1. Introduction
Pulmonary hypertension (PH)
PH is a condition with relatively high morbidity and
an annual global incidence of 3-10 cases per 1 million adults. In
individuals >65 years of age the prevalence of PH is estimated
to be as high as ~10%. The prognosis of PH is poor and the 5-year
survival rate in newly diagnosed patients is only 57% (1,2). The
main symptoms of PH include progressive dyspnea during exercise,
fatigue, including chest pain and syncope (3). However, patients with PH do not
usually exhibit specific symptoms, which may result in delays in
diagnosis, ranging from several months to years (4).
Regarding the pathogenesis of the disease, PH is
classified into the following five categories (4): i) pulmonary arterial hypertension
(PAH); ii) left heart disease-induced PH; iii) hypoxia- or lung
disease-induced PH; iv) chronic thromboembolic PH (CTEPH); and v)
PH caused by unclear or multifactorial mechanisms. The pathogenesis
of PH is very complex, and includes impaired angiogenesis,
metabolic disorders, chronic inflammation, abnormal proliferation,
and the resistance of pulmonary artery endothelial cells (PAECs)
and pulmonary artery smooth muscle cells (PASMCs) to apoptosis
(5). The abnormal proliferation of
VSMCs is considered to be the main cause of vascular remodeling.
Under hypoxic conditions, abnormal PASMC proliferation promotes
thickening of the media layer, pulmonary vasoconstriction and
pulmonary vascular remodeling, which together result in sustained
increased pulmonary artery pressure and right ventricular
hypertrophy (6). Although several
molecules and signaling pathways, including bone morphogenetic
protein receptor type 2, platelet-derived growth factor (PDGF),
Rho/Rho-associated coiled-coil containing protein kinase,
serotonin, endothelin, nitric oxide and NADPH oxidase, have been
found to be involved in the pathogenesis of PH, others may as yet
be unidentified (7-9).
Despite significant progress in understanding the
basic mechanisms of PH and the reduction in the number of
PH-associated hospitalizations that has occurred over the past few
decades (10), PH remains an
incurable disease with high treatment costs. In addition, an upward
trend in hospitalization time with no significant decrease in
mortality has been reported (11).
Therefore, further clarification of the pathophysiological
mechanisms of PH is important for understanding the disease and
developing novel treatments.
Long non-coding RNAs (lncRNAs)
Non-coding RNAs (ncRNAs) were originally considered
as ‘transcriptional noise’. However, in the past few decades, the
roles of ncRNAs, including microRNAs (miRNAs) and lncRNAs, in
normal physiology and disease pathology have been widely
investigated, and ncRNAs have been reported to participate in cell
homeostasis and disease processes (12). Several studies have confirmed the
importance of ncRNAs as transcriptional regulators and further
explored their potential molecular mechanisms, thereby
demonstrating the vital role of ncRNAs in several biological
processes (13). The development of
next-generation sequencing, particularly RNA-sequencing (RNA-seq),
has led to the discovery of numerous lncRNAs for which biological
functions have been identified (14). There is considerable evidence of
associations between lncRNAs and the onset of diverse human
diseases, including cancer, cardiovascular and neurodegenerative
diseases. Abnormal and uncontrolled cell proliferation and
migration, as well as an imbalance between cell growth and
apoptosis are characteristic pathological changes of PH. Several
studies on the roles of lncRNAs and their mechanisms of action in
the regulation of PH pathogenesis have indicated that lncRNAs act
as key regulators in the aforementioned biological changes.
lncRNAs are a class of ncRNAs >200 nucleotides in
length that are synthesized by RNA polymerase II and subjected to
post-transcriptional processing, including capping, splicing and
polyadenylation. The majority of lncRNAs are located in the cell
nucleus, expressed at low levels and show poor sequence
conservation (15). lncRNAs serve
an important epigenetic role by acting as activators or repressors
of gene transcription and mRNA translation, RNA stabilizers, miRNA
precursors and sponges (16). In
addition, the interaction of lncRNAs with RNA, DNA or proteins may
promote or inhibit protein-coding gene expression (17).
It is now recognized that lncRNAs regulate cell
differentiation and apoptosis, chromatin remodeling and
carcinogenesis at transcriptional and post-transcriptional levels
(18,19). In addition, lncRNAs are closely
associated with hypoxic diseases, and their abnormal expression has
been associated with different types of cancer and cardiovascular
diseases (20-22).
For example, it has been reported that the lncRNAs
Nkx2.1-associated non-coding intergenic RNA (NANCI) and growth
arrest-specific transcript 5 (GAS5) are associated with lung
diseases (23,24). PH and cancer share some common
features, such as excessive cell proliferation and apoptosis
resistance (25), and it has been
suggested that lncRNAs participate in the proliferation, apoptosis
and cell cycle of PASMCs and PAECs in PH (26). PAECs, PASMCs and inflammatory cells
contain lncRNAs, some of which serve important roles in the
pathological process of PH and are of great significance in its
occurrence and development. Abnormal levels of lncRNAs in the blood
have been suggested to be a novel diagnostic marker for PH.
lncRNAs and miRNAs
miRNAs are single stranded RNA molecules that are
~22 nucleotides in length, and so are shorter than lncRNAs. miRNAs
are derived from primary miRNAs, and when mature are mainly located
in the cytoplasm. By contrast, lncRNAs are derived from different
cells and are mainly localized in the cell nucleus (15).
miRNAs inhibit the translation of mRNA or promote
its degradation to silence downstream gene expression through
binding to the 3'untranslated region of the mRNA (27). Regarding the pathological mechanisms
of PH, lncRNAs mainly act as miRNA sponges (28). Each miRNA has been reported to
regulate the expression of >100 mRNAs (29). Studies using animal models of PH
have shown that miRNA mimics or inhibitors may delay pulmonary
vascular remodeling to some extent and exhibit therapeutic effects
by reducing pulmonary artery pressure and ameliorating right heart
failure (30,31). In addition, clinical studies have
identified a variety of differentially expressed miRNAs in the
peripheral blood of patients with PH, suggesting the diagnostic and
prognostic value of miRNAs in PH (32). lncRNAs interact with miRNAs to
regulate the expression and biological activities of the miRNA, and
also act as competitive endogenous RNAs by binding to miRNA,
thereby inhibiting the ability of the miRNA to bind to its target
genes (33). Thus, miRNAs are
regulated by lncRNAs. These two types of RNA molecule complement
and interact with each other to form a complex network of
interactions in which lncRNAs act as miRNA sponges, some miRNAs are
derived from lncRNAs while others degrade them, and lncRNAs and
miRNAs both bind to mRNAs, indicating a competitive relationship
between them (19,34).
2. lncRNAs and PH
Numerous studies have indicated that lncRNAs have
essential roles in the occurrence and development of PH. Therefore,
the present review summarizes the characteristics of lncRNAs that
have been identified to be involved in the pathogenesis of PH.
Maternally expressed gene 3
(MEG3)
The lncRNA MEG3 is located on human chromosome 14q32
and is 1.6 kb in length. It acts as a tumor suppressor and has a
vital role in several types of cancer, including breast, liver,
gastrointestinal and lung cancer (35-37).
It has been reported that the downregulation of MEG3 increases the
sensitivity of lung cancer to cisplatin treatment (38,39).
MEG3 also serves a key role in cardiovascular diseases and is
involved in hypoxia, abnormally expressed in cardiac fibroblasts
and downregulated during late cardiac remodeling (40). Furthermore, elevated MEG3 levels
have been detected in vascular endothelial cells (41).
A study conducted by Piccoli et al (40) demonstrated that MEG3 was mainly
localized in the cytoplasm of hypoxic PASMCs, and expressed at
significantly increased levels in a hypoxia-induced animal model of
PH and PASMCs from idiopathic PH patients. The increased expression
of MEG3 was further confirmed in hypoxia-induced human and mouse
PASMC cell lines. Furthermore, fluorescence in situ
hybridization analysis revealed that MEG3 was primarily localized
in PASMCs and the media layer of the vascular wall, and
translocated to the cytoplasm when exposed to hypoxia.
Additionally, the authors suggested that MEG3 associated with
miR-328-3p to regulate multiple targets, particularly insulin-like
growth factor 1 receptor (IGF1R), the upregulation of which further
induced PASMC proliferation and pulmonary vascular remodeling
(42). However, Zhu et al
(43) demonstrated that MEG3 was
downregulated in hypoxia-induced human PASMCs (HPASMCs), and that
the downregulation of MEG3 significantly promoted the proliferation
and migration of PASMCs under both normal and hypoxic conditions.
The underlying mechanism of MEG3 was suggested to involve a
MEG3/miR-21/PTEN axis. Similarly, a study by Sun et al
(44) detected the downregulation
of MEG3 in pulmonary arteries derived from PH patients and
demonstrated that MEG3 promoted PASMC proliferation and migration
via the p53 pathway.
In an animal model, experiments using a
lung-specific small interfering (siRNA)-loaded liposomal delivery
system demonstrated that hypoxic PH was significantly ameliorated
when MEG3 was knocked down, as the right ventricle systolic
pressure, right ventricular hypertrophy index and pulmonary artery
pressure were relieved. These findings indicate the potential of
MEG3 as a novel therapeutic target for the pharmaceutical treatment
of PH (42).
Notably, the findings regarding MEG3 up- or
downregulation in PH are not consistent in the aforementioned
studies. This may be associated with differences in the
distribution of MEG3 in cells, the time of measurement and study
objects. Further research is needed to clarify the role of MEG3 in
PH.
Hoxa cluster antisense RNA 3
(HOXA-AS3)
The HOX gene cluster is a group of highly homologous
transcription factors (45). It has
been reported that members of the HOXA cluster, namely HOTAIR and
HOTTIP, regulate the proliferation of lung cancer cells (46). A study by Zhang et al
(47) demonstrated that Hoxaas3, a
member of the HOX gene cluster, was upregulated in hypoxia- and
monocrotaline (MCT)-induced PH models as well as in PASMCs isolated
from patients with idiopathic PH. Furthermore, the knockdown of
Hoxaas3 reduced the expression of proliferating cell nuclear
antigen (PCNA), Ki-67 and cyclins A, D and E in PASMCs, suggesting
that Hoxaas3 promotes PASMC proliferation and accelerates the cell
cycle. In addition, histone acetyltransferase p300 inhibitors
downregulated Hoxaas3 expression under hypoxic conditions,
suggesting histone acetyltransferase was involved in Hoxaas3
upregulation under hypoxia. Another study demonstrated that HOX
genes are overexpressed in the lung tissues of patients with PH,
suggesting that they may have an important role in endothelial cell
proliferation and vascular remodeling (48). These studies provide new data that
improve our understanding of the role of Hoxaas3 lncRNA in PASMC
proliferation. However, further research is required to confirm the
role of Hoxaas3 in patients with different clinical subtypes of
PH.
MANTIS
In a study by Leisegang et al (49), exon array analyses were performed to
investigate the effect of histone demethylase JARID1B knockdown on
endothelial RNA expression. The expression levels of several
lncRNAs were found to be significantly altered, including MANTIS
which was significantly downregulated. The study also revealed that
MANTIS lncRNA was downregulated in the lung tissues of patients
with end-stage idiopathic PH and in MCT-induced rat models of PH,
in which endothelial dysfunction and apoptosis serve important
roles (49). MANTIS lncRNA has also
been reported to be overexpressed during the regression of
atherosclerosis in high-fat feeding monkeys restored to a normal
diet (50). These findings indicate
a novel and potent epigenetic regulatory mechanism for MANTIS in
endothelial cells.
Taurine upregulated gene 1 (TUG1)
TUG1 is a 7.1-kb lncRNA that is expressed in the
cytoplasm and nucleus of PASMCs (51). Studies have shown that TUG1 is
involved in the regulation of protein and miRNA expression, serves
an important role in the regulation of cell proliferation,
apoptosis and chromatin remodeling, and is associated with hypoxia
(52-54).
TUG1 is a tumor suppressor gene that is highly
conserved in mammals (55). Two
studies have demonstrated that TUG1 is upregulated in the pulmonary
arteries of hypoxic PH mice and in hypoxic PASMCs, where it leads
to pulmonary vascular remodeling via the regulation of PASMC
proliferation and apoptosis. In one study, Yang et al
(56) reported that TUG1 knockdown
downregulated Foxc1 expression through a TUG1/miR-374c/Foxc1/Notch
pathway. Furthermore, TUG1 knockdown inhibited the proliferation
and migration of HPASMCs, promoted apoptosis and regulated
pulmonary vascular remodeling via the regulation hypoxia-inducible
factor 1α (HIF-1α) and vascular endothelial growth factor
expression (57). In the other
study, Wang et al (58)
showed that TUG1 regulated the proliferation and cell cycle of
ΗPASMCs by directly binding to miR-328. However, whether TUG1 also
regulates PH via other mechanisms, such as phenotypic switch,
endothelial-to-mesenchymal transition (EndMT), immunological
dysregulation and inflammatory responses remains to be further
clarified.
Metastasis-associated lung
adenocarcinoma transcript 1 (MALAT1)
MALAT1 is a highly conserved lncRNA involved in the
pathogenesis of several types of cancer, including prostate,
gastric and non-small cell lung cancer (59,60).
The expression of MALAT1 is significantly increased in
hypoxia-induced human endothelial cells and is involved in the
phenotype switch of endothelial cells (41).
Brock et al (61) first reported that MALAT1 expression
was significantly increased in hypoxic HPASMCs and the lung tissues
of mice with hypoxia-induced PH. They also demonstrated that MALAT1
promoted the proliferation and migration of PASMCs, and regulated
their phenotype. Furthermore, MALAT1 knockdown was indicated to
inhibit PASMC proliferation in vitro and cardiac hypertrophy
in the mouse model via upregulation of the expression of
cyclin-dependent kinase inhibitors.
In addition to its role in PASMCs, MALAT1 also
serves a vital role in PAECs. In one study, the downregulation of
MALAT1 increased the migration of human umbilical vein endothelial
cells (HUVECs) and slightly upregulated their apoptosis under
hypoxic conditions. A microarray analysis performed to investigate
the mechanism of MALAT1 indicated that cell cycle inhibitory genes
were significantly increased in the HUVECs transfected with MALAT1
siRNA, suggesting that MALAT1 is involved in regulation of the cell
cycle (41). MALAT1 has also been
proposed to regulate the phenotypic transition of endothelial cells
(62).
A previous study has confirmed that MALAT1 binds to
hsa-miR-124-3p and the latter directly targets kruppel-like factor
5(63). Zhuo et al (64) conducted a case-control study
involving 587 patients with PH and 736 healthy individuals. The
results showed that the MALAT1 gene rs619586 A>G polymorphism
was significantly associated with increased PH risk. The study
further demonstrated that MALAT1 acts as a competitive endogenous
RNA for miR-124 and thereby regulates X-box binding protein 1
expression and participates in the pathogenesis of PH. Furthermore,
MALAT1 was significantly increased in the pulmonary artery tissues
and PASMCs of patients with PH (63). The knockdown of MALAT1 significantly
decreased the proliferation and migration of PASMCs, and the
expression levels of PCNA and cyclin in HPASMCs, suggesting that
the mechanism of MALAT1 in PH involves modulation of the
proliferation, migration and cell cycle of PASMCs. Furthermore,
bioinformatics analysis and luciferase reporter assays confirmed
that hsa-miR-124-3p.1 was a downstream target of MALAT1.
In summary, there is considerable evidence that
MALAT1 contributes to PH. MALAT1 is significantly differently
expressed in hypoxia-induced PAECs, PASMCs, hypoxia-induced animal
models and pulmonary arterial tissues isolated from patients with
PH. MALAT1 gene polymorphism is also significantly associated with
the risk of PH.
CPS1-intronic transcript 1
(CPS1-IT1)
It has been documented that CPS1-IT1 lncRNA is
involved in the pathogenesis of lung cancer and other malignancies.
In one study, the overexpression of CPS1-IT1 in lung cancer cell
lines restrained cell proliferation and migration, and induced cell
apoptosis, indicating that CPS1-IT1 has protective effects on lung
cancer. Therefore, it was suggested that CPS1-IT1 may be used for
the early diagnosis of lung cancer and could be applied as a
targeted therapy (65). With regard
to PH, a study found that CPS1-IT expression levels were decreased
in the pulmonary tissues of rats with obstructive sleep
apnea-induced PH. In addition, the overexpression of CPS1-IT1
significantly alleviated pulmonary arterial remodeling in the rat
model. Analysis of the mechanism of action in vitro
indicated that CPS1-IT overexpression attenuated PH by
downregulating interleukin (IL)-1β via the inhibition of the
nuclear factor κB (NF-κB) signaling pathway and HIF-1
transcriptional activity (66).
However, further in vitro studies are required to clarify
the cells in which CPS1-IT1 exerts biological functions, and
research of CPS1-IT1 in patients with PH is also necessary.
Cancer susceptibility candidate 2
(CASC2)
The lncRNA CASC2 is a tumor suppressor gene, located
on human chromosome 10(67). CASC2
is closely associated with PH. CASC2 upregulation has been shown to
inhibit cell proliferation and migration, promote apoptosis and
ultimately suppress pulmonary artery remodeling. The
contractile-to-synthetic phenotypic switching of PASMCs is vitally
important for the biological processes associated with
hypertension, atherosclerosis, PH and other cardiovascular diseases
(68). The upregulation of CASC2
has been demonstrated to reverse the phenotypic transition of
PASMCs, thereby delaying the progression of PH (25). The specific mechanism of CASC2 in
the regulation of PH, however, requires further exploration.
TCONS_00034812
Liu et al (26) performed a microarray analysis on the
pulmonary arteries of rats with PH and found that the expression of
TCONS_00034812 was 8.7881-fold higher in the control rats compared
with the rats with PH. Consistent with this, reverse
transcription-quantitative PCR (RT-qPCR) demonstrated that
TCONS_00034812 expression levels were 6.1-fold higher in the
controls compared with the rats with PH. In addition,
TCONS_00034812 knockdown was demonstrated to promote the
proliferation of PASMCs and inhibit their apoptosis. Gene Ontology
(GO) analysis was also performed to identify potential target genes
of TCONS_00034812. The results indicated that storkhead box 1
(Stox1), Met and neurotrophin 3 were upregulated in PH and have an
association with cell proliferation. The direct targeting of Stox1,
a transcription factor of the expanded forkhead box gene family, by
TCONS_00034812 was confirmed by RT-qPCR analysis.
The mitogen-activated protein kinase (MAPK)
signaling pathway is important for cell survival and apoptosis
(69) and is involved in pulmonary
vascular remodeling (70). In
PASMCs, TCONS_00034812 silencing activates the MAPK signaling
pathway and thereby increases Stox1 expression, which in turn
regulates PASMC proliferation and apoptosis, resulting in PH.
Therefore, TCONS_00034812 may be a potential novel therapeutic
target for PH (26).
H19
It is well documented that H19 serves a key role in
tumorigenesis and metastasis (71,72).
In addition, H19 is involved in pathophysiological processes
associated with hypoxia (73). A
study demonstrated that the expression levels of H19 were 5-fold
higher in the lung tissues of rats with MCT-induced PH compared
with those in healthy rats. H19 levels were also significantly
increased in the serum of the MCT-induced PH rats. The stimulation
of PASMCs with different concentrations of IL-1β and PDGF-BB
significantly increased H19 expression in a dose-dependent manner.
Furthermore, H19 upregulated angiotensin II (Ang II) type 1
receptor expression by sponging the miRNA let-7b to regulate PASMC
proliferation, resulting in PH. The knockout of H19 was
demonstrated to protect mice from MCT-induced pulmonary vascular
remodeling and alleviate PH (74).
Melatonin has been indicated to have a therapeutic
effect on PH, and may act via antiproliferative effects on PASMCs
and anti-inflammatory activity (75). Wang et al (76) suggested that H19 plays a significant
role in the pathological process of PH through the miR-675-3p/IGF1R
and miR-200a/programmed cell death 4 (PDCD4) signaling pathways. In
a rat model of MCT-induced PH, melatonin treatment upregulated the
expression of H19 and miR-675-3p and downregulated that of
miR-200a, resulting in increased PDCD4 and decreased IGFR1
expression. The differential expression levels of PDCD4 and IGFR1
ultimately led to the apoptosis of PASMCs and inhibition of their
proliferation, resulting in reduced vascular remodeling and PH.
Urothelial carcinoma associated 1
(UCA1)
There is evidence to suggest that the lncRNA UCA1 is
involved in the pathogenesis of lung cancer (77,78).
In addition, it has been reported that UCA1 is highly expressed in
hypoxia-induced HPASMCs and promotes cell proliferation under
hypoxic conditions (79). By
contrast, inhibitor of growth family 5 (ING5) promotes tumor cell
apoptosis and inhibits tumor growth, thus playing a protective role
in tumor development (80). ING5
also inhibits the proliferation and promotes the apoptosis of
PASMCs. However, UCA1 competes with ING5 for binding to
heterogeneous ribonucleoprotein I (HnRNP I), which contains an
RNA-binding domain with mRNA splicing activity (61), thus decreasing ING5-HnRNP I binding
and promoting PASMC proliferation, pulmonary vascular remodeling
and ultimately PH (79). Elevated
UCA1 might aid in the diagnosis of PH, and targeting UCA1 may
provide novel therapeutic approaches.
Lnc-Ang362
Lnc-Ang362 was first identified as a lncRNA that is
differentially upregulated in Ang II-induced VSMCs. It regulates
cell proliferation and the expression of miR-221 and
miR-222(81). In another study,
lnc-Ang362 was found to be abnormally elevated in the lung tissues
of patients with PH. Furthermore, the overexpression of lnc-Ang362
promoted HPASMC proliferation and migration, reduced apoptosis and
was involved in the pathological process of PH. In addition,
lnc-Ang362 upregulated miR-221 and miR-222, thereby activating the
NF-κB signaling pathway (82).
However, the expression of lnc-Ang362 in PASMCs and PAECs induced
by hypoxia requires further clarification.
lncRNA regulated by PDGF and
transforming growth factor β (LnRPT)
PDGF plays an important role in PASMC
hyperproliferation and pulmonary vascular remodeling (83). Using RNA sequencing, 95
differentially expressed lncRNAs were identified in PDGF-BB-induced
rat PASMCs. These included LnRPT, the expression of which was
significantly lower in rat PASMCs following PDGF-BB treatment
compared with that in the control PASMCs. Furthermore, LnRPT
knockdown promoted PASMC proliferation (84). These results suggest that LnRPT
participates in PH pathogenesis by inhibiting the proliferation of
PASMCs.
The Notch receptor is a cell surface receptor
responsible for cell signaling between adjacent cells (85). The Notch signaling pathway is also
involved in the development of PH (86). In one study, RNA sequencing and
RT-qPCR assays demonstrated that the downregulation of LnRPT
increased the expression of two important Notch signaling pathway
genes, namely notch3 and jag1, and that inhibition of the Notch
signaling pathway attenuated, to some extent, the proliferation of
PASMCs. These findings indicate that LnRPT regulates the Notch
signaling pathway and that PDGF-BB participates in PH by affecting
the expression of LnRPT. Furthermore, inhibition of
phosphoinositide 3-kinase (PI3K) diminished the PDGF-BB-induced
downregulation of LnRPT, indicating that a PDGF-BB/PI3K/LnRPT
pathway participates in the pathological process of PH (84). However, the involvement of LnRPT in
apoptosis and PAECs, and in animals and patients with PH require
further investigation.
NONRATT015587.2
In addition to hypoglycemic effects, it has been
reported that metformin has a vascular protective activity and may
delay cell senescence (87).
Studies have shown that metformin reverses hypoxia and has
therapeutic effects in MCT-induced PH (87,88).
To elucidate the specific mechanisms of metformin, microarray
analyses were performed to analyze the differential expression of
lncRNAs and mRNAs. The results showed that NORNATT015587.2 was
significantly increased in hypoxia-induced PASMCs. It was also
reported that NORNATT015587.2 promotes PASMC proliferation,
inhibits apoptosis and affects pulmonary vascular remodeling. In
addition, GO enrichment analysis, Kyoto Encyclopedia of Genes and
Genomes pathway analysis and western blot assays verified that p53
and HIF-1 participated in the NORNATT015587-induced vascular
remodeling (89). In vivo
experiments and clinical research are required to verify whether
NONRATT015587.2 is involved in PH.
Although RNA sequencing technology has identified
large numbers of lncRNAs, and lncRNA has been indicated to perform
key epigenetic modifications in PH, the role of lncRNA in PH
requires further elucidation. The lncRNAs involved in the
pathological process of PH reviewed in this article mainly exert an
influence through regulating the proliferation, migration and
apoptosis of PAECs and PASMCs. A series of studies have shown that
lncRNA can be involved in regulation of the phenotype switch of
smooth muscle cells, which contributes to cell migration and
proliferation (90). EndMT is
involved in the occurrence and development of a variety of
cardiovascular diseases, including PH. Three specific lncRNAs have
been found to be associated with EndMT: MALAT1, GATA6 antisense RNA
and H19(91). Immunological and
inflammatory disorders also play an important role. The activation
and release of a variety of cellular inflammatory factors are
involved in PH (92), but the roles
of lncRNA in the regulation of the vascular inflammation associated
with PH remain unclear. Therefore, in the future, researchers may
investigate whether lncRNAs participate in the regulation of PH
through phenotypic switch, EndMT and immunological effects and the
specific mechanisms.
Translational potential of the
reviewed lncRNAs
Numerous studies have shown that some ncRNAs,
including lncRNA, have the potential to translate into proteins
(93,94). Methodologies combining ribosome
profiling and scoring schemes have been developed for the
evaluation of lncRNA translation. When we used the Coding Potential
Assessment Tool 2.0.0 (http://lilab.research.bcm.edu/cpat/index.php) to
determine whether or not specific lncRNAs have translational
potential, the results indicated that only MANTIS and H19 have
translational potential.
lncRNA and PH with different
etiologies
PH can be divided into five categories according to
its etiologies, as briefly summarized in the Introduction. Table I shows the underlying causes for
each category (95). Basic research
on lncRNA and PH is mostly based on hypoxia-induced cell or animal
models. However, only data obtained on lncRNAs identified from
research using tissues from patients with PH are summarized in
Table I. The study populations were
all patients with PAH and CTEPH. No studies on lncRNAs in patients
with the other three classes of PH were identified, which may be
due to the small number of patients with these PH types. There have
been few clinical studies on lncRNAs in patients with PH, and the
included populations are all patients with PAH (64,96,97).
 | Table IUnderlying causes of PH and their
associated lncRNAs. |
Table I
Underlying causes of PH and their
associated lncRNAs.
| Classification | Underlying
cause | Associated
lncRNA | (Refs.) |
|---|
| 1. | Pulmonary arterial
hypertension | PAXIP1-AS1, MEG3,
HypERlnc, MALAT1, lncRNA-Ang362 | (42,44,63,64,82,100,101) |
| 2. | PH due to left
heart disease | | |
| 3. | PH due to lung
diseases and/or hypoxia | | |
| 4. | Chronic
thromboembolic PH | CTEPH1, NR_036693,
NR_027783, NR_033766, NR_001284, PAFAH1B1, lncRNA
NONHSAT073641 | (102-104) |
| 5. | PH with unclear
multifactorial mechanisms | | |
lncRNAs and the pathological stages of
PH
The pathological process of PH includes the
following stages: PAEC dysregulation, EndMT, PASMC proliferation,
migration and apoptosis, adventitial thickening of lexiform lesions
and perivascular inflammation. At present, research on the
pathological stages of PH in association with lncRNA expression has
mainly focused on PASMC proliferation, migration and apoptosis, and
PAEC dysfunction. However, no studies have been conducted on the
association of lncRNAs with the adventitial thickness of plexiform
lesions. The lncRNAs associated with each pathological stage are
listed in Table II.
 | Table IIInvolvement of lncRNAs in each
pathological stage of pulmonary hypertension. |
Table II
Involvement of lncRNAs in each
pathological stage of pulmonary hypertension.
| Pathological
stage | lncRNA | (Refs.) |
|---|
| PAEC dysregulation
and EndMT | NR_001284,
NR_036693, NR_033766, NR_027783, MIR22HG, MIR210HG, H19, MEG9,
MALAT1, GATA6-AS | (103,105-108) |
| PASMC proliferation
and migration | H19, MALAT1,
lnc-Ang362, PAXIP1-AS1, TUG1, HOXA-AS3, MEG3, LnRPT, CASC2 | (25,47,56,74,82,84,100,109) |
| PASMC
apoptosis | TCONS_00034812,
UCA1 | (26,79) |
| Adventitial
thickening | - | - |
| Plexiform
lesions | - | - |
| Perivascular
inflammation | H19 | (74) |
Therapeutic targets for lncRNAs in
PH
lncRNAs have the potential to be used in combination
with other PH-treating drugs such as endothelin receptor
antagonists, PDE-5 inhibitors and prostacyclin analogs as novel
treatments for PH; however, no studies have yet been performed to
investigate this. The use of a combination of lncRNA and
PH-treating drugs has the following theoretical basis: lncRNAs are
stably expressed in the circulation and have the potential to be
used as biomarkers, which is of great significance for the
treatment of PH and judgement of the curative effect. Taking into
account the tissue specificity of lncRNA, lncRNA-based therapies
should be tissue- and dose-specific interventions. However, as of
yet no clinical research has been conducted on targeted lncRNAs in
the treatment of PH as a number of obstacles remain. First, lncRNAs
are poorly conserved among different species, thus increasing the
difficulty of drug development and clinical application. Second,
the functions and underlying mechanisms of lncRNAs are
significantly more complex and diverse than those of miRNAs
(34). The use of siRNAs to silence
the expression of target lncRNA is not always possible, as most
lncRNAs are localized in the nucleus. Furthermore, the toxicity of
chemically modified siRNAs has not yet been fully clarified
(98). In addition, the secondary
structure of lncRNAs, their delivery into the human body, the speed
of onset and duration of their action, as well as the prevention of
off-target effects require further investigation.
Outlook
Using second generation sequencing, especially
RNA-seq and other methods, a number of studies have identified
lncRNA networks involved in the regulation of PH. However, the
sequences, structures and functions of lncRNAs are poorly conserved
among different species; therefore, the in vivo study of
lncRNAs is a challenging task. The number of large-scale clinical
studies regarding the diagnosis, assessment of disease severity and
prognosis of lncRNA-induced PH is limited. This may be explained by
the following: First, it is difficult to achieve a perfect match
between patients with and without PH as clinical patients may also
exhibit different comorbidities and complications. Second, lncRNAs
are poorly conserved among different species; therefore, the
results of in vitro animal experiments cannot be directly
applied to clinical practice. Third, lncRNA tests are more
complicated and expensive compared with commonly used clinical
detection methods. However, the screening of lncRNAs with excellent
sensitivity, specificity or predictive value and the development of
drugs targeting specific lncRNAs for PH treatment would be of great
significance in the future.
3. Conclusions
PH is a multifactorial disease characterized by
pulmonary vascular remodeling, resulting in sustained increased
pulmonary arterial pressure, right heart failure and even death.
lncRNAs are key molecules that control cellular biological
activities by regulating gene expression at the transcriptional and
post-transcriptional levels (99).
A considerable body of evidence has demonstrated that lncRNAs are
essential regulators of the pathogenesis and progression of PH.
Numerous studies have greatly improved our understanding of the
roles of lncRNAs in PH. Novel PH-associated lncRNAs, their
expression and targets are listed in Table III. The dysregulation mechanisms
of differentially expressed lncRNAs involved in cell proliferation,
migration and apoptosis, regulation of the cell cycle, and
phenotypic switching are shown in Fig.
1. lncRNAs have the potential to become novel diagnostic
markers in clinical practice and lncRNAs may become potential
pharmacological targets for PH treatment.
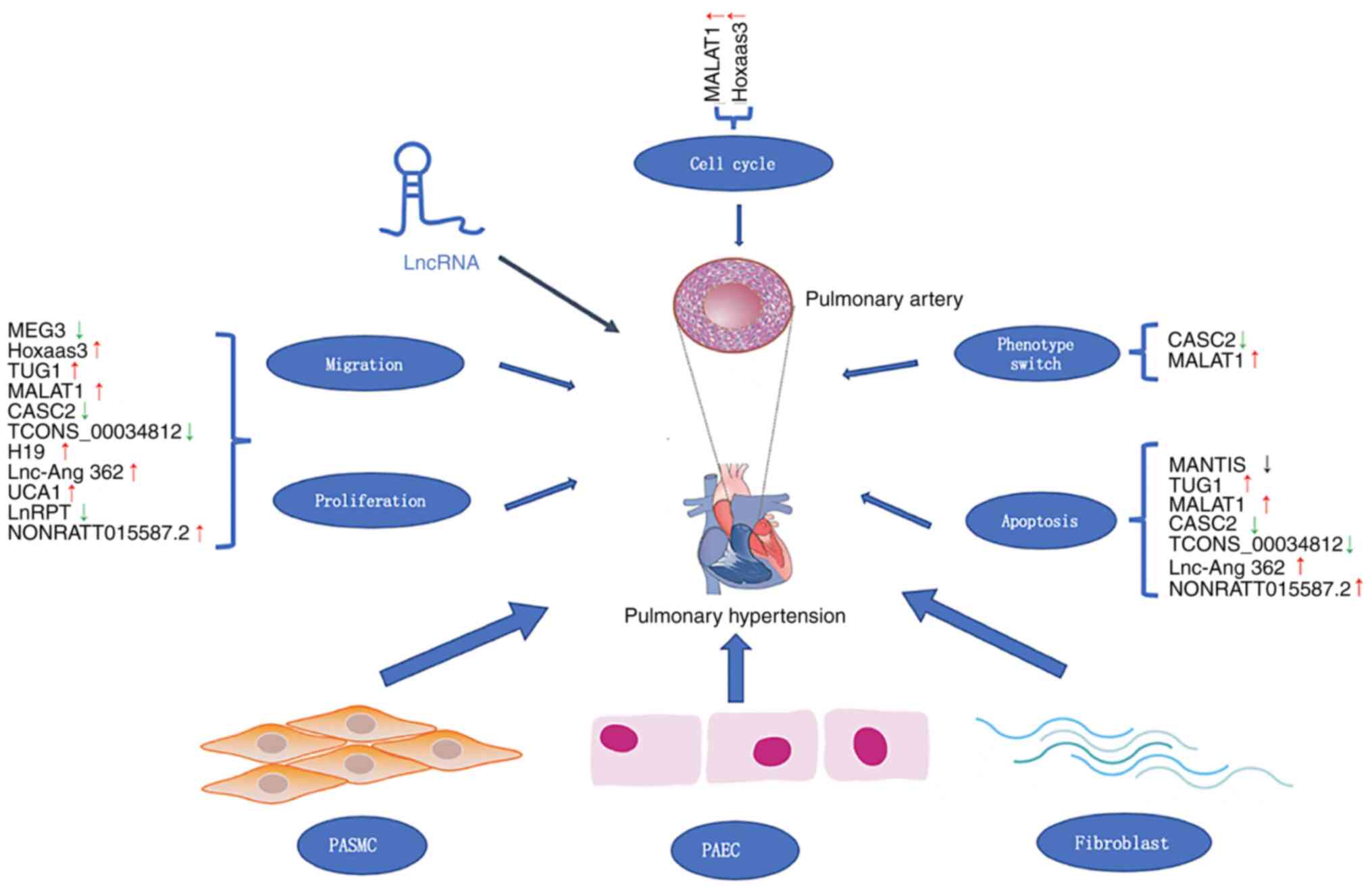 | Figure 1Roles of lncRNAs in PH. PH is caused
by thickening of the pulmonary blood vessel wall, narrowing of the
lumen and increasing pulmonary artery pressure. The dysregulation
of lncRNA expression is involved in cell proliferation, migration
and apoptosis, phenotype switch and regulation of cell cycle. Red
and green arrows indicate upregulated and downregulated lncRNAs in
PH, respectively. PH, pulmonary hypertension; lncRNA, long
non-coding RNA; PASMC, pulmonary artery smooth muscle cell; PAEC,
pulmonary artery endothelial cell; MEG3, maternally expressed 3;
Hoxaas3, Hoxa cluster antisense RNA 3; TUG1, taurine upregulated 1;
MALAT1, metastasis-associated lung adenocarcinoma transcript 1;
CASC2, cancer susceptibility candidate 2; UCA1, urothelial
carcinoma associated 1; LnRPT, lncRNA regulated by PDGF and
transforming growth factor β. |
 | Table IIIlncRNA expression, targets and
effects on PH. |
Table III
lncRNA expression, targets and
effects on PH.
| lncRNA | Expression | Target | Effect | (Refs.) |
|---|
| MEG3 | Upregulated |
miR-328-3p/IGF1R | Promotes
proliferation of PASMCs | (42) |
| MEG3 | Downregulated | miR-21/PTEN; p53
pathway | Inhibits
proliferation and migration of PASMCs | (43,44) |
| HOXA-AS3 | Upregulated | Hoxa3 | Promotes
proliferation and regulates the cell cycle | (47) |
| MANTIS | Downregulated | BRG1 | Facilitates
endothelial angiogenic function, promotes apoptosis | (49) |
| TUG1 | Upregulated |
miR-374c/Foxc1/Notch; miR-328 | Promotes
proliferation and migration, and inhibits apoptosis of HASMCs | (56) |
| MALAT1 | Upregulated | Cyclin-dependent
kinase inhibitors; cell cycle regulator; hsa-miR-124-3p.1/KLF5 | Promotes
proliferation and migration, slightly inhibits cell apoptosis,
regulates cell cycle and phenotype switch of PASMCs | (41,61,63) |
| CPS1-IT | Downregulated | IL-1β; inhibits
HIF-1 transcriptional activity and the NF-κB signaling pathway | Alleviates PH in a
rat model | (66) |
| CASC2 | Downregulated | Unknown | Inhibits
proliferation and migration, promotes apoptosis and inhibits the
phenotypic switch of hypoxia-induced PASMCs. | (25) |
| TCONS_00034812 | Downregulated | Stox1/MAPK
signaling | Promotes PASMC
proliferation and inhibits apoptosis | (26) |
| H19 | Upregulated | miRNA let-7b AT1R;
H19/miR-675-3p/IGF1R and H19/miR-200a/PDCD4 | Promotes PASMC
proliferation and pulmonary vascular remodeling | (74,76) |
| Lnc-Ang362 | Upregulated | miR-221,
miR-222 | Promotes PASMC
proliferation and migration, inhibits apoptosis | (82) |
| UCA1 | Upregulated | HnRNP I | Promotes PASMC
proliferation | (79) |
| LnRPT | Downregulated | Notch signaling
pathway | PASMC
proliferation | (84) |
| NONRATT0155
87.2 | Upregulated | p53 and HIF-1
signaling pathway | Promotes PASMC
proliferation, inhibits cell apoptosis | (89) |
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81970237 and
81600227).
Availability of data and materials
Not applicable.
Authors' contributions
All the authors (YHQ, GLY, YQ, DW, EFL, JTH and CCT)
contributed to the conception and design of the study. YHQ, EFL and
JTH searched the relevant literature, and YHQ wrote the manuscript.
GLY, YQ and DW provided advice and are responsible for revising the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benza RL, Miller DP, Barst RJ, Badesch DB,
Frost AE and McGoon MD: An evaluation of long-term survival from
time of diagnosis in pulmonary arterial hypertension from the
REVEAL Registry. Chest. 142:448–456. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hoeper MM, Humbert M, Souza R, Idrees M,
Kawut SM, Sliwa-Hahnle K, Jing ZC and Gibbs JS: A global view of
pulmonary hypertension. Lancet Respir Med. 4:306–322.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hoeper MM, Simon R and Gibbs J: The
changing landscape of pulmonary arterial hypertension and
implications for patient care. Eur Respir Rev. 23:450–457.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hoeper MM, Ghofrani HA, Grunig E, Klose H,
Olschewski H and Rosenkranz S: Pulmonary hypertension. Dtsch
Arztebl Int. 114:73–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Luna RCP, de Oliveira Y, Lisboa JVC,
Chaves TR, de Araújo TAM, de Sousa EE, Miranda Neto M, Pirola L,
Braga VA and de Brito Alves JL: Insights on the epigenetic
mechanisms underlying pulmonary arterial hypertension. Braz J Med
Biol Res. 51(e7437)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhao L, Oliver E, Maratou K, Atanur SS,
Dubois OD, Cotroneo E, Chen CN, Wang L, Arce C, Chabosseau PL, et
al: The zinc transporter ZIP12 regulates the pulmonary vascular
response to chronic hypoxia. Nature. 524:356–360. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Savai R, Al-Tamari HM, Sedding D,
Kojonazarov B, Muecke C, Teske R, Capecchi MR, Weissmann N,
Grimminger F, Seeger W, et al: Pro-proliferative and inflammatory
signaling converge on FoxO1 transcription factor in pulmonary
hypertension. Nat Med. 20:1289–1300. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Shintani M, Yagi H, Nakayama T, Saji T and
Matsuoka R: A new nonsense mutation of SMAD8 associated with
pulmonary arterial hypertension. J Med Genet. 46:331–337.
2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pullamsetti SS, Berghausen EM, Dabral S,
Tretyn A, Butrous E, Savai R, Butrous G, Dahal BK, Brandes RP,
Ghofrani HA, et al: Role of Src tyrosine kinases in experimental
pulmonary hypertension. Arterioscler Thromb Vasc Biol.
32:1354–1365. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
de-Miguel-Díez J, López-de-Andrés A,
Hernandez-Barrera V, Jimenez-Trujillo I, de-Miguel-Yanes JM,
Mendez-Bailón M and Jimenez-Garcia R: National trends and outcomes
of hospitalizations for pulmonary hypertension in Spain
(2001-2014). Int J Cardiol. 263:125–131. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Anand V, Roy SS, Archer SL, Weir EK, Garg
SK, Duval S and Thenappan T: Trends and outcomes of pulmonary
arterial hypertension-related hospitalizations in the United
States: Analysis of the nationwide inpatient sample database from
2001 through 2012. JAMA Cardiol. 1:1021–1029. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hon CC, Ramilowski JA, Harshbarger J,
Bertin N, Rackham OJ, Gough J, Denisenko E, Schmeier S, Poulsen TM,
Severin J, et al: An atlas of human long non-coding RNAs with
accurate 5'ends. Nature. 543:199–204. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang K, Shi ZM, Chang YN, Hu ZM, Qi HX
and Hong W: The ways of action of long non-coding RNAs in cytoplasm
and nucleus. Gene. 547:1–9. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ezkurdia I, Juan D, Rodriguez JM, Frankish
A, Diekhans M, Harrow J, Vazquez J, Valencia A and Tress ML:
Multiple evidence strands suggest that there may be as few as
19,000 human protein-coding genes. Hum Mol Genet. 23:5866–5878.
2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bunch H: Gene regulation of mammalian long
non-coding RNA. Mol Genet Genomics. 293:1–15. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Atianand MK, Caffrey DR and Fitzgerald KA:
Immunobiology of long noncoding RNAs. Annu Rev Immunol. 35:177–198.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sun W, Yang Y, Xu C and Guo J: Regulatory
mechanisms of long noncoding RNAs on gene expression in cancers.
Cancer Genet. 216-217:105–110. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yoon JH, Abdelmohsen K and Gorospe M:
Functional interactions among microRNAs and long noncoding RNAs.
Semin Cell Dev Biol. 34:9–14. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ferdin J, Nishida N, Wu X, Nicoloso MS,
Shah MY, Devlin C, Ling H, Shimizu M, Kumar K, Cortez MA, et al:
HINCUTs in cancer: Hypoxia-induced noncoding ultraconserved
transcripts. Cell Death Differ. 20:1675–1687. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bell RD, Long X, Lin M, Bergmann JH, Nanda
V, Cowan SL, Zhou Q, Han Y, Spector DL, Zheng D and Miano JM:
Identification and initial functional characterization of a human
vascular cell-enriched long noncoding RNA. Arterioscler Thromb Vasc
Biol. 34:1249–1259. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shi X, Sun M, Liu H, Yao Y, Kong R, Chen F
and Song Y: A critical role for the long non-coding RNA GAS5 in
proliferation and apoptosis in non-small-cell lung cancer. Mol
Carcinog. 54 (Suppl 1):E1–E12. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang Y, Cheng HP, Bao TP, Wang XG and
Tian ZF: Expression of long non-coding RNA NANCI in lung tissues of
neonatal mice with hyperoxia-induced lung injury and its regulatory
effect on NKX2.1. Zhongguo Dang Dai Er Ke Za Zhi. 19:215–221.
2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
25
|
Gong J, Chen Z, Chen Y, Lv H, Lu H, Yan F,
Li L, Zhang W and Shi J: Long non-coding RNA CASC2 suppresses
pulmonary artery smooth muscle cell proliferation and phenotypic
switch in hypoxia-induced pulmonary hypertension. Respir Res.
20(53)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu Y, Sun Z, Zhu J, Xiao B, Dong J and Li
X: LncRNA-TCONS_00034812 in cell proliferation and apoptosis of
pulmonary artery smooth muscle cells and its mechanism. J Cell
Physiol. 233:4801–4814. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lund E, Guttinger S, Calado A, Dahlberg JE
and Kutay U: Nuclear export of microRNA precursors. Science.
303:95–98. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Su Z, Zhi X, Zhang Q, Yang L, Xu H and Xu
Z: LncRNA H19 functions as a competing endogenous RNA to regulate
AQP3 expression by sponging miR-874 in the intestinal barrier. FEBS
Lett. 590:1354–1364. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rothman AM, Arnold ND, Pickworth JA,
Iremonger J, Ciuclan L, Allen RM, Guth-Gundel S, Southwood M,
Morrell NW, Thomas M, et al: MicroRNA-140-5p and SMURF1 regulate
pulmonary arterial hypertension. J Clin Invest. 126:2495–2508.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Caruso P, Dempsie Y, Stevens HC, McDonald
RA, Long L, Lu R, White K, Mair KM, McClure JD, Southwood M, et al:
A role for miR-145 in pulmonary arterial hypertension: Evidence
from mouse models and patient samples. Circ Res. 111:290–300.
2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Schlosser K, White RJ and Stewart DJ:
miR-26a linked to pulmonary hypertension by global assessment of
circulating extracellular microRNAs. Am J Respir Crit Care Med.
188:1472–1475. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lennox KA and Behlke MA: Cellular
localization of long non-coding RNAs affects silencing by RNAi more
than by antisense oligonucleotides. Nucleic Acids Res. 44:863–877.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ballantyne MD, McDonald RA and Baker AH:
lncRNA/MicroRNA interactions in the vasculature. Clin Pharmacol
Ther. 99:494–501. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Al-Rugeebah A, Alanazi M and Parine NR:
MEG3: An oncogenic long non-coding RNA in different cancers. Pathol
Oncol Res. 25:859–874. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhao Y, Zhu Z, Shi S, Wang J and Li N:
Long non-coding RNA MEG3 regulates migration and invasion of lung
cancer stem cells via miR-650/SLC34A2 axis. Biomed Pharmacother.
120(109457)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Guo W, Dong Z, Liu S, Qiao Y, Kuang G, Guo
Y, Shen S and Liang J: Promoter hypermethylation-mediated
downregulation of miR-770 and its host gene MEG3, a long non-coding
RNA, in the development of gastric cardia adenocarcinoma. Mol
Carcinog. 56:1924–1934. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xia Y, He Z, Liu B, Wang P and Chen Y:
Downregulation of Meg3 enhances cisplatin resistance of lung cancer
cells through activation of the WNT/β-catenin signaling pathway.
Mol Med Rep. 12:4530–4537. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhou Y, Zhang X and Klibanski A: MEG3
noncoding RNA: A tumor suppressor. J Mol Endocrinol. 48:R45–R53.
2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Piccoli MT, Gupta SK, Viereck J,
Foinquinos A, Samolovac S, Kramer FL, Garg A, Remke J, Zimmer K,
Batkai S and Thum T: Inhibition of the cardiac fibroblast-enriched
lncRNA Meg3 prevents cardiac fibrosis and diastolic dysfunction.
Circ Res. 121:575–583. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Michalik KM, You X, Manavski Y,
Doddaballapur A, Zörnig M, Braun T, John D, Ponomareva Y, Chen W,
Uchida S, et al: Long noncoding RNA MALAT1 regulates endothelial
cell function and vessel growth. Circ Res. 114:1389–1397.
2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xing Y, Zheng X, Fu Y, Qi J, Li M, Ma M,
Wang S, Li S and Zhu D: Long noncoding RNA-maternally expressed
gene 3 contributes to hypoxic pulmonary hypertension. Mol Ther.
27:2166–2181. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhu B, Gong Y, Yan G, Wang D, Qiao Y, Wang
Q, Liu B, Hou J, Li R and Tang C: Down-regulation of lncRNA MEG3
promotes hypoxia-induced human pulmonary artery smooth muscle cell
proliferation and migration via repressing PTEN by sponging miR-21.
Biochem Biophys Res Commun. 495:2125–2132. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sun Z, Nie X, Sun S, Dong S, Yuan C, Li Y,
Xiao B, Jie D and Liu Y: Long non-coding RNA MEG3 downregulation
triggers human pulmonary artery smooth muscle cell proliferation
and migration via the p53 signaling pathway. Cell Physiol Biochem.
42:2569–2581. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Duboule D: The rise and fall of Hox gene
clusters. Development. 134:2549–2560. 2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gong WJ, Yin JY, Li XP, Fang C, Xiao D,
Zhang W, Zhou HH, Li X and Liu ZQ: Association of
well-characterized lung cancer lncRNA polymorphisms with lung
cancer susceptibility and platinum-based chemotherapy response.
Tumour Biol. 37:8349–8358. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhang H, Liu Y, Yan L, Wang S, Zhang M, Ma
C, Zheng X, Chen H and Zhu D: Long noncoding RNA Hoxaas3
contributes to hypoxia-induced pulmonary artery smooth muscle cell
proliferation. Cardiovasc Res. 115:647–657. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Golpon HA, Geraci MW, Moore MD, Miller HL,
Miller GJ, Tuder RM and Voelkel NF: HOX genes in human lung:
Altered expression in primary pulmonary hypertension and emphysema.
Am J Pathol. 158:955–966. 2001.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Leisegang MS, Fork C, Josipovic I, Richter
FM, Preussner J, Hu J, Miller MJ, Epah J, Hofmann P, Günther S, et
al: Long noncoding RNA MANTIS facilitates endothelial angiogenic
function. Circulation. 136:65–79. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hathaway CA, Heistad DD, Piegors DJ and
Miller FJ Jr: Regression of atherosclerosis in monkeys reduces
vascular superoxide levels. Circ Res. 90:277–283. 2002.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Katsushima K, Natsume A, Ohka F, Shinjo K,
Hatanaka A, Ichimura N, Sato S, Takahashi S, Kimura H, Totoki Y, et
al: Targeting the Notch-regulated non-coding RNA TUG1 for glioma
treatment. Nat Commun. 7(13616)2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Li FP, Lin DQ and Gao LY: LncRNA TUG1
promotes proliferation of vascular smooth muscle cell and
atherosclerosis through regulating miRNA-21/PTEN axis. Eur Rev Med
Pharmacol Sci. 22:7439–7447. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Xie C, Chen B, Wu B, Guo J and Cao Y:
LncRNA TUG1 promotes cell proliferation and suppresses apoptosis in
osteosarcoma by regulating miR-212-3p/FOXA1 axis. Biomed
Pharmacother. 97:1645–1653. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Jiang L, Wang W, Li G, Sun C, Ren Z, Sheng
H, Gao H, Wang C and Yu H: High TUG1 expression is associated with
chemotherapy resistance and poor prognosis in esophageal squamous
cell carcinoma. Cancer Chemother Pharmacol. 78:333–339.
2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Cai H, Liu X, Zheng J, Xue Y, Ma J, Li Z,
Xi Z, Li Z, Bao M and Liu Y: Long non-coding RNA taurine
upregulated 1 enhances tumor-induced angiogenesis through
inhibiting microRNA-299 in human glioblastoma. Oncogene.
36:318–331. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yang L, Liang H, Shen L, Guan Z and Meng
X: LncRNA Tug1 involves in the pulmonary vascular remodeling in
mice with hypoxic pulmonary hypertension via the
microRNA-374c-mediated Foxc1. Life Sci. 237(116769)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhang J, Silva T, Yarovinsky T, Manes TD,
Tavakoli S, Nie L, Tellides G, Pober JS, Bender JR and Sadeghi MM:
VEGF blockade inhibits lymphocyte recruitment and ameliorates
immune-mediated vascular remodeling. Circ Res. 107:408–417.
2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wang S, Cao W, Gao S, Nie X, Zheng X, Xing
Y, Chen Y, Bao H and Zhu D: TUG1 regulates pulmonary arterial
smooth muscle cell proliferation in pulmonary arterial
hypertension. Can J Cardiol. 35:1534–1545. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ren S, Liu Y, Xu W, Sun Y, Lu J, Wang F,
Wei M, Shen J, Hou J, Gao X, et al: Long noncoding RNA MALAT-1 is a
new potential therapeutic target for castration resistant prostate
cancer. J Urol. 190:2278–2287. 2013.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Qi Y, Ooi HS, Wu J, Chen J, Zhang X, Tan
S, Yu Q, Li YY, Kang Y, Li H, et al: MALAT1 long ncRNA promotes
gastric cancer metastasis by suppressing PCDH10. Oncotarget.
7:12693–12703. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Brock M, Schuoler C, Leuenberger C,
Bühlmann C, Haider TJ, Vogel J, Ulrich S, Gassmann M, Kohler M and
Huber LC: Analysis of hypoxia-induced noncoding RNAs reveals
metastasis-associated lung adenocarcinoma transcript 1 as an
important regulator of vascular smooth muscle cell proliferation.
Exp Biol Med (Maywood). 242:487–496. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Potente M, Gerhardt H and Carmeliet P:
Basic and therapeutic aspects of angiogenesis. Cell. 146:873–887.
2011.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wang D, Xu H, Wu B, Jiang S, Pan H, Wang R
and Chen J: Long noncoding RNA MALAT1 sponges miR1243p.1/KLF5 to
promote pulmonary vascular remodeling and cell cycle progression of
pulmonary artery hypertension. Int J Mol Med. 44:871–884.
2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zhuo Y, Zeng Q, Zhang P, Li G, Xie Q and
Cheng Y: Functional polymorphism of lncRNA MALAT1 contributes to
pulmonary arterial hypertension susceptibility in Chinese people.
Clin Chem Lab Med. 55:38–46. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Xiaoguang Z, Meirong L, Jingjing Z,
Ruishen Z, Qing Z and Xiaofeng T: Long noncoding RNA CPS1-IT1
suppresses cell proliferation and metastasis in human lung cancer.
Oncol Res. 25:373–380. 2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhang Z, Li Z, Wang Y, Wei L and Chen H:
Overexpressed long noncoding RNA CPS1-IT alleviates pulmonary
arterial hypertension in obstructive sleep apnea by reducing
interleukin-1beta expression via HIF1 transcriptional activity. J
Cell Physiol. 234:19715–19727. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Yu X, Zheng H, Tse G, Zhang L and Wu WKK:
CASC2: An emerging tumour-suppressing long noncoding RNA in human
cancers and melanoma. Cell Prolif. 51(e12506)2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Jie W, Guo J, Shen Z, Wang X, Zheng S,
Wang G and Ao Q: Contribution of myocardin in the hypoxia-induced
phenotypic switching of rat pulmonary arterial smooth muscle cells.
Exp Mol Pathol. 89:301–306. 2010.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Ma C, Liu Y, Wang Y, Zhang C, Yao H, Ma J,
Zhang L, Zhang D, Shen T and Zhu D: Hypoxia activates 15-PGDH and
its metabolite 15-KETE to promote pulmonary artery endothelial
cells proliferation via ERK1/2 signalling. Br J Pharmacol.
171:3352–3363. 2014.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10(38)2011.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Matouk IJ, Halle D, Gilon M and Hochberg
A: The non-coding RNAs of the H19-IGF2 imprinted loci: A focus on
biological roles and therapeutic potential in lung cancer. J Transl
Med. 13(113)2015.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Matouk IJ, Mezan S, Mizrahi A, Ohana P,
Abu-Lail R, Fellig Y, Degroot N, Galun E and Hochberg A: The
oncofetal H19 RNA connection: Hypoxia, p53 and cancer. Biochim
Biophys Acta. 1803:443–451. 2010.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Su H, Xu X, Yan C, Shi Y, Hu Y, Dong L,
Ying S, Ying K and Zhang R: LncRNA H19 promotes the proliferation
of pulmonary artery smooth muscle cells through AT1R via sponging
let-7b in monocrotaline-induced pulmonary arterial hypertension.
Respir Res. 19(254)2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Jin H, Wang Y, Zhou L, Liu L, Zhang P,
Deng W and Yuan Y: Melatonin attenuates hypoxic pulmonary
hypertension by inhibiting the inflammation and the proliferation
of pulmonary arterial smooth muscle cells. J Pineal Res.
57:442–450. 2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Wang R, Zhou S, Wu P, Li M, Ding X, Sun L,
Xu X, Zhou X, Zhou L, Cao C and Fei G: Identifying involvement of
H19-miR-675-3p-IGF1R and H19-miR-200a-PDCD4 in treating pulmonary
hypertension with melatonin. Mol Ther Nucleic Acids. 13:44–54.
2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Wang ZQ, He CY, Hu L, Shi HP, Li JF, Gu
QL, Su LP, Liu BY, Li C and Zhu Z: Long noncoding RNA UCA1 promotes
tumour metastasis by inducing GRK2 degradation in gastric cancer.
Cancer Lett. 408:10–21. 2017.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Liu X, Huang Z, Qian W, Zhang Q and Sun J:
Silence of lncRNA UCA1 rescues drug resistance of cisplatin to
non-small-cell lung cancer cells. J Cell Biochem. 120:9243–9249.
2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Zhu TT, Sun RL, Yin YL, Quan JP, Song P,
Xu J, Zhang MX and Li P: Long noncoding RNA UCA1 promotes the
proliferation of hypoxic human pulmonary artery smooth muscle
cells. Pflugers Arch. 471:347–355. 2019.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Xing YN, Yang X, Xu XY, Zheng Y, Xu HM,
Takano Y and Zheng H: The altered expression of ING5 protein is
involved in gastric carcinogenesis and subsequent progression. Hum
Pathol. 42:25–35. 2011.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Leung A, Trac C, Jin W, Lanting L, Akbany
A, Sætrom P, Schones DE and Natarajan R: Novel long noncoding RNAs
are regulated by angiotensin II in vascular smooth muscle cells.
Circ Res. 113:266–278. 2013.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Wang H, Qin R and Cheng Y: LncRNA-Ang362
promotes pulmonary arterial hypertension by regulating miR-221 and
miR-222. Shock. 53:723–729. 2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Heldin CH and Westermark B: Mechanism of
action and in vivo role of platelet-derived growth factor. Physiol
Rev. 79:1283–1316. 1999.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Chen J, Guo J, Cui X, Dai Y, Tang Z, Qu J,
Raj JU, Hu Q and Gou D: The long noncoding RNA LnRPT is regulated
by PDGF-BB and modulates the proliferation of pulmonary artery
smooth muscle cells. Am J Respir Cell Mol Biol. 58:181–193.
2018.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Baeten JT and Lilly B: Differential
regulation of NOTCH2 and NOTCH3 contribute to their unique
functions in vascular smooth muscle cells. J Biol Chem.
290:16226–16237. 2015.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Li X, Zhang X, Leathers R, Makino A, Huang
C, Parsa P, Macias J, Yuan JX, Jamieson SW and Thistlethwaite PA:
Notch3 signaling promotes the development of pulmonary arterial
hypertension. Nat Med. 15:1289–1297. 2009.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Arunachalam G, Lakshmanan AP, Samuel SM,
Triggle CR and Ding H: Molecular interplay between microRNA-34a and
Sirtuin1 in hyperglycemia-mediated impaired angiogenesis in
endothelial cells: Effects of metformin. J Pharmacol Exp Ther.
356:314–323. 2016.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Dean A, Nilsen M, Loughlin L, Salt IP and
MacLean MR: Metformin reverses development of pulmonary
hypertension via aromatase inhibition. Hypertension. 68:446–454.
2016.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Sun Z, Liu Y, Yu F, Xu Y, Yanli L and Liu
N: Long non-coding RNA and mRNA profile analysis of metformin to
reverse the pulmonary hypertension vascular remodeling induced by
monocrotaline. Biomed Pharmacother. 115(108933)2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Lino Cardenas CL, Kessinger CW, Cheng Y,
MacDonald C, MacGillivray T, Ghoshhajra B, Huleihel L, Nuri S, Yeri
AS, Jaffer FA, et al: An HDAC9-MALAT1-BRG1 complex mediates smooth
muscle dysfunction in thoracic aortic aneurysm. Nat Commun.
9(1009)2018.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Ranchoux B, Antigny F, Rucker-Martin C,
Hautefort A, Péchoux C, Bogaard HJ, Dorfmüller P, Remy S, Lecerf F,
Planté S, et al: Endothelial-to-mesenchymal transition in pulmonary
hypertension. Circulation. 131:1006–1018. 2015.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Rabinovitch M, Guignabert C, Humbert M and
Nicolls MR: Inflammation and immunity in the pathogenesis of
pulmonary arterial hypertension. Circ Res. 115:165–175.
2014.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Jackson R, Kroehling L, Khitun A, Bailis
W, Jarret A, York AG, Khan OM, Brewer JR, Skadow MH, Duizer C, et
al: The translation of non-canonical open reading frames controls
mucosal immunity. Nature. 564:434–438. 2018.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Li LJ, Leng RX, Fan YG, Pan HF and Ye DQ:
Translation of noncoding RNAs: Focus on lncRNAs, pri-miRNAs, and
circRNAs. Exp Cell Res. 361:1–8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Thenappan T, Ormiston ML, Ryan JJ and
Archer SL: Pulmonary arterial hypertension: Pathogenesis and
clinical management. BMJ. 360(j5492)2018.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Schlosser K, Hanson J, Villeneuve PJ,
Dimitroulakos J, McIntyre L, Pilote L and Stewart D: Assessment of
circulating LncRNAs under physiologic and pathologic conditions in
humans reveals potential limitations as biomarkers. Sci Rep.
6(36596)2016.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Han B, Bu P, Meng X and Hou X: Microarray
profiling of long non-coding RNAs associated with idiopathic
pulmonary arterial hypertension. Exp Ther Med. 13:2657–2666.
2017.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Viereck J, Kumarswamy R, Foinquinos A,
Xiao K, Avramopoulos P, Kunz M, Dittrich M, Maetzig T, Zimmer K,
Remke J, et al: Long noncoding RNA Chast promotes cardiac
remodeling. Sci Transl Med. 8(326ra322)2016.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Mercer TR and Mattick JS: Structure and
function of long noncoding RNAs in epigenetic regulation. Nat
Struct Mol Biol. 20:300–307. 2013.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Jandl K, Thekkekara Puthenparampil H,
Marsh LM, Hoffmann J, Wilhelm J, Veith C, Sinn K, Klepetko W,
Olschewski H, Olschewski A, et al: Long non-coding RNAs influence
the transcriptome in pulmonary arterial hypertension: The role of
PAXIP1-AS1. J Pathol. 247:357–370. 2019.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Bischoff FC, Werner A, John D, Boeckel JN,
Melissari MT, Grote P, Glaser SF, Demolli S, Uchida S, Michalik KM,
et al: Identification and functional characterization of
hypoxia-induced endoplasmic reticulum stress regulating lncRNA
(HypERlnc) in pericytes. Circ Res. 121:368–375. 2017.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Wang M, Gu S, Liu Y, Yang Y, Yan J, Zhang
X, An X, Gao J, Hu X and Su P: miRNA-PDGFRB/HIF1A-lncRNA CTEPHA1
network plays important roles in the mechanism of chronic
thromboembolic pulmonary hypertension. Int Heart J. 60:924–937.
2019.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Gu S, Li G, Zhang X, Yan J, Gao J, An X,
Liu Y and Su P: Aberrant expression of long noncoding RNAs in
chronic thromboembolic pulmonary hypertension. Mol Med Rep.
11:2631–2643. 2015.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Josipovic I, Fork C, Preussner J, Prior
KK, Iloska D, Vasconez AE, Labocha S, Angioni C, Thomas D,
Ferreirós N, et al: PAFAH1B1 and the lncRNA NONHSAT073641 maintain
an angiogenic phenotype in human endothelial cells. Acta Physiol
(Oxf). 218:13–27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Voellenkle C, Garcia-Manteiga JM, Pedrotti
S, Perfetti A, De Toma I, Da Silva D, Maimone B, Greco S, Fasanaro
P, Creo P, et al: Implication of Long noncoding RNAs in the
endothelial cell response to hypoxia revealed by RNA-sequencing.
Sci Rep. 6(24141)2016.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Su Q, Sun Y, Ye Z, Yang H and Li L:
Oxidized low density lipoprotein induces endothelial-to-mesenchymal
transition by stabilizing Snail in human aortic endothelial cells.
Biomed Pharmacother. 106:1720–1726. 2018.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Neumann P, Jaé N, Knau A, Glaser SF,
Fouani Y, Rossbach O, Krüger M, John D, Bindereif A, Grote P, et
al: The lncRNA GATA6-AS epigenetically regulates endothelial gene
expression via interaction with LOXL2. Nat Commun.
9(237)2018.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Lin R, Roychowdhury-Saha M, Black C, Watt
AT, Marcusson EG, Freier SM and Edgington TS: Control of RNA
processing by a large non-coding RNA over-expressed in carcinomas.
FEBS Lett. 585:671–676. 2011.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Rivero Puente A, Asín Marcotegui J,
Reparaz B and Achutegui G: Malignant nephroangiosclerosis. Rev Clin
Esp. 140:251–255. 1976.PubMed/NCBI(In Spanish).
|















