Introduction
The incidence of diabetes mellitus (DM) ranks first
among all metabolic disorders and is still rising (1). In 2014, ~387 million patients were
suffering from diabetes and it is predicted that >200 million
new cases will be diagnosed by 2035(2). A hyperglycemic state affects cell
functions, which induce the development of macro- and microvascular
diseases, such as diabetic nephropathy, retinopathy,
atherosclerosis, neuropathy and dysregulation of other major organs
(3-5).
The lungs are also a target of diabetes, and altered lung volume,
ventilation control, capacity of pulmonary diffusing and
neuroadrenergic bronchial innervation were frequently observed in
diabetic patients with a long course of the disease (6). However, to the best of the authors'
knowledge, studies on diabetic lung (DL) are rare and the
pathogenesis is still relatively unknown.
Genetic factors contribute to the development of
diabetic complications, and aberrant gene expression patterns are
closely correlated with the pathological changes in diabetic
patients (3-6).
Genes are dysregulated under a hyperglycemic state and the altered
gene expression participates in the development of diabetic
complications (7). Long non-coding
RNAs (lncRNAs; >200 nt) have critical roles in controlling
development of pathological changes under a hyperglycemic state by
regulating gene expression at multiple levels (8,9). It
was identified that the activation of p53 in diabetic mice induced
a pro-apoptotic gene expression pattern, suggesting the enhancing
effect of the gene on the progression of diabetic complications
(10). p53 may interact with
lncRNAs to achieve its functions (11). In a previous study, Li et al
(12) demonstrated that lncRNA
ZEB1-antisense RNA 1 (AS1) promoted the development of non-small
cell lung cancer by regulating Wnt/β-catenin signaling, which is
known to be involved in diabetic complications (3-5).
There is a hypothesis that this lncRNA may also participate in
other types of lung disease. The present study investigated the
role of ZEB1-AS1 in DL.
Materials and methods
Research subjects
The patients of the present study included 78 cases
of DL, pneumonia with excluded causes other than diabetes (41 males
and 37 females; 38 to 62 years old, with a mean age of 51.8±7.7
years), 78 patients with diabetes (DB) with obvious complications
(DB group; 41 males and 37 females; 37 to 65 years old, with a mean
age of 52.1±7.9 years) and 78 healthy volunteers (control group; 41
males and 37 females; 36 to 63 years old, with a mean age of
52.2±7.0 years). All the participants were selected from The
Affiliated Huaian No. 1 People's Hospital of Nanjing Medical
University between October 2016 and October 2018. Inclusion
criteria for DL and DB group consisted of the following: i) Newly
diagnosed pneumonia cases; ii) normal conditions of major organs
except lung in the DL group; and iii) no therapies initiated before
admission. Exclusion criteria for the DL and DB groups consisted of
the following: i) Other obvious clinical disorders were observed;
and ii) lung diseases other than diabetic lung. The three groups of
participants showed similar sex and age distributions, while white
blood cell count and C-reactive protein were significantly higher
in the DL group compared with the DB and control groups (P<0.05;
Table I). The general clinical data
of the three groups of participants are presented in Table I. The Ethics Committee of The
Affiliated Huaian No. 1 People's Hospital of Nanjing Medical
University approved the present study (Nanjing, China). All
patients were informed of the experimental principle, and all
patients signed informed consent.
 | Table IGeneral clinical data of the three
groups of participants. |
Table I
General clinical data of the three
groups of participants.
| Characteristic | DL n=78 | DB n=78 | Control n=78 |
|---|
| Age, years | 51.8±7.7 | 52.1±7.9 | 52.2±7.0 |
| Sex | | | |
|
Male | 41 | 41 | 41 |
|
Female | 37 | 37 | 37 |
| Disease duration
(years) | 9.9±2.3 | 3.1±1.1 | N/A |
| White blood cell
count, 109/l | 12.2±1.9a,b | 8.9±2.1 | 7.1±2.5 |
| C-reactive protein,
mg/l | 55.4±9.2a,b | 3.4±1.7 | 9.5±3.1 |
Plasma and cells
Fasting blood (~5 ml) was extracted from the elbow
vein of each participant on the day of admission and before the
initiation of any therapies. Blood was transferred to EDTA-treated
tubes, followed by centrifugation at 1,200 x g for 10 min at room
temperature to prepare the plasma samples.
The BEAS-2B normal human lung cell line (American
Type Culture Collection) was used to perform all in vitro
cell experiments. Eagle's MEM (Sigma-Aldrich; Merck KGaA) was mixed
with 10% FBS (Sigma-Aldrich; Merck KGaA) and the mixture was used
to cultivate BEAS-2B cells. Cells were cultivated under conditions
of 37˚C and 5% CO2.
Reverse transcription-quantitative PCR
(RT-qPCR)
Plasma and BEAS-2B cells were mixed with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) to extract total RNA. Following DNase I digestion, AMV
Reverse Transcriptase XL (Takara Bio, Inc.) was used to synthesize
cDNA by reverse transcription through the following temperature
protocol: 25˚C for 10 min, 55˚C for 10 min and 85˚C for 10 min.
SYBR®-Green Realtime PCR Master Mix (Toyobo Life
Science) was used to prepare all PCR mixtures to detect the
expression of ZEB1-AS1 and p53 mRNA with 18S rRNA, and GAPDH was
used as an endogenous control. PCR reactions were performed using
the following thermocycling conditions: Initial denaturation at
95˚C for 1 min, followed by 40 cycles of 95˚C for 10 sec and 55˚C
for 40 sec. Primer sequences were as follows: ZEB1-AS1: 5'-CCG
TGGGCACTGCTGAA-3' (forward) and 5'-CTGCTGGCA AGCGGAAC-3' (reverse);
p53: 5'-CTGTCATCTTCTGTC CCTT-3' (forward) and
5'-TGGAATCAACCCACAGCTG-3' (reverse); 18S rRNA:
5'-CTACCACATCCAAGGAAGC-3' (forward) and 5'-TTTTTCGTCACTACCTCCCC-3'
(reverse); and GAPDH: 5'-GGTCTCCTCTGACTTCAAC-3' (forward) and
5'-TGAGGGTCTCTCTCTTCCT-3' (reverse). All reactions were repeated
three times and Cq values were processed using the
2-ΔΔCq method (13).
ELISA
The Human p53 ELISA kit (cat. no. ab46067; Abcam)
was used to measure the levels of p53 in plasma of all three groups
of participants. Plasma p53 levels were normalized to pg/ml.
Transient cell transfection
ZEB1-AS1 and p53 expression vectors were constructed
by Sangon Biotech Co., Ltd. using pcDNA3.1. BEAS-2B cells were
harvested at a confluence of 70-90%, and 10 nM ZEB1-AS1
(NR_024284.1) and p53 (AB082923.1) expression vector or 10 nM empty
pcDNA3.1 (negative control; NC) were transfected into
105 BEAS-2B cells using Lipofectamine® 2000
reagent (Thermo Fisher Scientific, Inc.). The control group
included cells without transfections. All subsequent experiments
were performed using cells collected 24 h after transfections.
Cell apoptosis assay
BEAS-2B cells (3x104) were harvested 24 h
after transfections and mixed with 1 ml Eagle's MEM, which was
supplemented with 10% FBS to prepare single cell suspensions. Cell
suspensions were transferred to 6-well plates with 2 ml per well,
followed by the addition of 0, 10 and 30 mM D-glucose. A total of
three replicates were set for each concentration. Cells were
cultivated under the conditions of 37˚C and 5% CO2 for
48 h. After that, 25% trypsin digestion was performed and Annexin
V-FITC (Dojindo Molecular Technologies, Inc.) and propidium iodide
(PI; Dojindo Molecular Technologies, Inc.) were used to stain the
cells. All operations were performed in strict accordance with the
manufacturer's instructions. Finally, apoptotic cells were detected
by performing flow cytometry using BD Biosciences 2 Laser
FACSCalibur Flow Cytometer w/HTS Autosampler (BD Biosciences). Data
were processed using BD FACSDiva™ software (version 8.0; BD
Biosciences).
Western blotting
BEAS-2B cells (105) were collected 24 h
after transfections and mixed with 1 ml RIPA solution (Thermo
Fisher Scientific, Inc.) to extract total protein. Bicinchoninic
Acid assay (Thermo Fisher Scientific, Inc.) was performed to
determine the protein concentration. Following protein denaturing
and 10% SDS-PAGE, proteins (30 µg per lane) were transferred to
PVDF membranes. After blocking in 5% non-fat milk (2 h at 25˚C),
p53 (1:1,200; cat. no. ab131442; Abcam) and GAPDH (1:1,000; cat.
no. ab9485; Abcam) rabbit polyclonal primary antibodies were used
to incubate with membranes (4˚C overnight), followed by incubation
with IgG-HRP goat anti-rabbit secondary antibody (1:1,000; cat. no.
MBS435036; MyBioSource, Inc.) for 2 h at 25˚C. Signals were
developed using ECL (Thermo Fisher Scientific, Inc.) and
densitometry was performed using ImageJ v1.46 software (National
Institutes of Health).
Statistical analysis
Sample sizes in the present study provide sufficient
statistical power in all cases (0.87-0.93). Statistical analysis
was performed using GraphPad Prism 6 software (GraphPad Software,
Inc.). All mean ± SD values presented in the present study
represent the data from three biological replicates. Differences
among different patient and cell groups were analyzed by performing
one-way ANOVA and Tukey's test. Correlations were analyzed by
performing Pearson's correlation coefficient. Diagnostic analyses
were performed using receiver operating characteristic (ROC) curve
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
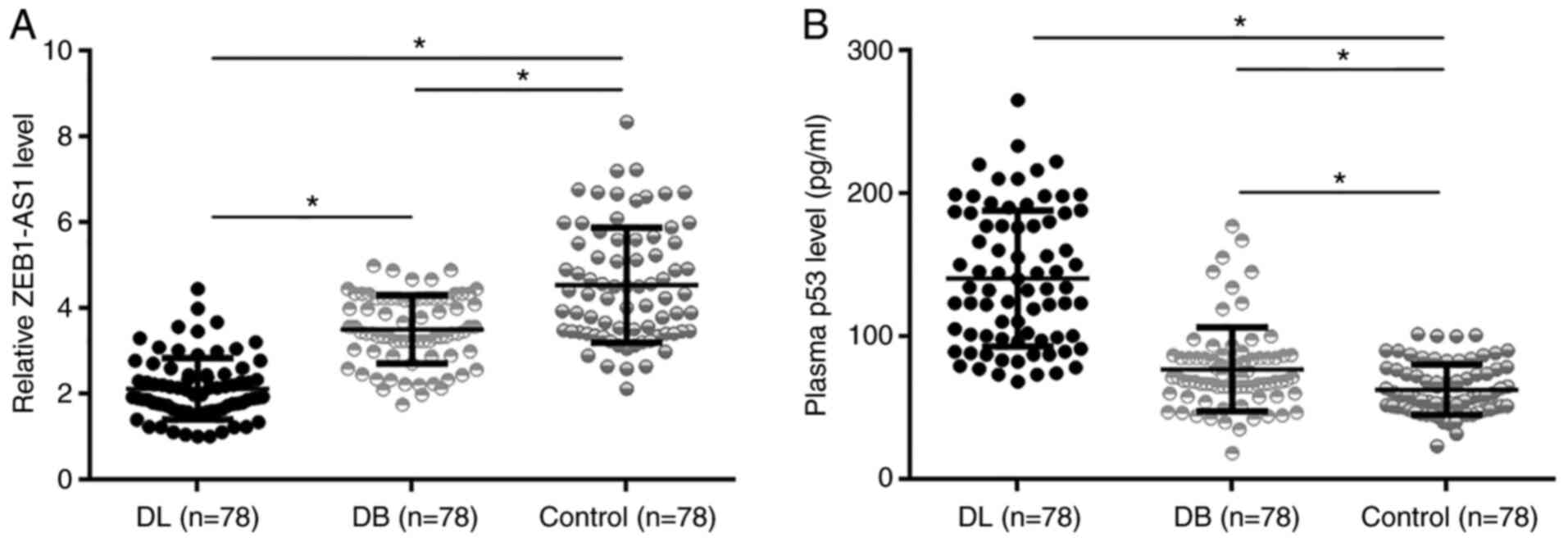
ZEB1-AS1 and p53 are dysregulated in
the DL group
ZEB1-AS1 and p53 in plasma were detected using
RT-qPCR and ELISA, respectively. The data were compared among the
DL (n=78), DB (n=78) and control (n=78) groups by performing
one-way ANOVA and Tukey's test. It was identified that ZEB1-AS1
levels were significantly lower in the DL group than in the other
two groups (1.6- and 2.4-fold, respectively), and the DB group also
showed lower ZEB1-AS1 levels than the control group (1.5-fold,
Fig. 1A; P<0.05). In contrast,
p53 levels were significantly higher in the DL group than in the
other two groups (1.9 and 2.9-fold, respectively), and the DB group
also showed higher p53 levels than the control group (1.5-fold;
Fig. 1B; P<0.05).
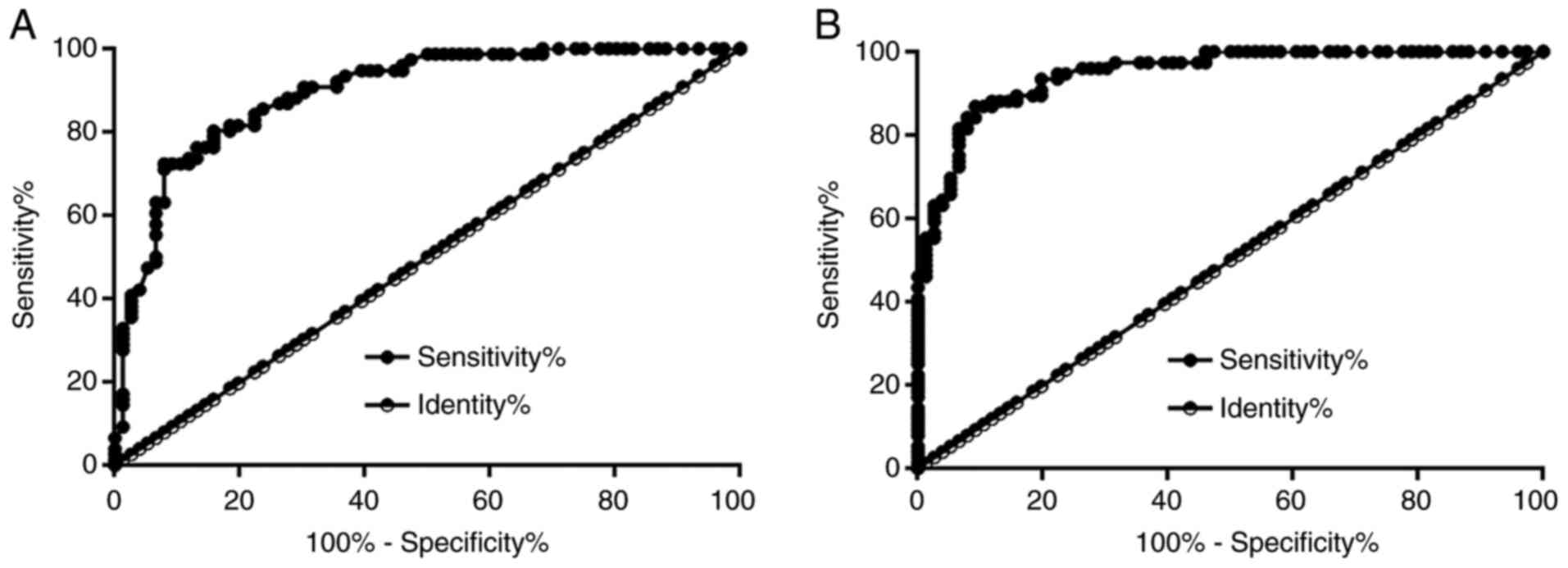
Downregulated plasma ZEB1-AS1
distinguishes patients with DL from the DB and control groups
The diagnostic value of ZEB1-AS1 for DL was
evaluated by performing ROC curve analysis. In the present study,
participants in the DB or the control group were used as true
negative cases and the patients with DL were true positive cases.
With DB as true negative cases, the area under the curve (AUC) was
0.89, with a standard error of 0.025 and 95% confidence interval of
0.84-0.94 (Fig. 2A). With the
control and the true negative cases, the AUC was 0.95, with a
standard error of 0.016 and 95% confidence interval of 0.92-0.98
(Fig. 2B).
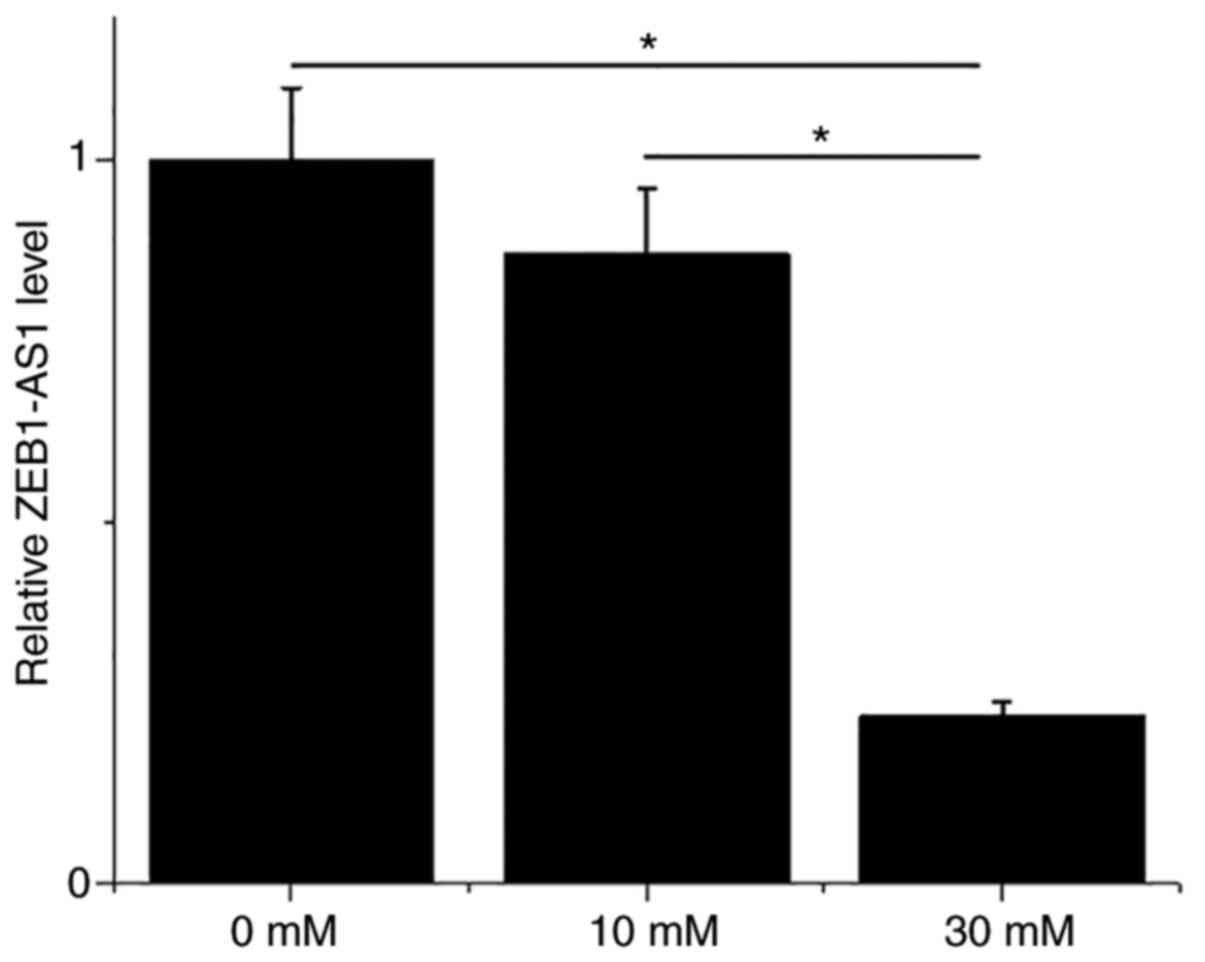
D-glucose treatment downregulates
ZEB1-AS1 in BEAS-2B cells
BEAS-2B cells were treated with 0, 10 and 30 mM
glucose for 48 h, followed by the detection of ZEB1-AS1 by
performing RT-qPCR. It was observed that compared with 0 and 10 nM
glucose, treatment with 30 mM glucose significantly downregulated
the expression levels of ZEB1-AS1 (Fig.
3; P<0.05). However, treatment with 10 nM D-glucose only led
to slightly downregulated ZEB1-AS1 in BEAS-2B cells compared with
the untreated cells.
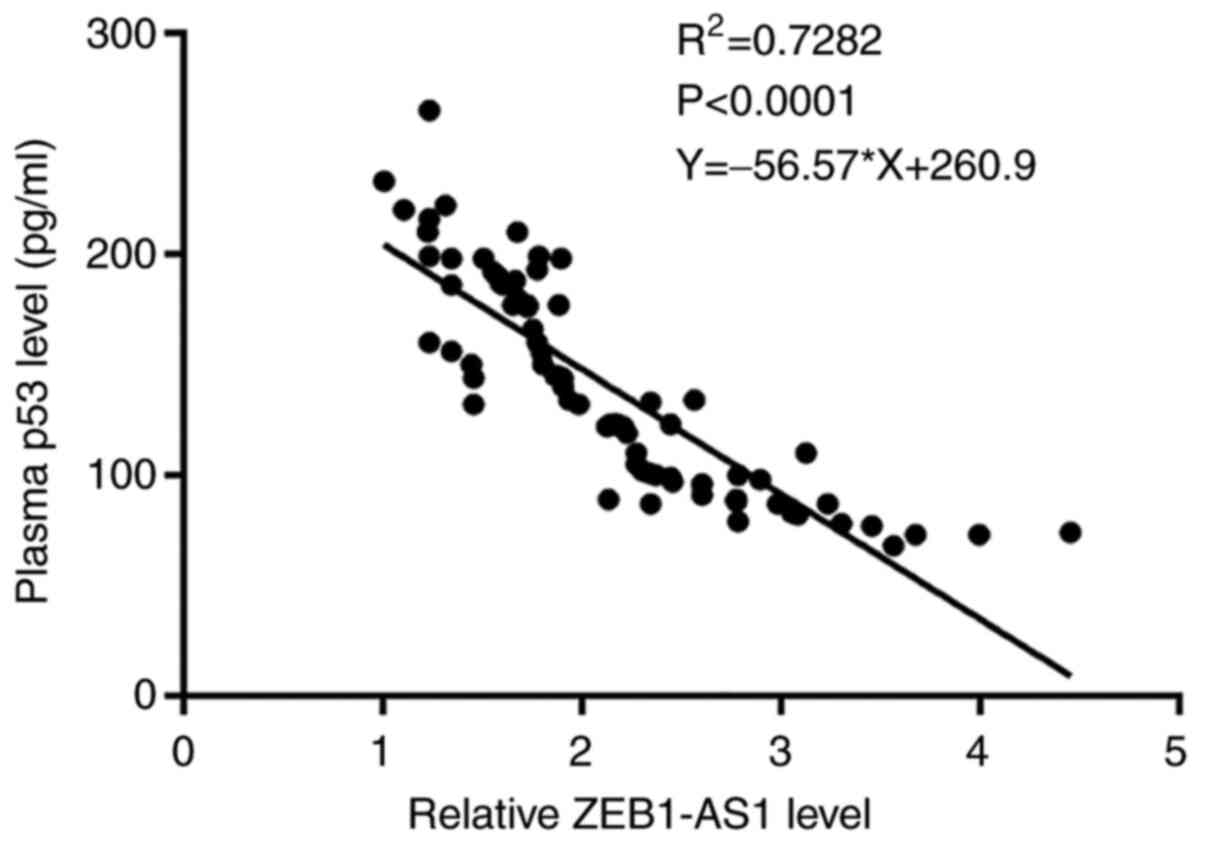
Plasma levels of ZEB1-AS1 and p53 are
negatively correlated in the DL group
The correlation between the plasma levels of
ZEB1-AS1 and p53 in the DL group was analyzed by performing
Pearson's correlation coefficient. R2>0.65 indicates
a promising linear correlation. It was observed that the plasma
levels of ZEB1-AS1 were negatively and significantly correlated
with the plasma levels of p53 (Fig.
4; R2=0.7282; P<0.0001).
ZEB1-AS1 downregulates p53 to inhibit
BEAS-2B cell apoptosis under glucose treatment
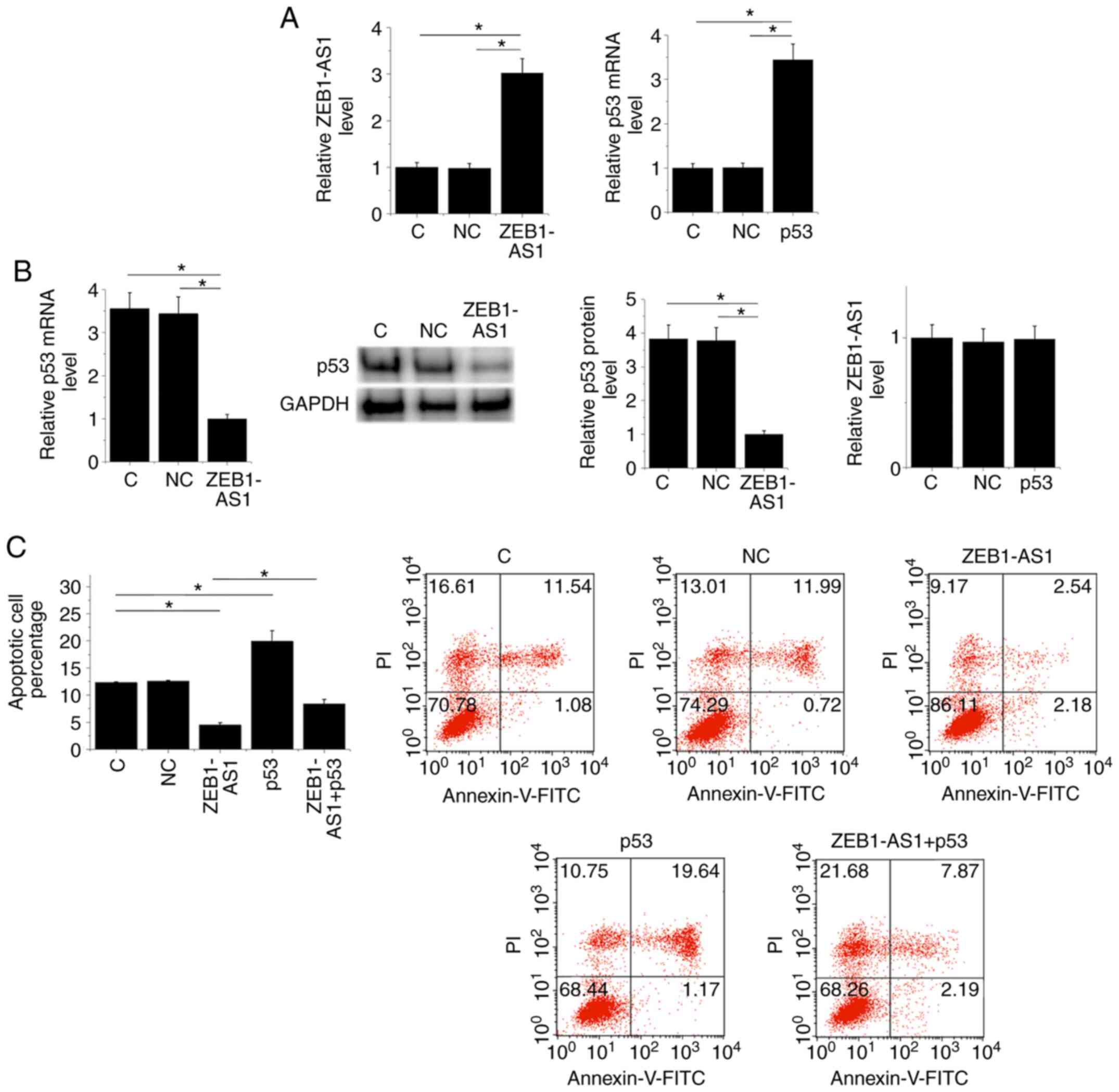
The ZEB1-AS1 and p53 expression vectors were
transfected into BEAS-2B cells. At 24 h after transfections,
expression levels of ZEB1-AS1 and p53 were significantly increased
compared with the control (C) and negative control (NC) groups
(Fig. 5A; P<0.05). Moreover,
ZEB1-AS1 overexpression led to the downregulation of p53 at both
the mRNA and protein levels, while p53 overexpression failed to
affect ZEB1-AS1 (Fig. 5B;
P<0.05). A cell apoptosis assay was performed to analyze the
effects of transfections on cell apoptosis. Cells apoptosis data
were compared among the groups by performing one-way ANOVA and
Tukey's test. Compared with the two controls, overexpression of
ZEB1-AS1 inhibited apoptosis, while p53 overexpression promoted
lung cancer cell apoptosis. Moreover, p53 overexpression attenuated
the effects of ZEB1-AS1 overexpression on lung cell apoptosis
(Fig. 5C; P<0.05).
Discussion
The expression pattern and functions of ZEB1-AS1 in
DL were investigated in the present study. ZEB1-AS1 was
downregulated in DL and it was identified that ZEB1-AS1
overexpression may inhibit the apoptosis of lung cells in a high
glucose environment by downregulating p53.
p53 is a well-characterized tumor suppressor that
inhibits the progression of different types of cancer by inducing
the apoptosis of cancer cells (14). However, the pro-apoptotic roles of
p53 may not be beneficial for use in humans. In the development of
other types of diseases, such as diabetic complications, the
activation of p53 in patients with diabetes promotes the apoptosis
of cells in many major organs, including the lungs, which thereby
promotes the development of diabetic complications (15). In effect, inhibition of p53 could
provide a novel approach for the prevention and treatment of
diabetic complications (16). In
the present study, upregulated p53 in the DL group and the
increased apoptotic rate of lung cells in a high glucose
environment after p53 overexpression was observed. Therefore, p53
may promote the development of DL by inducing lung cell
apoptosis.
ZEB1-AS1 is upregulated in patients with lung cancer
and it may promote cancer development by interacting with the
Wnt/β-catenin pathway (12). It is
widely accepted that Wnt/β-catenin signaling participates in the
development of diabetic complications (3-5).
Therefore, ZEB1-AS1 may also participate in the development of
diabetic lung.
The present study demonstrated that ZEB1-AS1 was
downregulated in patients with DL, and downregulated ZEB1-AS1 may
serve as a marker for the diagnosis of DL. ZEB1-AS1 was identified
as an inhibitor of p53 in lung cells, and the inhibition of p53 by
ZEB1-AS1 was involved in the regulation of lung cell apoptosis in a
high glucose environment. Therefore, the overexpression of ZEB1-AS1
may be a potential target to inhibit the apoptosis of lung cells in
patients with diabetes. It is known that Wnt/β-catenin has
cross-talk with p53 in many types of diseases (17,18).
Therefore, Wnt/β-catenin may be a mediator for the interactions
between Wnt/β-catenin and p53; however, this requires further
investigation.
The present study investigated the involvement of an
lncRNA in DL. To the best of the authors' knowledge, studies on
lncRNAs in DL are rare. However, the present study is limited by
the small sample size and the lack of animal model studies. In
conclusion, ZEB1-AS1 was downregulated in DL, and ZEB1-AS1
overexpression may improve DL by downregulating p53.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LG designed all experiments. LG and HS performed
experiments. ZY collected and analyzed data. LG drafted the
manuscript. All authors reviewed and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of The Affiliated Huaian No. 1
People's Hospital of Nanjing Medical University (approval no.
2016-181; Nanjing, China) approved the present study. All patients
were informed of the experimental principle, and all patients
signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ingelfinger JR and Jarcho JA: Increase in
the incidence of diabetes and its implications. N Engl J Med.
376:1473–1474. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
da Rocha Fernandes J, Ogurtsova K,
Linnenkamp U, Guariguata L, Seuring T, Zhang P, Cavan D and
Makaroff LE: IDF Diabetes Atlas estimates of 2014 global health
expenditures on diabetes. Diabetes Res Clin Pract. 117:48–54.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Forbes JM and Cooper ME: Mechanisms of
diabetic complications. Physiol Rev. 93:137–188. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kato M, Castro NE and Natarajan R:
MicroRNAs: Potential mediators and biomarkers of diabetic
complications. Free Radic Biol Med. 64:85–94. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Reddy MA, Zhang E and Natarajan R:
Epigenetic mechanisms in diabetic complications and metabolic
memory. Diabetologia. 58:443–455. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pitocco D, Fuso L, Conte EG, Zaccardi F,
Condoluci C, Scavone G, Incalzi RA and Ghirlanda G: The diabetic
lung - a new target organ? Rev Diabet Stud. 9:23–35.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ahlqvist E, van Zuydam NR, Groop LC and
McCarthy MI: The genetics of diabetic complications. Nat Rev
Nephrol. 11:277–287. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Leung A and Natarajan R: Long noncoding
RNAs in diabetes and diabetic complications. Antioxid Redox Signal.
29:1064–1073. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Leung A, Amaram V and Natarajan R: Linking
diabetic vascular complications with LncRNAs. Vascul Pharmacol.
114:139–144. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kung CP and Murphy ME: The role of the p53
tumor suppressor in metabolism and diabetes. J Endocrinol.
231:R61–R75. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Grossi E, Sánchez Y and Huarte M:
Expanding the p53 regulatory network: LncRNAs take up the
challenge. Biochim Biophys Acta. 1859:200–208. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li L, Wang QF, Zou ML and He XA:
Overexpressed lncRNA ZEB1-AS1 promotes cell invasion and
angiogenesis through Wnt/β-catenin signaling in non-small cell lung
cancer. Int J Clin Exp Pathol. 10:3990–3997. 2017.
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Labuschagne CF, Zani F and Vousden KH:
Control of metabolism by p53 - Cancer and beyond. Biochim Biophys
Acta Rev Cancer. 1870:32–42. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Peng J, Li X, Zhang D, Chen JK, Su Y,
Smith SB and Dong Z: Hyperglycemia, p53, and mitochondrial pathway
of apoptosis are involved in the susceptibility of diabetic models
to ischemic acute kidney injury. Kidney Int. 87:137–150.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gu J, Wang S, Guo H, Tan Y, Liang Y, Feng
A, Liu Q, Damodaran C, Zhang Z, Keller BB, et al: Inhibition of p53
prevents diabetic cardiomyopathy by preventing early-stage
apoptosis and cell senescence, reduced glycolysis, and impaired
angiogenesis. Cell Death Dis. 9(82)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang DY, Wang HJ and Tan YZ:
Wnt/β-catenin signaling induces the aging of mesenchymal stem cells
through the DNA damage response and the p53/p21 pathway. PLoS One.
6(e21397)2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gu Z, Tan W, Feng G, Meng Y, Shen B, Liu H
and Cheng C: Wnt/β-catenin signaling mediates the senescence of
bone marrow-mesenchymal stem cells from systemic lupus
erythematosus patients through the p53/p21 pathway. Mol Cell
Biochem. 387:27–37. 2014.PubMed/NCBI View Article : Google Scholar
|