1. Introduction
Coronaviruses (CoV) are enveloped viruses with a
single-stranded, positive-sense RNA genome 43 known to cause
respiratory infections in humans (1). Given the phylogenetic similarity to
the previously isolated severe acute respiratory syndrome
coronavirus (SARS-CoV), the new virus has been named severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease it
causes is called coronavirus disease 2019 (COVID-19) (2). SARS-CoV-2 was reported in people who
had been exposed to a seafood market in Wuhan, China, where live
animals were sold. Since then, there has been rapid spread of the
virus, leading to a global pandemic of COVID-19(3).
The COVID-19 outbreak continues to cause severe
morbidity worldwide and has now infected over 6 million people
worldwide, with a death toll of >350,000 people (https://www.worldometers.info/coronavirus).
Worldwide data from 2020 have shown that COVID-19 is
associated with different co-morbidities such as diabetes,
hypertension, cardiovascular and lung diseases. Moreover, risk
factors including age, male gender were associated with worse
outcome in this disease (4). It is
widely known that diabetes is associated with an increased risk of
severe bacterial (5) and viral
respiratory tract infections, including influenza and pneumonia
(6). Diabetic patients with
SARS-CoV-2 infection are at higher risk of experiencing a severe
form of COVID-19 disease.
In the present review we are addressing several
hypotheses that may explain why diabetic patients with SARS-CoV-2
infection are at higher risk of experiencing a severe form COVID-19
disease.
Diabetes by itself is unlikely to be a risk factor
for SARS-CoV-2 infection. According to several studies, the
prevalence of diabetes in people infected with the virus is about
the same as in the general population, even slightly lower
(2). However, poorly controlled
blood sugar subjects are at increased risk for all infections and
so there is no reason to think that this would be different for
SARS-CoV-2. Moreover, any sickness that may pose a physiological
stress could cause a difficult management of blood glucose levels
in diabetics and especially type 1 diabetes mellitus (T1DM)
patients.
Cell entry of CoV depends on binding of the viral
spike (S) proteins to cellular receptors and on S protein priming
by host cell proteases. Unravelling which cellular factors are used
by SARS-CoV-2 for entry might provide insights into viral
transmission and reveal therapeutic targets. SARS-CoV-2 uses the
cell receptor for angiotensin converting enzyme 2 (ACE2) for cell
entry and the serine protease TMPRSS2 for viral S (spike) protein
priming (https://www.diabetesatlas.org/en) (7,8).
2. Interference of coronaviruses with
diabetes
More than 463 million people worldwide have diabetes
(https://www.diabetesatlas.org/en)
(9). Recent studies demonstrate a
high rate of mortality for people with diabetes compared to healthy
subjects of similar age infected with SARS-CoV-2(10).
The link between cell-mediated
immunity, diabetes and SARS-CoV-2 infection
Diabetes is a disorder that appears after the
accumulation of activated innate immune cells in metabolic tissues
thus leading to a release of inflammatory cytokines, such as IL-1β
and tumor necrosis factor (TNF)-α that promote insulin resistance
and the β cell destruction (11).
SARS-CoV-2 infection associated with diabetes can
trigger stress conditions with higher secretion of hyperglycemic
hormones (glucocorticoid and catecholamines) that can result in
insulin resistance, hyperglycemia and other complications (11-13).
Pancreatic β cells, which are the only insulin producing cells in
the body, could be also a target for infection and subsequent
destruction as elaborated below, thus worsening the glucose
homeostasis.
Respiratory viral infections including SARS-CoV-2
exhibit increased expression of interleukins (ILs), chemokines,
interferon (IFN), tumor necrosis factors that were identified as
cytokine storm in other studies (14). In this sense, obesity which is
associated with diabetes mellitus may also worsen the outcome of
SARS-CoV-2 infection, due to ‘pre-activated’ immune systems and
resident cytokine signaling pathways such as TNF-α, IL-1 and
IL-6(15). IL-10 that acts as a
negative regulator of inflammation process, could be at lower
levels in diabetic patients (16).
Thus, it is critical to maintain a balance between pro- and
anti-inflammatory mechanisms in order to have a balanced lung
homeostasis. Studies have shown that IL-6 can sustain the
activation of several cytokine pathways for days after initial
immune insult (17). Also, in
initial studies of COVID-19, IL-6 was a strong predictor of
mortality (18).
A link between SARS-CoV-2 cell entry
and the endocrine pathway of diabetes
Angiotensin converting enzyme 2 (ACE2),
transmembrane serine protease 2 (TMPRRSS2) and DPP4 gained interest
for the clinical relevance that they exert in CoV infection
(2,3,8).
ACE22
ACE2, a transmembrane carboxypeptidase enzyme is
expressed within epithelial tissues and represents the functional
receptor for S1 of SARS-CoV and SARS-CoV-2 (19,20).
ACE2 is expressed in all tissues, with major activity in the ileum
and kidney followed by respiratory system, type I and II alveolar
epithelial cells in lungs, heart, endothelial cells, kidney tubular
epithelium, enterocytes, and both endocrine and exocrine pancreas
(19-21).
ACE2 is a key enzymatic component of the
renin-angiotensin-aldosterone system (RAAS); ACE catalyzes the
conversion of the prohormone, angiotensin I (AngI) to the
octapeptide, angiotensin II (AngII), whereas ACE2 converts AngII to
Ang 1-7. AngII, through activation of Ang II type 1a receptors
induces vasoconstriction and proliferation, whereas angiotensin 1-7
stimulates vasodilatation and suppresses cell growth (13).
Low expression of ACE2 within the vasculature can
promote endothelial dysfunction and inflammation and exacerbate
existing atherosclerosis and diabetes (13,22,23).
Both type I and type II diabetic patients have
increased urinary ACE2 protein, enzymatic activity (24,25)
and values for urinary ACE2/Creatinine ratios that correlate
positively with fasting glucose and hemoglobin A1C (17). Additionally, dysglycemia development
is mechanistically linked to ACE2 and complications in subjects
with diabetes remains uncertain. RNA and protein expression of ACE2
and Ang (https://www.worldometers.info/coronavirus) (1-6)
is upregulated in jejunal enterocytes from the experiments with
diabetic rats induced with streptozotocin. Whether hyperglycemia
and insulin deficiency regulate ACE2 expression in human tissues
has not been studied yet (26).
A relevant question to be analyzed through clinical
studies is whether the virus can induce diabetes, by direct
infection of pancreatic beta cells (27). Some signs of acinar pancreatic
assault in COVID-19 patients was documented by increased serum
elastase. The hypothesis underlying these observations is based on
the fact that ACE2 has been identified in the liver and in the
endocrine pancreas and is involved the development of insulin
resistance (28,29).
TMPRSS2
TMPRSS2, a transmembrane serine protease is highly
expressed within the lung and gastrointestinal tissues, including
stomach, bowel, pancreas and liver. TMPRSS2 is implicated in viral
infection as it cleaves and activates influenza A and influenza B
virus hemagglutinin envelope glycoproteins and also the spike
protein of SARS-CoV and Middle East respiratory syndrome
(MERS)-CoV, enabling virus-membrane fusion (30,31).
TMPRSS2 after binding to ACE2 is involved in S
protein priming and cleavage of the spike. The viral pathogenicity
importance of TMPRSS2 is analyzed in studies employing TMPRSS2
inhibitors, such as camostat mesylate, which attenuates SARS-CoV-2
infection of human lung cells cultured ex vivo (7,8).
Human dipeptidyl peptidase-4 (DPP4) in
coronavirus infection and diabetes
DPP4 (also known as CD26) was identified as receptor
for human coronavirus at Erasmus Medical Center (hCoV-EMC)
(32). However, during the
pandemic, specific clinical data regarding the influence of DPP4
inhibitors (DPP4i) on COVID-19 in people with diabetes are under
investigation. In patients with type 2 diabetes, DPP4 is involved
in insulin metabolism and inflammation by breaking the circulating
glucagon-like peptide-1 (GLP1), upregulating the CD86 expression
and lowering the infiltrate with macrophages (33). DPP4i, such as sitagliptin,
vitagliptin, saxagliptin, alogliptin and linagliptin are currently
used in therapy for patients with type 2 diabetes mellitus (T2DM).
In addition to their glucose lowering effect they have been shown
to decrease inflammation directly via GLP-1 dependent
signaling.
A study from UK Clinical Practice Research Datalink
based on a cohort study with diabetes mellitus patients found no
increased risk of pneumonia in 22,435 subjects that were treated
with DPP4i compared to 188,614 individuals treated with non-insulin
glucose lowering agents (34).
Another study had similar results also with no increased risk of
infections detected in patients treated with DPP4i (35). A set of large clinical trials
focused on the safety of saxagliptin, alogliptin, sitagliptin, and
linagliptin in patients with diabetes mellitus at risk for
cardiovascular or renal disease did not reveal clinically relevant
safety issues in relation with immune or inflammatory disorders
(36-39).
However, in-depth investigation of the DPP4 role in SARS-CoV-2
infection is needed.
3. Could anti-diabetes drugs impact on
disease progression?
To answer this question, we need to know if drugs,
such as pioglitazone or rosiglitazone used for treatment of insulin
resistance or related inflammation could impact on prognosis of
patients with diabetes and SARS-CoV-2 infection (40).
Administration of insulin diminishes ACE2 expression
(41,42) while hypoglycemic agents such as
GLP-1 agonists (liraglutide) and thiazolidinediones (pioglitazone),
anti-hypertensives such as ACE inhibitors, and statins upregulate
ACE2 (41,43-45).
In a study by Pfutzner et al (46), the anti-inflammatory effect induced
by pioglitazone was assessed by highly sensitive C-reactive protein
(CRP), level after starting therapy. It was also reported that
pioglitazone induced the IL-6 and TNF-α increased expression in
insulin resistant individuals without manifest hyperglycaemia, and
inhibits the secretions of IL-1b, IL-6, and IL-8.
Another anti-inflammatory molecule that can be used
in glucose-lowering therapies in subjects with diabetes mellitus
and obesity is glucagon-like peptide-1 receptor (GLP-1R) agonist
that reduces biomarkers of systemic inflammation in experimental
studies (47). Other studies
demonstrated that GLP-1R agonists attenuate pulmonary inflammation,
reduces cytokine production and also preserve lung function in mice
and rats with experimental lung injuries (48,49).
It has also been observed that circulating GLP-1 levels are
increased in human subjects with sepsis and critical illness and
correlated with illness severity and mortality (50,51).
This effect is important for COVID-19 patients with
advanced disease that had elevated levels of CRP at admission.
Metformin has anti-inflammatory proprieties and
reduces circulating biomarkers of inflammation in people with
diabetes mellitus (52). Metformin
has also been used in patients with stable hepatitis or HIV
infections and its immunomodulatory actions of metformin in the
context of CoV infection are still to be studied (53,54).
4. Are diabetes related complications
expected to worsen upon SARS-CoV-2 infection?
Diabetes can affect many different organ systems in
the body and, over time, can lead to serious complications.
Complications from diabetes can be classified as microvascular or
macrovascular. Microvascular complications include nervous system
damage (neuropathy), renal system damage (nephropathy) and eye
damage (retinopathy). Macrovascular complications include
cardiovascular disease, stroke, and peripheral vascular disease.
These complications of which many are related to damaged blood
vessels are a significant cause of increased morbidity and
mortality among people with diabetes (55). Many of the diabetes affected organs
are also a target for SARS-CoV-2 infection, which may further
augment the severity of the COVID-19 related disease. Moreover, all
these complications are expected to worsen upon SARS-CoV-2
infection.
Effective onset of type 2 diabetes can precede
clinical diagnosis by many years. In the first 10-15 years from the
diagnosis of diabetes, the incidence of diabetic nephropathy in
patients with diabetes mellitus is low, after which it increases
rapidly to a maximum of approximately 18 years (56,57)
which may explain the high prevalence of nephropathy in the
diagnosis of diabetes when either a kidney transplant or a chronic
dialysis treatment is needed (55).
Special attention is needed on control of classic risk factors for
kidney damage as recent studies show that in patients confirmed
with COVID-19 renal disturbances are common (58). Due to acute tubular necrosis induced
by sepsis, hydration, cytokine storm syndrome, rhabdomyolysis and
hypoxia, renal disorders caused by COVID-19 consist of acute renal
damage, and have been detected direct invasion of the virus in
renal tubular cells and interstitial or glomeruli (59). Moreover, acute renal damage in
COVID-19 is strongly associated with a higher level mortality and
morbidity, renal evolution being an indicator for survival with CoV
infection (59). In a study on
renal function of 59 infected SARS-CoV-2 patients, 63% of patients
reported proteinuria. In addition, they recorded elevated serum
values for creatinine (19%) and urea nitrogen (27%), respectively.
In addition, all patients for whom a computerized tomography was
performed to scan the kidneys, had inflammation and edema of the
renal parenchyma.
Possible mechanisms may be due to dehydration, which
may be related to fever or decreased fluid intake having various
consequences on the kidneys, leading to a reduction in glomerular
filtration rate and acute kidney damage (59). Other proposed mechanisms include
COVID-19 sepsis, which leads to cytokine storm syndrome. In
addition, direct invasion of the virus on the renal tubular and
interstitial cells or glomeruli is possible due to direct
cytopathic effects on different renal cells, and could be detected
due to increased ACE2 in kidneys at much higher level than in the
lung (19,60). The finding that SARS-CoV-2 uses ACE2
explains why the kidney cells are targeted and infected with
COVID-19. This strengthens the hypothesis that renal dysfunction
could accelerate the progression of inflammation started in the
lungs, not just as a side effect of lung inflammation. Thus,
inflammatory reactions through lung deficiencies can damage the
kidney while the damage and death of renal tubular epithelial cells
could also cause severe damage to the lungs and other organs
through a large amount of inflammatory substances released. Above a
certain critical point, the kidney-lung intersection could lead to
an irreversible self-amplification storm of cytokines that quickly
induces failure and death for multiple organs (61,62).
Neurologic injury has been confirmed in the
infection of other CoV infections such as in SARS-CoV and MERS-CoV,
the SARS-CoV nucleic acid has been found in the cerebrospinal fluid
of these patients and also in their brain tissue on autopsy
(63,64). One of the first reports centered on
detailed neurologic manifestations of the hospitalized patients
with COVID-19 had reported higher neutrophil counts, lower
lymphocyte counts, higher CRP and D-dimer levels in patients with
severe infection than that of patients with non-severe infection
(65). In addition, multiorgan
damage has been reported for patients with increased inflammation
and blood coagulation. Of these patients, 14% had diabetes, 23.8%
hypertension, 7% cardiac or cerebrovascular disease, and 6.1%
malignancy, and 2.8% of diabetic patients had associated chronic
kidney disease (65). Such aspects
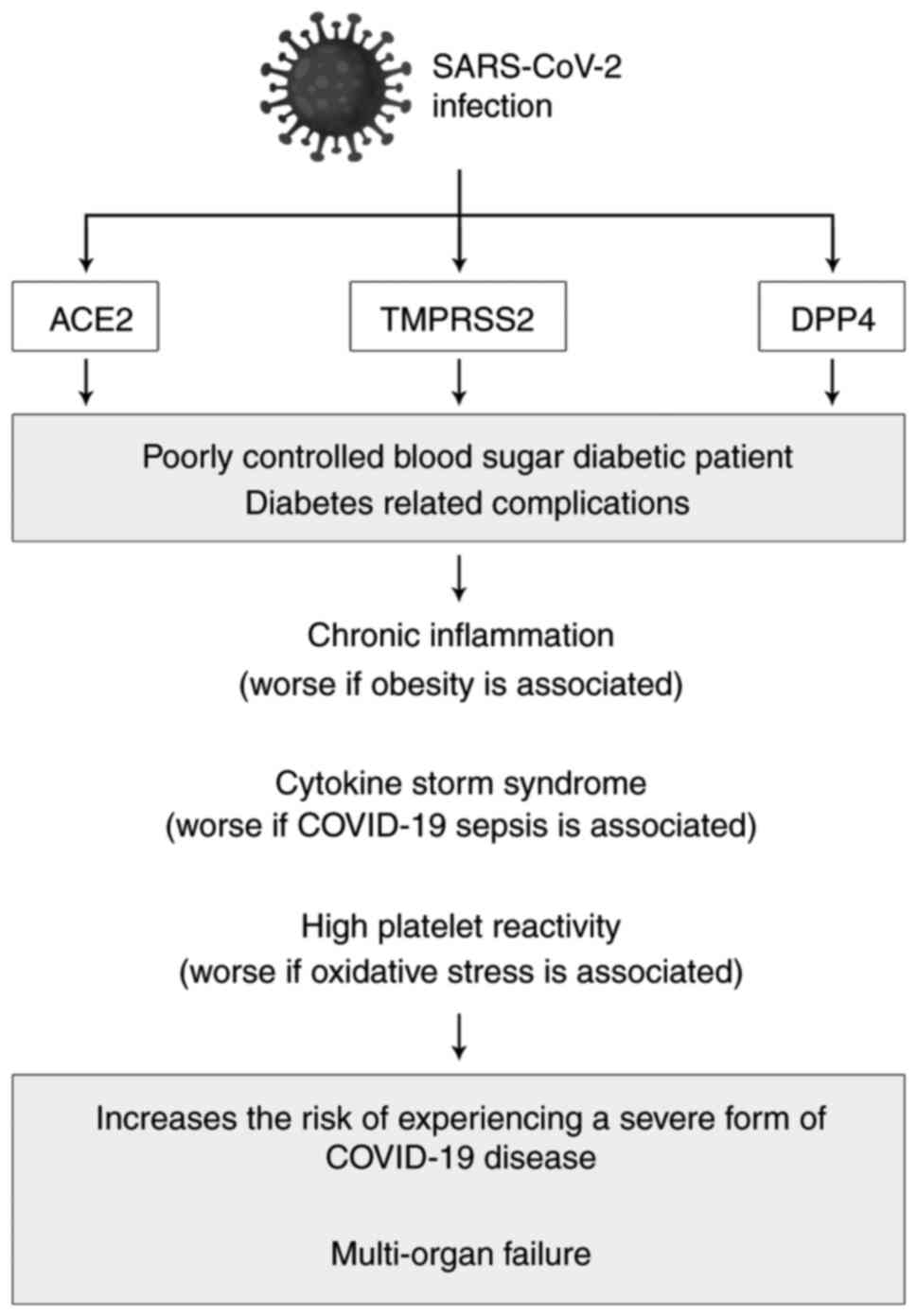
are illustrated in Fig. 1.
5. Platelet destruction in patients with
diabetes and COVID-19
Higher platelet reactivity in diabetic patients is
due to increased platelet functions, including an increased
response to stimulation by platelet aggregation agonists, adhesion
to thrombogenic surfaces and platelet aggregation (66). Oxidative stress and reduced
antioxidant activity induced by hyperglycemia are significantly
augmented in diabetic patients, subsequently leading to platelet
activation and hyper-reactivity.
Increased blood coagulation could affect the
severity of SARS-CoV-2 infection outcome on lung and additional
critical organs function (67).
Diabetes mellitus patients have increased platelet
reactivity, manifested by increased tendency to respond
aggressively to particular stimuli (68). Consistent with this observation,
hyperglycemia and hypertriglyceridemia contribute to increased
platelet reactivity through direct effects and by promoting
nonenzymatic glycation of proteins by decreasing membrane fluidity.
Moreover, both decreased insulin production and insulin resistance
have been shown to be a stimulator of platelet reactivity (69). Thus, both relative or absolute
insulin deficiency is expected to increase platelet reactivity. If
diabetes mellitus is associated with obesity, then increased
oxidative stress and associated inflammation may promote the
endothelial dysfunction. Oxidative stress exacerbates this effect
by attenuating NO activity and promoting platelet activation.
Inflammation favors the activation of platelets which, in turn,
favors inflammation (70).
Consequently, improved metabolic and glycemic control improves
insulin sensitivity and maintains pancreatic β-cell function, and
is likely to decrease platelet reactivity and increase the effects
of antiplatelet agents. Thus, people treated with ACE2 and
angiotensin 2 receptor type I (ARB) blockers, for high blood
pressure that is associated with metabolic syndrome have been shown
to display reduced inflammation in lungs (71). Previous pathogenic CoV, such as
SARS-CoV, have been shown to bind to their target cells through
ACE2, which is expressed by the epithelial cells of the lungs,
kidneys, intestines, pancreas and blood vessels (72). Hence, drugs that increase ACE2
expression including pioglitazone and liraglutide or ACE2
polymorphisms may further worsen virus infection prognosis
(71).
Inflammatory state associated with metabolic
syndrome, T2DM, and visceral obesity, plays a role also in
imprinting the cluster of cardiovascular risk factors. These
factors lead to an approximately 3-fold increase in the risk of
coronary heart disease, stroke, and venous thromboembolism
(73,74) that might be among the underlying
pathophysiological mechanisms contributing to the increased
morbidity and mortality of COVID-19 infected people that suffer
from obesity-associated diseases.
The early hypothesis was that SARS is associated
with cytokine dysregulation (75-81).
Subjects with SARS have high levels of pro-inflammatory cytokines
and chemokines associated with T cell depletion, lung inflammation,
and extensive lung injury (80).
Increased levels of IL-6 are associated with severe disease and are
found in patients with respiratory syncytial virus (RSV) infection
(79). Circulating levels of IL-8,
IL-1β, and IL-6 undergo modifications in chronic inflammatory
conditions, and are critically involved in abnormal clot formation,
erythrocyte pathology and platelet hyperactivation (82). Decreased cytokine levels cause an
increased hypercoagulability of whole blood and affect both
erythrocytes and platelets. If there was an association of the
three cytokines then it caused hyperactivation and platelet spread
resulted in the largest changes (73,83-85).
6. Skin complications of diabetes mellitus -
relevance for SARS-CoV-2 infection
The most recognized cutaneous manifestation in
diabetes, present in 74% of obese patients with diabetes mellitus
is Acanthosis nigricans (AN), a hyperpigmented velvety
thickening of skin folds (86).
Moreover, the presence of AN could be a good prognostic indicator
for development of hyperinsulinemia in newly diagnosed diabetes
mellitus with a possible genetic predisposition or increased
sensitivity of the skin to increased plasma insulin levels
(87,88). Benign AN type 2 is linked to T2DM,
initially starting insidiously with hyperpigmentation while
pseudo-AN type 3 is associated with metabolic syndrome. Both
underlying conditions are associated with insulin resistance
(86). Among other skin
manifestations that accompany diabetes is chronic psoriasis,
commonly in areas of the scalp, elbows and nails, a complex
interplay between an inflammatory process, polygenic skin disorders
with environmental triggers such as infections (89). Recent research shows that psoriasis
can increase the predisposition for the development of diabetes,
strong arguments being brought in a study of 52,000 participants,
which concluded that people with psoriasis have a 49-56% higher
risk of development diabetes mellitus later in life (90). Furthermore, being partly
immune-depressed the psoriatic patients have an increased risk for
infections, and there is no data for the beneficial effect of
treatment with drugs such as cyclosporine, methotrexate and
anti-TNF-α for COVID-19(91).
Malum perforans pedis is a long-lasting
trauma accompanied by various metabolic and infectious pathologies
with a chronic ulceration in the sole of the foot (92). Malum perforans pedis is a
neurovascular disease for the most part on the skin with abnormal
innervations with damage infusion, the development of ulceration in
the accompanied foot, loss of sensation of pain having a
predominance of the appearance in the elderly and with a severe
form in diabetic neuropathies (93). Some findings indicate that a common
cause of morbidity and mortality in diabetic patients could be
changes in the healing of skin wounds. The effects of hyperglycemia
on skin keratinocytes have been proved to enhance the
proliferation-differentiation balance in an in vitro model
of wound healing. Indeed, a decrease in the basal glucose update
rate is associated with the induction of keratinocyte
differentiation. These changes have been associated with enhanced
GLUT1 expression, changes in cells morphology, as well as with a
low proliferation and improving Ca2+-induced
keratinocyte differentiation, and it has been demonstrated that
hyperglycemia and disruption of the insulin signaling pathway could
be directly involved in the development of chronic complications of
diabetes by affecting the use of glucose by keratinocytes, as well
as by proliferating and differentiating the skin (94). However, the molecular mechanisms by
which diabetes changes the structure of the skin has not been
elucidated.
Currently, data in the literature on skin
manifestations in COVID-19 is limited. One of the first cases that
have been reported with observed cutaneous manifestations, skin
rashes manifestations related with the COVID-19 disease, involving
mainly the trunk, is a case with no drug intake for the previous 10
days. The observed skin manifestations are similar to those found
in common viral infection (95).
All of these skin complications can worsen the prognosis of people
with diabetes, increasing the need for intensive care depending on
severity of SARS-CoV-2 infection.
7. Treatment of SARS-CoV-2 infection
Recent studies explored the feasibility of
convalescent plasma therapy for SARS-CoV-2 infections.
Convalescent plasma contains neutralizing antibodies
that are capable of neutralizing SARS-CoV-2 from blood circulation
and pulmonary tissues (96). Also,
studies on COVID-19 showed that lymphocyte levels in the peripheral
blood were decreased and cytokines levels in the plasma from
patients with severe complication, IL-6, IL-10, TNF-α, and
granulocyte-macrophage colony-stimulating factor, were
significantly higher than in those who had mild symptoms (58,97).
The results of Duan et al (98) suggested that antibodies contained in
convalescent plasma lowered the inflammation and overreaction of
the immune system. All analyzed patients reached serum SARS-CoV-2
RNA negativity after convalescent plasma transfusion, accompanied
by an increase of oxygen saturation and lymphocyte counts, and the
improvement of liver function and CRP, 10 severe patients showed
that only one dose of 200 ml of convalescent plasma increased
significantly or maintained the neutralizing antibodies at a high
level, leading to disappearance of viremia in one week.
Convalescent plasma shows a potential therapeutic effect reducing
the viral load and lowering risk in the treatment of severe
COVID-19 patients. Combined with other treatments, such as
antiviral therapy and other supportive care, convalescent plasma
improved clinical outcomes. Certain strategies used for vaccines
development stimulate neutralizing antibodies for S proteins and
T-cell responses which are together required in convalescence. The
challenge for protective immunity against CoV at its start
(99).
Use of 2019 nCoV receptor binding inhibitors with
high affinity for ACE-2, and angiotensin-converting enzyme
inhibitors (ACE-I) or angiotensin receptor antagonists (ARA), drugs
that inhibit the renin-angiotensin system (RAS) are gaining
increasing popularity and could play a role in treating severe
respiratory diseases (100,101).
Efforts are being made to develop a vaccine, which will be a major
tool in the fight against COVID-19 (https://www.who.int/blueprint/priority-diseases/key-action/list-of-candidate-vaccines-developed-against-ncov.pdf).
8. Conclusions
COVID-19 represents an unattended threat for health
and life, having so far over 6,000,000 infected and about 300.000
deaths. The present review brought hypotheses that should be
clinically analyzed of why diabetic patients, represent a
population of patients which is prone to be affected by a more
severe disease manifestation of this viral infection. As the human
endocrine pancreas expresses ACE-2, the coronavirus might enter
islets and cause acute β-cell dysfunction, leading to acute
hyperglycemia and transient T2DM. Moreover, ACE-2 activity levels
could be enhanced in diabetic patients making the SARS-CoV
infections more efficient also in peripheral, non-pancreatic
tissues. This may explain why diabetes mellitus can contribute
mechanistically to multi-organ failure in SARS-CoV infections. Some
skin complications can worsen the prognosis of people with
diabetes, increasing the need for intensive care depending on
severity of SARS-CoV-2 infection. On the other hand, some of the
drugs used to ameliorate diabetes mellitus and its complications,
can also affect rate of SARS-CoV infections, such as GLP-1 analogs
and others.
Acknowledgements
Not applicable.
Funding
This study was supported by the Research Grant:
Dia-Cure P_37_794, POC-A. 1- A.1.1.4-E-2015 (2016-2020) and the
projects PN 19-29.01.04 and P19-41-05-01.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RA, SOD, IRF, DL, AMS, VMA, CT, IP and SF
contributed to the data acquisition, manuscript drafting and
critical revision of the manuscript for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cui J, Li F and Shi ZL: Origin and
evolution of pathogenic coronaviruses. Nat Rev Microbiol.
17:181–192. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hussain A, Bhowmik B and do Vale Moreira
NC: COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin
Pract. 162(108142)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gandhi RT, Lynch JB and Del Rio C: Mild or
moderate covid-19. N Engl J Med: Apr 24, 2020 (Epub ahead of
print). doi: 10.1056/NEJMcp2009249.
|
|
4
|
Ryan PM and Caplice NM: Is adipose tissue
a reservoir for viral spread, immune activation and cytokine
amplification in Coronavirus disease 2019. Obesity (Silver Spring).
28:1191–1194. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hodgson K, Morris J, Bridson T, Govan B,
Rush C and Ketheesan N: Immunological mechanisms contributing to
the double burden of diabetes and intracellular bacterial
infections. Immunology. 144:171–185. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Allard R, Leclerc P, Tremblay C and
Tannenbaum TN: Diabetes and the severity of pandemic influenza A
(H1N1) infection. Diabetes Care. 33:1491–1493. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Cell.
181:271–280.e8. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Docea AO, Tsatsakis A, Albulescu D,
Cristea O, Zlatian O, Vinceti M, Moschos SA, Tsoukalas D, Goumenou
M, Drakoulis N, et al: A new threat from an old enemy: Reemergence
of coronavirus (Review). Int J Mol Med. 45:1631–1643.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Saeedi P, Petersohn I, Salpea P, Malanda
B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA,
Ogurtsova K, et al: Global and regional diabetes prevalence
estimates for 2019 and projections for 2030 and 2045: Results from
the International Diabetes Federation Diabetes Atlas, 9(th)
edition. Diabetes Res Clin Pract. 157(107843)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian
C, Qin R, Wang H, Shen Y, Du K, et al: Diabetes is a risk factor
for the progression and prognosis of COVID-19. Diabetes Metab Res
Rev: Mar 31, 2020 (Epub ahead of print). doi:
10.1002/dmrr.3319.
|
|
11
|
Odegaard JI and Chawla A: Connecting type
1 and type 2 diabetes through innate immunity. Cold Spring Harb
Perspect Med. 2(a007724)2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang A, Zhao W, Xu Z and Gu J: Timely
blood glucose management for the outbreak of 2019 novel coronavirus
disease (COVID-19) is urgently needed. Diabetes Res Clin Pract.
162(108118)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lovren F, Pan Y, Quan A, Teoh H, Wang G,
Shukla PC, Levitt KS, Oudit GY, Al-Omran M, Stewart DJ, et al:
Angiotensin converting enzyme-2 confers endothelial protection and
attenuates atherosclerosis. Am J Physiol Heart Circ Physiol.
295:H1377–H1384. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tisoncik JR, Korth MJ, Simmons CP, Farrar
J, Martin TR and Katze MG: Into the eye of the cytokine storm.
Microbiol Mol Biol Rev. 76:16–32. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kern L, Mittenbuhler MJ, Vesting AJ,
Ostermann AL, Wunderlich CM and Wunderlich FT: Obesity-induced TNFα
and IL-6 signaling: The missing link between obesity and
inflammation-driven liver and colorectal cancers. Cancers (Basel).
11(24)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Park WY, Goodman RB, Steinberg KP,
Ruzinski JT, Radella F, II Park DR, Pugin J, Skerrett SJ, Hudson LD
and Martin TR: Cytokine balance in the lungs of patients with acute
respiratory distress syndrome. Am J Respir Crit Care Med.
164:1896–1903. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yiu HH, Graham AL and Stengel RF: Dynamics
of a cytokine storm. PLoS One. 7(e45027)2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ruan Q, Yang K, Wang W, Jiang L and Song
J: Clinical predictors of mortality due to COVID-19 based on an
analysis of data of 150 patients from Wuhan, China. Intensive Care
Med. 46:846–848. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li W, Moore MJ, Vasilieva N, Sui J, Wong
SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough
TC, et al: Angiotensin-converting enzyme 2 is a functional receptor
for the SARS coronavirus. Nature. 426:450–454. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Raj VS, Mou H, Smits SL, Dekkers DH,
Muller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, et
al: Dipeptidyl peptidase 4 is a functional receptor for the
emerging human coronavirus-EMC. Nature. 495:251–254.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu F, Long X, Zhang B, Zhang W, Chen X
and Zhang Z: ACE2 expression in pancreas may cause pancreatic
damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol.
18:2128–2130.e2. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yousif MH, Dhaunsi GS, Makki BM, Qabazard
BA, Akhtar S and Benter IF: Characterization of Angiotensin-(1-7)
effects on the cardiovascular system in an experimental model of
type-1 diabetes. Pharmacol Res. 66:269–275. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang C, Zhao YX, Zhang YH, Zhu L, Deng
BP, Zhou ZL, Li SY, Lu XT, Song LL, Lei XM, et al:
Angiotensin-converting enzyme 2 attenuates atherosclerotic lesions
by targeting vascular cells. Proc Natl Acad Sci USA.
107:15886–15891. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Burns KD, Lytvyn Y, Mahmud FH, Daneman D,
Deda L, Dunger DB, Deanfield J, Dalton RN, Elia Y, Har R, et al:
The relationship between urinary renin-angiotensin system markers,
renal function, and blood pressure in adolescents with type 1
diabetes. Am J Physiol Renal Physiol. 312:F335–F342.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gutta S, Grobe N, Kumbaji M, Osman H,
Saklayen M, Li G and Elased KM: Increased urinary angiotensin
converting enzyme 2 and neprilysin in patients with type 2
diabetes. Am J Physiol Renal Physiol. 315:F263–F274.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wong TP, Ho KY, Ng EK, Debnam ES and Leung
PS: Upregulation of ACE2-ANG-(1-7)-Mas axis in jejunal enterocytes
of type 1 diabetic rats: Implications for glucose transport. Am J
Physiol Endocrinol Metab. 303:E669–E681. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bornstein SR, Rubino F, Khunti K, Mingrone
G, Hopkins D, Birkenfeld AL, Boehm B, Amiel S, Holt RI, Skyler JS,
et al: Practical recommendations for the management of diabetes in
patients with COVID-19. Lancet Diabetes Endocrinol. 8:546–550.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bindom SM and Lazartigues E: The sweeter
side of ACE2: Physiological evidence for a role in diabetes. Mol
Cell Endocrinol. 302:193–202. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Maddaloni E and Buzzetti R: Covid-19 and
diabetes mellitus: Unveiling the interaction of two pandemics.
Diabetes Metab Res Rev: Mar 31, 2020 (Epub ahead of print). doi:
10.1002/dmrr.3321.
|
|
30
|
Iwata-Yoshikawa N, Okamura T, Shimizu Y,
Hasegawa H, Takeda M and Nagata N: TMPRSS2 contributes to virus
spread and immunopathology in the airways of murine models after
coronavirus infection. J Virol. 93:e01815–e1818. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Limburg H, Harbig A, Bestle D, Stein DA,
Moulton HM, Jaeger J, Janga H, Hardes K, Koepke J, Schulte L, et
al: TMPRSS2 is the major activating protease of influenza a virus
in primary human airway cells and influenza B virus in human type
ii pneumocytes. J Virol. 93:e00649–19. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Iacobellis G: COVID-19 and diabetes: Can
DPP4 inhibition play a role? Diabetes Res Clin Pract.
162(108125)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ceriello A, Stoian AP and Rizzo M:
COVID-19 and diabetes management: What should be considered?
Diabetes Res Clin Pract. 163(108151)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
van der Zanden R, de Vries F, Lalmohamed
A, Driessen JH, de Boer A, Rohde G, Neef C and den Heijer C: Use of
dipeptidyl-peptidase-4 inhibitors and the risk of pneumonia: A
population-based cohort study. PLoS One.
10(e0139367)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gooßen K and Gräber S: Longer term safety
of dipeptidyl peptidase-4 inhibitors in patients with type 2
diabetes mellitus: Systematic review and meta-analysis. Diabetes
Obes Metab. 14:1061–1072. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Green JB, Bethel MA, Armstrong PW, Buse
JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, et al:
Effect of sitagliptin on cardiovascular outcomes in type 2
diabetes. N Engl J Med. 373:232–242. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rosenstock J, Perkovic V, Johansen OE,
Cooper ME, Kahn SE, Marx N, Alexander JH, Pencina M, Toto RD,
Wanner C, et al: Effect of Linagliptin vs placebo on major
cardiovascular events in adults with type 2 diabetes and high
cardiovascular and renal risk: The CARMELINA Randomized Clinical
Trial. JAMA. 321:69–79. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Scirica BM, Bhatt DL, Braunwald E, Steg
PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD,
Hoffman EB, et al: Saxagliptin and cardiovascular outcomes in
patients with type 2 diabetes mellitus. N Engl J Med.
369:1317–1326. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
White WB, Cannon CP, Heller SR, Nissen SE,
Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S,
et al: Alogliptin after acute coronary syndrome in patients with
type 2 diabetes. N Engl J Med. 369:1327–1335. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Carboni E and Carta AR: Can pioglitazone
be potentially useful therapeutically in treating patients with
COVID-19? Med Hypotheses. 140(109776)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ferrario CM, Jessup J, Chappell MC,
Averill DB, Brosnihan KB, Tallant EA, Diz DI and Gallagher PE:
Effect of angiotensin- converting enzyme inhibition and angiotensin
II receptor blockers on cardiac angiotensin-converting enzyme 2.
Circulation. 111:2605–2610. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao
HD, Bernstein KE, Coffman TM, Chen S and Batlle D: ACE and ACE2
activity in diabetic mice. Diabetes. 55:2132–2139. 2006.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Romani-Perez M, Outeirino-Iglesias V, Moya
CM, Santisteban P, Gonzalez-Matias LC, Vigo E and Mallo F:
Activation of the GLP-1 Receptor by liraglutide increases ACE2
expression, reversing right ventricle hypertrophy, and improving
the production of SP-A and SP-B in the lungs of type 1 diabetes
rats. Endocrinology. 156:3559–3569. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wosten-van Asperen RM, Lutter R, Specht
PA, Moll GN, van Woensel JB, van der Loos CM, van Goor H, Kamilic
J, Florquin S and Bos AP: Acute respiratory distress syndrome leads
to reduced ratio of ACE/ACE2 activities and is prevented by
angiotensin-(1-7) or an angiotensin II receptor antagonist. J
Pathol. 225:618–627. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang W, Xu YZ, Liu B, Wu R, Yang YY, Xiao
XQ and Zhang X: Pioglitazone upregulates angiotensin converting
enzyme 2 expression in insulin-sensitive tissues in rats with
high-fat diet-induced nonalcoholic steatohepatitis.
ScientificWorldJournal. 2014(603409)2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Pfutzner A, Schondorf T, Hanefeld M and
Forst T: High-sensitivity C-reactive protein predicts
cardiovascular risk in diabetic and nondiabetic patients: Effects
of insulin-sensitizing treatment with pioglitazone. J Diabetes Sci
Technol. 4:706–716. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Drucker DJ: Mechanisms of action and
therapeutic application of glucagon-like peptide-1. Cell Meta.
27:740–756. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Toki S, Goleniewska K, Reiss S, Zhang J,
Bloodworth MH, Stier MT, Zhou W, Newcomb DC, Ware LB, Stanwood GD,
et al: Glucagon-like peptide 1 signaling inhibits allergen-induced
lung IL-33 release and reduces group 2 innate lymphoid cell
cytokine production in vivo. J Allergy Clin Immunol. 142:1515–1528
e8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Viby NE, Isidor MS, Buggeskov KB, Poulsen
SS, Hansen JB and Kissow H: Glucagon-like peptide-1 (GLP-1) reduces
mortality and improves lung function in a model of experimental
obstructive lung disease in female mice. Endocrinology.
154:4503–4511. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kahles F, Meyer C, Mollmann J, Diebold S,
Findeisen HM, Lebherz C, Trautwein C, Koch A, Tacke F, Marx N and
Lehrke M: GLP-1 secretion is increased by inflammatory stimuli in
an IL-6-dependent manner, leading to hyperinsulinemia and blood
glucose lowering. Diabetes. 63:3221–3229. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lebherz C, Schlieper G, Mollmann J, Kahles
F, Schwarz M, Brunsing J, Dimkovic N, Koch A, Trautwein C, Flöge J,
et al: GLP-1 levels predict mortality in patients with critical
illness as well as end-stage renal disease. Am J Med.
130:833–841.e3. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Cameron AR, Morrison VL, Levin D, Mohan M,
Forteath C, Beall C, McNeilly AD, Balfour DJ, Savinko T, Fagerholm
SC, et al: Anti-inflammatory effects of metformin irrespective of
diabetes status. Circ Res. 119:652–665. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Agarwal D, Schmader KE, Kossenkov AV,
Doyle S, Kurupati R and Ertl HCJ: Immune response to influenza
vaccination in the elderly is altered by chronic medication use.
Immun Ageing. 15(19)2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Saenwongsa W, Nithichanon A,
Chittaganpitch M, Buayai K, Kewcharoenwong C, Thumrongwilainet B,
Butta P, Palaga T, Takahashi Y, et al: Metformin-induced
suppression of IFN-alpha via mTORC1 signalling following seasonal
vaccination is associated with impaired antibody responses in type
2 diabetes. Sci Rep. 10(3229)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Deshpande AD, Harris-Hayes M and Schootman
M: Epidemiology of diabetes and diabetes-related complications.
Phys Ther. 88:1254–1264. 2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Bojestig M, Arnqvist HJ, Hermansson G,
Karlberg BE and Ludvigsson J: Declining incidence of nephropathy in
insulin- dependent diabetes mellitus. N Engl J Med. 330:15–18.
1994.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Harris MI, Klein R, Welborn TA and Knuiman
MW: Onset of NIDDM occurs at least 4-7 yr before clinical
diagnosis. Diabetes Care. 15:815–819. 1992.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Valizadeh RBA, Mirzazadeh A and Bhaskar
LVKS: Coronavirus-nephropathy; renal involvement in COVID-19. J
Renal Inj Prev. 9(e18)2020.
|
|
60
|
Zhou P, Yang XL, Wang XG, Hu B, Zhang L,
Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al: A pneumonia outbreak
associated with a new coronavirus of probable bat origin. Nature.
579:270–273. 2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Faubel S and Edelstein CL: Mechanisms and
mediators of lung injury after acute kidney injury. Nat Rev
Nephrol. 12:48–60. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Wang H and Ma S: The cytokine storm and
factors determining the sequence and severity of organ dysfunction
in multiple organ dysfunction syndrome. Am J Emerg Med. 26:711–715.
2008.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Arabi YM, Balkhy HH, Hayden FG, Bouchama
A, Luke T, Baillie JK, Al-Omari A, Hajeer AH, Senga M, Denison MR,
et al: Middle East respiratory syndrome. N Engl J Med. 376:584–594.
2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Desforges M, Le Coupanec A, Brison E,
Meessen-Pinard M and Talbot PJ: Human respiratory coronaviruses:
Neuroinvasive, neurotropic and potentially neurovirulent pathogens.
Virologie (Montrouge). 18:5–16. 2014.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q,
Chang J, Hong C, Zhou Y, Wang D, et al: Neurologic manifestations
of hospitalized patients with coronavirus disease 2019 in Wuhan,
China. JAMA Neurol. 77:1–9. 2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Maiocchi S, Alwis I, Wu MCL, Yuan Y and
Jackson SP: Thromboinflammatory functions of platelets in
ischemia-reperfusion injury and its dysregulation in diabetes.
Semin Thromb Hemost. 44:102–113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Demirtunc R, Duman D, Basar M, Bilgi M,
Teomete M and Garip T: The relationship between glycemic control
and platelet activity in type 2 diabetes mellitus. J Diabetes
Complications. 23:89–94. 2009.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Angiolillo DJ, Fernandez-Ortiz A, Bernardo
E, Ramirez C, Sabate M, Jimenez-Quevedo P, Hernandez R, Moreno R,
Escaned J, Alfonso F, et al: Platelet function profiles in patients
with type 2 diabetes and coronary artery disease on combined
aspirin and clopidogrel treatment. Diabetes. 54:2430–2435.
2005.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Vaidyula VR, Boden G and Rao AK: Platelet
and monocyte activation by hyperglycemia and hyperinsulinemia in
healthy subjects. Platelets. 17:577–585. 2006.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Schneider DJ: Factors contributing to
increased platelet reactivity in people with diabetes. Diabetes
Care. 32:525–527. 2009.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Fang L, Karakiulakis G and Roth M: Are
patients with hypertension and diabetes mellitus at increased risk
for COVID-19 infection? Lancet Respir Med. 8(e21)2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wan Y, Shang J, Graham R, Baric RS and Li
F: Receptor recognition by the novel coronavirus from Wuhan: An
analysis based on decade-long structural studies of SARS
coronavirus. J Virol. 94:e00127–e00220. 2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Hubert HB, Feinleib M, McNamara PM and
Castelli WP: Obesity as an independent risk factor for
cardiovascular disease: A 26-year follow-up of participants in the
Framingham Heart Study. Circulation. 67:968–977. 1983.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Tsai AW, Cushman M, Rosamond WD, Heckbert
SR, Polak JF and Folsom AR: Cardiovascular risk factors and venous
thromboembolism incidence: The longitudinal investigation of
thromboembolism etiology. Arch Intern Med. 162:1182–1189.
2002.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Nicholls JM, Poon LL, Lee KC, Ng WF, Lai
ST, Leung CY, Chu CM, Hui PK, Mak KL, Lim W, et al: Lung pathology
of fatal severe acute respiratory syndrome. Lancet. 361:1773–1778.
2003.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Chen J and Subbarao K: The immunobiology
of SARS*. Annu Rev Immunol. 25:443–472. 2007.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Kalliolias GD and Ivashkiv LB: Overview of
the biology of type I interferons. Arthritis Res Ther. 12 (Suppl
1)(S1)2010.PubMed/NCBI View
Article : Google Scholar
|
|
78
|
Law HK, Cheung CY, Ng HY, Sia SF, Chan YO,
Luk W, Nicholls JM, Peiris JS and Lau YL: Chemokine up-regulation
in SARS-coronavirus-infected, monocyte-derived human dendritic
cells. Blood. 106:2366–2374. 2005.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Okabayashi T, Kariwa H, Yokota S, Iki S,
Indoh T, Yokosawa N, Takashima I, Tsutsumi H and Fujii N: Cytokine
regulation in SARS coronavirus infection compared to other
respiratory virus infections. J Med Virol. 78:417–424.
2006.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Zhang Y, Li J, Zhan Y, Wu L, Yu X, Zhang
W, Ye L, Xu S, Sun R, Wang Y and Lou J: Analysis of serum cytokines
in patients with severe acute respiratory syndrome. Infect Immun.
72:4410–4415. 2004.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Lo AW, Tang NL and To KF: How the SARS
coronavirus causes disease: Host or organism? J Pathol.
208:142–151. 2006.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Choi G, Schultz MJ, Levi M and van der
Poll T: The relationship between inflammation and the coagulation
system. Swiss Med Wkly. 136:139–144. 2006.PubMed/NCBI
|
|
83
|
Bester J and Pretorius E: Effects of
IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot
viscoelasticity. Sci Rep. 6(32188)2016.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Gupta R, Ghosh A, Singh AK and Misra A:
Clinical considerations for patients with diabetes in times of
COVID-19 epidemic. Diabetes Metab Syndr. 14:211–212.
2020.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Lixandru D BE, Vîrgolici B, Vîrgolici H,
Alexandru P, Băcanu ME, Gagniuc P, Ionescu-Tîrgovişte C and
Serafinceanu C: Changes in the serum proinflammatory cytokines in
patients with elevated HOMA-IR and type 2 diabetes mellitus.
Farmacia. 63:132–139. 2015.
|
|
86
|
Kalus AA CA and Oleraud JE: Diabetes
mellitus and other endocrine diseases. In: Fitzpatrick's
Dermatology in General Medicine. Goldsmith LA, Katz S, Gilchrest
BA, Paller AS, Leffel DJ and Wolff K (eds.). McGrawHill, New York,
NY, 2012.
|
|
87
|
Popa ML, Popa AC, Tanase C and
Gheorghisan-Galateanu AA: Acanthosis nigricans: To be or not to be
afraid. Oncol Lett. 17:4133–4138. 2019.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Hud JA Jr, Cohen JB, Wagner JM and Cruz PD
Jr: Prevalence and significance of acanthosis nigricans in an adult
obese population. Arch Dermatol. 128:941–944. 1992.PubMed/NCBI
|
|
89
|
Duff M, Demidova O, Blackburn S and
Shubrook J: Cutaneous manifestations of diabetes mellitus. Clin
Diabetes. 33:40–48. 2015.
|
|
90
|
Khalid U, Hansen PR, Gislason GH,
Lindhardsen J, Kristensen SL, Winther SA, Skov L, Torp-Pedersen C
and Ahlehoff O: Psoriasis and new-onset diabetes: A Danish
nationwide cohort study. Diabetes Care. 36:2402–2407.
2013.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Conforti C, Giuffrida R, Dianzani C, Di
Meo N and Zalaudek I: COVID-19 and psoriasis: Is it time to limit
treatment with immunosuppressants? A call for action. Dermatol
Ther: Mar 11, 2020 (Epub ahead of print). doi:
10.1111/dth.13298.
|
|
92
|
Tesch M: Spinal claudication and malum
perforans pedis. Late sequela of ankylosing spondylitis (Bechterew
disease) with cystic lumbosacral arachnopathy. Der Nervenarzt.
65:874–877. 1994.PubMed/NCBI(In German).
|
|
93
|
Begolli Gerqari A, Ferizi M, Halimi S,
Aferdita Daka A, Hapciu S, Begolli I, Begolli M and Gerqari I:
Malum perforans pedis - case report. Sci J Clin Med. 5:29–31.
2016.
|
|
94
|
Spravchikov N, Sizyakov G, Gartsbein M,
Accili D, Tennenbaum T and Wertheimer E: Glucose effects on skin
keratinocytes: Implications for diabetes skin complications.
Diabetes. 50:1627–1635. 2001.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Recalcati S: Cutaneous manifestations in
COVID-19: A first perspective. J Eur Acad Dermatol Venereol.
35:e212–e213. 2020.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Marano G, Vaglio S, Pupella S, Facco G,
Catalano L, Liumbruno GM and Grazzini G: Convalescent plasma: New
evidence for an old therapeutic tool? Blood Transfus. 14:152–157.
2016.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Chen N, Zhou M, Dong X, Qu J, Gong F, Han
Y, Qiu Y, Wang J, Liu Y, Wei Y, et al: Epidemiological and clinical
characteristics of 99 cases of 2019 novel coronavirus pneumonia in
Wuhan, China: A descriptive study. Lancet. 395:507–513.
2020.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Duan K, Liu B, Li C, Zhang H, Yu T, Qu J,
Zhou M, Chen L, Meng S, Hu Y, et al: Effectiveness of convalescent
plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA.
117:9490–9496. 2020.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Calina D, Docea AO, Petrakis D, Egorov AM,
Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Carvalho F,
Vinceti M, et al: Towards effective COVID-19 vaccines: Updates,
perspectives and challenges (Review). Int J Mol Med. 46:3–16.
2020.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Kickbusch I and Leung G: Response to the
emerging novel coronavirus outbreak. BMJ. 368(m406)2020.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Sun ML, Yang JM, Sun YP and Su GH:
Inhibitors of RAS might be a good choice for the therapy of
COVID-19 pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 43:219–222.
2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|