Introduction
Osteoarthritis (OA) is a chronic multifactorial
condition characterized by progressive joint degeneration and
subchondral bone sclerosis, which may result in the formation of
bone cysts and marginal osteophytes (1). OA has been indicated to be the most
recurrent health problem in the middle age (45-65 years) and
elderly (>65 years) (2). Of
note, chronic pain remains the primary concern and predominant
clinical feature of patients with OA and is typically poorly
treated using occupational therapy (3,4).
Current preclinical studies, which are largely
reliant on animal models and laboratory pain testing, are widely
used to develop more suitable analgesics for patients with OA
(5,6). Indeed, a number of animal models have
been used to investigate OA, and have effectively been found to
predict the clinical efficacy of therapies, that are universally
used against pain, including ibuprofen and acetaminophen (7). However, a gold standard animal model,
that may aid in standardization across clinical groups, has not yet
been established.
Numerous models of OA, including the monoiodoacetate
(MIA) model, and behavioral tests, such as von Frey filament
testing and static or dynamic analysis of weight distribution, have
been used to investigate potential analgesic compounds (8). Of note, false positive results in
evoked and non-evoked pain measures may also be observed owing to
effects other than analgesia, such as sedation, motor side effects
or drug-induced anxiety (9). The
negative contradictory findings between rodents and humans have
guided a number of researchers to question whether rodents should
be used to model human chronic pain and examine the efficiency of
analgesic compounds (10). To
overcome these concerns, an incorporation of suitable additional
assays, such as locomotor activity, may assist with the
identification of these issues in OA models and reduce false
positives (11). Indeed, a benefit
of using an open field locomotor activity analysis may be the
recognition of non-specific side effects, including sedation, by
the computed animal activity system (12).
Therefore, using traditional methods to measure
pain, such as von Frey and weight bearing, in combination with
novel pain-related measures, such as the locomotor test, may
potentially decrease the possibility of obtaining false positive
results and would provide an improved understanding of the
translational capability of preclinical findings.
To decrease the translational gap between rodent and
human pain research, the present study aimed to evaluate the rat
locomotor activity in the MIA model of OA, which includes
alterations that are at least partially pain-related. Using a
computed animal activity system, the current study aimed to measure
both horizontal (X axis) and vertical (Z axis) locomotor activity,
which represent both the overall locomotor activity and repetitive
small-scale movements, such as scratching and grooming.
Furthermore, ibuprofen was used, as a positive control, and its
effects were evaluated using well-established techniques of
mechanical allodynia evaluation (von Frey and weight bearing) and
the newly proposed locomotor activity system.
Materials and methods
Animals
A total of 29 Adult, male, Sprague-Dawley rats
(180-250 g) were used for behavioral experiments. Rats (4-6 weeks
old) were purchased from Jordan University of Science and
Technology (Irbid, Jordan). The rats were housed in a
temperature-controlled environment (22±1˚C; 12 h light/dark cycle;
relative humidity, 40-60%) at the animal house unit of The
University of Jordan. Animals had free access to food and water,
their bedding was altered twice a week and their health status was
monitored daily, with no mortalities observed. All experiments were
performed in The University of Jordan laboratories. The protocols
were approved by the Ethics Committee of The University of Jordan
(approval no. 19/2018/322). Behavioral experiments were performed
in agreement with the Animals (Scientific Procedure) Act 1986 and
the guidelines of the International Association for the Study of
Pain (13).
Induction of the osteoarthritic pain
model
For the induction of OA, the rats received
intra-articular injections of MIA (1 mg in 50 µl saline) in the
left knee joint. The selection of the MIA dose was based on
previous studies, in which MIA exhibited significant effects in
different assays (14,15). This procedure was performed under
transient isoflurane (3%) inhalation anesthesia. In the control
group, the rats received intra-articular injections of vehicle (3%
Tween-20 in saline), in an equivalent amount (50 µl). After
determining the baseline nociceptive responses, behavioral testing
was performed every other day for 21 days, following MIA injection.
At the end of each experiment (day 21), the rats were deeply
anesthetized using diethyl ether inhalation and following a
negative toe-pinch reflex, rats were euthanized using
decapitation.
Assessment of mechanical
allodynia
The responsiveness to a punctate pressure stimulus
was evaluated using the von Frey filament test. The rats were
individually placed in plastic cages, which grant full access to
the paws using a wire mesh bottom. Behavioral acclimatization to
the testing room was then permitted until cage inspection, and main
grooming activities had ceased for at least 25 min. Subsequently,
the ‘up-down’ method was used when applying the von Frey filaments
(2-15 g, with logarithmically incremental stiffness; Bioseb; cat.
no. BIO-VF-M) to the mid-plantar surface of the left hind paw to
evaluate the withdrawal threshold. Every time, the von Frey
filament was held for 6-8 sec perpendicularly to the planter aspect
of the hind paw. The data are presented as paw withdrawal
thresholds (PWT) measured in grams (16,17).
Assessment of weight bearing
difference
To assess the postural equilibrium, the difference
in weight distribution between the two hind paws was calculated in
each rat, using a static weight bearing instrument (Static Weight
Bearing Touch: Incapacitance test; Bioseb; cat. no.
BIO-SWB-TOUCH-M). The weight was normally equally distributed
between both hind paws. The level of pain was assessed by
evaluating the weight bearing difference between the injured and
uninjured paws. The incapacitance test consists of a plexiglass
chamber, in which each rat was allowed to move freely until settled
in an appropriate position without leaning on either side of the
chamber. The rat then adapted to a suitable weight distribution
between the hind paws depending on the degree of pain. The
distribution of the weight between the hind paws was measured over
a period of 10 sec and the values which applied to each sensor were
indicated on the screen in the control unit. These data were
subsequently used to calculate the ratio of left hind paw
contribution in total weight bearing (18).
Assessment of open field locomotor
activity
To evaluate the alterations in locomotor activity, a
computed animal activity system (Opto-M4; Columbus Instruments
International) was used (18,19).
This open field system consists of a 45x25x20 cm arena with two
horizontal planes of detector-emitter pairs across the width of the
arena, which are positioned 5 and 10 cm above the cage floor. Each
horizontal plane is monitored by 16 infrared beams, that are spaced
2.54 cm apart. The total number of beam interruptions occurring due
to rat movements were calculated every 5 min using the infrared
beams, and sent to a central computer. The animals were placed
individually 60 min following the drug injections. The total number
of beam interruptions was recorded for 20 min and stored every 5
min. This allows the system to continuously monitor the horizontal
(X axis) and vertical (Z axis) activity. The horizontal activity is
represented by the lower plane, while the vertical activity is
represented by the upper plane. This system also calculates both
the total and ambulatory counts. X total counts register a count
every time an infrared beam is broken in the lower plane
(horizontal counts). This represents both the overall locomotor
activity and repetitive small-scale movements, such as scratching
and grooming. X ambulatory counts register a count only when a new
beam is broken, allowing it to measure actual locomotion (distance
traveled in beams rather than centimeters or inches). Z total
counts register a count every time an infrared beam is broken in
the upper plane (vertical counts) and is utilized to detect rearing
or standing on the hind paws.
Pharmacological treatments
The effects of different doses of ibuprofen (50 and
100 mg/kg) on MIA-induced nociceptive behavior were assessed.
Ibuprofen was diluted in 3% Tween-20 in saline. The rats were
treated with ibuprofen by intraperitoneal injections at day 7
following MIA injection, when mechanical allodynia had developed.
The control groups, which received MIA injections without ibuprofen
treatment, received intraperitoneal injections of vehicle (3%
Tween-20 in saline) in equal amounts to the ibuprofen treatment.
Blinded behavioral experiments were performed 1 h post-injection to
evaluate the nociceptive behavior. The behavior was assessed based
on alterations in the mechanical PWT and the ratio of the left hind
paw contribution in total weight bearing.
Statistical analysis
Data regarding MIA-induced mechanical allodynia are
expressed as the mean ± SEM of PWT in grams. The ratio of left hind
paw contribution in total weight bearing is also presented as the
mean ± SEM. Regarding the locomotor activity, data are presented as
the mean ± SEM of X total counts, X ambulatory counts and Z total
counts in percentage, and were calculated using the following
equation: Percentage counts=counts following MIA/saline/baseline
counts. These data were subsequently analyzed using two-way ANOVA
with treatment and time as the main factors, allowing both between
and within group comparisons, followed by a Holm-Sidak post hoc
test, as appropriate. Data regarding the effects of ibuprofen on
MIA-induced mechanical allodynia are presented as the mean ± SEM of
percentage antinociception, and were calculated according to the
following equation: Percentage antinociception=(reading following
drug application-reading before drug application)/(reading before
MIA injection-reading before drug application). Data regarding the
recovery effects of ibuprofen on MIA-induced reduction in locomotor
activity are presented as the mean ± SEM of percentage locomotor
activity recovery and were calculated as follows: Percentage
locomotor activity recovery=(reading following drug
application-reading before drug application)/(reading before MIA
injection-reading before drug application). These data were
analyzed using one-way ANOVA followed by Bonferroni's post hoc test
as appropriate. Statistical analysis was performed using GraphPad
Prism v6 statistical program (GraphPad Software, Inc.).
Results
Effects of MIA on nociceptive
behavior
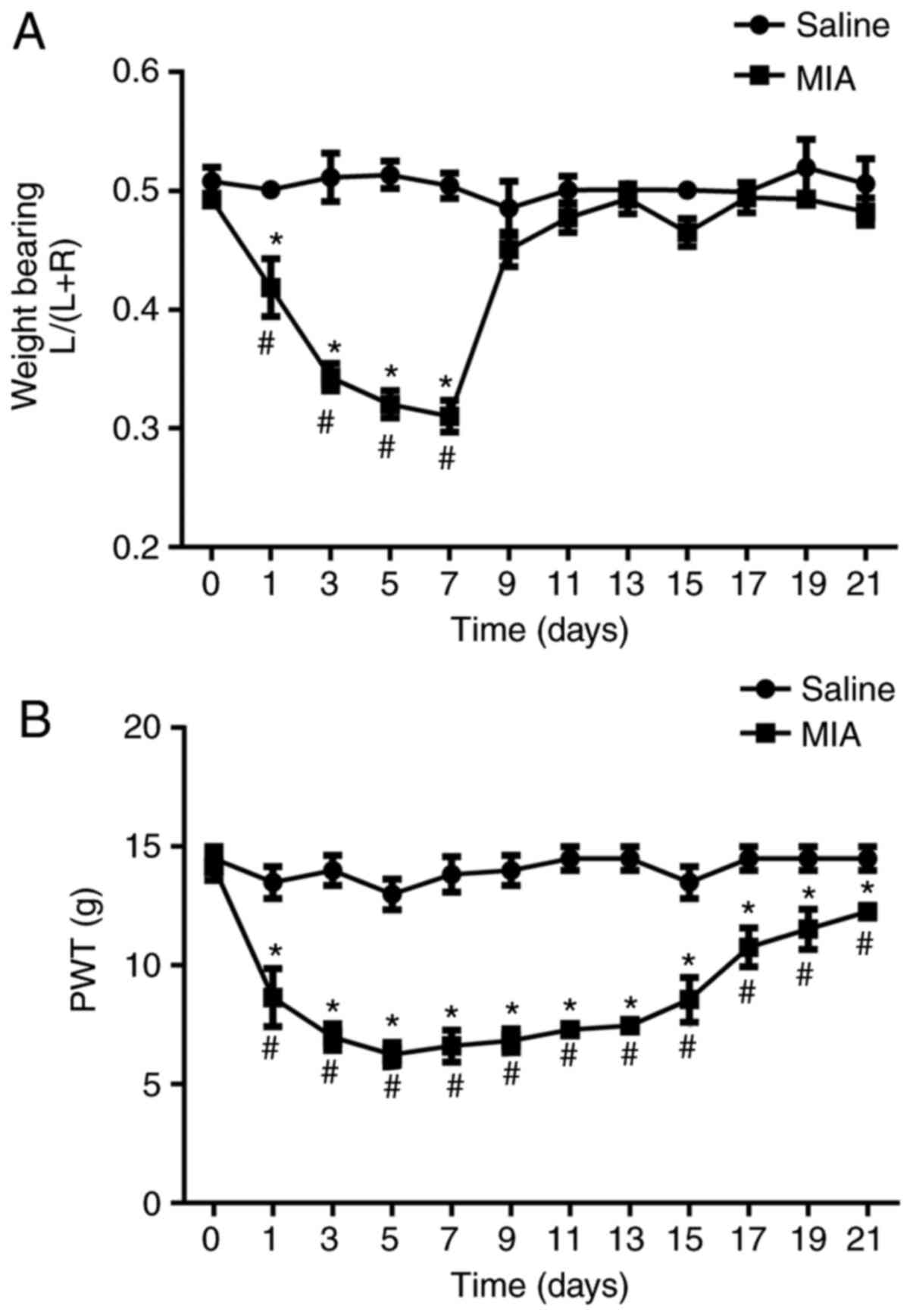
At day 1 post MIA injection, the left hind paw
contribution in total weight bearing was significantly decreased
compared with that in the vehicle-treated control group (0.5±0.01
vs. 0.42±0.02; P<0.05; Fig. 1A).
This effect persisted for ~1 week (Fig.
1A). Furthermore, the mechanical PWT was significantly
decreased compared with that in the vehicle-treated control group,
at day 1 post MIA injection (13.5±0.74 vs. 8.4±0.84; P<0.05;
Fig. 1B). This reduction persisted
for 21 days, suggesting that the development of nociceptive
behavior following MIA treatment.
Effects of MIA on open field locomotor
activity
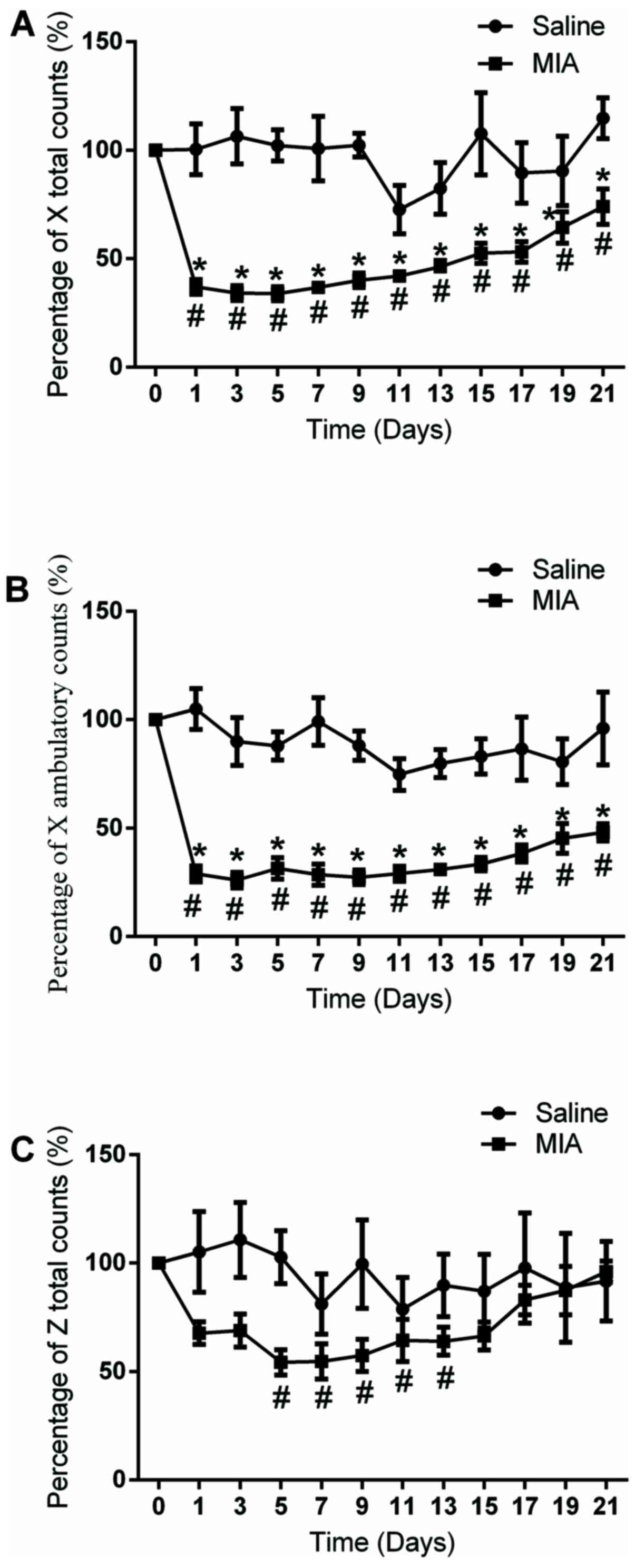
At day 1 post MIA injection, the X total and X
ambulatory counts were significantly decreased compared with that
in the vehicle-treated control group (100.4±16.3% vs. 37±2.9% and
104.9±10.6% vs. 31.5±5.1%, respectively; P<0.05; Fig. 2A and B). At day 5, the Z total counts were also
significantly decreased compared with that in the vehicle-treated
control group (102.2±11.2 vs. 54.8±8.5%; P<0.05; Fig. 2C). The reduction of the X total and
X ambulatory counts persisted for ~21 days, while the reduction of
Z total counts persisted from day 5 to day 13 post-MIA injection
(Fig. 2A-C). These data suggested
that the rats in the osteoarthritic pain model exhibited a deficit
in non-evoked measures (locomotor activity), in addition to the
alterations in evoked sensory sensitivity (PWT and weight bearing),
which may suggest a nociceptive behavior.
Effects of ibuprofen on the
osteoarthritic pain model
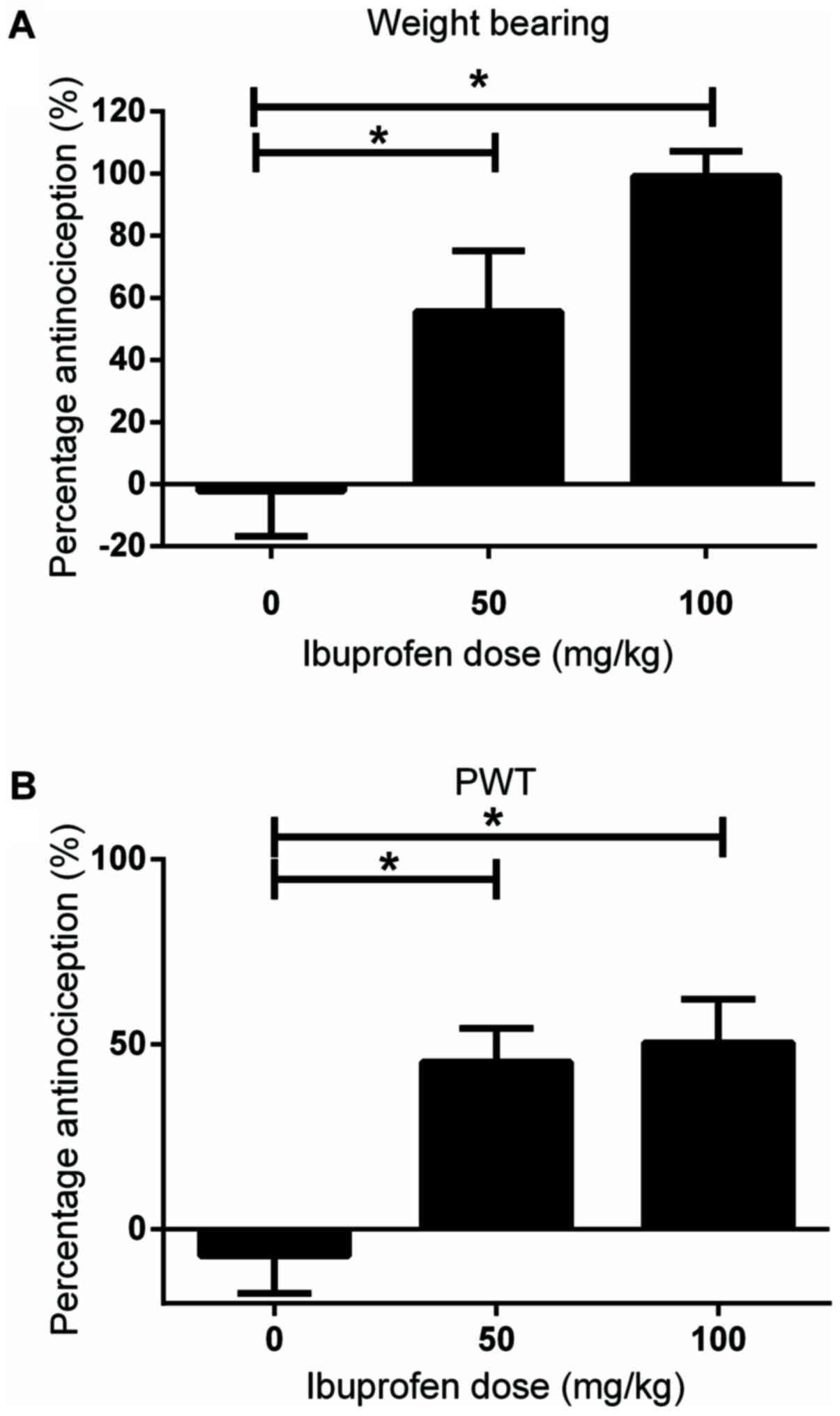
At 1-week post MIA injection, when the PWT and left
hind paw contribution in total weight bearing were significantly
decreased compared with that in the control group, different doses
of ibuprofen (50 and 100 mg/kg) were injected intraperitoneally.
Behavioral experiments were performed 1 h post-injection to assess
the analgesic properties of the drug. Both doses significantly
restored the left hind paw contribution in total weight bearing
compared with that in the vehicle-treated control group (-2.2±6.6
vs. 55.59±8.7% and -2.2±6.6 vs. 99.2±3.6%, respectively; both
P<0.05; Fig. 3A). With respect
to mechanical allodynia, both doses of ibuprofen significantly
restored the PWT compared with that in the vehicle-treated control
group (-7.1±11.3 vs 45.3±10.1% and -7.1±11.3 vs. 50.4±13%,
respectively; both P<0.05; Fig.
3B).
Effects of ibuprofen on open field
locomotor activity
To determine if the inhibitory effects of MIA
injections on locomotor activity influenced the interpretation of
the analgesic effects of ibuprofen, additional experiments were
performed.
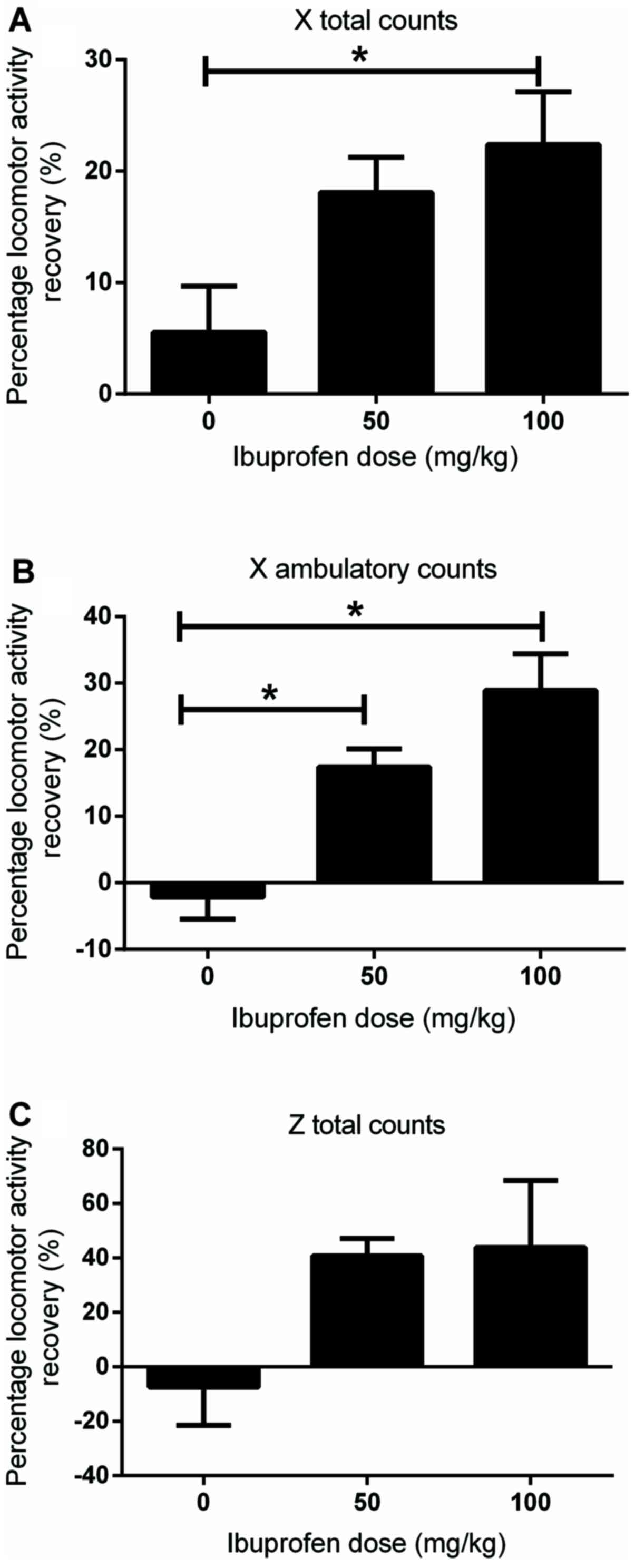
At 1 week post MIA injection, when the locomotor
activity was significantly reduced compared with that in the
control group, different doses of ibuprofen (50 and 100 mg/kg) were
injected intraperitoneally. At 1 h post-injection, the analgesic
properties of the drug were assessed. With respect to the X total
counts, only the 100 mg/kg dose significantly restored the counts
compared with that in the vehicle-treated control group (22.4±5.2
vs. 5.5±4.6%; P<0.05; Fig. 4A).
Both doses (50 and 100 mg/kg) significantly restored the X
ambulatory counts compared with that in the vehicle-treated control
group (17.4±3 vs. -2.1±3.7% and 28.9±6.1 vs. -2.1±3.7%,
respectively; both P<0.05; Fig.
4B). These data suggested that the antinociceptive behavior,
that was observed following drug injection, may attribute to the
analgesic effects of ibuprofen rather than the reduction in
locomotor activity, and also nullified any sedative effect of the
drug influencing the nociceptive behavior of the rats. On the other
hand, neither dose of ibuprofen restored the Z total counts
compared with that in the vehicle-treated control group (40.7±4.5
and 43.8±21.5, respectively, vs. -7.5±12.5%; Fig. 4C).
Discussion
The results of the present study indicated that
MIA-treated rats exhibited a significant decrease in the locomotor
activity compared with that in the untreated control group. These
data suggested that a decline in animal activity may correspond to
the inflammatory stage of the animals. This may reflect the
association that has been observed between clinical OA and
locomotion in rodent models or may indicate that locomotor activity
was altered based on the inflammatory stage of the animals
(20).
The MIA model was selected to evaluate the activity
of analgesic drugs, as it has been found to induce reproducible
behavioral alterations (21).
Therefore, the MIA model may be used to examine the efficacy of
analgesic drugs, with novel mechanisms of action for potential use
in OA (22). Indeed, the
MIA-induced OA model has been regularly used to evaluate the pain
behavior and drug efficacy to resolve the pain in animals (5,6), and
may be more indicative of the drug efficacy compared with that in
other pain models that are used to test osteoarthritis drugs such
as the naturally occurring models (such as elderly hamsters) and
genetically modified models (23).
The present study revealed that the decrease in
locomotion, which was characterized by the reduction in X total and
X ambulatory counts, was associated with the decrease in the PWT,
as revealed by the von Frey test. Notably, the reduced PWT remained
longer compared with that in the decrease in the weight bearing of
the ipsilateral paw (21 days vs. 7 days, respectively). In contrast
to this finding, a previous study reported that MIA, at a dose of 1
or 3 mg, induced a significant reduction in the weight bearing of
the ipsilateral hind limb from days 3-28 compared with control rats
(14). The difference in the
duration between the reduced PWT and the weight bearing may be
attributed to various potential mechanisms, such as the chondrocyte
degeneration, that has been observed at days 1-7 post MIA injection
(24) or a potential transient
inflammation that has been indicated at days 1-4 post MIA injection
(25). The finding that both von
Frey and weight bearing tests are not always consistent may suggest
the presence of different pain mechanisms. The weight bearing test
has been found to be more effective in measuring spontaneous pain
(non-evoked painful behaviors), that is often associated with joint
degeneration or inflammation arising from peripheral sensitization
(15), while the von Frey test has
been revealed to be more effective in identifying the evoked
reflexive responses and eventually evaluating both peripheral and
central sensitization (26).
Similarly, the MIA-induced reduction in locomotor activity (X total
counts and X ambulatory counts) lasted for the duration of the
experiment, indicating that locomotion impairment in the horizontal
direction was dependent on both peripheral and central
sensitization. On the other hand, the MIA-induced reduction of Z
total counts, which represented the vertical movement such as
rearing, lasted for 13 days compared with 21 days in the case of X
counts, indicating that locomotion impairment in the vertical
direction was similar to the deficit in weight bearing and was
primarily dependent on peripheral sensitization. On the contrary, a
previous study has demonstrated that MIA injection produced
prolonged impairment (21 days) in the locomotor activity both in
horizontal and vertical directions (20). This discrepancy may be attributed to
the higher dose of MIA used in the previous study (3 mg/kg)
compared with that used in the preset study (1 mg/kg).
To examine the predictive validity of the open field
test for the evaluation of pain and analgesia, the effects of
ibuprofen treatment on locomotion were detected in the MIA-treated
rats. The results indicated that the MIA-treated rats exhibited a
significant recovery of locomotor activity in the X ambulatory
counts, following ibuprofen administration, in a dose-dependent
manner, while only the higher dose of ibuprofen (100 mg/kg) induced
a significant effect on the X total counts. Neither dose of
ibuprofen (50 and 100 mg/kg) significantly reversed the reduced
locomotion, with respect to the Z total counts; however, the
locomotion recoveries in the presence of ibuprofen were higher
compared with that in the control animals. This may be attributed
to using only 5-6 rats in each experimental protocol in the present
study. It can be suggested that ibuprofen may also exhibit a
beneficial effect in restoring locomotion in vertical movement,
such as rearing. By contrast, Bryden et al (27) indicated that clinically used
analgesics, such as ibuprofen and morphine, did not exhibit any
reversal effects on MIA-induced locomotion deficit, and
additionally reported that the burrowing deficit was a more
sensitive method of the analgesic effect of a drug than deficits in
locomotion. The discrepancy between the results of the present
study and those of the aforementioned study may be attributed to
the application of a unilateral injection of MIA and a higher dose
of ibuprofen in the current study vs. a bilateral injection of MIA
and a lower dose of ibuprofen (30 mg/kg) in the aforementioned
study (25).
The locomotion test is one of the most known primary
behavioral tests and is a common method to evaluate locomotion and
potentially other behaviors, such as pain, in rodents (28). The results of the current study are
in agreement with those of other studies that have used different
pain models and different assays of locomotion. For example,
Complete Freund's adjuvant (CFA) has been found to decrease the
voluntary wheel running in male and female rats (29-32).
Furthermore, rotarod performance was decreased in MIA-treated rats
(33) and CFA-treated mice compared
with that in the control groups (34).
Measuring sedation and motor dysfunction may limit
the use of certain potential antinociceptive compounds, such as
cannabinoids, for pain relief (35). Indeed, the false positive effects of
measuring a pain-stimulated response (von Frey test) and a
pain-suppressed behavior (locomotor test) in animal models are
likely to be reduced in clinical trials.
In conclusion, the results of the present study
indicated that MIA-induced OA reduced motor activity, and ibuprofen
significantly restored the locomotion impairment in the horizontal
direction, but not in the vertical direction, suggesting that
impairment of locomotion in the horizontal direction was a more
sensitive method of the analgesic drug effects. Evaluation of
locomotion may aid in the differentiation between the valid
analgesic effects and the drug-induced motor impairment or sedative
effects. This simple non-invasive quantitative and qualitative
method may also aid in developing novel therapeutic strategies to
treat OA in humans, and may successfully be used to predict the
analgesic efficacy of compounds in models of joint inflammation and
OA.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Deanship of
Scientific Research at The University of Jordan (grant no.
1748).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MA, AA and MH conceived the study and acquired
funding. MH and AA developed the methodology and validated the
data. MA, HK and MH analyzed the data. KES, MH and MA performed the
weight bearing testing and analyzed its results. AMA and MH
performed the open field testing and analyzed its results. MA and
MH drafted the manuscript. MA, MH and AA critically revised and
edited the manuscript. KES and SAA critically revised the
manuscript and designed the experiments. MH and AMA performed the
von Fry testing and its analysis. KES and MA supervised the study.
All authors have made substantial contributions to the conception
and design of the work. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The protocols were approved by the Ethics Committee
of The University of Jordan (approval no. 19/2018/322).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chard JA, Tallon D and Dieppe PA:
Epidemiology of research into interventions for the treatment of
osteoarthritis of the knee joint. Ann Rheum Dis. 59:414–418.
2000.PubMed/NCBI View Article : Google Scholar
|
|
2
|
O'Neill TW, McCabe PS and McBeth J: Update
on the epidemiology, risk factors and disease outcomes of
osteoarthritis. Best Pract Res Clin Rheumatol. 32:312–326.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schaible HG: Osteoarthritis pain Recent
advances and controversies. Curr Opin Support Palliat Care.
12:148–153. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fu K, Robbins SR and McDougall JJ:
Osteoarthritis: The genesis of pain. Rheumatology (Oxford). 57
(Suppl 4):iv43–iv50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Malfait AM and Little CB: On the
predictive utility of animal models of osteoarthritis. Arthritis
Res Ther. 17(225)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kuyinu EL, Narayanan G, Nair LS and
Laurencin CT: Animal models of osteoarthritis: Classification,
update, and measurement of outcomes. J Orthop Surg Res.
11(19)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Negus SS, Vanderah TW, Brandt MR, Bilsky
EJ, Becerra L and Borsook D: Preclinical assessment of candidate
analgesic drugs: Recent advances and future challenges. J Pharmacol
Exp Ther. 319:507–514. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Malfait AM, Little CB and McDougall JJ: A
commentary on modelling osteoarthritis pain in small animals.
Osteoarthritis Cartilage. 21:1316–1326. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cobos EJ and Portillo-Salido E:
‘Bedside-to-Bench’ behavioral outcomes in animal models of pain:
Beyond the evaluation of reflexes. Curr Neuropharmacol. 11:560–591.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Berge OG: Predictive validity of
behavioural animal models for chronic pain. Br J Pharmacol.
164:1195–1206. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Matson DJ, Broom DC and Cortright DN:
Locomotor activity in a novel environment as a test of inflammatory
pain in rats. Methods Mol Biol. 617:67–78. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Niikura K, Takahashi Y, Iino M, Funatsu Y
and Matsuda R: An automated method by which effects of compounds on
locomotor activity and spontaneous neuropathic pain-specific
movements can be simultaneously evaluated in rats with
chronic-constriction nerve injury. Eur J Pharm Sci. 96:551–559.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tannenbaum J: Ethics and pain research in
animals. ILAR J. 40:97–110. 1999.
|
|
14
|
Ma Y, Guo H, Bai F, Zhang M, Yang L, Deng
J and Xiong L: A rat model of knee osteoarthritis suitable for
electroacupuncture study. Exp Anim. 67:271–280. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bove SE, Calcaterra SL, Brooker RM, Huber
CM, Guzman RE, Juneau PL, Schrier DJ and Kilgore KS: Weight bearing
as a measure of disease progression and efficacy of
anti-inflammatory compounds in a model of monosodium
iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage.
11:821–830. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Alsalem M, Altarifi A, heba K, Heba AZ,
Belal A and El-Salem K: Role of PPARα and PPARγ in mediating the
analgesic properties of Ibuprofen in vivo and the effects of dual
PPARα/γ activation in inflammatory pain model in the rat. Int J
Pharmacol. 12(8)2016.
|
|
18
|
Alsalem M, Haddad M, Aldossary SA,
Kalbouneh H, Altarifi A, Jaffal SM, Abbas MA, Aldaoud N and
El-Salem K: Role of cannabinoid receptor 1 and the peroxisome
proliferator-activated receptor α in mediating anti-nociceptive
effects of synthetic cannabinoids and a cannabinoid-like compound.
Inflammopharmacology. 27:1131–1142. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rorabaugh BR, Rose MJ, Stoops TS, Stevens
AA, Seeley SL and D'Souza MS: Regulators of G-protein signaling 2
and 4 differentially regulate cocaine-induced rewarding effects.
Physiol Behav. 195:9–19. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
More AS, Kumari RR, Gupta G, Lingaraju MC,
Balaganur V, Pathak NN, Kumar D, Kumar D, Sharma AK and Tandan SK:
Effect of iNOS inhibitor S-methylisothiourea in monosodium
iodoacetate-induced osteoathritic pain: Implication for
osteoarthritis therapy. Pharmacol Biochem Behav. 103:764–772.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kobayashi K, Imaizumi R, Sumichika H,
Tanaka H, Goda M, Fukunari A and Komatsu H: Sodium
iodoacetate-induced experimental osteoarthritis and associated pain
model in rats. J Vet Med Sci. 65:1195–1199. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fernihough J, Gentry C, Malcangio M, Fox
A, Rediske J, Pellas T, Kidd B, Bevan S and Winter J: Pain related
behaviour in two models of osteoarthritis in the rat knee. Pain.
112:83–93. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
de Sousa Valente J: The pharmacology of
pain associated with the monoiodoacetate model of osteoarthritis.
Front Pharmacol. 10(974)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Stevenson GW, Mercer H, Cormier J, Dunbar
C, Benoit L, Adams C, Jezierski J, Luginbuhl A and Bilsky EJ:
Monosodium iodoacetate-induced osteoarthritis produces
pain-depressed wheel running in rats: Implications for preclinical
behavioral assessment of chronic pain. Pharmacol Biochem Behav.
98:35–42. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Orita S, Ishikawa T, Miyagi M, Ochia N,
Inoue G, Eguchi Y, Kamoda H, Arai G, Toyone T, Aoki Y, et al:
Pain-related sensory innervation in monoiodoacetate-induced
osteoarthritis in rat knees that gradually develops neuronal injury
in addition to inflammatory pain. BMC Musculoskelet Disord.
12(134)2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Muley MM, Krustev E and McDougall JJ:
Preclinical assessment of inflammatory pain. CNS Neurosci Ther.
22:88–101. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bryden LA, Nicholson JR, Doods H and
Pekcec A: Deficits in spontaneous burrowing behavior in the rat
bilateral monosodium iodoacetate model of osteoarthritis: An
objective measure of pain-related behavior and analgesic efficacy.
Osteoarthr Cartil. 23:1605–1612. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fraser LM, Brown RE, Hussin A, Fontana M,
Whittaker A, O'Leary TP, Lederle L, Holmes A and Ramos A: Measuring
anxiety- and locomotion-related behaviours in mice: A new way of
using old tests. Psychopharmacology (Berl). 211:99–112.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kandasamy R, Calsbeek JJ and Morgan MM:
Home cage wheel running is an objective and clinically relevant
method to assess inflammatory pain in male and female rats. J
Neurosci Methods. 263:115–122. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cobos EJ, Ghasemlou N, Araldi D, Segal D,
Duong K and Woolf CJ: Inflammation-induced decrease in voluntary
wheel running in mice: A nonreflexive test for evaluating
inflammatory pain and analgesia. Pain. 153:876–884. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kandasamy R, Calsbeek JJ and Morgan MM:
Analysis of inflammation-induced depression of home cage wheel
running in rats reveals the difference between opioid
antinociception and restoration of function. Behav Brain Res.
317:502–507. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Grace PM, Strand KA, Maier SF and Watkins
LR: Suppression of voluntary wheel running in rats is dependent on
the site of inflammation: Evidence for voluntary running as a
measure of hind paw-evoked pain. J Pain. 15:121–128.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Vonsy JL, Ghandehari J and Dickenson AH:
Differential analgesic effects of morphine and gabapentin on
behavioural measures of pain and disability in a model of
osteoarthritis pain in rats. Eur J Pain. 13:786–793.
2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Altarifi A, Alsalem M and Mustafa A:
Effects of intraplantar administration of Complete Freund's
Adjuvant (CFA) on rotarod performance in mice. Scand J Pain: Jul 2,
2019 (Epub ahead of print).
|
|
35
|
Bonfa L, Vinagre RC and de Figueiredo NV:
Cannabinoids in chronic pain and palliative care. Rev Bras
Anestesiol. 58:267–279. 2008.PubMed/NCBI View Article : Google Scholar
|