Introduction
Implantation is defined as the organized process
through which the blastocyst attaches to the endometrium and
invades the epithelium to form the placenta. Implantation is
directly dependent on the synchronization of the fertilized egg's
progression into a blastocyst and the specific differentiation of
the endometrium through molecular and cellular changes regulated by
agents with an endocrine, paracrine or autocrine activity (1,2). This
synchronization occurs over a certain period of time, called the
‘window of implantation’, and requires a molecular dialogue of
sorts between the secretory activity of the endometrium and that of
the blastocyst (1-3).
Numerous studies have implicated adhesion molecules, extracellular
matrix proteins, growth factors, extracellular substrate
degradation proteins and pro-inflammatory agents in the
implantation process of the blastocyst into the endometrium
(4). Collectively these data point
towards a complex molecular process, whose underlying mechanisms
have not been fully elucidated.
Progesterone and estrogen are the steroid hormones
responsible for the regulation of the implantation window.
Progesterone induces changes in signalling pathways that lead to
the establishment of a receptive endometrium (5). Estrogen receptors (ER)α and β and
progesterone receptors (PR)-A and -B are expressed in the
epithelium and stroma of the human endometrium. ER mediates most of
the biological effect of estrogens by interacting with its
site-specific DNA and with other coregulatory proteins, while ER
and PR signalling during implantation is carried out through
paracrine and autocrine factors mediated by growth factors, as well
as cytokines (6). Progesterone
exerts its effects by activating the canonical PRs to act in a
genomic fashion to regulate transcriptional responses of
implantation-related genes (5). For
example, progesterone drives an increase in the gene expression of
integrin αvβ3 in epithelial cells (7). Integrins are a family of
transmembrane binding glycoproteins consisting of two protein
subunits (α and β). Integrins function as receptors for
extracellular matrix molecules, glycoproteins and other cells, and
their concentration in adhesion points leads to the creation of a
network of cytoskeletal proteins and intracellular signalling
(8,9). The importance of these adhesion
molecules has been widely studied in mice with blastocysts lacking
the β1 subunit that fail to implant (10,11).
Other adhesion molecules also play a crucial role in
this dialogue underlying the adhesion and attachment of the
blastocyst in the adequately prepared endometrium (12). E-cadherin, for example, is critical
to the creation and maintenance of blastocyst adhesion ligands
(12-14).
Since E-cadherin has been found in the trophoblast and endometrium,
it has been suggested to participate in the initial adhesion and
attachment of the blastocyst during implantation (15).
On the other hand, estrogens exert their effects by
activating primarily the nuclear steroid hormone receptor. ERα
appears to be upregulated during the proliferative phase and
downregulated during the implantation window, an event driven
primarily by progesterone (16). Of
note, elevated levels of ERα during implantation were associated
with a decrease in β3 integrin expression in patients with
polycystic ovarian syndrome and endometriosis (17). It has been suggested that the
disappearance of ERα at the time of implantation may disturb the
expression pattern of proteins that regulate endometrial
receptivity.
Despite the existence of a plethora of studies on
the role of these steroid hormones and their receptors at the
endometrial level, knowledge around their protein expression and
tissue distribution during the implantation window (days 0-5) in
humans remains limited. One of the main reasons for this lack of
knowledge is that participants in such studies are required to
undergo endometrial biopsy, which obviously affects endometrial
receptivity and subsequently the success of in vitro
fertilisation (IVF). Therefore, it is not feasible to carry out a
study in women undergoing IVF, since the procedure will have an
adverse effect on the outcome. The patient population of the
present study consisted of women undergoing ovarian stimulation for
egg donation.
In this study the following question was addressed:
Can morphological and functional markers be used to evaluate the
changes in the endometrium during implantation in women undergoing
IVF?
In this study we investigated whether morphological
and functional markers (i.e. ER and PR) can be used to evaluate the
changes in the endometrium during implantation in women undergoing
IVF.
Materials and methods
Study population and design
The study was conducted at the 1st Dept. of OB-GYN,
Centre for Human Reproduction of the Aristotle University of
Thessaloniki, ‘Papageorgiou’ General Hospital and the ‘Biogenesis’
Assisted Reproduction Centre, (both in Thessaloniki, Greece). The
participants recruited for this prospective study included 15
oocyte donors (age range, 25-32 years; mean age, 28.9±2.89)
undergoing IVF treatment. The inclusion criteria were white race,
no uterine-ovarian pathology, age <35 years and no prior known
medical pathology. All donors had undergone extensive preoperative
work-up, which included common blood tests, karyotyping, specific
test for cystic fibrosis and pap smear. All donors were non-smokers
and had given their informed consent (NP: Α 13032 15/7/10).
Informed consent was written, and patients agreed to the use of
their samples in scientific research.
Participants underwent ovarian stimulation with
gonadotrophin-releasing hormone antagonist and recombinant
follicle-stimulating hormone (18-20).
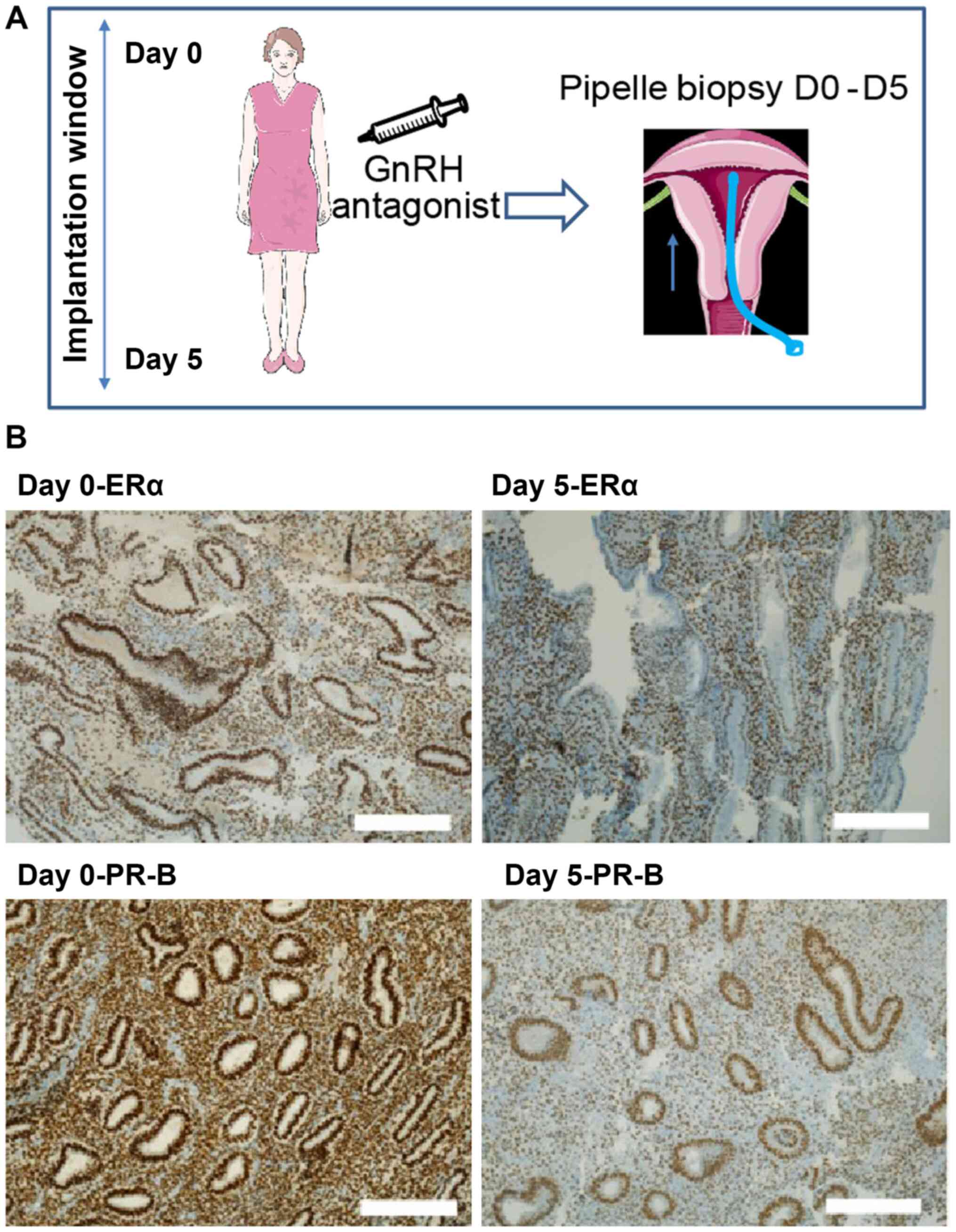
Endometrial aspiration biopsy was performed using a Z-Sampler
(Gynétics) on the day of oocyte retrieval and 5 days later
(Fig. 1A).
Immunohistochemistry
Endometrial histology was evaluated blindly, using
the Noyes criteria by a single specialized pathologist (20). The tissue was fixed in neutral
buffered formalin 10% and followed the usual technical procedure
for histological samples and embedding in paraffin blocks. Sections
(3 µm thickness) were sliced from the blocks placed on slides and
then stained with hematoxylin and eosin for the evaluation of
histologic characteristic. Immunohistochemistry was used to
evaluate expression of ERα and PR-B, using monoclonal antibodies
(ER: Clone 4f11, PR:clone 16+SAN27; Leica). Immunohistochemistry
was performed on Ventana Benchmark XT automatic immunostainer
(Ventana Medical Systems Inc.) using OptiView DAB IHC detection kit
(Roche) as a detection method. As part of the automated service
positive and negative controls (immunoglobulin G control) were
tested simultaneously with the test slides (Fig. S1). The percentage of epithelial
cell nuclei positive for ERα and PR-B receptors was recorded on
days 0 and 5. Slide photos were captured using a x10-magnification
lens on a Leica DMi1 Inverted Microscope (Leica Microsystems,
Inc.), with all scale bars set to 0.25 mm.
Bioinformatic analysis
GeneMANIA (http://genemania.org/), a user-friendly web interface
that provides large datasets to perform analyses for gene and
protein interactions and prioritise genes for functional assays.
Using this software, the relationship between ER and PR with 43
previously identified genes (21)
involved with unexplained infertility (UIF) and recurrent
implantation failure (RIF) were used to generate PPI networks using
GeneMANIA.
Statistical analysis
Statistical analysis was performed using the SPSS
version 23 (IBM Corp.). The data were non-parametric (Shapiro-Wilk
normality test P<0.005), and as such, the Wilcoxon signed-rank,
Mann-Whitney U and Fisher's exact tests were used for analysis. A
two-tailed P<0.05 was considered to indicate a statistically
significant difference. Sample stratification (for ER staining
only) was performed for ages (±30 years old) and to determine
whether there was differential gene expression between the two days
(yes, no).
Results
Differential expression of steroid
hormone receptors during the implantation window
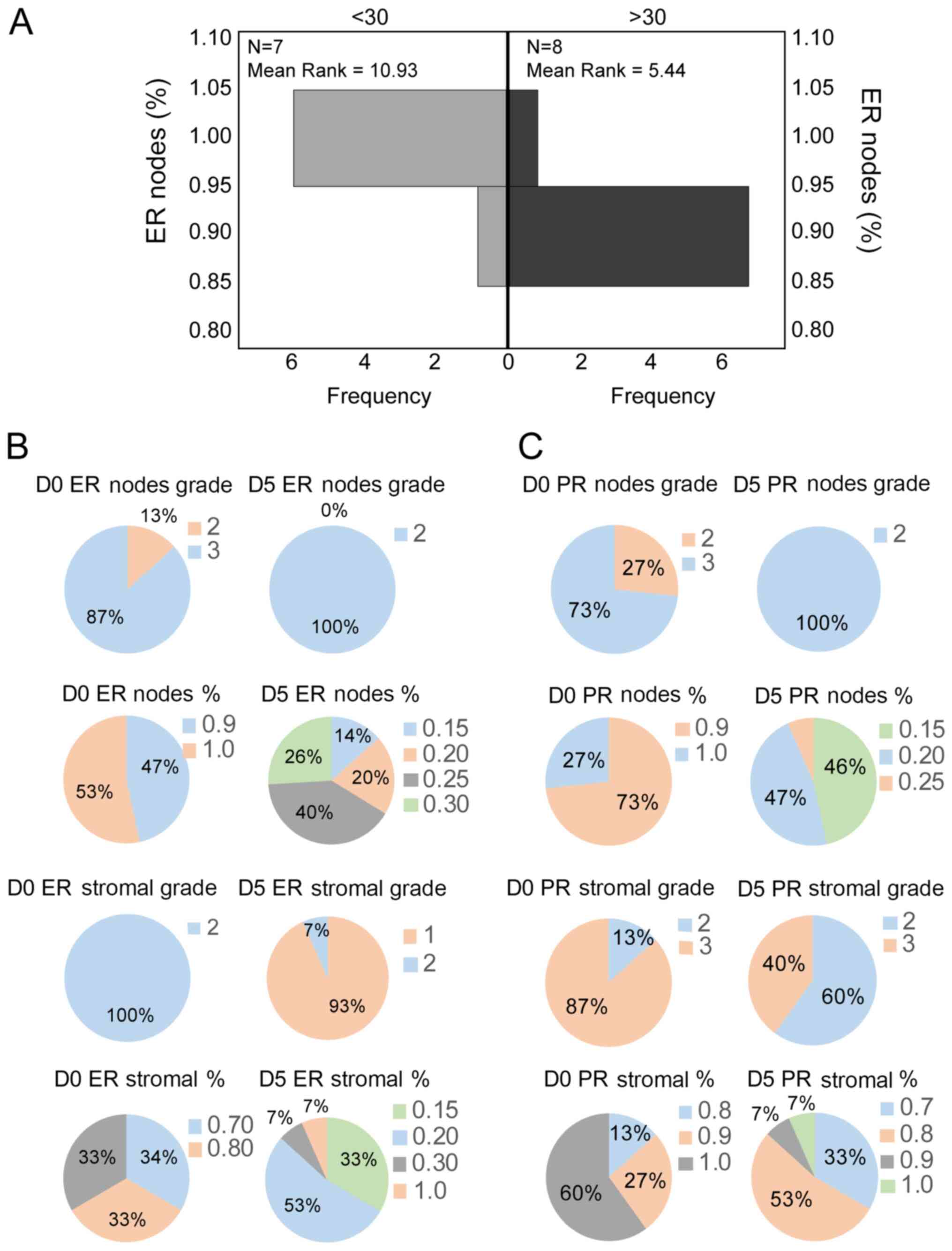
Both ERα and PR-B were expressed abundantly on both
days (0 and 5; Fig. 1B). The ERα
nodal staining percentage on day 0 was age-related, with patients
aged <30 years showing 100% staining and those aged >30 years
showing 90% staining (Mann-Whitney U test; P=0.014; Fig. 2A).
Both steroid hormone receptors showed significant
variation between days 0 and 5, both in the nodal and stromal
preparations. According to Wilcoxon signed-rank test; for ER (nodes
% and stromal %) Day 0/5, P=0.0001; for PR (nodes % and stromal %)
Day 0/5, P=0.0001 and P=0.035, respectively; for ER (Grade nodes
and stromal) Day 0/5, P=0.0001; and for PR (Grade nodes and
stromal) Day 0/5, P=0.0001 and P=0.016, respectively (Fig. 2B and C; Table
I).
 | Table IDescriptive statistics of
immunohistochemical analysis. |
Table I
Descriptive statistics of
immunohistochemical analysis.
| Parameter | N | Minimum | Maximum | Mean | SDEV |
|---|
| Age (years) | 15 | 25 | 32 | 28.93 | 2.89 |
| A, Day 0 | | | | | |
|
ER nodal
grade | 15 | 2.00 | 3.00 | 2.87 | 0.35 |
|
ER stromal
grade | 15 | 2.00 | 2.00 | 2.00 | 0.00 |
|
ER nodal
% | 15 | 0.90 | 1.00 | 0.95 | 0.05 |
|
ER stromal
% | 15 | 0.70 | 0.90 | 0.80 | 0.08 |
|
PR nodal
grade | 15 | 2.00 | 3.00 | 2.73 | 0.05 |
|
PR stromal
grade | 15 | 2.00 | 3.00 | 2.87 | 0.35 |
|
PR nodal
% | 15 | 0.90 | 1.00 | 0.93 | 0.05 |
|
PR stromal
% | 15 | 0.80 | 1.00 | 0.95 | 0.07 |
| B, Day 5 | | | | | |
|
ER nodal
grade | 15 | 1.00 | 1.00 | 1.00 | 0.00 |
|
ER stromal
grade | 15 | 1.00 | 2.00 | 1.07 | 0.26 |
|
ER nodal
% | 15 | 0.15 | 0.30 | 0.24 | 0.05 |
|
ER stromal
% | 15 | 0.15 | 1.00 | 0.24 | 0.21 |
|
PR nodal
grade | 15 | 1.00 | 1.00 | 1.00 | 0.00 |
|
PR stromal
grade | 15 | 2.00 | 3.00 | 2.40 | 0.51 |
|
PR nodal
% | 15 | 0.15 | 0.25 | 0.18 | 0.03 |
|
PR stromal
% | 15 | 0.70 | 1.00 | 0.89 | 0.06 |
Involvement of ER and PR-B in
endometrial gene networks: An in-silico analysis
In a recent meritorious study, microarray datasets
collected during the time of human uterine receptivity and
implantation were compared to the transcriptome signature of women
with unexplained infertility (UIF) and recurrent implantation
failure (RIF) (21). In that study,
the authors have identified 24 and 21 shared differentially
expressed genes (DEGs) between the transcriptome of women with UIF
and RIF and those of normal endometrial receptivity samples
(Table II) (21).
 | Table IIFull names of genes studied in
silico including genes that have been identified from the
previous referenced study (21). |
Table II
Full names of genes studied in
silico including genes that have been identified from the
previous referenced study (21).
| Official gene
symbol | Name |
|---|
| POSTN | Periostin,
osteoblast specific factor |
| RBP4 | Retinol binding
protein 4, plasma |
| MMP26 | Matrix
metallopeptidase 26 |
| WT1 | Wilms tumor 1 |
| LRRC17 | Leucine rich repeat
containing 17 |
| OSR2 | Odd-skipped related
transcription factor 2 |
| HABP2 | Hyaluronan binding
protein 2 |
| HSD17B2 | Hydroxysteroid
(17-β) dehydrogenase 2 |
| PTGER3 | Prostaglandin E
receptor 3 |
| SOD3 | Superoxide
dismutase 3, extracellular |
| PAEP |
Progestogen-associated endometrial
protein |
| THBS4 | Thrombospondin
4 |
| EFNB2 | Ephrin-B2 |
| TRHR |
Thyrotropin-releasing hormone
receptor |
| ECEL1 | Endothelin
converting enzyme-like 1 |
| GLI1 | GLI family zinc
finger 1 |
| MMP7 | Matrix
metallopeptidase 7 (matrilysin, uterine) |
| COL9A1 | Collagen type IX, α
1 |
| SERPINA1 | Serpin peptidase
inhibitor, clade A, member 1 |
| CTNNA2 | Catenin
(cadherin-associated protein), α 2 |
| KLK3 | Kallikrein-related
peptidase 3 |
| JDP2 | Jun dimerization
protein 2 |
| IGFBP1 | Insulin-like growth
factor binding protein 1 |
| KLF9 | Kruppel-like factor
9 |
| C20orf103 | Chromosome 20 open
reading frame 103 |
| NID2 | Nidogen 2
(osteonidogen) |
| CAPN6 | Calpain 6 |
| OVGP1 | Oviductal
glycoprotein 1, 120kDa |
| NID1 | Nidogen 1 |
| SLC9A3 | Solute carrier
family 9 (sodium/hydrogen exchanger), member 3 |
| PGR | Progesterone
receptor |
| SF1 | Splicing factor
1 |
| CLU | Clusterin |
| COL4A6 | Collage type IV α 6
chain |
| RRM2B | Ribonucleotide
reductase regulatory TP53 inducible subunit M2B |
| TRH |
Thyrotropin-releasing hormone |
| NFIA | Nuclear factor
I/A |
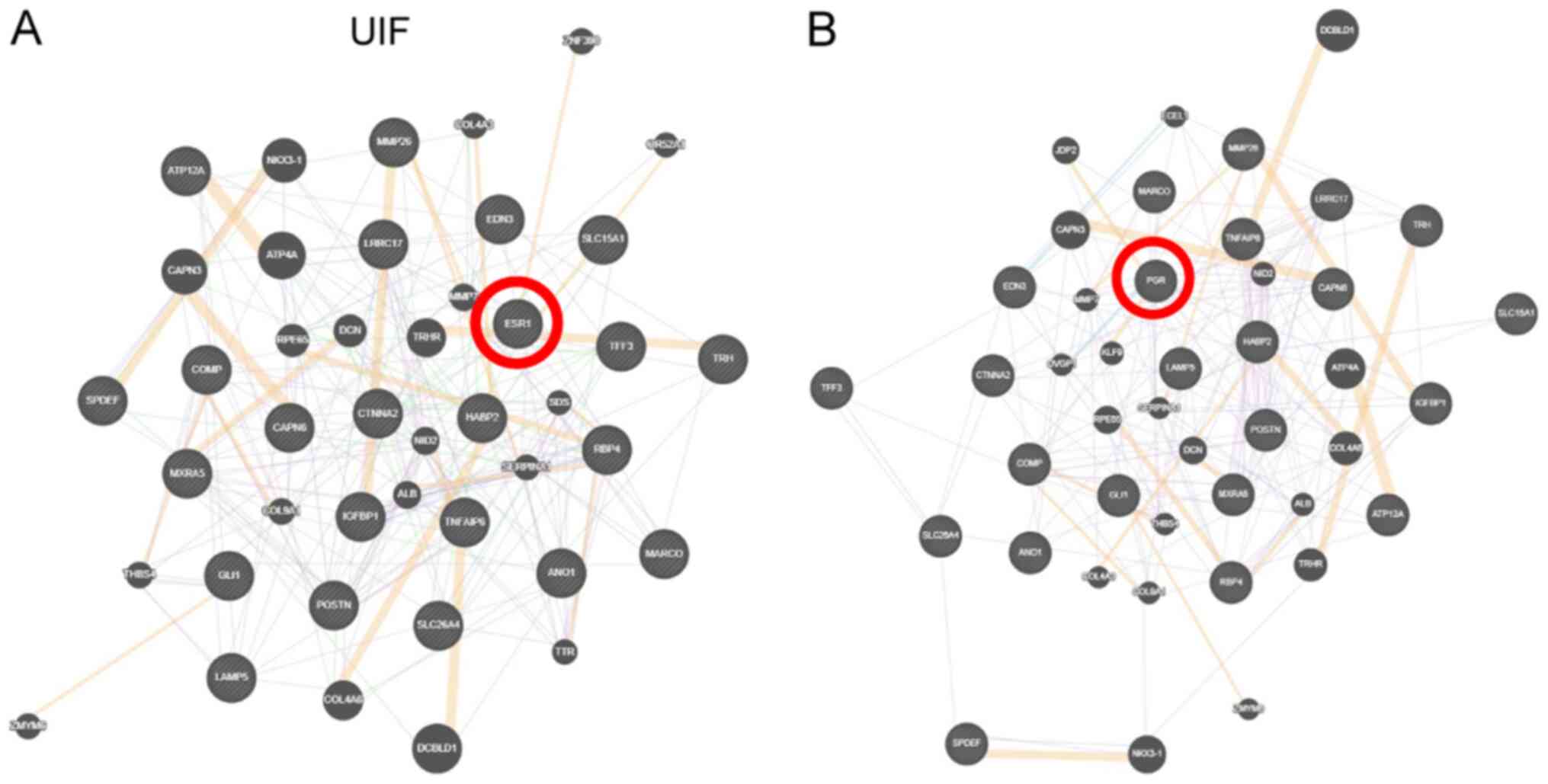
In view of the differential expression of ER and
PR-B during the implantation window, their potential involvement
was investigated further by studying potential interactions between
the common genes for UIF and RIF using GeneMANIA software. The
previously identified UIF genes (21) that potentially interact with ER are
as follows: Thyrotropin releasing hormone (TRH), TRH receptor
(TRHR), GLI family zinc finger 1, matrix metallopeptidase 26
(MMP26), retinol binding protein 4, serpin family A member 1, MMP7,
catenin α 2, hyaluronan binding protein 2, collagen type IX α 1
(Fig. 3A). PR-B appears to interact
with the following genes: Chromosome 20 open reading frame 103,
calpain 6, thrombospondin 4, leucine rich repeat containing 17,
periostin, collagen type IV α 6 chain, TRH, TRHR, nidogen 2, MMP7,
MMP26, oviductal glycoprotein 1 (OVGP1), Jun dimerization protein
2, endothelin converting enzyme like 1, insulin like growth factor
binding protein 1 (IGFBP1), Kruppel-like factor 9 (Fig. 3B).
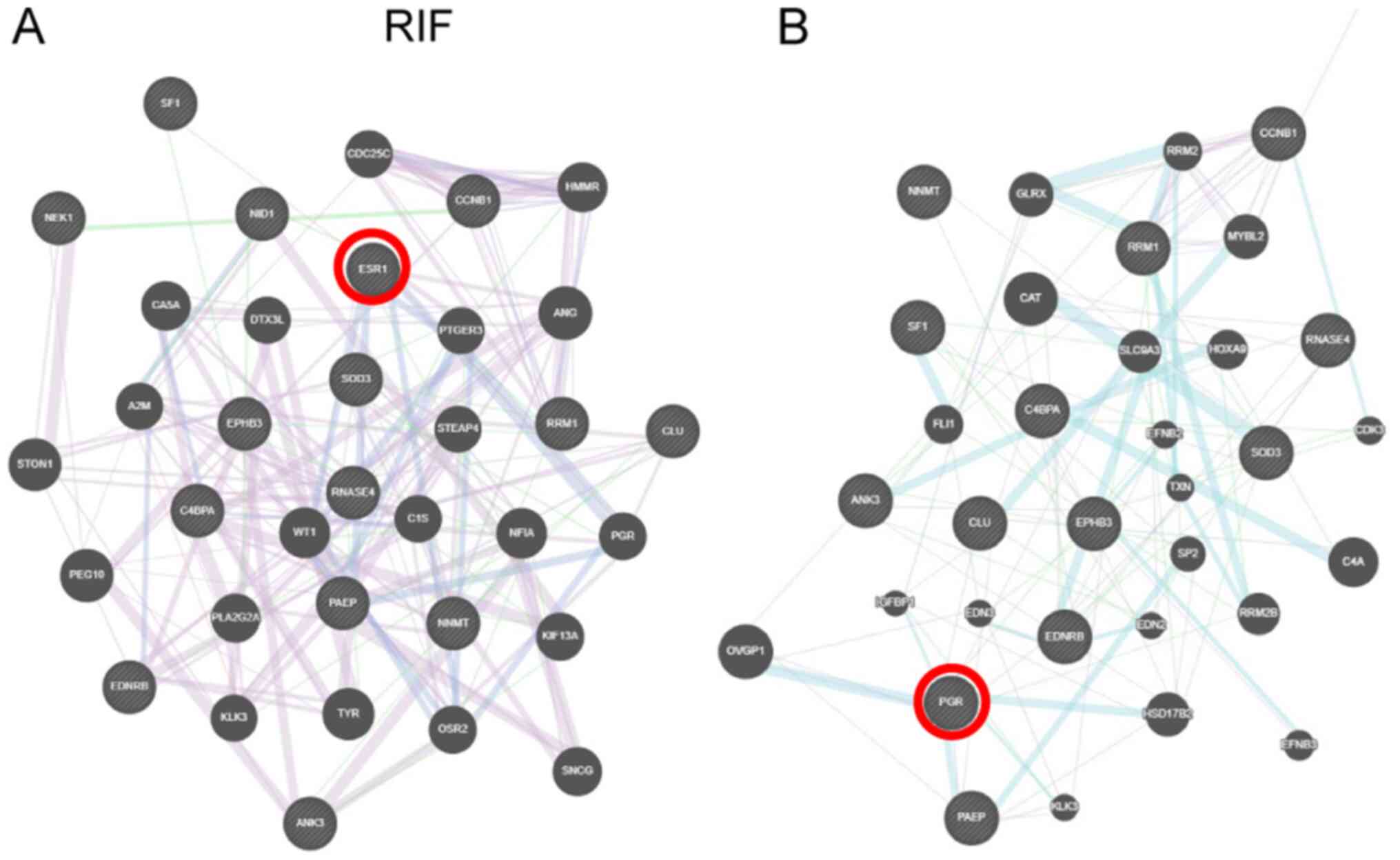
In the case of previously identified RIF overlapping
genes (21), potential interactions
with ER include: Prostaglandin E receptor 3, Wilms tumor 1 (WT1),
progestogen-associated endometrial protein (PAEP), odd-skipped
related transcription factor 2, progesterone receptor, nidogen 1,
nuclear factor I/A, splicing factor 1 (SF1; Fig. 4A). PR-B interacts with the following
genes: Clusterin, solute carrier family 9 member A3, OVGP1, PAEP,
superoxide dismutase 3, extracellular, ribonucleotide reductase
regulatory TP53 inducible subunit M2B, ephrin B2, hydroxysteroid
17-β dehydrogenase 2 (HSD17B2), kallikrein related peptidase 3, and
IGFBP1 (Fig. 4B).
Discussion
Successful implantation following IVF is a complex
procedure that is very much dependent on the fertilized egg's
progression into a blastocyst, synchronized with the
differentiation of the endometrium (1,2). This
‘implantation window’ initiates a molecular dialogue of sorts that
has not yet been fully clarified (1-4).
The aim of the present study was to elucidate a
small part of this dialogue. These results, in combination with the
number of ERs and PRs, play an important role in the success of
IVF, since their expression causes a series of paracrine and
autocrine signals, which through adhesion molecule processes
ultimately lead to the successful adhesion and penetration of the
endometrium by blastocysts (22).
As far as permeability is concerned, the effect of ovarian steroids
on the uterus is achieved through their receptors (23). Immunohistochemistry results for ER
receptors showed a significant reduction in D5, as compared to D0,
while an equally significant increase in the PR receptors was
observed on the same days. Nodal ER and PR receptors with a strong
presence on day 0 showed a very limited presence on day 5, while
only PR receptors were strongly represented in the stroma.
Estrogens in the follicular phase prepare the endometrium for the
action of progesterone in the subsequent secretory phase of the
cycle (24). Having stratified our
samples into two age-groups (<30 and >30 years), it was found
that the ER receptor nodal staining percentage on day 0 was
age-related, with patients aged <30 years showing 100% staining
and those aged >30 years showing 90% staining. This was
consistent with potential age-related complications associated with
maternity and successful pregnancy. Progesterone is another
determining factor in the creation of the implantation window and
the maintenance of pregnancy. The stromal cells differentiate into
progesterone-responsive peristaltic cells during the perforation
process, which is characterized by morphological changes (25). In the present study, changes in the
expression of PR-B were observed between days 0 and 5. This was
consistent with a study showing that stimulation of the endometrium
with ganirelix acetate (a GnRH antagonist) and gonadotropins led to
the increase of PR-B gene expression at the time of embryo transfer
(26). Of note, in the same study
it was only the expression of PR-B that changed, while PR-A, the
other splice isoform of the PR, was undetectable. It should be
noted that, in addition to the two well studied variants of PR-A
and -B, other splicing isoforms have also been detected including
PR-C and PR-M (27). Moreover, it
is now well accepted that both steroids can activate membrane-bound
receptors acting in a non-genomic manner (28,29).
Future studies should also concentrate on elucidating the
expression of these receptors during the implantation window.
Finally, evidence of a potential crosstalk between these receptors
and genes implicated in RIF and UIF during the implantation window
was provided herein, using data previously generated (21). This is of interest, given that these
genes are involved in key physiological processes, such as the
regulation of the protein activation cascade, complement
activation, humoral immune response, acute inflammatory response or
protein processing in the case of the gene cluster, which is common
in RIF. One of the most interesting interactions of both ER and PR
is with TRH and TRHR in UIF. Previous studies have corroborated
these in silico findings in other in vitro or in
vivo systems. For example, 17β-estradiol (E2) was found to
modulate the prolactin secretion induced by TRH in a female
anterior pituitary primary cell culture. This response was mediated
by a membrane-ER, but this finding provided an insight into a
potential crosstalk (30).
Similarly, E2 appears to inhibit TRH expression in the hypothalamic
paraventricular nucleus of female rats (31). Similarly, TRHR immunoreactivity in
the myometrium of cynomolgus macaques was increased when they were
treated with conjugated equine estrogens alone or in combination
with medroxyprogesterone acetate (32). Another common pathway that estrogen
and progesterone appear to modulate is that of MMP7. Again,
previous studies have corroborated this interaction. There has been
an association between cellular and molecular responses in the rat
mammary gland and E2, including MMP7 and MMP9(33). When female sheep pups were treated
with P4, it led to a reduced expression of MMP7. Moreover, P4
inhibited uterine gland development in the uterus of a neonatal
mouse; a process that involved the downregulation of MMP7(34). Endometriotic cells have been found
to contain the full complement of steroidogenic genes such as SF1
and WT-1, which can influence the transcription of steroidogenic
genes necessary for E2 synthesis in endometriosis (35). A similar interplay between
ER-SF1-WT1 was produced as interactions under RIF conditions. On
the other hand, P4 inhibited the stimulatory effect of E2 on the
expression of oviductin in the cervix of rhesus macaques (36). In addition, HSD17B2 expression in
endometrial epithelial cells was found to be regulated by
downstream molecules of progesterone (37).
Finally, we have shown using qRT-PCR that patients
that were E-Cadherin-positive between days 0 and 5 were also all PR
Grade 3. The small sample of this study clearly limits its
scientific value, particularly with regards to very low gene
expression, and lack of any statistical strength. We appreciate
that this is a limitation of the current study. However, it should
be noted that endometrial sampling at Day 5 is considered an
invasive procedure and a cause of some discomfort to the
individual, so it is very hard to recruit large numbers. Despite
close follow-ups of the oocyte recipients, matching the
morphological-structural changes of the donor endometria and the
IVF result was not feasible, due to the anonymous nature of the
oocyte donation procedure. It should be noted that the present the
present study is limited to young women (due to better quality of
oocytes and lower incidence of trisomy 21) and future studies
should aim to test whether the present findings would hold true in
other age ranges, such as women >35 years old.
Another limitation of the present study is that
immunohistochemistry is less quantitative than western blotting.
Performing this analysis using a housekeeping protein as a loading
control, such as GAPDH or β-actin, would have been useful.
Alternatively, ELISA or a gene expression assessment of both
receptors using RT-qPCR could have been conducted. However, due to
ethical restrictions, sufficient tissue for protein extraction
could not be obtained in order to pursue this further.
As mentioned, synchronization between blastocyst
development and the acquisition of endometrial receptivity is a
prerequisite for the success of IVF, a process that appears to be
dependent on a number of different events at the hormonal and
cellular levels. In this study, novel evidence of differential
expression and potential involvement of two key steroid hormone
receptors in this process was provided; namely, in women undergoing
treatment during an oocyte donation program. The present results,
combined with those of in silico analyses, suggested that
the changes in ER and PR expression and cellular distribution are
crucial events that can impact implantation. This should be
investigated further in studies with a larger population, which
should further validate the original findings using alternative,
more quantitative approaches, and further explore changes between
D0-5 in a ‘non-biased’ way through RNAseq or proteomic analysis
instead. Expanding our knowledge on this field beyond the two
steroid hormone receptors described herein, would augment our
understanding of the signalling mechanisms implicated in
infertility, and potentially provide novel therapeutic targets. It
would have been useful to investigate the downstream signalling as
well. However, for this to happen we would need to generate primary
cell cultures from the biopsies. This was impossible due to the
small size of the study. There are no commercially available cell
lines to mimic this pharmacological milieu. We mention this under
limitations in our discussion. It is for the very reason that we
embarked on bioinformatic analysis to we provide novel evidence for
a potential crosstalk of these receptors during the implantation
window with genes that are implicated in RIF and UIF.
In conclusion, successful implantation implies
synchronization between a blastocyst and the endometrium, which
undergoes structural and functional remodelling. It was shown
herein that both ER-a and PR-B were expressed abundantly on days 0
and 5, showing significant variation in the nodal and stromal
preparations. Age appeared to be a critical factor, since ER-a
nodal staining showed higher values in the age group of oocyte
donors <30 years old. Therefore, focusing on the functional
characteristics of the endometrium will provide a better insight
into successful embryo implantation, thus improving IVF
results.
Supplementary Material
Immunohistochemistry controls. (A) NST
exhibiting strong positive staining for estrogen receptors. (B)
Invasive micropapillary breast carcinoma stained negative for
estrogenreceptors. (C) NST exhibiting strong positive staining for
progesterone receptors. (D) Invasive micropapillary breast
carcinoma staining negative for progesterone receptors.
Magnification, x200. NST, Invasive breast carcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EKa, EP, BT and GP conceived the current study. EKl,
PK and EKa performed the experiments. EKl, PK, Eka and GP performed
in silico, bioinformatics and statistical analyses. EKa, EP,
BT and GP performed the investigation provided funding and
materials. EKl and PK integrated the data and created figures. EKl,
PK, Eka and GP wrote the original draft. writing review and
editing, EKl, EKa, EP, BT and GP reviewed and edited the
manuscript. EKl and GP supervised the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Committee for
Medical Ethics and Deontology, School of Medicine, Aristotle
University of Thessaloniki (approval no. Α 13032 15/7/10). Written
informed consent was obtained from patients prior to enrolment.
Patient consent for publication
Consent for publication was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krüssel JS, Bielfeld P, Polan ML and Simón
C: Regulation of embryonic implantation. Eur J Obstet Gynecol
Reprod Biol. 110 (Suppl 1):S2–S9. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Teh WT, McBain J and Rogers P: What is the
contribution of embryo-endometrial asynchrony to implantation
failure? J Assist Reprod Genet. 33:1419–1430. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sharma A and Kumar P: Understanding
implantation window, a crucial phenomenon. J Hum Reprod Sci. 5:2–6.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fox C and Lessey BA: Signaling between
embryo and endometrium: Normal implantation. In: Recurrent
Implantation Failure: Etiologies and Clinical Management, pp1-19,
2018.
|
|
5
|
Young SL: Oestrogen and progesterone
action on endometrium: A translational approach to understanding
endometrial receptivity. Reprod Biomed Online. 27:497–505.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kumar R, Zakharov MN, Khan SH, Miki R,
Jang H, Toraldo G, Singh R, Bhasin S and Jasuja R: The dynamic
structure of the estrogen receptor. J Amino Acids.
2011(812540)2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sharkey AM and Smith SK: The endometrium
as a cause of implantation failure. Best Pract Res Clin Obstet
Gynaecol. 17:289–307. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schwartz MA: Integrins and extracellular
matrix in mechanotransduction. Cold Spring Harb Perspect Biol.
2(a005066)2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Arnaout MA, Goodman SL and Xiong JP:
Structure and mechanics of integrin-based cell adhesion. Curr Opin
Cell Biol. 19:495–507. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang J and Armant DR: Integrin-mediated
adhesion and signaling during blastocyst implantation. Cells
Tissues Organs. 172:190–201. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Basak S, Dhar R and Das C: Steroids
modulate the expression of alpha4 integrin in mouse blastocysts and
uterus during implantation. Biol Reprod. 66:1784–1789.
2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stemmler MP: Cadherins in development and
cancer. Mol Biosyst. 4:835–850. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Gumbiner BM: Cell adhesion: the molecular
basis of tissue architecture and morphogenesis. Cell. 84:345–357.
1996.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Poncelet C, Leblanc M, Walker-Combrouze F,
Soriano D, Feldmann G, Madelenat P, Scoazec JY and Daraï E:
Expression of cadherins and CD44 isoforms in human endometrium and
peritoneal endometriosis. Acta Obstet Gynecol Scand. 81:195–203.
2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jha RK, Titus S, Saxena D, Kumar PG and
Laloraya M: Profiling of E-cadherin, beta-catenin and Ca(2+) in
embryo-uterine interactions at implantation. FEBS Lett.
580:5653–5660. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lessey BA, Killam AP, Metzger DA, Haney
AF, Greene GL and McCarty KS Jr: Immunohistochemical analysis of
human uterine estrogen and progesterone receptors throughout the
menstrual cycle. J Clin Endocrinol Metab. 67:334–340.
1988.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gregory CW, Wilson EM, Apparao KB,
Lininger RA, Meyer WR, Kowalik A, Fritz MA and Lessey BA: Steroid
receptor coactivator expression throughout the menstrual cycle in
normal and abnormal endometrium. J Clin Endocrinol Metab.
87:2960–2966. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Papanikolaou EG, Bourgain C, Kolibianakis
E, Tournaye H and Devroey P: Steroid receptor expression in late
follicular phase endometrium in GnRH antagonist IVF cycles is
already altered, indicating initiation of early luteal phase
transformation in the absence of secretory changes. Hum Reprod.
20:1541–1547. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Papanikolaou EG, D'haeseleer E, Verheyen
G, Van de Velde H, Camus M, Van Steirteghem A, Devroey P and
Tournaye H: Live birth rate is significantly higher after
blastocyst transfer than after cleavage-stage embryo transfer when
at least four embryos are available on day 3 of embryo culture. A
randomized prospective study. Hum Reprod. 20:3198–3203.
2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Noyes RW, Hertig AT and Rock J: Dating the
endometrial biopsy. Am J Obstet Gynecol. 122:262–263.
1975.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Herington JL, Guo Y, Reese J and Paria BC:
Gene profiling the window of implantation: Microarray analyses from
human and rodent models. J Reprod Heal Med. 2 (Suppl 2):S19–S25.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fox C, Morin S, Jeong JW, Scott RT Jr and
Lessey BA: Local and systemic factors and implantation: What is the
evidence? Fertil Steril. 105:873–884. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Carpenter KD and Korach KS: Potential
biological functions emerging from the different estrogen
receptors. Ann N Y Acad Sci. 1092:361–373. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kodaman PH and Taylor HS: Hormonal
regulation of implantation. Obstet Gynecol Clin North Am.
31:745–66, ix. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dunn CL, Kelly RW and Critchley HO:
Decidualization of the human endometrial stromal cell: An enigmatic
transformation. Reprod Biomed Online. 7:151–161. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Detti L, Saed GM, Fletcher NM, Kruger ML,
Brossoit M and Diamond MP: Endometrial morphology and modulation of
hormone receptors during ovarian stimulation for assisted
reproductive technology cycles. Fertil Steril. 95:1037–1041.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zachariades E, Foster H, Goumenou A,
Thomas P, Rand-Weaver M and Karteris E: Expression of membrane and
nuclear progesterone receptors in two human placental
choriocarcinoma cell lines (JEG-3 and BeWo): Effects of
syncytialization. Int J Mol Med. 27:767–774. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Foster H, Reynolds A, Stenbeck G, Dong J,
Thomas P and Karteris E: Internalisation of membrane progesterone
receptor-alpha after treatment with progesterone: Potential
involvement of a clathrin-dependent pathway. Mol Med Rep. 3:27–35.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Karteris E, Zervou S, Pang Y, Dong J,
Hillhouse EW, Randeva HS and Thomas P: Progesterone signaling in
human myometrium through two novel membrane G protein-coupled
receptors: Potential role in functional progesterone withdrawal at
term. Mol Endocrinol. 20:1519–1534. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sosa LD, Gutiérrez S, Petiti JP, Palmeri
CM, Mascanfroni ID, Soaje M, De Paul AL and Torres AI:
17β-Estradiol modulates the prolactin secretion induced by TRH
through membrane estrogen receptors via PI3K/Akt in female rat
anterior pituitary cell culture. Am J Physiol Endocrinol Metab.
302:E1189–E1197. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Uribe RM, Zacarias M, Corkidi G, Cisneros
M, Charli JL and Joseph-Bravo P: 17β-Oestradiol indirectly inhibits
thyrotrophin-releasing hormone expression in the hypothalamic
paraventricular nucleus of female rats and blunts thyroid axis
response to cold exposure. J Neuroendocrinol. 21:439–448.
2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hulchiy M, Zhang H, Cline JM, Hirschberg
AL and Sahlin L: Receptors for thyrotropin-releasing hormone,
thyroid-stimulating hormone, and thyroid hormones in the macaque
uterus: Effects of long-term sex hormone treatment. Menopause.
19:1253–1259. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ding L, Zhao Y, Warren CL, Sullivan R,
Eliceiri KW and Shull JD: Association of cellular and molecular
responses in the rat mammary gland to 17β-estradiol with
susceptibility to mammary cancer. BMC Cancer.
13(573)2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Filant J, Zhou H and Spencer T:
Progesterone inhibits uterine gland development in the neonatal
mouse uterus. Biol Reprod. 86: 146:1–9. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Attar E, Tokunaga H, Imir G, Yilmaz MB,
Redwine D, Putman M, Gurates B, Attar R, Yaegashi N, Hales DB and
Bulun SE: Prostaglandin E2 via steroidogenic factor-1 coordinately
regulates transcription of steroidogenic genes necessary for
estrogen synthesis in endometriosis. J Clin Endocrinol Metab.
94:623–631. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Slayden OD, Friason FKE, Bond KR and
Mishler EC: Hormonal regulation of oviductal glycoprotein 1 (OVGP1;
MUC9) in the rhesus macaque cervix. J Med Primatol. 47:362–370.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cheng YH, Imir A, Suzuki T, Fenkci V,
Yilmaz B, Sasano H and Bulun SE: SP1 and SP3 mediate
progesterone-dependent induction of the 17beta hydroxysteroid
dehydrogenase type 2 gene in human endometrium. Biol Reprod.
75:605–614. 2006.PubMed/NCBI View Article : Google Scholar
|