Introduction
Hydroxyurea (HU) is a urea hydroxylated derivate
used for nearly 60 years as a chemotherapeutic agent, effective in
the treatment of certain neoplasias: Hematological (chronic myeloid
leukemia resistant to treatment), inoperable brain, kidney, breast,
cervical and cutaneous cancers (squamous cell carcinoma, malignant
melanoma). Besides, HU can also be used in the treatment of
non-neoplastic diseases (sickle cell anemia, psoriasis, HIV
infection) (1).
In terms of pharmacokinetics, HU is intestinally
absorbed, metabolized by the liver and urinary excreted (1). After absorption, it is transformed
into a nitroxide free radical and transported by diffusion to the
cells presenting the active site of the M2 protein subunit of the
ribonucleotide reductase, which is inactivated. DNA synthesis is
selectively inactivated by apoptosis in the S phase of the cellular
cycle. HU inhibits DNA repair, having synergistic action with
ultraviolet radiation or with alkylating agents. HU also shows
radiosensitization activity by maintaining the cells in
radiation-sensitive G1 phase and interfering with DNA repair
(2).
The anti-neoplastic mechanism of action is not fully
elucidated. Some studies indicate that HU interferes with DNA
synthesis without interfering with RNA or protein synthesis.
Although it has numerous sites of action, it most likely inhibits
the incorporation of thymidine into DNA by its direct action and
also the free tyrosyl radical residue (the catalytic center of
ribonucleotide diphosphate reductase, the enzyme which catalyzes
the transformation of ribonucleotides into deoxyribonucleotides).
HU is an inhibitor of the S phase of the cellular cycle,
determining cell sequestration in G1-S phase, decreasing cell
progression rate in S phase, and/or determining accumulation of
cells in S phase as a result of DNA synthesis inhibition (2).
Studies conducted on animals have shown that the
cytotoxic effects of HU are limited to the tissues with high
cellular proliferation rates and the effects are noticeable in
cells with active synthesis of DNA (3). Thus, the teratogenic effects of the
chemotherapeutic agent are explained.
HU also presents a number of systemic side effects,
of which the most important are hematological (pancytopenia or
cytopenia on certain hematological lines), gastrointestinal
(dyspeptic syndromes), neurological (headache, vertigo,
hallucinations, seizures), renal (hyperuricemia, renal
lithiasis).
Skin reactions occur in 10-30% of patients
chronically treated with HU. The most common are pruritus, facial
and acral erythema and xeroderma, skin hyperpigmentation, leg
ulcers and stomatitis. Exacerbation of post-radiotherapy erythema
(in case of associated radiotherapy), affection of the nails
(melanonychia, onycholysis, blue lunula), skin atrophy and alopecia
may also occur.
The increasing number of new therapies used to
stabilize oncological diseases determines the increment of the
common mucocutaneous side effects in dermatological practice. The
greatest use of HU in the hospital is in hemato-oncology clinic.
Most frequently we have controllable side effects, only with
compensatory dermatological therapy. Rarely, in selected cases, the
severity of the skin manifestations requires the discontinuation of
HU therapy. We present two cases with severe skin adverse events
(leg, submammary and palmoplantar ulcers) in patients undergoing
chronic HU treatment for chronic granulocytic leukemia (CGL),
respectively primary thrombocythemia, which required the
discontinuation of HU therapy and the modification of oncological
therapy.
Case reports
Case 1
A 63-year-old female patient with type 2 diabetes,
chronic venous insufficiency (venous ulcer epithelized in 2016),
severe psoriasis vulgaris since 2001 with many episodes of
erythroderma, treated with Methotrexate then with Acitretin, was
diagnosed with CGL (2012) for which HU treatment was followed for 5
years. For CGL, the patient initially followed a treatment with
Imatinib which was not tolerated due to severe thrombocytopenia.
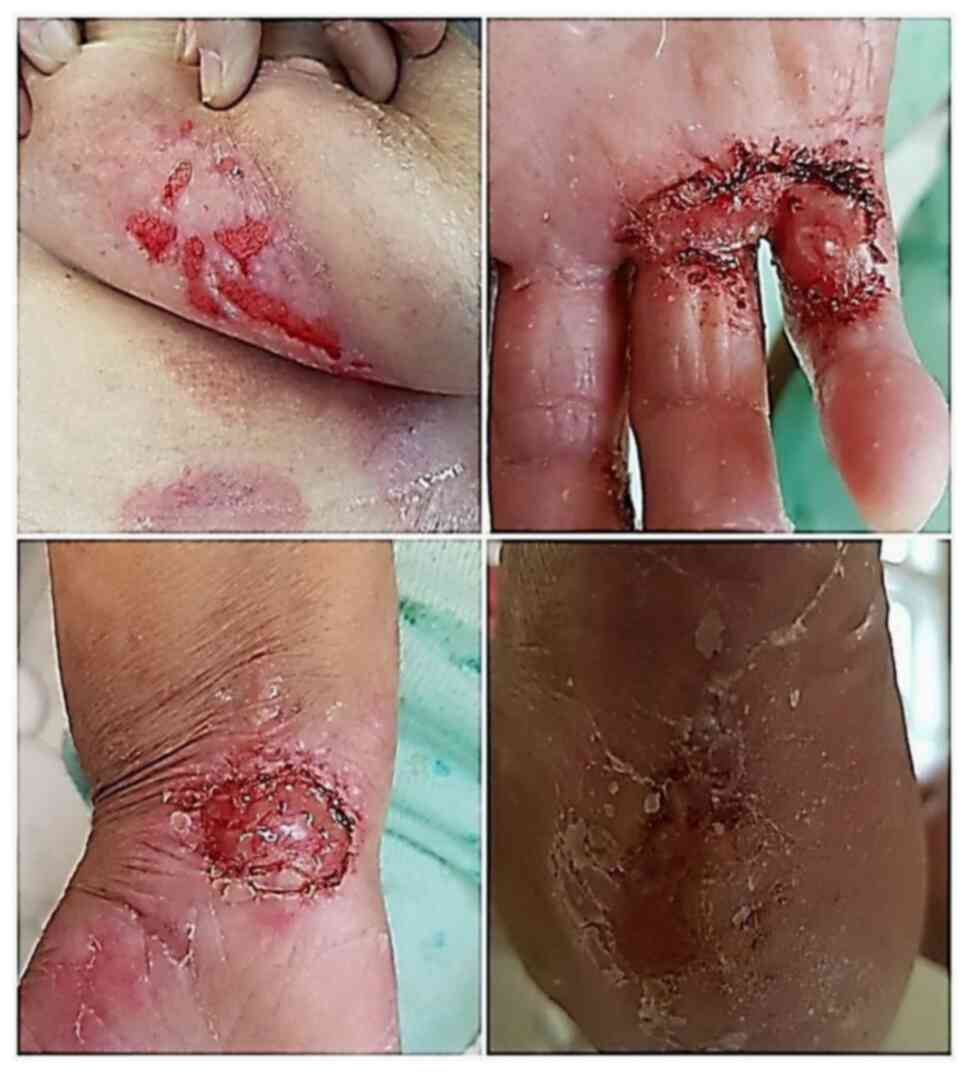
After 2 months she presented with multiple palmoplantar and
submammary, painful, bleeding ulcers, which progressively
aggravated with depth extensions (Fig.
1). Of note is the complete remission of psoriasis lesions over
the last 4 years without specific therapy for psoriatic disease. On
admission the patient presented also skin atrophy, generalized
xerosis and melanonychia.
Laboratory investigations showed moderate anemia (Hb
9.3 g/dl, Ht 27.7%), inflammatory syndrome (ESR 63 mm/h),
superinfection of ulcers with MRSA Staphylococcus aureus and
Proteus mirabilis and positive urine cultures with E.
Coli.
In order to control the adverse events of the skin,
the hematologist recommended a temporary discontinuation of HU.
Superinfection of ulcers was treated with Cefuroxime 3 g/day.
Topical treatment of ulcers was performed with disinfection and
application of epithelizing agents. The xerosis was controlled with
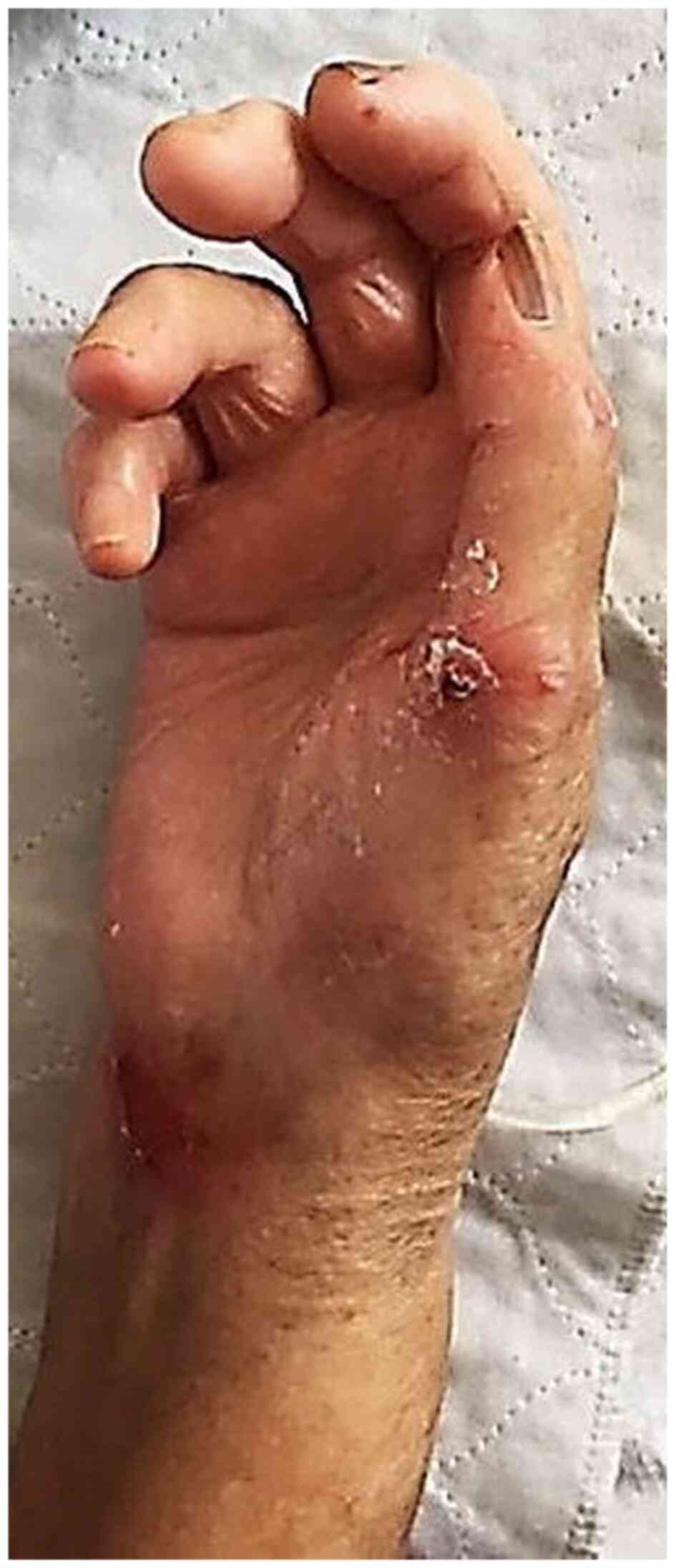
emollients. In evolution, the ulcers slowly epithelialized and the
skin texture improved (Fig. 2).
Case 2
A 72-year-old female, diagnosed in 2014 with primary
thrombocytemia (treated with Thromboreductin 2.5 mg/day and HU 500
mg/day for 3 years) was admitted for giant, necrotic leg ulcers,
extremely painful, appeared in the previous 2 months. A
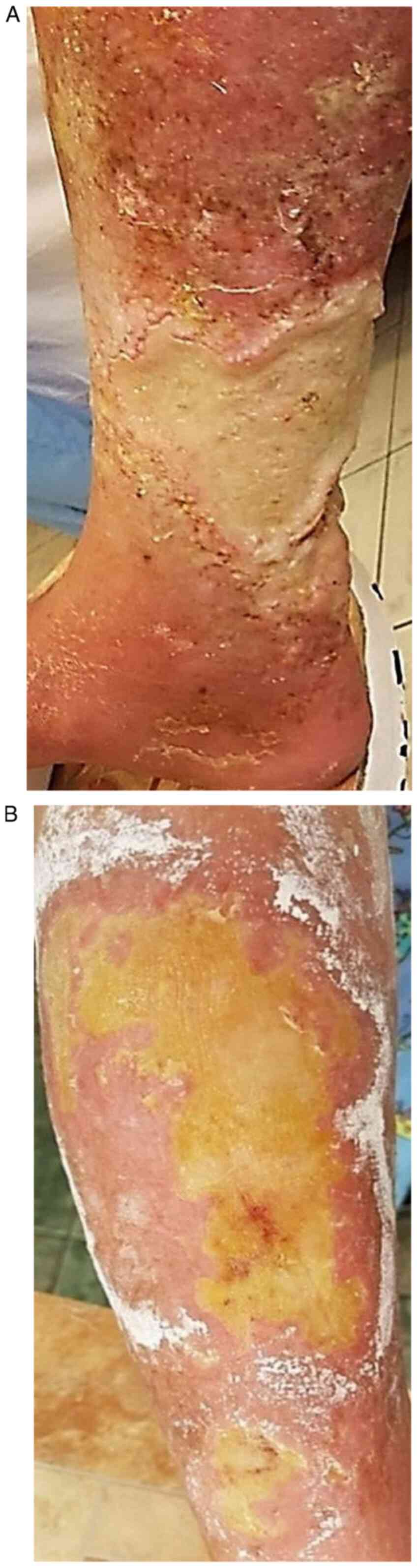
well-defined, pruriginous, erythematous plaques covered with
squamo-crusts, edema (Fig. 3A) and
marked skin xerosis were present around the ulcer.
On admission we had an underweight patient (body
mass index 18.3 kg/m²), with a kyphoscoliotic thorax, with
abolished vesicular murmur in the right lung base and with slightly
elevated blood pressure (160/80 mmHg). The laboratory
investigations showed a hypochromic macrocytic anemia (Hb 9.6 g/dl,
Ht 30, l1%), leukocytosis with neutrophilia (L 24.440/mm³, Ne
89.6%), mild thrombocytosis (Tr 484000/mm³). The bacteriological
exam of the ulcer identified a triple association of germs
[Pseudomonas aeruginosa, Enterobacter ESBL (+) and
E. Coli ESBL (+)]. Chest radiography revealed a right basal
pneumonia with pleural effusion.
Due to the triple association of germs and pulmonary
congestive process, we decided to start the treatment with
Cefuroxime 3 g/day, 10 days, with probiotics, antimycotics,
analgesics and diuretics. The therapeutic approach regarding the
ulcers was a classical one with disinfectants and silver nitrate
epithelizing creams. Hematologist decided to discontinue the HU
administration and increasing the dose of Thromboreductin, which
allowed a slow epithelialization of ulcers (Fig. 3B).
Both patients or their family gave written inform
consent for publication.
Discussion
Leg ulcers appear in approximately 9% of patients
who receive high doses of HU, administered chronically. Chaine
et al reported a higher incidence of HU induced leg ulcers
(29%) (4). The ulcers are more
frequent in the malleolus region and more painful for women who
have been treated with HU for at least 1 year (65%). The skin
around the ulcer usually presents atrophies blanche
(histopathological-epidermal atrophy, dermal fibrosis with scar
tissue but without vascular damage) (5).
Leg ulcers occur by reducing tissue viability when
the chemotherapeutic agent disrupts the S phase of the cellular
cycle. HU inhibits DNA synthesis, damaging the basal keratinocytes
and disruption of collagen production (5). From the pathophysiological point of
view, leg ulcers induced by chronic HU therapy appear by lowering
the blood flow at microcirculation level. The anoxia secondary to
chemotherapy induced macrocytosis and the formation of
microthrombi. Healing occurs within 2-9 months after stopping the
HU treatment. Some authors consider it necessary to stop the
treatment only in cases of refractory, progressive leg ulcers
(6). Other methods for ulcer
treatment are textile biomaterials impregnated with active
principles (phlebotonics, heparin-drugs, capillarotrophic drugs)
(7).
In the first case, after 5 years, the HU therapy
induced the occurrence of ulcers with particular submammary and
acral disposition and marked cutaneous xerosis and melanonychia. In
this case, the HU therapy was beneficial in psoriasis evolution
with the disappearance of psoriatic lesions after 1 year of
treatment. Even if the patient had experienced challenging, hardly
manageable episodes of psoriasis erythroderma in the past, before
taking HU, over the last 4 years she has not had any psoriasis
lesions (she reached PASI 0-absolut psoriasis area and severity
index). In psoriasis the interconnections between the immune
system, the nervous system (expressed by stress that triggers the
first or the new episodes of skin lesions) and the skin are very
complex (8). To control this
disease, a variety of treatment schemes were tested, including HU.
The therapeutic effect of HU in psoriasis is based on the
antimitotic effect of HU (reduces DNA replication in the basal
layer).
Oakley (9) showed
that in half of the patients with psoriasis, resistant to some
conventional therapies (Methotrexate, Acitretin or phototherapy),
HU therapy determines a favorable response. The first results
appear after 2 weeks of treatment and the response is within 8
weeks. In our case, PASI 0 was reached after 1 year of HU
treatment. It has also been found that doses of 500 mg up to 1
g/day of HU have therapeutic effects in selected cases of
psoriasis. In our case, 1 g/day of HU was the therapeutic dose for
both psoriasis and CGL but unfortunately with the occurrence of
adverse events (ulcerations with particular sites, xerosis,
melanonychia) after 5 years of treatment. In the second case, skin
reactions secondary to HU treatment appeared after 3 years of
therapy and were among the most commonly encountered: Leg ulcers
and xeroderma.
The mucocutaneous and nail hyperpigmentation induced
by chronic HU therapy is due to genetic predisposition,
photosensitivity with focal stimulation of melanocytes and
cytotoxic effect on the nail bed and matrix. A plausible hypothesis
is that the activation of melanocytes inducted by drugs increased
the production of melanin (6). Hoff
et al (10) observed that in
patients chronically treated with a chemotherapeutic agent, skin
hyperpigmentation is more common, without nail damage, and the
isolated presence of melanonychia is rarely encountered. The
pigmentation changes induced by HU occur especially in adult
patients with dark phototype and sickle cell anemia (11).
The nail changes that may occur are longitudinal
nail bands with varying pigmentation intensity, usually on a few
nails, especially those on the hand. In specialized literature only
4 cases were reported in which nail damage was observed in all 20
nails in the same patient (12).
Other changes that may sometimes occur simultaneously are
transversal bands and diffuse pigmentation. Also, melanonychia
appeared in 4% of the patients who underwent chronic HU treatment
over a long period of time (13).
In our cases, nail hyperpigmentation remitted after few months of
HU therapy discontinuation.
Rarely, in the course of chronic therapy with HU,
dermatomyositis-like lesions, skin carcinomas (squamous cell
carcinomas, rarely basal cell carcinoma) (3%) (4) or precancerous lesions may occur. Skin
carcinomas occur due to the mutagenic effect of the
chemotherapeutic agent that determines the inhibition of DNA
repair, damaged by external factors (especially ultraviolet
radiation). The appearance of skin carcinomas requires a surgical
treatment, general measures with clothing and cream
photo-protection with high sunscreen protection factor. In our
case, the decision to discontinue the HU therapy was taken by the
hematologist, balancing the benefits and risks of this therapy
(14).
In conclusion, in over 1 year of HU treatments, the
mucocutaneous adverse effects are frequent. The mechanism by which
these side effects occur is not fully known. In the presented
cases, the mucocutaneous manifestations secondary to chronic HU
therapy were leg ulcers with particular localizations (submammar,
palmoplantar), xeroderma, skin atrophy, melanonychia. In the first
case, the beneficial effect of HU on the evolution of psoriatic
disease should be mentioned, obtaining PASI absolute after 1 year
of treatment. In the cases presented by us, leg ulcers and those
with particular localization (palmoplantar and submammary) occurred
after 5 years, respectively, 3 years of HU therapy initiation. The
healing process required HU discontinuation, association of
systemic antibiotics for superinfected ulcers, and topical
epithelizing treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GMI and MR contributed in the conception and design
of the study, analysis and interpretation of patient data,
manuscript drafting and critical revision of the manuscript for
important intellectual content. AO contributed in the data
acquisition, analysis and interpretation of patient data,
manuscript drafting and design of the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patients or their family gave written inform
consent for publication.
Competing interests
The authors declare that they have no competing
interests and they have no financial relationships to disclose.
References
|
1
|
Gwilt PR and Tracewell WG:
Pharmacokinetics and pharmacodynamics of hydroxyurea. Clin
Pharmacokinet. 34:347–358. 1998.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yarbro JW: Mechanism of action of
hydroxyurea. Semin Oncol. 19 (3 Suppl 9):S1–S10. 1992.PubMed/NCBI
|
|
3
|
National Library of Medicine (US),
National Center for Biotechnology Information: PubChem Compound
Summary for CID 3657, Hydroxyurea. urihttps://pubchem.ncbi.nlm.nih.gov/compound/Hydroxyureasimplehttps://pubchem.ncbi.nlm.nih.gov/compound/Hydroxyurea.
Accessed August 4, 2020.
|
|
4
|
Chaine B, Neonato MG, Girot R and
Aractingi S: Cutaneous adverse reactions to hydroxyurea in patients
with sickle cell disease. Arch Dermatol. 137:467–470.
2001.PubMed/NCBI
|
|
5
|
Dissemond J and Körber A:
Hydroxyurea-induced ulcers on the leg. CMAJ.
180(1132)2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
França ER, Teixeira MA, Matias Kde F,
Antunes DE, Braz Rde A and Silva CE: Cutaneous effects after
prolonged use of hydroxyurea in polycythemia Vera. An Bras
Dermatol. 86:751–754. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Branisteanu DE, Nichifor M, Dorobat CM,
Branisteanu DC, Petrariu FD, Molodoi AD, Radu DC and Boda D: Use of
textile biomaterials for the topic treatment of chronic venous
disease. Rom Biotechnol Lett. 20:10618–10625. 2015.
|
|
8
|
Grigore O, Mihailescu AI, Solomon I, Boda
D and Caruntu C: Role of stress in modulation of skin neurogenic
inflammation. Exp Ther Med. 17:997–1003. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Oakley A: Hydroxyurea. DermNet NZ, New
Zealand, 2001. urihttps://www.dermnetnz.org/topics/hydroxyureasimplehttps://www.dermnetnz.org/topics/hydroxyurea.
Accessed June 23, 2019.
|
|
10
|
Hoff NP, Akanay-Diesel S, Pippirs U,
Schulte KW and Hanneken S: Cutaneous side effects of hydroxyurea
treatment for polycythemia Vera. Hautarzt. 60:783–787.
2009.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
11
|
Soutou B and Aractingi S:
Myeloproliferative disorder therapy: Assessment and management of
adverse events - a dermatologist's perspective. Hematol Oncol. 27
(Suppl 1):S11–S13. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Gropper CA, Don PC and Sadjadi MM: Nail
and skin hyperpigmentation associated with hydroxyurea therapy for
polycythemia Vera. Int J Dermatol. 32:731–733. 1993.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Neynaber S, Wolff H, Plewig G and Wienecke
R: Longitudinal melanonychia induced by hydroxyurea therapy. J
Dtsch Dermatol Ges. 2:588–5891. 2004.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
14
|
Wiechert A, Reinhard G, Tüting T, Uerlich
M, Bieber T and Wenzel J: Multiple skin cancers in a patient
treated with hydroxyurea. Hautarzt. 60:651–652, 654.
2009.PubMed/NCBI View Article : Google Scholar : (In German).
|