Introduction
Hyperlipidemia is a high risk factor for
cardiovascular disease (CVD), either by eroding large elastic
arteries or causing damage to endotheliocytes (1,2). In
ApoE-/- mice, hyperlipidemia induces lipid deposition
and foam cell formation, which ultimately leads to atherosclerosis
(3,4). Decreasing the blood lipid levels,
particularly low-density lipoprotein cholesterol (LDL-C) levels,
lowers the risk of CVD (5). Statins
are a class of cholesterol-lowering agents that significantly
decrease the severity of CVDs (6).
For example, rosuvastatin is prescribed to lower cholesterol levels
and thereby decrease the risk of CVD (7). Although statins are extensively used
to prevent hyperlipidemia and CVD, concerns have been raised
regarding their association with an increased risk of new-onset
diabetes and other adverse effects, such as liver toxicity
(8) and myopathy (9), leading to termination of treatment
(10). In addition, previous
studies have reported that a subset of patients who receive
statins, even those with well-controlled LDL-C levels, still
experience CVD events due to changes in the levels of other
lipids/lipoproteins (11-13).
Thus, novel strategies are currently being developed to improve the
therapeutic effects and minimize the side effects.
Sarpogrelate is a selective 5-hydroxytryptamine type
2A serotonin receptor antagonist that possesses an extensive range
of antiplatelet effects and can prevent arterial thrombosis
(14). Acyl-coenzyme A cholesterol
acyltransferase-1 is inhibited by sarpogrelate, which decreases the
accumulation of lipid droplets in macrophages and blocks
atherosclerosis (15). However, the
underlying molecular mechanism of sarpogrelate remains unclear, as
only two previous studies on rabbits have been published (16,17).
In rabbits, sarpogrelate delays the progression of atherosclerosis
by upregulating endothelial nitric oxide synthase expression
(17). Several clinical studies
have demonstrated that sarpogrelate improves atherosclerosis
(18-21).
These studies focused on patients with peripheral artery disease
(18-20)
or cerebrovascular disease (21);
however, the role of sarpogrelate on major arteries remains poorly
understood. Thus, the present study aimed to investigate whether
sarpogrelate synergistically protects against aortic damage in
ApoE-/- HF mice, when combined with rosuvastatin
treatment.
Materials and methods
Mice and diets
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Dalian Medical
University (approval no. L20160153; Dalian, China), and all animal
experiments were performed in accordance with the Guide for the
Care and Use of Laboratory Animals (17). A total of 22 male ApoE-/-
mice (8 weeks old) were provided by Beijing Vital River Laboratory
Animal Technology Co., Ltd. The body weights of mice ranged from 22
to 26 g. All animals were housed at 24˚C and 60% relative humidity
with a 12 h light and dark cycle with free access to water and
food. Mice were randomly divided into four treatment groups. The
control group was fed a normal diet (NF; n=5; 20.3% protein, 66%
carbohydrate, 5% fat; D10001; Research Diets, Inc.), whilst the
other three groups were fed a high-fat diet (HFD), containing 1.5%
cholesterol and 15% fat (HF group; n=5; Shanghai SLAC Laboratory
Animal Co., Ltd.). Furthermore, two of these groups were
additionally treated with rosuvastatin calcium (40 mg/kg/day) and
sarpogrelate (50 mg/kg/day; both purchased from Mitsubishi Tanabe
Pharma Corporation; HF+RS group; n=6), or rosuvastatin calcium
alone (40 mg/kg/day; HF+R group; n=6), respectively. Following 8
weeks (22) of treatment, the mice
were euthanized to analyze and characterize aortic injury.
Biochemical measurements
Following 8 weeks of treatment, all mice were
sacrificed by a 1% sodium pentobarbital overdose. After fasting for
12 h, the heart was exposed and blood samples were taken by left
ventricular puncture. The serum was subsequently separated, all
samples were centrifuged at 1,200 x g for 5 min at 4˚C, and the
expression levels of total cholesterol (TC), triglyceride (TG) and
LDL-C were determined using an automatic analyzer (Hitachi,
Ltd.).
Histological analysis
After the mice were euthanized, the complete aorta
(from the aortic root to the abdominal aorta) was fixed with 4%
formaldehyde at room temperature for 24 h and embedded in paraffin.
The paraffin-embedded aortas were cut into 4 µm thick sections and
dewaxed. Subsequently, the sections were stained with hematoxylin
for 6 min and eosin for another 1 min. Resinene were fixed on glass
slides and observed using a light microscope (Olympus Corporation;
magnification, x40).
Immunohistochemistry (IHC) was performed using the
Histofine Simple Stain kit (cat. no. 414142F; Nichirei) according
to the manufacturer's protocol. Briefly, the sections were
deparaffinized in xylene (3 times, 5 min each) and rehydrated (100,
90, 85, and 75% alcohol, 5 min each) following removal of the
excess tissue outside the aorta at room temperature. Sections were
incubated with 3% hydrogen peroxide at room temperature for 15 min
to inhibit endogenous peroxidase activity. The tissue sections were
incubated at 1% blocking solution (cat. no. P0220; Beyotime
Institute of Biotechnology) at room temperature for 10 min.
Subsequently, sections were incubated with primary antibodies
against cluster of differentiation 68 (CD68; 1:500; cat. no.
ab213363) and lectin-like oxidized low-density lipoprotein
receptor-1 (LOX-1; 1:250; cat. no. ab60178; both purchased from
Abcam) at room temperature for 1 h. Following the primary
incubation, sections were incubated with goat anti-rabbit IgG
secondary antibody (1:2,000; cat. no. ab205718; Abcam) at 37˚C for
30 min. The slides were observed under a light microscope (Olympus
Corporation; magnification, x40).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from the aorta using
TRIzol® reagent (Nippon Gene, Co., Ltd.) and reverse
transcribed into cDNA using the SuperScript VILO cDNA synthesis kit
(cat. no. 11756050; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. qPCR was subsequently performed using
SYBR Green (Light Cycler; Roche Molecular Diagnostics) and in
accordance with the manufacturer's instructions. The primer
sequences used for qPCR primers are listed in Table I. The following thermocycling
conditions were used for qPCR: 95˚C for 30 sec, 38 cycles at 95˚C
for 10 sec, 60˚C for 20 sec and 72˚C for 15 sec. Relative mRNA
levels were calculated using the 2-ΔΔCq method (23) and normalized to the internal
reference gene β-actin.
 | Table IPrimer sequences used for
quantitative PCR. |
Table I
Primer sequences used for
quantitative PCR.
| Gene | Primer
sequence |
|---|
| TNF-α |
F:5'-TCTCATGCACCACCATCAAGGACT-3' |
| |
R:5'-ACCACTCTCCCTTTGCAGAACTCA-3' |
| IL-1β |
F:5'-TGCCACCTTTGACAGTGAT-3' |
| |
R:5'-TGTGCTGCTGCGAGATTTGA-3' |
| IL-6 |
F:5'-TACCAGTTGCCTTCTTGGGACTGA-3' |
| |
R:5'-TAAGCCTCCGACTTGTGAAGTGGT-3' |
| β-actin |
F:5'-CGATGCCCTGAGGGTCTTT-3' |
| |
R:5'-TGGATGCCACAGGATTCCAT-3' |
Western blotting
The aorta was washed three times with PBS (cat. no.
C0221A; Beyotime Institute of Biotechnology) and subsequentl y
lysed using tissue lysis fluid (P0013G; Beyotime Institute of
Biotechnology). The mixture was centrifuged at 12,000 x g for 7 min
at 4˚C, the suspension after centrifugation was absorbed and total
protein was quantified using a bicinchoninic acid assay. Equal
amounts of protein (35 µg) were subjected to electrophoresis using
10% SDS-PAGE gels, transferred onto polyvinylidene difluoride
membranes (EMD Millipore) and blocked with 5% skimmed milk at 37˚C
for 1 h. The membranes were incubated with primary antibodies
against LOX-1 (rabbit anti-LOX-1; 1:250; cat. no. ab60178; Abcam),
phospho (p)-ERK (rabbit anti-p-ERK; 1:1,000; cat. no. 9101; Cell
Signaling Technology, Inc.), total-ERK (rabbit anti-ERK; 1:1,000;
cat. no. 4695; Cell Signaling Technology, Inc.), β-tubulin (rabbit
anti-β-tublin; 1:1,000; cat. no. 2148; Cell Signaling Technology,
Inc.) and β-actin (rabbit anti-β-actin; 1:1,000; cat. no. 4970S;
Cell Signaling Technology, Inc.) overnight at 4˚C. Following the
primary incubation, membranes were incubated with anti-rabbit IgG
secondary antibody (1:1,000; cat. no. 7074P2; Cell Signaling
Technology, Inc.) at room temperature for 1 h. A and B chromogenic
solutions (cat. no. P0013G; Beyotime Institute of Biotechnology)
were mixed in 1:1 ratio, and 2 ml of the solution was added to the
films. Protein signal intensity was determined using ImageJ 2.0
software (National Institutes of Health).
Statistical analysis
Each experiment was repeated three times.
Statistical analysis was performed using SPSS 23.0 software (IBM
Corp.) and all data are presented as the mean ± standard error of
the mean. One-way analysis of variance followed by Tukey's post hoc
test were used to compare differences between multiple groups. If
the data did not show homogeneity of variance, a Tamhane's T2 test
was performed. P<0.05 was considered to indicate a statistically
significant difference.
Results
Metabolic characterization
To determine the effects of the combined therapy on
metabolism, the serum levels of lipids were assayed and presented
in Table II, including TC, TG and
LDL-C. Following 8 weeks of dietary treatment, ApoE-/-
mice fed an HFD exhibited significantly increased lipid levels.
Conversely, sarpogrelate combined with rosuvastatin treatment
significantly decreased the levels of TC and LDL-C (P<0.05),
whereas the levels of TG only moderately decreased (P=0.51).
Although the levels of TC and LDL-C also decreased following
treatment with rosuvastatin alone, the differences were not as
notable compared with the HF+RS group (P<0.05). Furthermore,
there were no significant differences in the body weights between
the four groups. Taken together, these results suggest that
sarpogrelate may accentuate the effects of rosuvastatin by
effectively lowering lipid levels, without affecting the body
weight.
 | Table IIMetabolic data from the four groups
following treatment for 8 weeks. |
Table II
Metabolic data from the four groups
following treatment for 8 weeks.
| Groups | Body weight
(g) | TC (mmol/l) | TG (mmol/l) | LDL-C (mmol/l) |
|---|
| NF | 23.78±0.66 | 8.03±1.57 | 5.93±0.78 | 3.95±0.18 |
| HF | 24.04±0.83 | 33.58±2.79 | 7.42±0.73 | 23.73±2.01 |
| HF+R | 22.08±0.50 | 22.43±1.36 | 7.75±1.16 | 14.13±1.64 |
| HF+RS | 22.83±1.16 | 14.45±0.77 | 5.45±1.30 | 6.83±1.07 |
| P-Value | 0.351 | 0.000 | 0.000 | 0.357 |
Sarpogrelate combined with
rosuvastatin suppresses aortic histopathological damage in
ApoE-/- HFD mice
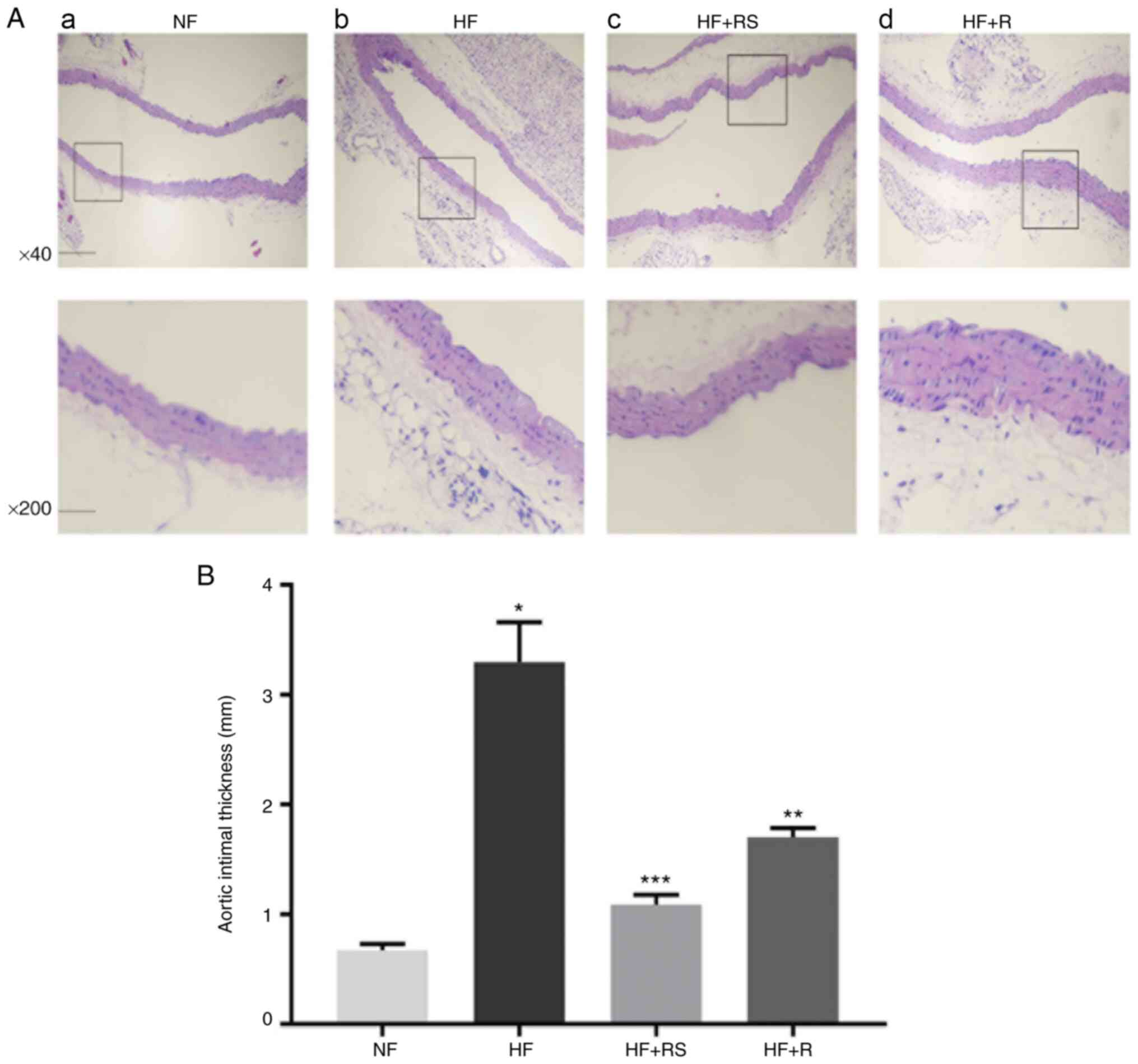
Aortic tissue damage was assessed via H&E
staining. The results demonstrated that the increased intima
thickness, lipid deposition and inflammatory cell infiltration
induced by the HFD were reversed following combined treatment with
sarpogrelate and rosuvastatin (Fig.
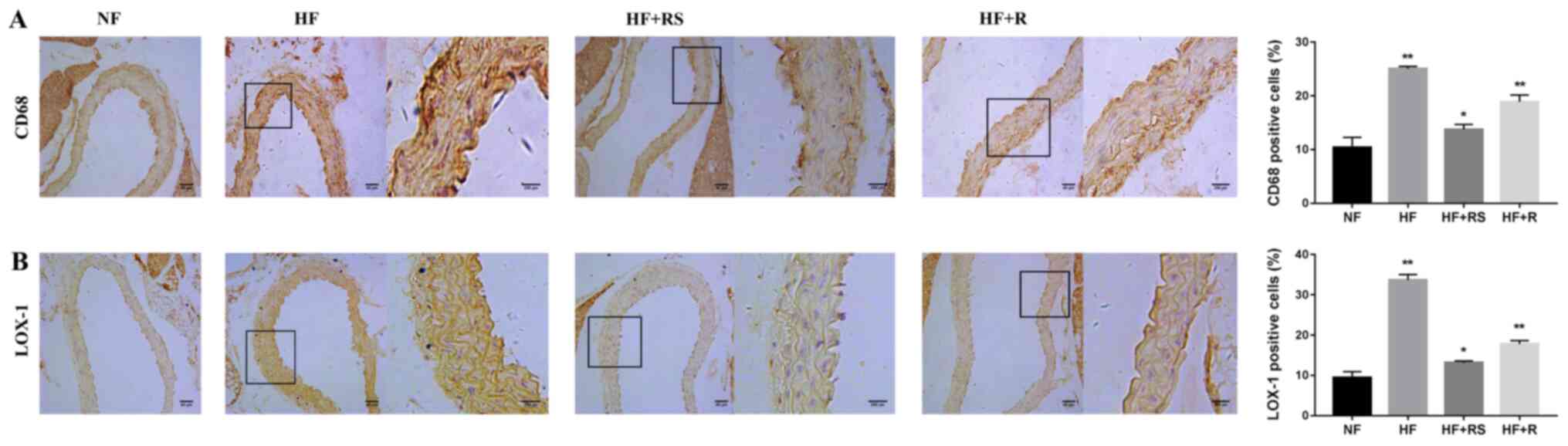
1). LOX-1 was analyzed via IHC analysis. The results
demonstrated that LOX-1 staining significantly decreased in the
HF+RS group compared with the HF+R group (P<0.05; Fig. 2B). The interaction between oxidized
(ox)-LDL and LOX-1 results in ox-LDL uptake by macrophages and foam
cell transformation (24,25). Collectively, these results indicate
that sarpogrelate and rosuvastatin can synergistically prevent
aortic damage induced by hyperlipidemia and reverse
atherosclerosis, which may be associated with the regulation of
LOX-1 expression.
Sarpogrelate combined with
rosuvastatin inhibits macrophage infiltration and significantly
decreases pro-inflammatory cytokine production
It is well known that the uptake of ox-LDL by
macrophages is dependent on several scavenger receptors (SRs)
(26). SR class A (SR-A), SR class
BI (SR-BI), LOX-1, cluster of differentiation 36 (CD36), and CD68
are relatively specific for ox-LDL (27) and CD68 is predominantly expressed in
macrophages (28). It was
hypothesized that, amongst others, CD68 plays a crucial role in the
formation of fatty-streaks. Thus, the present study set out to
determine whether the regulation of CD68 improved aortic injury in
HFD ApoE-/- mice. IHC analysis demonstrated that the
expression of CD68+ cells was upregulated in the HF group, while
the accumulation of CD68 decreased following combined treatment
with sarpogrelate and rosuvastatin (P<0.01; Fig. 2A), and the expression of foam cells
also significantly decreased (Fig.
1). Taken together, these results suggest that CD68 plays a key
role in the formation of foam cells, and that combined treatment
with sarpogrelate and rosuvastatin may reverse this effect.
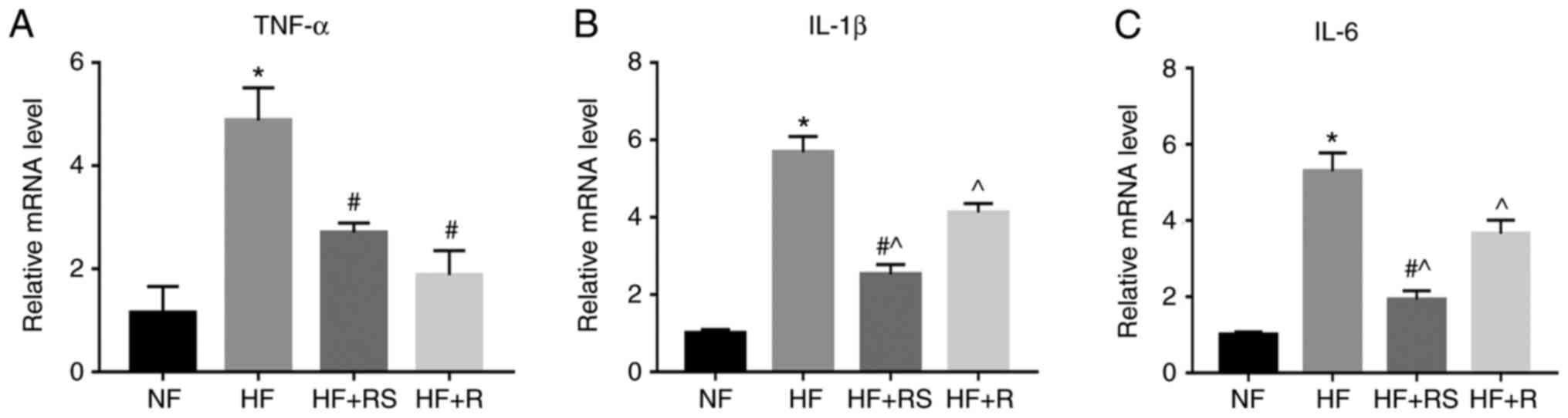
Furthermore, the expression levels of tumor necrosis factor-α
(TNF-α), interleukin (IL)-1β and IL-6 increased in the HF group,
the effects of which were significantly reversed following combined
treatment with sarpogrelate and rosuvastatin (P<0.05; Fig. 3). Collectively, these results
indicate that sarpogrelate and rosuvastatin synergistically inhibit
macrophage infiltration into the aorta, and inflammatory cytokine
release.
Sarpogrelate combined with
rosuvastatin decreases LOX-1 protein expression in
ApoE-/- HFD mice
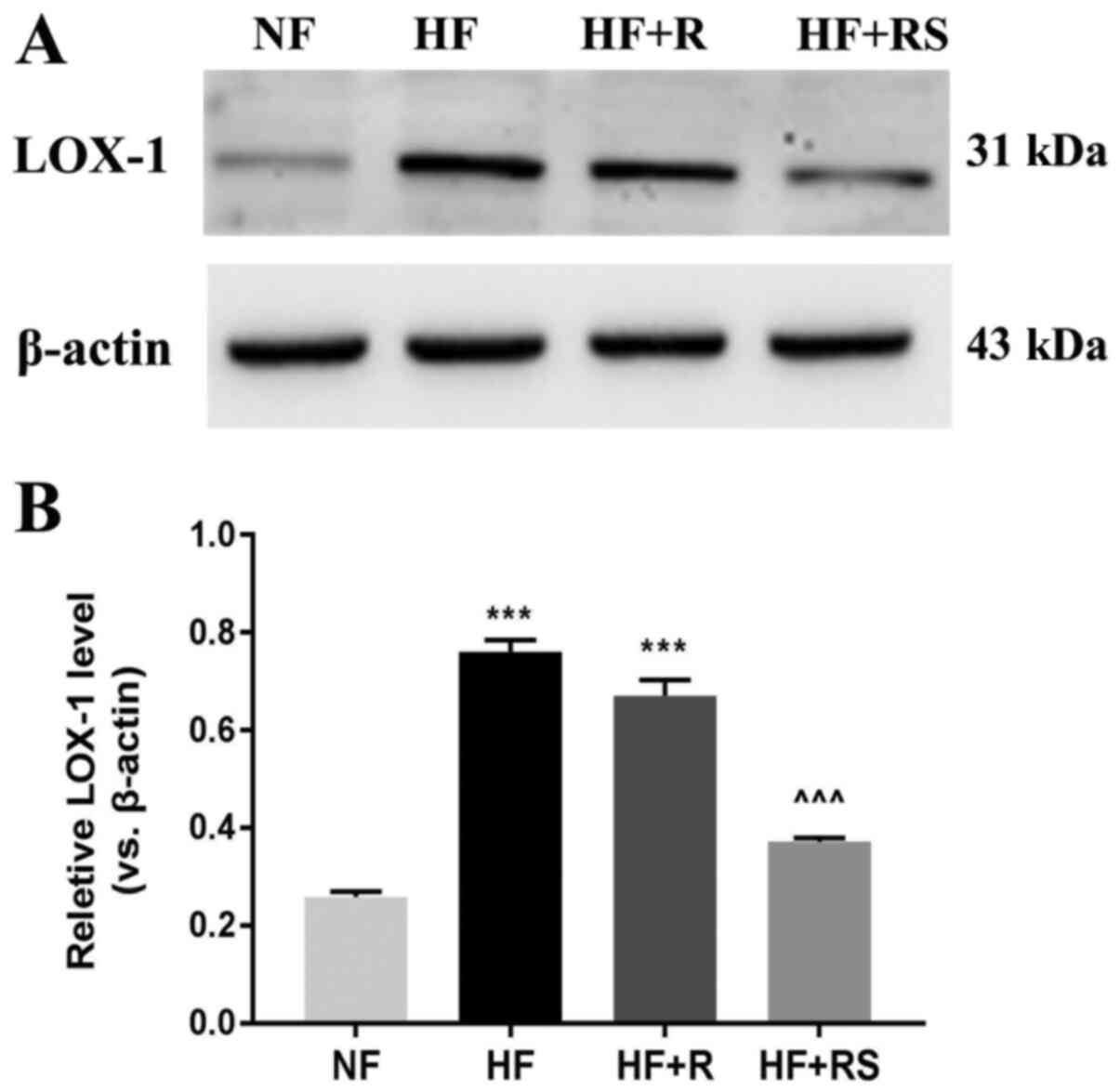
IHC analysis demonstrated that combined treatment
with sarpogrelate and rosuvastatin significantly decreased
hyperlipemia-induced LOX-1-positive staining in the aorta. In order
to verify these results, LOX-1 protein expression was determined
via western blot analysis. The results demonstrated that LOX-1
protein expression significantly decreased in the HF+RS group
compared with the HF+R group and the HF group, respectively (both
P<0.001; Fig. 4).
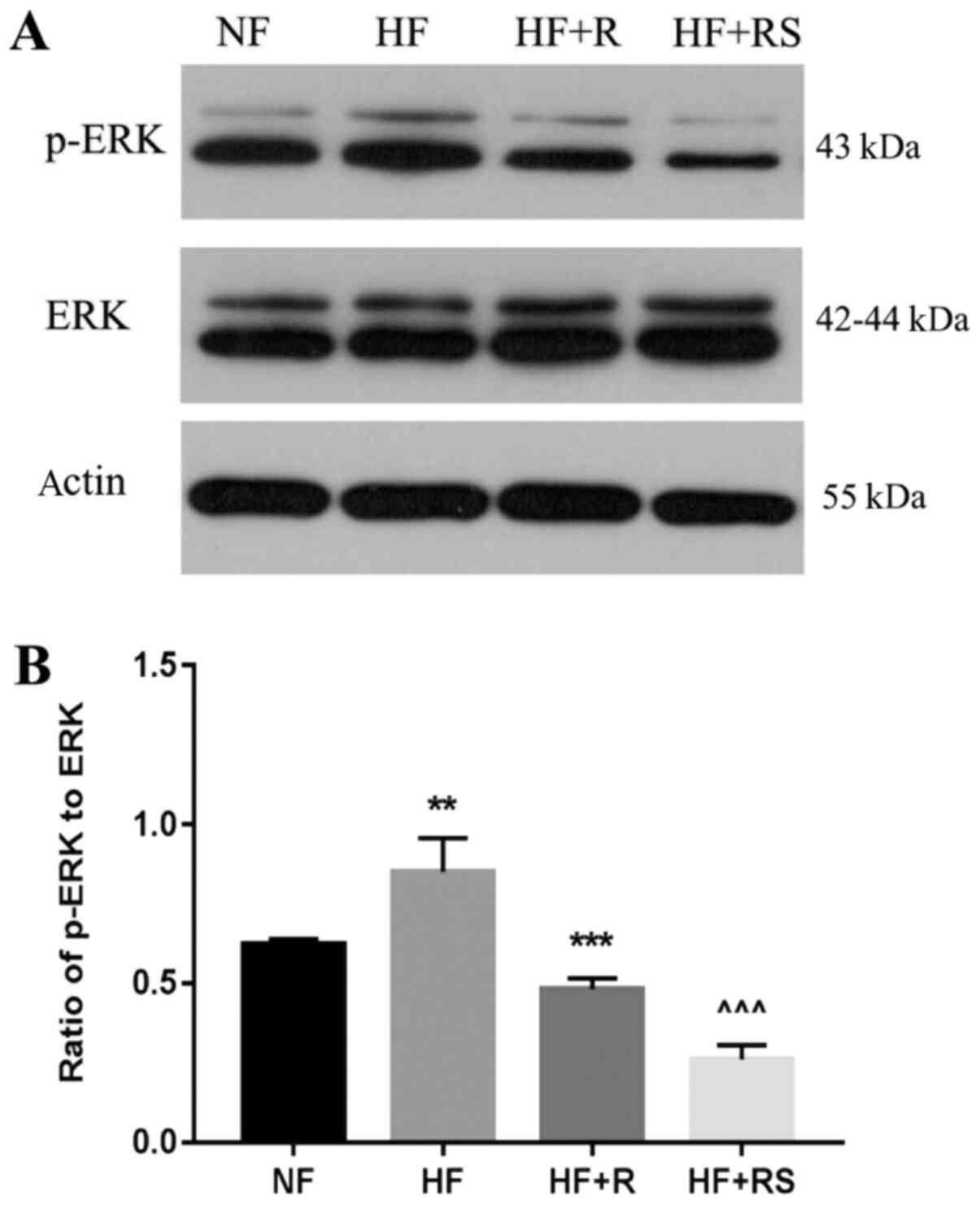
Sarpogrelate combined with
rosuvastatin decreases p-ERK expression in the aorta of
ApoE-/- HFD mice
The ox-LDL-LOX-ERK signaling pathway is involved in
atherosclerosis formation (29). To
the best of our knowledge, the effects of sarpogrelate and statins
on the hyperlipid-induced p-ERK pathway activation in aorta have
not yet been investigated. Western blot analysis demonstrated that
p-ERK levels were significantly higher in the HF group compared
with the NF group (P<0.01; Fig.
5). Rosuvastatin monotherapy slightly decreased p-ERK levels
compared with HF+RS group. Furthermore, combined treatment with
sarpogrelate and rosuvastatin significantly decreased p-ERK levels
compared with the HF and HF+R groups, respectively (both
P<0.001; Fig. 5). Taken
together, these results suggest that sarpogrelate and rosuvastatin
may have a direct inhibitory effect on ox-LDL-induced p-ERK
activation.
Discussion
The results of the present study demonstrated that
combined treatment with sarpogrelate and rosuvastatin decreased
hyperlipid-induced aortic injury by inhibiting p-ERK pathway
activation and downregulating expression of the scavenger receptor
protein, LOX-1. Furthermore, combined treatment with sarpogrelate
and rosuvastatin prevented CD68+ macrophage recruitment and
inflammatory cytokine release in ApoE-/- HFD mice.
A previous study reported a significant reduction in
coronary stent restenosis in patients with stable angina
administered sarpogrelate compared with the placebo group (30). Another study confirmed that
sarpogrelate effectively decreases restenosis in patients with
stable effort angina (31).
Although these studies demonstrated the therapeutic effects of
sarpogrelate in atherosclerotic heart disease, most studies focus
on the thrombosis-inhibiting effects, whereas the effects of
sarpogrelate on blood lipids have not yet been investigated.
Statins are well recognized as lipid-lowering agents and are used
to prevent atherosclerotic disease. Although statins have a certain
therapeutic efficacy in patients with atherosclerosis, monotherapy
is often insufficient to achieve the desired therapeutic outcomes
(32). In the present study,
combined treatment with sarpogrelate and rosuvastatin enhanced the
protective effects of rosuvastatin alone. Combined treatment
effectively decreased serum lipid levels, particularly TC and
LDL-C, compared with rosuvastatin alone, and alleviated aortic
injury in ApoE-/- HFD mice. In addition, combined
treatment synergistically decreased intima thickness, lipid
deposition and inflammatory cell infiltration induced by a HFD.
Several possible reasons may underly these effects; a recent study
demonstrated that sarpogrelate inhibits the accumulation of lipid
droplets in macrophages and improves arteriosclerosis (15). Another study also indicated that
5-HT increased the uptake of LDL via LDL receptors, and that of
ox-LDL via scavenger receptors in murine macrophages (33). Conversely, inhibition of 5-HT may
decrease the uptake of LDL-C to prevent the formation of foam cells
(33). To assess the potential
involvement of such a mechanism, the morphological changes
associated with aortic injury in hyperlipidemic ApoE-/-
mice were assessed via immunohistochemical staining. LOX-1 and CD68
are relatively specific for ox-LDL (27), and the latter is expressed primarily
in macrophages (28). LOX-1 and
CD68-stained areas in cross-sectional aortic roots significantly
decreased following combined treatment compared with either
rosuvastatin alone or HFD. Thus, it is hypothesized that the
significant synergistic lipid-lowering effects of sarpogrelate and
rosuvastain may be associated with the regulation of ox-LDL
scavenging receptors.
In aortic diseases, hyperlipidemia is a key factor
in the development of atherosclerosis as it increases the quantity
of circulating inflammatory cells and induces inflammatory pathways
(34,35). Reportedly, sarpogrelate also
modulates inflammatory-macrophage accumulation and inflammatory
responses (36). In a recent
experimental study, treatment with sarpogrelate decreased
inflammatory macrophage markers and inflammatory mediators in mice
with type 2 diabetes and diabetic nephropathy (36). In the present study, combined
treatment with sarpogrelate and rosuvastatin resulted in a
significant decrease in the mRNA expression levels of inflammatory
cytokines levels compared with rosuvastatin alone.
LOX-1 is a detrimental factor in hyperlipid-induced
aortic injury (37). Physiological
basal cellular expression of LOX-1 is low; however, LOX-1
expression is rapidly increased in response to proinflammatory
cytokines (38,39). In turn, LOX-1 stimulates the release
of inflammatory cytokines and activates inflammatory responses,
aggravating disease pathogenesis (40,41).
Thus, it was hypothesized that the key to blocking this positive
feedback loop is to prevent LOX-1 expression. Administration of
LOX-1 blockers or LOX-1 knockout can inhibit the binding of
inflammatory factors to LOX-1 and prevent the progress of
atherosclerosis (42). To verify
the results of IHC, LOX-1 expression was measured via western
blotting, which confirmed that sarpogrelate combined with
rosuvastatin significantly suppressed hyperlipidemia-induced LOX-1
protein expression. This suggests that sarpogrelate and
rosuvastatin can inhibit ox-LDL uptake in the arterial wall by
interfering with LOX-1 activation. The 5-HT2A receptor
is known to modulate both the MAPK/ERK and the PI3K/PDK/AKT
pathways, which serve prominent roles in cell survival (41,43).
Inhibitors of ERK, PKC and NF-κB attenuate LOX-1 expression,
indicating that activation of the ERK/PKC/MAPK pathway is an
initial signaling event in LOX-1 expression regulation (44). Other circumstantial evidence
suggests that LDL induces inflammation via LOX-1 and increases
phosphorylation of members of the ERK signaling pathway (45). Thus, it is hypothesized that p-ERK,
which is downstream of LOX-1, is the target of aortic injury.
Following 8 weeks of combined treatment with sarpogrelate and
rosuvastatin, p-ERK levels significantly decreased in
ApoE-/- HFD mice. A possible explanation for this may be
that increased blood lipids level result in an increase in ox-LDL
levels in artery walls. As a LOX-1 ligand, ox-LDL activates LOX-1
and its downstream signaling molecules, including p-ERK (30,29,46).
Activated p-ERK results in LOX-1 upregulation and promotes
arteriosclerosis (47), thereby
resulting in aortic injury. This effect on ox-LDL/LOX-1/p-ERK
signaling was more prominent in the HF+RS group compared with the
HF+R group. Thus, it is hypothesized that by blocking LOX-1 or
downstream p-ERK signaling, sarpogrelate and rosuvastatin may
improve hyperlipid-induced vascular remodeling and aortic
injury.
Dyslipidaemia, characterized by increased plasma
levels of LDL-C, VLDL-C, TG, and decreased plasma levels of HDL-C,
is a key factor associated with atherosclerotic disease (48). The effects of sarpogrelate and
rosuvastatin on VLDL-C, HDL-C and inflammatory factors in plasma of
HFD ApoE-/- mice were not assessed in the present study.
However, the results of the present study suggest that sarpogrelate
may enhance the lipid-lowering effect of statins, improve the
elevation of TG, TC and LDL-C caused by hyperlipidaemia (Table II), and improve the formation of
foam cells in aortic tissues and the infiltration of inflammatory
cells (Fig. 1). Further studies are
required to confirm the association between sarpogrelate enhanced
statin therapy and cardiovascular outcomes to better understand the
benefits of sarpogrelate in CVD.
In conclusion, the novel effects of sarpogrelate in
synergistically acting with rosuvastatin to inhibit
hyperlipid-induced aortic damage through the LOX-1/p-ERK pathway
were determined. These findings may provide novel insight into the
roles of sarpogrelate and rosuvastatin in vascular protection and
highlight the potential of a novel therapeutic intervention for the
treatment of aortic lesions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
HL, YL and SX conceived and designed the present
study. XF analyzed the data. GL, DL, QL, LH, and JZ have been
involved in acquiring the data, analyzing and interpreting the
data, and drafting the manuscript. HL, YL, JM and ZS performed the
experiments. YL and HL revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Dalian Medical
University (approval no. L20160153; Dalian, China), and all animal
experiments were performed in accordance with the Guide for the
Care and Use of Laboratory Animals (21).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wijeysundera DN, Duncan D, Nkonde-Price C,
Virani SS, Washam JB, Fleischmann KE and Fleisher LA: Perioperative
beta blockade in noncardiac surgery: A systematic review for the
2014 ACC/AHA guideline on perioperative cardiovascular evaluation
and management of patients undergoing noncardiac surgery: A report
of the; American college of cardiology American heart association
task force on practice guidelines. J Am Coll Cardiol. 64:2406–2425.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arsenault BJ, Kritikou EA and Tardif JC:
Regression of atherosclerosis. Curr Cardiol Rep. 14:443–449.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Karshovska E, Zhao Z, Blanchet X, Schmitt
MM, Bidzhekov K, Soehnlein O, von Hundelshausen P, Mattheij NJ,
Cosemans JM, Megens RT, et al: Hyperreactivity of junctional
adhesion molecule A-deficient platelets accelerates atherosclerosis
in hyperlipidemic mice. Circ Res. 116:587–599. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pei Z, Okura T, Nagao T, Enomoto D, Kukida
M, Tanino A, Miyoshi K, Kurata M and Higaki J: Osteopontin
deficiency reduces kidney damage from hyperlipidemia in
Apolipoprotein E-deficient mice. Sci Rep. 6(28882)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Collins R, Reith C, Emberson J, Armitage
J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets
D, et al: Interpretation of the evidence for the efficacy and safey
of statin therapy. Lancet. 388:2532–2561. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Prospective Studies Collaboration.
Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey
J, Qizilbash N, Peto R and Collins R. Blood cholesterol and
vascular mortality by age, sex, and blood pressure. A meta analysis
of individual data from 61 prospective studies with 55000 vascular
deaths. Lancet. 370:1829–1839. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rosenson RS: Rosuvastatin: A new inhibitor
of HMG-coA reductase for the treatment of dyslipidemia. Expert Rev
Cardiovasc Ther. 1:495–505. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Alqahtani SA and Sanchez W: Statins are
safe for the treatment of hypercholesterolemia in patients with
chronic liver disease. Gastroenterology. 135:702–704.
2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Phillips PS, Haas RH, Bannykh S, Hathaway
S, Gray NL, Kimura BJ, Vladutiu GD and England JD: Scripps Mercy
Clinical Research Center. Statin-associated myopathy with normal
creatine kinase levels. Ann Intern Med. 137:581–585.
2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chogtu B, Magazine R and Bairy KL: Statin
use and risk of diabetes mellitus. World J Diabetes. 6:352–357.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ahn CH and Choi SH: New drugs for treating
dyslipidemia: Beyond statins. Diabetes Metab J. 39:87–94.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fruchart JC, Davignon J, Hermans MP,
Al-Rubeaan K, Amarenco P, Assmann G, Barter P, Betteridge J,
Bruckert E, Cuevas A, et al: Residual macrovascular risk in 2013:
What have we learned? Cardiovasc Diabetol. 13(26)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nordestgaard BG: Triglyceride-rich
lipoproteins atherosclerotic cardiovascular disease: New insights
from epidemiology, genetics, and biology. Circ Res. 118:547–563.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Saini HK, Takeda N, Goyal RK, Kumamoto H,
Arneja AS and Dhalla NS: Therapeutic potentials of sarpogrelate in
cardiovascular disease. Cardiovasc Drug Rev. 22:27–54.
2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Suguro T, Watanabe T, Kanome T, Kodate S,
Hirano T, Miyazaki A and Adachi M: Serotonin acts as an
up-regulator of acyl-coenzyme A: Cholesterol acyltransferase-1 in
human monocyte-macrophages. Atherosclerosis. 186:275–281.
2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu YJ, Zhang M, Ji L, Elimban V, Chen L
and Dhalla NS: Suppression of high lipid diet induced by
atherosclerosis sarpogrelate. J Cell Mol Med. 16:2394–2400.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hayashi T, Sumi D, Matsui-Hirai H, Fukatsu
A, Arockia Rani PJ, Kano H, Tsunekawa T and Iguchi A: Sarpogrelate
HCl, a selective 5-HT2A antagonist, retards the progression of
atherosclerosis through a novel mechanism. Atherosclerosis.
168:23–31. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yamakawa J, Takahashi T, Saegusa S, Moriya
J, Itoh T, Kusaka K, Kawaura K, Wang XQ and Kanda T: Effect of the
serotonin blocker sarpogrelate on circulating interleukin-18 levels
in patients with diabetes and arteriosclerosis obliterans. J Int
Med Res. 32:166–169. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Takahara M, Kaneto H, Katakami N, Iida O,
Matsuoka TA and Shimomura I: Effect of sarpogrelate treatment on
the prognosis after endovascular therapy for critical limb
ischemia. Heart Vessels. 29:563–567. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Miyazaki M, Higashi Y, Goto C, Chayama K,
Yoshizumi M, Sanada H, Orihashi K and Sueda T: Sarpogrelate
hydrochloride, a selective 5-HT2A antagonist, improves vascular
function in patients with peripheral arterial disease. J Cardiovasc
Pharmacol. 49:221–227. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Carbone L: Pain management standards in
the eighth edition of the guide for the care and use of laboratory
animals. J Am Assoc Lab Anim Sci. 51:322–328. 2012.PubMed/NCBI
|
|
22
|
Shinohara Y, Nishimaru K, Sawada T,
Terashi A, Handa S, Hirai S, Hayashi K, Tohgi H, Fukuuchi Y,
Uchiyama S, et al: Sarpogrelate-aspirin comparative clinical study
for efficacy and safety in secondary prevention of cerebral
infarction (S-ACCESS): A randomized, double-blind,
aspirin-controlled trial. Stroke. 39:1827–1833. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang HY, Bian YF, Zhang HP, Gao F, Xiao
CS, Liang B, Li J, Zhang NN and Yang ZM: LOX 1 is implicated in
oxidized low density lipoprotein induced oxidative stress of
macrophages in atherosclerosis. Mol Med Rep. 12:5335–5341.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang X, Ding Z, Lin J, Guo Z and Mehta JL:
LOX-1 in macrophage migration in response to ox-LDL and the
involvement of calpains. Biochem Biophys Res Commun. 467:135–139.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yu XH, Fu YC, Zhang DW, Yin K and Tang CK:
Foam cells in atherosclerosis. Clin Chim Acta. 424:245–252.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Graeves DR and Gordon S: The macrophage
scavenger receptor at 30 years of age: Current knowledge and future
challenges. J Lipid Res. 50 (Suppl):S282–S286. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ramprasad MP, Terpstra V, Kondratenko N,
Quehenberger O and Steinberg D: Cell surface expression of mouse
macrosialin and human CD68 and their role as macrophage receptors
for oxidized low density lipoprotein. Proc Natl Acad Sci USA.
93:14833–14838. 1996.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang Z, Zhang M, Li Y, Liu S, Ping S,
Wang J, Ning F, Xie F and Li C: Simvastatin inhibits the additive
activation of ERK1/2 and proliferation of rat vascular smooth
muscle cells induced by combined mechanical stress and oxLDL
through LOX-1 pathway. Cell Signal. 25:332–340. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fujita M, Mizuno K, Ho M, Tsukahara R,
Miyamoto A, Miki O, Ishii K and Miwa K: Sarpogrelate treatment
reduces restenosis after coronary stenting. Am Heart J.
145(E16)2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kajiwara I, Soejima H, Miyamoto S and
Ogawa H: Effects of additional treatment of sarpogrelate to aspirin
therapy on platelet aggregation and plasma plasminogen activator
inhibitor activity in patients with stable effort angina. Thromb
Res. 128:547–551. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Katsiki N, Athyros VG and Karagiannis A:
Exploring the management of statin intolerant patients: 2016 and
beyond. Curr Vasc Pharmacol. 14:523–533. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Aviram M, Fuhrman B, Maor I and Brook GJ:
Serotonin increases macrophage uptake of oxidized low density
lipoprotein. Eur J Clin Chem Clin Biochem. 30:55–61.
1992.PubMed/NCBI
|
|
34
|
Tannock LR: Advances in the management of
hyperlipidemia-induced atherosclerosis. Expert Rev Cardiovasc Ther.
6:369–383. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Siasos G, Tousoulis D, Oikonomou E,
Zaromitidou M, Stefanadis C and Papavassiliou AG: Inflammatory
markers in hyperlipidemia: From experimental models to clinical
practice. Curr Pharm Des. 17:4132–4146. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lee ES, Lee MY, Kwon MH, Kim HM, Kang JS,
Kim YM, Lee EY and Chung CH: Sarpogrelate hydrochloride ameliorates
diabetic nephropathy associated with inhibition of macrophage
activity and inflammatory reaction in db/db mice. PLoS One.
12(e0179221)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Metha JL, Chen J, Hermonat PL, Romeo F and
Novelli G: Lectin-like, oxidized low-density lipoprotein receptor-1
(LOX-1): A critical player in the development of atherosclerosis
and related disorders. Cardiovasc Res. 69:36–45. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Morawietz H: LOX-1 and atherosclerosis:
Proof of concept in LOX-1-knockout mice. Circ Res. 100:1534–1536.
2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Moriwaki H, Kume N, Kataoka H, Murase T,
Nishi E, Sawamura T, Masaki T and Kita T: Expression of lectin-like
oxidized low density lipoprotein receptor-1 in human and murine
macrophages: Upregulated expression by TNF-alpha. FEBS Lett.
440:29–32. 1998.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li D and Mehta JL: Antisense to LOX-1
inhibits oxidized LDL-mediated upregulation of monocyte
chemoattractant protein-1 and monocyte adhesion to human coronary
artery endothelial cells. Circulation. 101:2889–2895.
2000.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xu S, Ogura S, Chen J, Little PJ, Moss J
and Liu P: LOX-1 in atherosclerosis: Biological functions and
pharmacological modifiers. Cell Mol Life Sci. 70:2859–2872.
2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lu J, Mitra S, Wang X, Khaidakov M and
Methta JL: Oxidative stress and lectin-like ox-LDL-receptor LOX-1
in atherogenesis and tumorigenesis. Antioxid Redox Signal.
15:2301–2333. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zamani A and Qu Z: Serotonin activates
angiogenic phosphorylation signaling in human endothelial cells.
FEBS Lett. 586:2360–2365. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li L, Sawamura T and Renier G: Glucose
enhances human macrophage LOX-1 expression: Role for LOX-1 in
glucose-induced macrophage foam cell formation. Circ Res.
94:892–901. 2004.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chang PY, Pai JH, Lai YS and Lu SC:
Electronegative LDL from rabbits fed with atherogenic diet is
highly proinflammatory. Mediators Inflamm.
2019(6163130)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yang TC, Chang PY, Kuo TL and Lu SC:
Electronegative L5-LDL induces the production of G-CSF and GM-CSF
in human macrophages through LOX-1 involving NF-κB and ERK2
activation. Atherosclerosis. 267:1–9. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ran X, Zhao W, Li W, Shi J and Chen X:
Cryptotanshinone inhibits TNF-α-induced LOX-1 expression by
suppressing reactive oxygen species (ROS) formation in endothelial
cells. Korean J Physiol Pharmacol. 20:347–355. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Su X and Peng D: The exchangeable
apolipoproteins in lipid metabolism and obesity. Clin Chim Acta.
503:128–135. 2020.PubMed/NCBI View Article : Google Scholar
|