type of treatment schedule (14,16,21,52).
Certain limitations of our study should be noted: i)
Due to the small sample size, rare/uncommon AE(s) were unlikely to
be detected in the present cohort (53); ii) follow-up of each patient lasted
6 months and considering that life-long treatment is required for
nAMD (54), a long-term outcome
assessment is not available; iii) no formal or specific assessment
on the psychological impact and quality of life of anti-VEGF
therapy was performed (55); iv)
the present study followed a pro re nata treatment strategy,
according to which patients were given 3 injections on a monthly
basis, followed by a decision of whether to continue treatment,
based on the evolution of VA and retinal thickening, safety
outcomes and any other clinical finding judged by the investigator
as relevant for the treatment decision. However, there are other
published therapeutic strategies (e.g., treat-and-extend) that may
also be applied in clinical practice, which were not pursued in the
present study (56,57).
In conclusion, the present study indicated that
intravitreal injection of bevacizumab led to an improvement of VA
in eyes with nAMD and with a favorable safety profile. Clinical
effectiveness has been observed within 6 months of therapy onset.
The sustainability of changes in retinal thickness and VA in
response to bevacizumab treatment warrant further investigation and
long-term follow up.
Acknowledgements
The authors thank Dr Silvia Andonian (Oftalmología
Parque Chas, Ciudad Autónoma de Buenos Aires, C1431DVC, Argentina)
and Dr Ricardo Zaldúa (IOFA Instituto Oftalmólogico Argentino,
Ciudad Autónoma de Buenos Aires, C1012, Argentina) for their
scientific advice and valuable feedback during the study design and
execution.
Funding
The present study was funded by Laboratorio
Elea-Phoenix S.A. (Argentina).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DAB, JM and DS led the clinical trial at their study
sites. AMRA, AAA, DGR, RQ, MRG, MACT and MLS evaluated the
candidates according to the inclusion and exclusion criteria,
treated the patients and collected the data. ES and MAT designed
the protocol, implemented QA and QC systems, monitored study
performance and prepared the study report. MD, MIP, FF and CL
provided medical advice and pharmacovigilance services, and have
also been involved in drafting and revising the manuscript for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki and was approved by the FEFyM ethics
committee from the Autonomous City of Buenos Aires (Buenos Aires,
Argentina) with the reference number CI.AS-0000000933/2016.
Informed consent for participation in the study was obtained from
all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Friedman DS, O'Colmain BJ, Muñoz B, Tomany
SC, McCarty C, de Jong PT, Nemesure B, Mitchell P and Kempen J: Eye
Diseases Prevalence Research Group. Prevalence of age-related
macular degeneration in the United States. Arch Ophthalmol.
122:564–572. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Flaxel CJ, Adelman RA, Bailey ST, Fawzi A,
Lim JI, Vemulakonda GA and Ying Gs: Age-Related Macular
Degeneration Preferred Practice Pattern®, Ophthalmology
(2019), doi: https://doi.org/10.1016/j.ophtha.2019.09.024.
|
|
3
|
Ferris FL III, Fine SL and Hyman L:
Age-related macular degeneration and blindness due to neovascular
maculopathy. Arch Ophthalmol. 102:1640–1642. 1984.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zarbin MA: Age-related macular
degeneration: Review of pathogenesis. Eur J Ophthalmol. 8:199–206.
1998.PubMed/NCBI
|
|
5
|
Beatty S, Koh H, Phil M, Henson D and
Boulton M: The role of oxidative stress in the pathogenesis of
age-related macular degeneration. Surv Ophthalmol. 45:115–134.
2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Holz FG, Schmitz-Valckenberg S and
Fleckenstein M: Recent developments in the treatment of age-related
macular degeneration. J Clin Invest. 124:1430–1438. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Heinemann V and Hoff PM: Bevacizumab plus
irinotecan-based regimens in the treatment of metastatic colorectal
cancer. Oncology. 79:118–128. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Johnson DR, Leeper HE and Uhm JH:
Glioblastoma survival in the United States improved after food and
drug administration approval of bevacizumab: A population-based
analysis. Cancer. 119:3489–3495. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ferrara N, Hillan KJ, Gerber HP and
Novotny W: Discovery and development of bevacizumab, an anti-VEGF
antibody for treating cancer. Nat Rev Drug Discov. 3:391–400.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Rosenfeld PJ, Moshfeghi AA and Puliafito
CA: Optical coherence tomography findings after an intravitreal
injection of bevacizumab (avastin) for neovascular age-related
macular degeneration. Ophthalmic Surg Lasers Imaging. 36:331–335.
2005.PubMed/NCBI
|
|
11
|
Modi YS, Tanchon C and Ehlers JP:
Comparative safety and tolerability of anti-VEGF therapy in
age-related macular degeneration. Drug Saf. 38:279–293.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jan S, Nazim M, Karim S and Hussain Z:
Intravitreal bevacizumab: Indications and complications. J Ayub Med
Coll Abbottabad. 28:364–368. 2016.PubMed/NCBI
|
|
13
|
Bro T, Derebecka M, Jørstad ØK and
Grzybowski A: Off-label use of bevacizumab for wet age-related
macular degeneration in Europe. Graefes Arch Clin Exp Ophthalmol.
258:503–511. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Martin DF, Maguire MG, Ying GS, Grunwald
JE, Fine SL and Jaffe GJ: CATT Research Group. Ranibizumab and
bevacizumab for neovascular age-related macular degeneration. N
Engl J Med. 364:1897–1908. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chakravarthy U, Harding SP, Rogers CA,
Downes SM, Lotery AJ, Wordsworth S and Reeves BC: IVAN Study
Investigators. Ranibizumab versus bevacizumab to treat neovascular
age-related macular degeneration: one-year findings from the IVAN
randomized trial. Ophthalmology. 119:1399–1411. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schauwvlieghe AM, Dijkman G, Hooymans JM,
Verbraak FD, Hoyng CB, Dijkgraaf MG, Peto T, Vingerling JR and
Schlingemann RO: Comparing the effectiveness of bevacizumab to
ranibizumab in patients with exudative age-related macular
degeneration. The BRAMD study. PLoS One.
11(e0153052)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Krebs I, Schmetterer L, Boltz A, Told R,
Vécsei-Marlovits V, Egger S, Schönherr U, Haas A, Ansari-Shahrezaei
S and Susanne Binder: MANTA Research Group. A randomised
double-masked trial comparing the visual outcome after treatment
with ranibizumab or bevacizumab in patients with neovascular
age-related macular degeneration. Br J Ophthalmol. 97:266–271.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kodjikian L, Souied EH, Mimoun G,
Mauget-Faÿsse M, Behar-Cohen F, Decullier E, Huot L and Aulagner G:
GEFAL Study Group. Ranibizumab versus bevacizumab for neovascular
age-related macular degeneration: Results from the GEFAL
noninferiority randomized trial. Ophthalmology. 120:2300–2309.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Berg K, Pedersen TR, Sandvik L and
Bragadóttir R: Comparison of ranibizumab and bevacizumab for
neovascular age-related macular degeneration according to LUCAS
treat-and-extend protocol. Ophthalmology. 122:146–152.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Poku E, Rathbone J, Wong R, Everson-Hock
E, Essat M, Pandor A and Wailoo A: The safety of intravitreal
bevacizumab monotherapy in adult ophthalmic conditions: Systematic
review. BMJ Open. 4(e005244)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Solomon SD, Lindsley K, Vedula SS,
Krzystolik MG and Hawkins BS: Anti-vascular endothelial growth
factor for neovascular age-related macular degeneration. Cochrane
Database Syst Rev. 3(CD005139)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Moja L, Lucenteforte E, Kwag KH, Bertele
V, Campomori A, Chakravarthy U, D'Amico R, Dickersin K, Kodjikian
L, Lindsley K, et al: Systemic safety of bevacizumab versus
ranibizumab for neovascular age-related macular degeneration.
Cochrane Database Syst Rev. 2014(CD011230)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jain P, Sheth J, Anantharaman G and
Gopalakrishnan M: Real-world evidence of safety profile of
intravitreal bevacizumab (Avastin) in an Indian scenario. Indian J
Ophthalmol. 65:596–602. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Foss AJ, Childs M, Reeves BC, Empeslidis
T, Tesha P, Dhar-Munshi S, Mughal S, Culliford L, Rogers CA, Tan W
and Montgomery A: Comparing different dosing regimens of
bevacizumab in the treatment of neovascular macular degeneration:
Study protocol for a randomised controlled trial. Trials.
16(85)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ross EL, Hutton DW, Stein JD, Bressler NM,
Jampol LM and Glassman AR: Diabetic Retinopathy Clinical Research
Network. Cost-effectiveness of aflibercept, bevacizumab, and
ranibizumab for diabetic macular edema treatment: Analysis from the
diabetic retinopathy clinical research network comparative
effectiveness trial. JAMA Ophthalmol. 134:888–896. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Age-Related Eye Disease Study System for
Classifying Age-related Macular Degeneration From Stereoscopic
Color Fundus Photographs. AREDS Report No. 6. Am J Ophthalmol 132:
668-681, 2001.
|
|
27
|
International Conference on Harmonization
of Technical Requirements for Registration of Pharmaceuticals for
Human Use (ICH). MedDRA® the Medical Dictionary for
Regulatory Activities. A registered trademark of the International
Federation of Pharmaceutical Manufacturers and Associations
(IFPMA); Chantilly, VA: Northrop Grumman MSSO (distributors). March
13, 2019. http://www.meddra.org/.
|
|
28
|
Bakri SJ, Thorne JE, Ho AC, Ehlers JP,
Schoenberger SD, Yeh S and Kim SJ: Safety and efficacy of
anti-vascular endothelial growth factor therapies for neovascular
age-related macular degeneration: A report by the american academy
of ophthalmology. Ophthalmology. 126:55–63. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Etminan M, Maberley DA, Babiuk DW and
Carleton BC: Risk of myocardial infarction and stroke with single
or repeated doses of intravitreal bevacizumab in age-related
macular degeneration. Am J Ophthalmol. 166:53–58. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ng WY, Tan GS, Ong PG, Cheng CY, Cheung
CY, Wong DW, Mathur R, Chow KY, Wong TY and Cheung GC: Incidence of
myocardial infarction, stroke, and death in patients with
age-related macular degeneration treated with intravitreal
anti-vascular endothelial growth factor therapy. Am J Ophthalmol.
159:557–64.e1. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Beato J, Pedrosa AC, Pinheiro-Costa J,
Freitas-da-Costa P, Falcão MS, Melo A, Estrela-Silva S,
Falcão-Reism F and Carneiro ÂM: Long-term effect of anti-VEGF
agents on intraocular pressure in age-related macular degeneration.
Ophthalmic Res. 56:30–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Matsuyama K, Ogata N, Matsuoka M, Wada M,
Takahashi K and Nishimura T: Plasma levels of vascular endothelial
growth factor and pigment epithelium-derived factor before and
after intravitreal injection of bevacizumab. Br J Ophthalmol.
94:1215–1218. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Carneiro AM, Costa R, Falcão MS,
Barthelmes D, Mendonça LS, Fonseca SL, Gonçalves R, Gonçalves C,
Falcão-Reis FM and Soares R: Vascular endothelial growth factor
plasma levels before and after treatment of neovascular age-related
macular degeneration with bevacizumab or ranibizumab. Acta
Ophthalmol. 90:e25–e30. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zehetner C, Kirchmair R, Huber S,
Kralinger MT and Kieselbach GF: Plasma levels of vascular
endothelial growth factor before and after intravitreal injection
of bevacizumab, ranibizumab and pegaptanib in patients with
age-related macular degeneration, and in patients with diabetic
macular oedema. Br J Ophthalmol. 97:454–459. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kwon JW, Jee D and La TY: The association
between myocardial infarction and intravitreal bevacizumab
injection. Medicine (Baltimore). 97(e0198)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Enseleit F, Michels S, Sudano I, Stahel M,
Zweifel S, Schlager O, Becker M, Winnik S, Nägele M, Flammer AJ, et
al: SAVE-AMD: Safety of VEGF inhibitors in age-related macular
degeneration. Ophthalmologica. 238:205–216. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Domigan CK, Warren CM, Antanesian V,
Happel K, Ziyad S, Lee S, Krall A, Duan L, Torres-Collado AX,
Castellani LW, et al: Autocrine VEGF maintains endothelial survival
through regulation of metabolism and autophagy. J Cell Sci.
128:2236–2248. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zechariah A, ElAli A, Doeppner TR, Jin F,
Hasan MR, Helfrich I, Mies G and Hermann DM: Vascular endothelial
growth factor promotes pericyte coverage of brain capillaries,
improves cerebral blood flow during subsequent focal cerebral
ischemia, and preserves the metabolic penumbra. Stroke.
44:1690–1697. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen HX and Cleck JN: Adverse effects of
anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol.
6:465–477. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kiss S, Dugel PU, Khanani AM, Broder MS,
Chang E, Sun GH and Turpcu A: Endophthalmitis rates among patients
receiving intravitreal anti-VEGF injections: A USA claims analysis.
Clin Ophthalmol. 12:1625–1635. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Adelman RA, Zheng Q and Mayer HR:
Persistent ocular hypertension following intravitreal bevacizumab
and ranibizumab injections. J Ocul Pharmacol Ther. 26:105–110.
2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Choi DY, Ortube MC, McCannel CA, Sarraf D,
Hubschman JP, McCannel TA and Gorin MB: Sustained elevated
intraocular pressures after intravitreal injection of bevacizumab,
ranibizumab, and pegaptanib. Retina. 31:1028–1035. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Good TJ, Kimura AE, Mandava N and Kahook
MY: Sustained elevation of intraocular pressure after intravitreal
injections of anti-VEGF agents. Br J Ophthalmol. 95:1111–1114.
2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hoang QV, Tsuang AJ, Gelman R, Mendonca
LS, Della Torre KE, Jung JJ and Freund KB: Clinical predictors of
sustained intraocular pressure elevation due to intravitreal
anti-vascular endothelial growth factor therapy. Retina.
33:179–187. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mathalone N, Arodi-Golan A, Sar S, Wolfson
Y, Shalem M, Lavi I and Geyer O: Sustained elevation of intraocular
pressure after intravitreal injections of bevacizumab in eyes with
neovascular age-related macular degeneration. Graefes Arch Clin Exp
Ophthalmol. 250:1435–1440. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Segal O, Ferencz JR, Cohen P, Nemet AY and
Nesher R: Persistent elevation of intraocular pressure following
intravitreal injection of bevacizumab. Isr Med Assoc J. 15:352–355.
2013.PubMed/NCBI
|
|
47
|
Sniegowski M, Mandava N and Kahook MY:
Sustained intraocular pressure elevation after intravitreal
injection of bevacizumab and ranibizumab associated with
trabeculitis. Open Ophthalmol J. 4:28–29. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Dedania VS and Bakri SJ: Sustained
elevation of intraocular pressure after intravitreal anti-VEGF
agents: What is the evidence? Retina. 35:841–858. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wen JC, Reina-Torres E, Sherwood JM,
Challa P, Liu KC, Li G, Chang JY, Cousins SW, Schuman SG, Mettu PS,
et al: Intravitreal anti-VEGF injections reduce aqueous outflow
facility in patients with neovascular age-related macular
degeneration. Invest Ophthalmol Vis Sci. 58:1893–1898.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sengul A, Rasier R, Ciftci C, Artunay O,
Kockar A, Bahcecioglu H and Yuzbasioglu E: Short-term effects of
intravitreal ranibizumab and bevacizumab administration on 24-h
ambulatory blood pressure monitoring recordings in normotensive
patients with age-related macular degeneration. Eye (Lond).
31:677–683. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Sagiv O, Zloto O, Moroz I and Moisseiev J:
Different clinical courses on long-term follow-up of age-related
macular degeneration patients treated with intravitreal
anti-vascular endothelial growth factor injections.
Ophthalmologica. 238:217–225. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Tufail A, Patel PJ, Egan C, Hykin P, da
Cruz L, Gregor Z, Dowler J, Majid MA, Bailey C, Mohamed Q, et al:
Bevacizumab for neovascular age related macular degeneration (ABC
trial): Multicentre randomised double masked study. BMJ.
340(c2459)2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang W and Zhang X: Systemic adverse
events after intravitreal bevacizumab versus ranibizumab for
age-related macular degeneration: A meta-analysis. PLoS One.
9(e109744)2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Müller S, Ehlken C, Bauer-Steinhusen U,
Lechtenfeld W, Hasanbasic Z, Agostini H and Wilke T: Treatment of
age-related neovascular macular degeneration: The patient's
perspective. Graefes Arch Clin Exp Ophthalmol. 255:2237–2246.
2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Senra H, Ali Z, Balaskas K and Aslam T:
Psychological impact of anti-VEGF treatments for wet macular
degeneration-a review. Graefes Arch Clin Exp Ophthalmol.
254:1873–1880. 2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Gianniou C, Dirani A, Ferrini W,
Marchionno L, Decugis D, Deli A, Ambresin A and Mantel I: Two-year
outcome of an observe-and-plan regimen for neovascular age-related
macular degeneration: How to alleviate the clinical burden with
maintained functional results. Eye (Lond). 29:450–451.
2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Alexandru MR and Alexandra NM: Wet
age-related macular degeneration management and follow-up. Rom J
Ophthalmol. 60:9–13. 2016.PubMed/NCBI
|