Introduction
Dry eye (DE) is a multifactorial disease of the
ocular surface that is characterized by loss of homeostasis of the
tear film and is accompanied by ocular symptoms, in which tear film
instability and hyperosmolarity, ocular surface inflammation and
damage, as well as neurosensory abnormalities, play etiological
roles (1). DE is one of the most
prevalent ocular disorders and is characterized by discomfort
symptoms, such as burning, tearing, foreign body sensation and
ocular fatigue (2). Patients with
DE experience difficulties in daily routine activities that
compromise their quality of life (3). The incidence of DE continues to
increase, which may be partly associated with changes in lifestyle
and working environments.
There is currently no cure or universally effective
treatment for DE. The main standard treatment for DE is the topical
administration of artificial tears, such as those containing sodium
hyaluronate (SH), to provide additional lubrication, but the
expected results are usually not optimal and the efficacy is
limited. Other therapeutic alternatives consist of topical
cyclosporine, topical corticosteroids and punctal occlusion, but
their use is limited due to the drawbacks and related side effects
(2,4). The currently available treatments are
mainly palliative, intended to supplement patients' natural tears
or improve the residence time of the limited volume of tears
present, as restoring the physiological lacrimal secretion is
difficult (5). In addition, a
number of patients report less improvement in chronic ocular pain
and photophobia with topical therapies (6). Evaluating the potential of low-risk
adjuvant treatments in these patients has been attracting
increasing interest (7).
Transcutaneous electrical stimulation (TES) is a
well-established therapeutic strategy for activating peripheral
nerve pathways directly, in order to correct organ dysfunction and
manage disease symptoms (8-11).
TES involves the transmission of electrical current to the
peripheral nervous system through electrodes placed on the skin
surface. It is a non-invasive form of neuromodulation that has
demonstrated effectiveness in numerous pain conditions and has been
widely used over recent decades (12-15).
TES also has been used for treating ocular diseases with promising
results (7,16,17).
Recently, TES was reported to have potential
efficacy as a novel option for treating DE (18). However, clinical researches have
been insufficient and no controlled comparative studies
demonstrating the superiority of TES for DE have been conducted to
date. Hence, the aim of the present study was to prospectively
investigate the efficacy of TES combined with artificial tears in
the treatment of DE.
Materials and methods
Participants
This randomized controlled trial was performed to
compare TES + SH with SH. The study was performed between February
2019 and December 2019 and was approved by the Ethics Committee of
the Ninth People's Hospital of Chongqing. Overall, 138 eyes of 69
patients who met the clinical diagnosis of bilateral DE according
to the definitions set out by the International Dry Eye WorkShop
were recruited for this study (1).
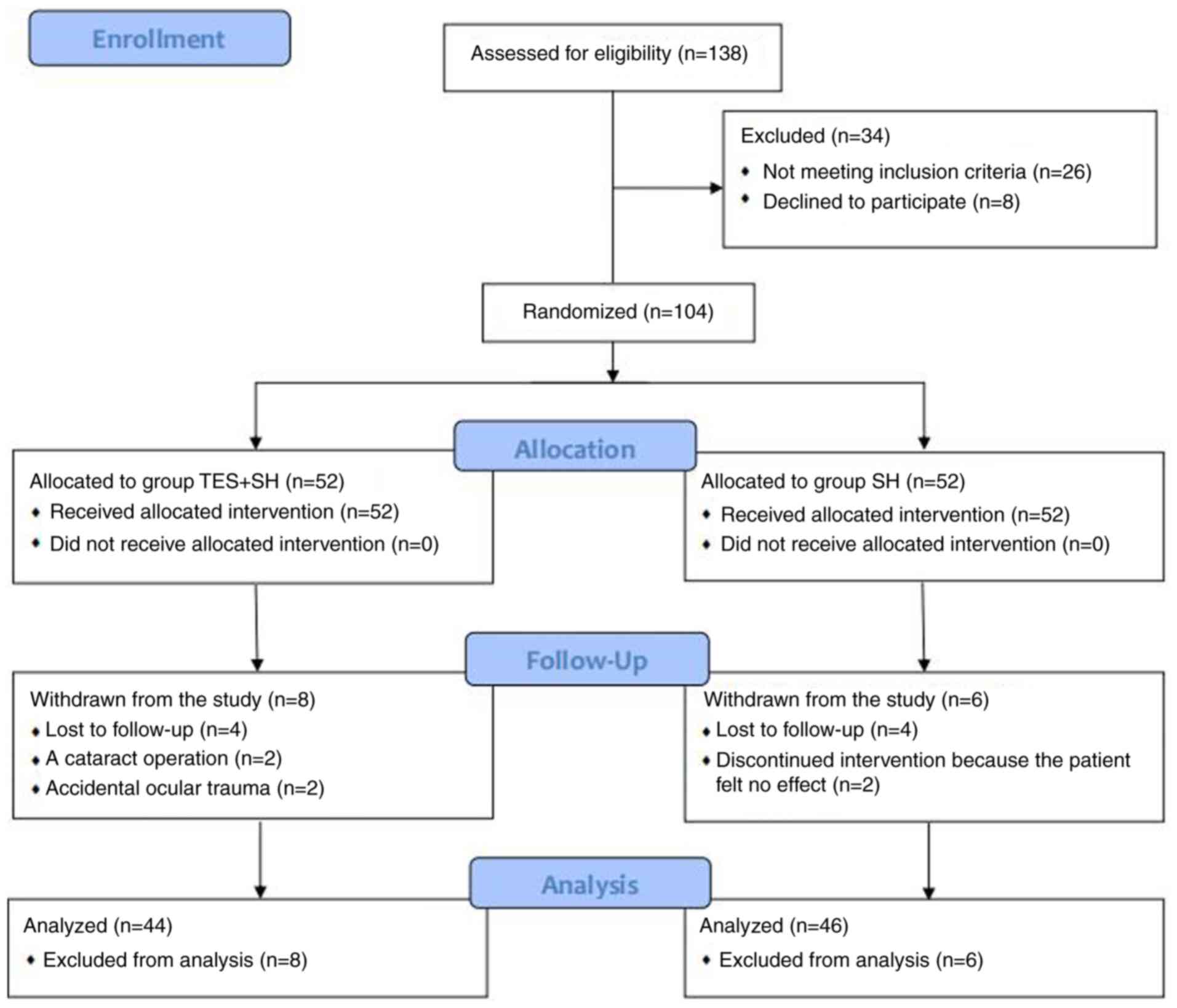
Based on the inclusion/exclusion criteria, 104 eyes of 52 patients
who matched the DE criteria were enrolled in the present study by a
trained ophthalmologist (Fig.
1).
The inclusion criteria were as follows: i) Patients
aged 18-65 years; ii) patients who volunteered to join the study
and signed the informed consent form; and iii) patients who
conformed to the following DE diagnostic criteria: Schirmer's I
test outcomes of ≤10 mm, tear film breakup time (BUT) <10 sec,
and presence of DE symptoms evaluated using the Ocular Surface
Disease Index (OSDI) questionnaire (OSDI ≥13).
The exclusion criteria were as follows: i)
Psychiatric and severe systemic diseases; ii) DE complicated by
active ocular infection, corneal abnormalities or any other ocular
pathologies; iii) history of punctal occlusion; iv) history of
biomedical electronic device implantation, including cardiac
pacemaker or automatic internal defibrillator, cerebrovascular
condition, epilepsy, pregnancy, acute pain of unknown etiology, and
skin lesion or injury at the site of electrode placement; v) other
previous treatments except artificial tears in the last 2 months;
and vi) wearing contact lenses.
Sample size
Based on previous data (18), a power analysis was performed to
determine the sample size required to obtain significant effects
after the treatment. A dropout rate of 15% was predicted.
Therefore, a total sample size of 104 eyes was deemed
sufficient.
Randomization
Washout was carried out in all patients with
preservative-free saline eye drops instilled four times per day for
2 weeks. After washout, all patients were randomized 1:1 into two
groups, each with 52 eyes of 26 patients, using a
computer-generated list of random numbers by a special
statistician. Patients in the SH group used only SH eye drops
(URSAPHARM Arzneimittel GmbH) four times per day, while patients in
the TES + SH group used TES combined with SH therapy. The treatment
was continued for 4 weeks in all cases. The patients were made
aware of the treatment group assignment, but the examiner was
blinded to the grouping of the patients.
Application of electrical
stimulation
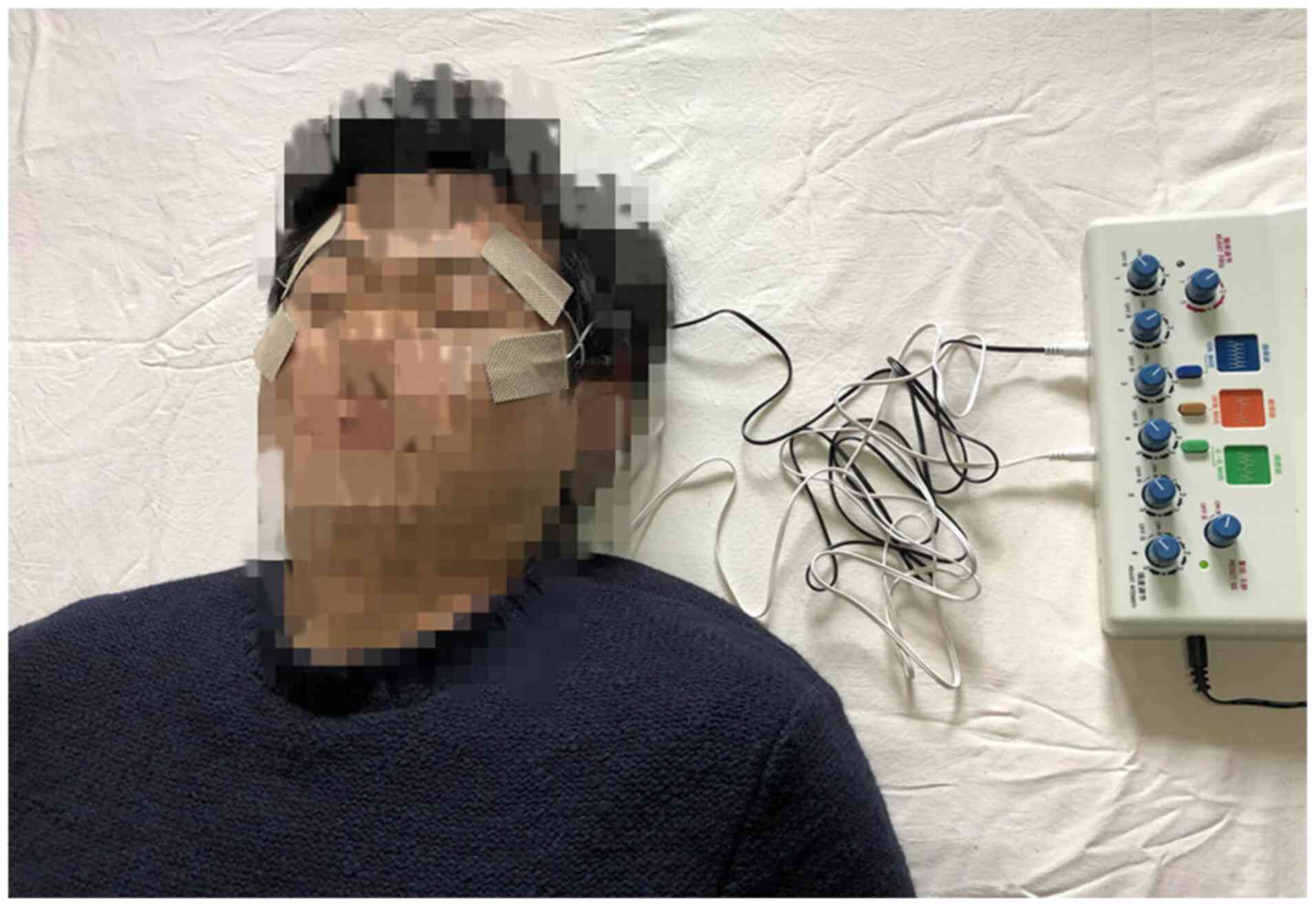
Electrical stimulation was applied with a device
(Huatuo brand SDZ-II Electrical Stimulator, Suzhou Medical Supply
& Equipment). Its function was based on the resonance effect,
with the possibility of maximizing the delivery of energy to
biological tissues by oscillating electric fields without
increasing the temperature and eliciting biological responses, both
pathophysiological and potentially therapeutic (19).
The participants were asked to lie down comfortably
and relax in a quiet environment. The skin overlying the sites of
electrode placement was first cleaned with alcohol pads and allowed
to dry. Treatment was administered as previously described
(7,18). Two rectangular electrodes sized
50x30 mm2 were placed in the periorbital area of each
eye: One over the temporal area and one near the lower lid, so that
they were in proximity to the ophthalmic (V1) and maxillary (V2)
branches of the trigeminal nerve (Fig.
2).
Each patient underwent 20 sessions (5 sessions per
week for 4 weeks), and each session lasted for 20 min, with a
frequency of 20 Hz and a power of no more than 2 mA. The amplitude
of each eye was increased manually until the point of discomfort
and then set to one level below this point.
Safety evaluation
The adverse effects included dizziness, edema and
severe pain during or after electrical stimulation. Any other
adverse events were also recorded.
Ocular examinations
Improvement in OSDI, BUT, Schirmer's Ⅰ test and
corneal fluorescein staining were assessed and compared before and
after treatment. A single examiner performed all ocular
examinations.
OSDI scores were obtained according to a subjective
questionnaire, and the patients gave their impressions on the
status of their eyes before and after treatment. Symptoms such as
itching, dryness and foreign body sensation were evaluated
(20). BUT was observed under a
slit lamp biomicroscope. Three measurements were performed and the
mean value was calculated. The Schirmer's Ⅰ test was performed
using standard strips (Alcon) kept in the lower conjunctival sac
for 5 min. Corneal fluorescein staining was scored according to the
grading system recommended by the National Eye Institute/Industry
Workshop on Clinical Trials in Dry Eyes (21). The cornea was divided into five
zones: Central, superior, temporal, nasal and inferior. For each
zone, the amount of corneal fluorescein staining was graded on a
scale of 0-3 as follows: 0, normal or negative slit lamp findings;
1, mild or superficial stippling; 2, moderate or punctate staining,
including superficial abrasion of the cornea; and 3, severe
abrasion or corneal erosion, deep corneal abrasion, or recurrent
erosion. The maximum score was 15(22).
The primary outcome measure was the differences in
the OSDI. The secondary outcome measures were the differences in
BUT, Schirmer's I test and corneal fluorescein staining.
Statistical analysis
The data are presented as mean ± SD and were
analyzed using SPSS 17.0 software for Windows (SPSS, Inc.). The
Student's t-test was used to assess the differences between the TES
+ SH and SH groups before and after treatment. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 52 patients (104 eyes) were included in
the present study. No significant differences were found between the
two groups in terms of basic characteristics, including age, sex
and duration of the disease (Table
I; P>0.05). A total of 90 eyes completed all aspects of the
study, 22 patients (44 eyes) in the TES + SH group and 23 patients
(46 eyes) in the SH group. The flow chart of the study is shown in
Fig. 1. A total of 4 patients in
the TES + SH group were withdrawn from the analysis: 2 patients (4
eyes) were lost to follow-up, 1 patient (2 eyes) underwent cataract
surgery, and 1 patient (2 eyes) was withdrawn due to accidental
ocular trauma. A total of 3 patients in the SH group were withdrawn
from the analysis: 2 patients (4 eyes) were lost to follow-up, and
1 patient (2 eyes) discontinued treatment as she experienced no
subjective improvement.
 | Table IDemographics and baseline
characteristics of patients with dry eye. |
Table I
Demographics and baseline
characteristics of patients with dry eye.
| Characteristics | SH group | TES + SH group | P-value |
|---|
| Total no. of patients
(eyes) | 23(46) | 22(44) | |
| Male:female
ratio | 10:13 | 9:13 | 0.862 |
| Age (years) | | | 0.497 |
|
Mean ±
SD | 42.9±15.1 | 41.2±14.2 | |
|
Range | 18-65 | 19-63 | |
| Course of DE
(years) | | | 0.255 |
|
Mean ±
SD | 3.9±2.7 | 4.2±3.4 | |
|
Range | 1-10 | 1-12 | |
Primary outcome
With respect to the OSDI, no statistically
significant difference in the OSDI was found between the TES + SH
and SH groups before treatment (42.3±7.6 vs. 43.2±6.2, P=0.106).
The OSDI scores declined significantly in the two groups after
treatment (P<0.05); a significant difference was observed
between the two groups after 4 weeks (24.5±4.8 vs. 31.3±8.6,
P=0.004). The OSDI scores were markedly better in the TES + SH
group compared with those in the SH group 4 weeks after treatment.
The differences between the two groups are summarized in Table II.
 | Table IIChanges in the outcomes of patients
with dry eye before and after treatment. |
Table II
Changes in the outcomes of patients
with dry eye before and after treatment.
| Outcomes | Baseline | 4 weeks | P-value |
|---|
| OSDI | | | |
|
SH group
(n=23 patients) | 43.2±6.2 | 31.3±8.6 | 0.013 |
|
TES + SH
group (n=22 patients) | 42.3±7.6 | 24.5±4.8 | 0.000 |
|
P-value | 0.106 | 0.004 | |
| BUT | | | |
|
SH group
(n=46 eyes) | 4.2±2.0 | 5.3±2.2 | 0.333 |
|
TES + SH
group (n=44 eyes) | 4.0±1.7 | 6.5±3.0 | 0.001 |
|
P-value | 0.658 | 0.039 | |
| Schirmer's I
test | | | |
|
SH group
(n=46 eyes) | 4.4±1.9 | 5.1±2.2 | 0.193 |
|
TES + SH
group (n=44 eyes) | 4.5±1.8 | 6.9±3.1 | 0.000 |
|
P-value | 0.523 | 0.016 | |
| Corneal
staining | | | |
|
SH group
(n=46 eyes) | 3.5±1.9 | 2.5±1.4 | 0.030 |
|
TES + SH
group (n=44 eyes) | 3.1±1.8 | 1.2±1.0 | 0.008 |
|
P-value | 0.385 | 0.029 | |
Secondary outcome
Regarding the objective DE measures, BUT and
Schirmer's I test findings did not differ between the two study
groups before treatment (P>0.05). Significant increases in BUT
and Schirmer's I test were found in the TES + SH group 4 weeks
after the treatment (P<0.05), but not in the SH group
(P>0.05). Significant differences in OSDI, BUT, Schirmer's I
test and corneal fluorescein scores were found between the two
groups 4 weeks after treatment (Table
II, P<0.05).
No statistically significant difference in corneal
fluorescein staining scores was found between the TES + SH and SH
groups before treatment (3.1±1.8 vs. 3.5±1.9, P=0.385). The scores
in both groups were markedly decreased after treatment (P<0.05).
The scores were markedly lower in the TES + SH group compared with
those in the SH group 4 weeks after treatment (1.2±1.0 vs. 2.5±1.4,
P=0.029). Therefore, the TES + SH group exhibited better epithelial
healing. The scores of fluorescein staining in the two groups are
presented in Table II.
Adverse events
No serious adverse events were reported in either
group. A total of 4 patients reported minor pain in the skin
overlying the site of electrode placement. No other adverse effect
was observed in all patients. No patients withdrew from the study
due to adverse effects.
Discussion
The present study demonstrated that TES
significantly affected subjective outcomes as well as objective
measures in patients with DE. Significant improvements in OSDI,
BUT, Schirmer's I test and corneal staining scores were observed in
patients treated with TES + SH compared with patients treated with
SH alone. No serious adverse events occurred in either group.
Patients in the TES + SH group generally exhibited a reduction in
symptoms and reported a high degree of overall satisfaction. The
majority stated that they would recommend TES therapy to friends or
family members with DE.
TES was initially described as an effective
treatment for DE by Pedrotti et al in 2016(18). A total of 27 patients with DE
underwent TES with electrodes placed onto the periorbital region of
both eyes. TES was shown to improve DE, both subjectively and
objectively, without any associated adverse effects, and may prove
to be of value for the treatment of DE. However, this was a pilot
study with only 27 patients and no control group. During medical
procedures, it was not considered practical to only treat patients
with TES without artificial tears as most patients were unwilling
to receive TES treatment only without eye drops. Sivanesan et
al (7) found that the
non-invasive electrical stimulation of the trigeminal nerve
achieved a short-term reduction in DE-related chronic ocular pain
and photophobia. The use of TENS reduced pain intensity in both
eyes by a mean of 57% and decreased light sensitivity by 27-28%.
However, this was also a pilot study with a small population and no
control group. It only studied subjective symptoms but did not
analyze objective indicators, such as BUT, Schirmer's I test and
corneal staining score. A prospective, open-label, non-randomized
clinical trial, using neurostimulation of the nasal sensory nerves,
was conducted by Friedman et al in 40 subjects with
mild-to-severe DE (4). The results
revealed a significant increase in tear production based on the
difference in Schirmer's I test scores, as well as an improvement
in OSDI scores, along with corneal and conjunctival staining. The
authors concluded that the neurostimulation of the nasolacrimal
pathway was an effective means for increasing tear production and
reducing symptoms among patients with DE. More recent studies also
demonstrated that intranasal tear neurostimulation exerted an
effect on the aqueous, lipid and mucin components of the tears
(23-26).
However, several patients did not approve of intranasal tear
neurostimulation due to discomfort and concerns regarding hygiene.
Compared with intranasal tear neurostimulation, TES is less
invasive, easy to perform and more tolerable.
Compared with previous studies, the present study
had several advantages: i) TES is an easy to perform, safe,
cost-effective and non-invasive procedure. Treatment is applied
transcutaneously; therefore, TES is less invasive compared with
surgical therapy and acupuncture, and relatively inexpensive
compared with pharmaceutical therapies. ii) Previous studies were
mainly pilot studies with a small population and no control group.
This was a randomized controlled trial on the use of TES + SH for
DE that covered the limitations of previous studies and helped
determine the best approach to the management of this frequent
ocular surface disease. iii) During actual clinical procedures, it
is not practical to only administer TES treatment to patients
without artificial tears. As a result, TES was combined with SH as
the experimental group in this study and compared with the control
group using artificial tears alone to explore the effect of TES in
treating DE.
The OSDI score was selected as the primary outcome
of the present study, as the main goal of the treatment was to
improve the symptoms of DE. The OSDI (27) is a 12-item questionnaire designed to
provide a rapid assessment of the symptoms of ocular irritation
consistent with DE (28). It is a
standardized and validated instrument for evaluating the symptoms
of the ocular surface disease and can be easily performed. A
significant reduction in the OSDI scores and a more marked effect
on patients treated with TES + SH were observed at the end of the
treatment. The results of the present study were attributed to the
application of TES.
The exact mechanisms underlying the beneficial
effect of TES treatment on DE remain unclear. However, two possible
hypotheses may explain the positive results obtained in the present
study.
The first hypothesis is that TES can effectively
stimulate the trigeminal nerve to relieve DE symptoms such as pain
and photophobia (7,29); this hypothesis may account for OSDI
results. Previous studies reported that the mechanism of TES for DE
may be associated with the modulation of neuroanatomical pain
pathways within the trigeminal system (22,30,31).
TES functions by a phenomenon referred to as ‘gate control theory’
(32). It stimulates vibration
receptors by electrical current, thereby reducing the transmission
of painful stimuli to the brain (33). Moreover, it relieves pain through
repeated application to an area and an increase in the secretion of
endogenous endorphins (34). In
addition, it is possible that TES can desensitize retinal cells
directly and reduce their ability to respond to light, thereby
reducing photophobia. Another possible site of action is the
trigeminal-cervical complex, where the photophobia and pain
pathways converge (35).
The second hypothesis may account for both the
subjective and objective results. This hypothesis is that TES can
produce quantum molecular resonance (QMR), stimulate the lacrimal
system, and reactivate the lacrimal and meibomian gland tissue
(18), thus promoting the secretion
of tears and increasing the thickness of the lipid and mucin layers
(36). QMR creates energy to break
the molecular bonds without increasing the kinetic energy of the
hit molecules, thus not increasing the temperature and limiting the
damage to the surrounding tissue. It can also produce a mechanical
stimulation, an electrical interaction with the cellular membrane,
and a biochemical interaction that involves the internal structures
of the cells. The metabolism and biochemical stimulation of
cellular structures are achieved through a series of contractions
and relaxations. The stimulation leads to self-renewal of tissues
and improvements in structure and function. The improvements appear
following applications repeated at intervals of 1 or more days. In
addition, QMR may induce deformation of cell membranes and lead to
a cascade of reactions at the cellular level that are capable of
increasing the normal metabolism. These potential mechanisms can
explain the positive effects achieved by TES in physiotherapy
medicine.
The improvements in objective DE measures (BUT,
Schirmer's I test and corneal fluorescein staining scores) were
related to the improvements in the secretion of tears and the
thickness of the lipid and mucin layers achieved by TES. The
corneal fluorescein staining scores in both groups were markedly
lower after treatment, but BUT and Schirmer's I test exhibited
significant improvements in the TES + SH group. This was likely due
to the increase in both tear secretion and the thickness of the
lipid and mucin layers in the TES + SH group. However, only SH was
administered to the SH group, and the water layer was supplemented
without a simultaneous improvement in the lipid and mucin layers.
With the help of artificial tears, the corneal epithelium healed
significantly within 4 weeks. Therefore, BUT and Schirmer's I test
scores may improve significantly in the SH group over a longer
study period. However, the exact underlying mechanisms require
further investigation.
The basis for selecting the sites of electrode
placement is the anatomical location of the trigeminal nerve and
lacrimal gland. TES can effectively stimulate the trigeminal nerve
to relieve DE symptoms including pain and photophobia, and produce
QMR to stimulate the lacrimal system and reactivate the lacrimal
and meibomian gland tissue. Whether this effect is associated with
the acupuncture points of Chinese medicine is unclear.
The present study yielded promising initial results.
However, there were certain limitations. First, it was only a
randomized controlled trial. Conventional eye drops in clinical
trials can be administered in a double-blinded manner, but this is
not possible with TES. Second, this study used a single waveform
and electrode location; whether these were optimal was not known.
It should be possible to increase the treatment effect through
further optimization of stimulation parameters and dosing interval.
Third, patients in the TES + SH group used TES combined with SH
therapy. We consider that the placebo effect was not significant.
However, as the control group only used SH treatment, a potential
placebo effect may be a limitation. Furthermore, the study was
performed in only one hospital and over a short period. Therefore,
a large-sample multicenter study with a long-term follow-up is
required to confirm the benefits of TES for DE.
In conclusion, TES combined with artificial tears
was found to be more effective in treating DE compared with
artificial tears alone. Therefore, TES may represent a promising
novel treatment option for DE, provided that its benefits are
confirmed and mechanism of action elucidated in prospective studies
using electrical stimulation for DE.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All datasets generated and/or analyzed during the
present study are included in this published article.
Authors' contributions
JZ was responsible for the design of the study and
interpretation of the analysis results; MC undertook data analysis
and drafted the manuscript. Both authors have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted following approval
by the Ethics Committee of the Ninth People's Hospital of
Chongqing. This study was registered with the Chinese Clinical
Trial Registry: ChiCTR1900021036, registered on January 25,
2019.
Patient consent for publication
All the patients consented to the publication of
their data and any associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Craig JP, Nichols KK, Akpek EK, Caffery B,
Dua HS, Joo CK, Liu Z, Nelson JD, Nichols JJ, Tsubota K and
Stapleton F: TFOS DEWS II defnition and classifcation report. Ocul
Surf. 15:276–283. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Merayo-Lloves J, Sanchez-Avila RM, Riestra
AC, Anitua E, Begoña L, Orive G and Fernandez-Vega L: Safety and
efficacy of autologous plasma rich in growth factors eye drops for
the treatment of evaporative dry eye. Ophthalmic Res. 56:68–73.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
García-Conca V, Abad-Collado M,
Hueso-Abancens JR, Mengual-Verdú E, Piñero DP, Aguirre-Balsalobre F
and Molina JC: Efficacy and safety of treatment of hyposecretory
dry eye with platelet-rich plasma. Acta Ophthalmol. 97:e170–e178.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Friedman NJ, Butron K, Robledo N, Loudin
J, Baba SN and Chayet A: A nonrandomized, open-label study to
evaluate the effect of nasal stimulation on tear production in
subjects with dry eye disease. Clin Ophthalmol. 10:795–804.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kossler AL, Wang J, Feuer W and Tse DT:
Neurostimulation of the lacrimal nerve for enhanced tear
production. Ophthal Plast Reconstr Surg. 31:145–151.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rosenthal P and Borsook D: The corneal
pain system Part I: The missing piece of the dry eye puzzle. Ocul
Surf. 10:2–14. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sivanesan E, Levitt RC, Sarantopoulos CD,
Patin D and Galor A: Noninvasive electrical stimulation for the
treatment of chronic ocular pain and photophobia. Neuromodulation.
21:727–734. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gofeld M: New horizons in neuromodulation.
Curr Pain Headache Rep. 18(397)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Goroszeniuk T and Pang D: Peripheral
neuromodulation: A review. Curr Pain Headache Rep.
18(412)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kacker R, Lay A and Das A: Electrical and
mechanical offce-based neuromodulation. Urol Clin North Am.
40:581–589. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Luan S, Williams I, Nikolic K and
Constandinou TG: Neuromodulation: Present and emerging methods.
Front Neuroeng. 7(27)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Park C, Choi JB, Lee YS, Chang HS, Shin
CS, Kim S and Han DW: The effect of intra-operative transcutaneous
electrical nerve stimulation on posterior neck pain following
thyroidectomy. Anaesthesia. 70:434–439. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Santana LS, Gallo RB, Ferreira CH, Duarte
G, Quintana SM and Marcolin AC: Transcutaneous electrical nerve
stimulation (TENS) reduces pain and postpones the need for
pharmacological analgesia during labour: A randomised trial. J
Physiother. 62:29–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Engen DJ, Carns PE, Allen MS, Bauer BA,
Loehrer LL, Cha SS, Chartrand CM, Eggler EJ, Cutshall SM and
Wahner-Roedler DL: Evaluating efficacy and feasibility of
transcutaneous electrical nerve stimulation for postoperative pain
after video-assisted thoracoscopic surgery: A randomized pilot
trial. Complement Ther Clin Pract. 23:141–148. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rakel BA, Zimmerman MB, Geasland K, Embree
J, Clark CR, Noiseux NO, Callaghan JJ, Herr K, Walsh D and Sluka
KA: Transcutaneous electrical nerve stimulation for the control of
pain during rehabilitation after total knee arthroplasty: A
randomized, blinded, placebo-controlled trial. Pain. 155:2599–2611.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Whitacre MM: The effect of transcutaneous
electrical nerve stimulation on ocular pain. Ophthalmic Surg.
22:462–466. 1991.PubMed/NCBI
|
|
17
|
Ghaffariyeh A, Peyman A, Puyan S,
Honarpisheh N, Bagheri B and Peyman M: Evaluation of transcutaneous
electrical simulation to improve recovery from corneal hypoesthesia
after LASIK. Graefes Arch Clin Exp Ophthalmol. 247:1133–1138.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pedrotti E, Bosello F, Fasolo A, Frigo AC,
Marchesoni I, Ruggeri A and Marchini G: Transcutaneous periorbital
electrical stimulation in the treatment of dry eye. Br J
Ophthalmol. 101:814–819. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pall ML: Electromagnetic fields act via
activation of voltage-gated calcium channels to produce beneficial
or adverse effects. J Cell Mol Med. 17:958–965. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Amparo F, Schaumberg DA and Dana R:
Comparison of two questionnaires for dry eye symptom assessment:
The ocular surface disease index and the symptom assessment in dry
eye. Ophthalmology. 122:1498–1503. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lemp MA: Report of the national eye
institute/industry workshop on clinical trials in dry eyes. CLAO J.
21:221–232. 1995.PubMed/NCBI
|
|
22
|
Sook Chun Y and Park IK: Reliability of 4
clinical grading systems for corneal staining. Am J Ophthalmol.
157:1097–1102. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cohn GS, Corbett D, Tenen A, Coroneo M,
McAlister J, Craig JP, Gray T, Kent D, Murray N, Petsoglou C, et
al: Randomized, controlled, double-masked, multicenter, pilot study
evaluating safety and effificacy of intranasal neurostimulation for
dry eye disease. Invest Ophthalmol Vis Sci. 60:147–153.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gumus K and Pflugfelder SC: Intranasal
tear neurostimulation: an emerging concept in the treatment of dry
eye. Int Ophthalmol Clin. 57:101–108. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pattar GR, Jerkins G, Evans DG, Torkildsen
GL, Ousle GW, Hollander DA, Holdbrook M and Senchyna M: Symptom
improvement in dry eye subjects following intranasal tear
neurostimulation: Results of two studies utilizing a controlled
adverse environment. Ocul Surf. 18:249–257. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Farhangi M, Cheng AM, Baksh B,
Sarantopoulos CD, Felix ER, Levitt RC and Galor A: Effect of
non-invasive intranasal neurostimulation on tear volume, dryness
and ocular pain. Br J Ophthalmol. 104:1310–1316. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ozcura F, Aydin S and Helvaci MR: Ocular
surface disease index for the diagnosis of dry eye syndrome. Ocul
Immunol Inflamm. 15:389–393. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Schiffman RM, Christianson MD, Jacobsen G,
Hirsch JD and Reis BL: Reliability and validity of the ocular
surface disease index. Arch Ophthalmol. 118:615–621.
2000.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chiou YF, Yeh ML and Wang YJ:
Transcutaneous electrical nerve stimulation on acupuncture points
improves myofascial pain, moods, and sleep quality. Rehabil Nurs.
45:225–233. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Galor A, Levitt RC, Felix ER, Martin ER
and Sarantopoulos CD: Neuropathic ocular pain: An important yet
underevaluated feature of dry eye. Eye (Lond). 29:301–312.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ainsworth L, Budelier K, Clinesmith M,
Fiedler A, Landstrom R, Leeper BJ, Moeller L, Mutch S, O'Dell K,
Ross J, et al: Transcutaneous electrical nerve stimulation (TENS)
reduces chronic hyperalgesia induced by muscle inflammation. Pain.
120:182–187. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Grover CA, McKernan MP and Close RJH:
Transcutaneous Electrical Nerve Stimulation (TENS) in the emergency
department for painrelief: A preliminary study of feasibility and
efficacy. West J Emerg Med. 19:872–876. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gozani SN: Fixed site high-frequency
transcutaneous electrical nerve stimulation for treatment of
chronic low back and lower extremity pain. J Pain Res. 9:469–479.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhu Y, Feng Y and Peng L: Effect of
transcutaneous electrical nerve stimulation for pain control after
total knee arthroplasty: A systematic review and meta analysis. J
Rehabil Med. 49:700–704. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Garrison DW and Foreman RD: Decreased
activity of spontaneous and noxiously evoked dorsal horn cells
during transcutaneous electrical nerve stimulation (TENS). Pain.
58:309–315. 1994.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dieckmann G, Fregni F and Hamrah P:
Neurostimulation in dry eye disease-past, present, and future. Ocul
Surf. 17:20–27. 2019.PubMed/NCBI View Article : Google Scholar
|