Introduction
Epidemiological studies have revealed that ischemic
cerebrovascular disease (ICD) is the second leading cause of
mortality in humans worldwide, with a continually rising incidence
rate (1,2). Currently, thrombolysis and bypass
grafting are the two most commonly used therapeutic strategies for
the treatment of ICD; however, they are often unsuccessful,
resulting in a lack of effective treatment strategies for cerebral
ischemia-reperfusion (I/R) injury (CIRI) (3). Therefore, understanding the specific
mechanisms underlying reperfusion injury is of clinical importance.
Numerous studies have demonstrated that inflammation is an
important pathological factor contributing to CIRI (4-6).
It has also been reported that a particular type of proinflammatory
programmed cell death, pyroptosis, also serves an important role in
the pathogenesis of I/R injury (7).
The main biological characteristics of pyroptosis
are dependent on caspase-1(8).
Cookson and Brennan (9) were the
first to propose the concept of pyroptosis, which has subsequently
been reported to occur during I/R injury in numerous organs, with
the levels of pyroptosis reflecting the degree of I/R to a certain
extent (10-12).
Therefore, the identification of novel therapeutic targets to
inhibit pyroptosis may lead to novel therapeutic strategies for
CIRI.
Almost all in vivo physiological and
pathological processes, such as growth and development,
proliferation, inflammation, tumorigenesis and neurodegenerative
diseases, are subject to microRNA (miRNA/miR)-mediated target gene
expression regulation (13-15).
In particular, miRNA-124 is highly expressed in the central nervous
system, with 100 times higher expression levels in the central
nervous system compared with other tissues (16). The increased expression levels of
miRNA-124 exert important neuroprotective effects; for example,
during the early stages of ischemic stroke, miR-124 injection
decreased both oxygen deprivation- and glucose deprivation-induced
neuronal damage (17). In addition,
Ponomarev et al (18)
demonstrated that increased miRNA-124 expression levels prevented
allergic cerebrospinal meningitis by opposing microglial
activation. The aforementioned study suggested that the
neuroprotective effects of miRNA-124 may be closely linked to its
effects on inflammation. Therefore, it was hypothesized that
pyroptosis, as a proinflammatory form of programmed cell death, may
be modulated by miRNA-124 during CIRI; however, the specific
mechanisms by which this occurs are not completely understood.
The present study aimed to investigate miRNA-124
expression levels during the early stage of CIRI in a rat model, as
well as the level of pyroptosis, which was determined by analyzing
the expression levels of caspase-1, gasdermin D, interleukin
(IL)-18 and IL-1β. Furthermore, the effects of altered miRNA-124
expression on STAT3 activation and the degree of pyroptosis were
also investigated. The results of the present study provided a
basis for determining the possible early pathogenic mechanisms
underlying CIRI and for the development of improved clinical
strategies.
Materials and methods
Animal studies
Rats were purchased from Beijing Huafukang
Biotechnology Co., Ltd. and housed at the Animal Experimental
Center of North China University of Technology at 20±2˚C with 55±5%
humidity, 12-h light/dark cycles, and access to food and water
ad libitum. All rats were acclimatized for a week with food
and water before experimental manipulation. The present study was
approved by the Animal Ethics Committee of North China University
of Technology (approval no. LX201901).
Middle cerebral artery occlusion
(MCAO) model preparation and experimental grouping
A total of 58 adult male Sprague-Dawley rats (age,
50-60 days; weight, 250-280 g) were randomly divided into four
groups: i) Control; ii) sham operation, iii) I/R; and iv) drug
treatment. The I/R group was further divided into four subgroups:
3, 6, 12 and 24 h post-reperfusion, for sample collection. The drug
treatment group was further divided into three subgroups: i) I/R +
negative control (NC); ii) I/R + miRNA-124 agonist (agomiRNA-124);
iii) and I/R + miRNA-124 antagonist (antagomiRNA-124). Rats were
used for the following experiments: 2-3-5 triphenyl tetrazolium
chloride (TTC) staining (n=14; sham (n=4), NC (n=3), agomiRNA-124
(n=3) and antagomiRNA-124 groups (n=4)); 30 were used for western
blotting and reverse transcription-quantitative PCR (RT-qPCR; n=30;
sham (n=4), control (n=4), I/R (n=12) and drug (n=10) groups); and
immunohistochemistry (n=14; sham (n=4) and drug (n=10) groups).
Rats in the I/R and drug groups were subjected to CIRI, rats in the
sham operation group underwent the same surgical operation but
without an inserted suture and rats in the control group were not
subjected to surgical operation. MCAO was performed in the left
hemisphere to induce CIRI as previously described (19,20).
Briefly, rats were anesthetized with an intraperitoneal injection
of 2% sodium pentobarbital (30 mg/kg) (21). Subsequently, the common carotid
artery (CCA) was separated from the nerves and tissue to gently
expose the internal carotid artery (ICA) and the external carotid
artery (ECA). The ECA was clipped and the ECA stump was stretched
to align it with the ICA. A length of 3.0 cm plugging-up suture
(cat. no. 3600AAA; Guangzhou Jialing Biological Technology Co.,
Ltd.) with its rounded tip was inserted to cut off the origin of
the middle cerebral artery. Finally, the microvascular clip was
removed and the thread was fastened. The length of insertion was
18.5-19.5 mm from the CCA bifurcation. Following ischemia for 2 h,
the monofilament was removed to induce CIRI.
Drug administration and neurological
deficit scoring
At 2 days prior to MCAO establishment, rats received
an intracerebroventricular injection of 20 nM miRNA-124 agonist (5
µl; Guangzhou RiboBio Co., Ltd.), 20 nM miRNA-124 antagonist (5 µl;
Guangzhou RiboBio Co., Ltd.) or 20 nM miRNA-124 negative control (5
µl; Guangzhou RiboBio Co., Ltd.) for 5 consecutive days. All
experiments were performed under anesthetic and the lateral
ventricle was located [coordinates from the bregma:
Anterior-posterior (AP)=-0.8 mm, lateral (L)=-1.5 mm, ventral
(V)=-4.8 mm] based on a stereotaxic atlas. Animals with hind limb
paralysis or paresis following surgery were excluded from the study
to rule out the effects of anesthetics and operative failures.
Three rats died from operation (sham operation group excluded two
rats in TTC straining and immunohistochemistry respectively;
control group excluded one rat).
Neurological deficits were scored by one
investigator after the rats awakened. The scores were evaluated
using the 5-point scale described by Longa et al (19) as follows: 0, no neurological
deficit; 1, failure to adequately extend the left forepaw; 2,
circling to the left; 3, falling to the left; 4, depressed level of
consciousness without spontaneous walking; and 5, death. Rats
subjected to MCAO without detectable neurological deficits or death
were removed from the following experiments to exclude the
interference arising from operative failures (Three rats that died
were excluded).
RT-qPCR
Total RNA was extracted from brain tissues using the
Total RNA Purification kit (Suzhou GenePharma Co., Ltd.). Total RNA
was reverse transcribed into cDNA using an miRNA RT primer and MMLV
reverse transcriptase (contained in Hairpin-it™ miRNA RT-PCR
Quantitative kit; cat. no. E22007; Suzhou GenePharma Co., Ltd.), at
25˚C for 30 min, 42˚C for 30 min and 85˚C for 5 min, then stored at
4˚C, according to the manufacturer's protocol. Subsequently, qPCR
was performed using the QuantStudio 6 Flex Detection system (Thermo
Fisher Scientiic, Inc.) and a rno-miRNA-124 probe Hairpin-it™ miRNA
and U6 snRNA Normalization RT-PCR Quantitation kit (cat. no.
E22007; Suzhou Jima Gene Co., Ltd.), according to the
manufacturer's protocol. The following primers were used for qPCR:
miRNA-124 forward, 5'-TGTCTCTAAGGCACGCGGT-3' and reverse,
5'-TATGGTTGTTCACGACTCCTTCAC-3'; and miRNA-124 probe,
5'-ATGCC+GTCT+GTATGGTTGGATAGGGA-3', U6 forward,
5'-CGCTTCGGCAGCACATATACTAA-3' and reverse,
5'-TATGGAACGCTTCACGAATTTGC-3'. The following thermocycling
conditions were used for qPCR: Initial denaturation at 95˚C for 3
min; followed by 40 cycles of 12 sec at 95˚C and 40 sec at 62˚C;
the fluorescence signal was collected after 40 cycles. miRNA
expression levels were quantified using the 2-ΔΔCq
method and normalized to the internal reference gene U6(22).
Western blotting
Total protein was extracted from fresh cerebral
cortex in the ischemic penumbra. Total protein was quantified using
a bicinchoninic acid assay kit (cat. no. PT0001; Beijing Leagene
Biotechnology Co., Ltd.). Proteins (30 µg/lane) were separated via
a 10% SDS-PAGE, transferred onto PVDF membranes and blocked with 5%
BSA for 1 h at room temperature. Subsequently, the membranes were
incubated at 4˚C overnight with the following primary antibodies:
Anti-gasdermin D (1:500; cat. no. sc-393581; Santa Cruz
Biotechnology, Inc.), anti-STAT3 (1:1,000; cat. no. 12640; Cell
Signaling Technology, Inc.), phosphorylated (p)-STAT3 (1:1,000;
cat. no. 52075; Cell Signaling Technology, Inc.),
anti-pro-caspase-1 (1:1,000; cat. no. ab179515; Abcam),
anti-caspase-1 p20 (1:1,000; 2225, Cell Signaling Technology,
Inc.), anti-IL-1β (1:1,000, cat. no. 12242; Cell Signaling
Technology, Inc.), anti-IL-18 (1:1,000; cat. no. ab71495; Abcam) or
anti-β-actin (1:2,000; cat. no. T0023; BIOSS). Following primary
incubation, the membranes were incubated with horseradish
peroxidase-conjugated anti-rabbit or anti-mouse secondary
antibodies (1:5,000; ZB2307, Zsbio Commerce Store) for 2 h at 37˚C.
The membranes were rinsed three times with TBS-0.1% Tween-20 for 10
min. Protein bands were visualized using EXPlus ECL reagent
(Beijing Zoman Biotechnology Co., Ltd.) and the expression levels
were quantified using ImageJ software (version 1.6.0; National
Institutes of Health) with β-actin as the loading control.
TargetScan and Dual-luciferase
reporter assay
The online target gene prediction software
TargetScan (http://www.targetscan.org/vert_72/) was used to
analyze the potential target genes of miRNA-124 and find the STAT3
is one of the target genes of miRNA-124. Dual-luciferase reporter
plasmids containing the wild-type (WT) or mutant (MT)
3'-untranslated region (UTR) of STAT3 (pGL3-Promoter Vector, R0531,
Promega Corporation) were constructed. PC12 cells (1x105
cells/ml; Chinese Academy of Sciences Cell Bank) were
co-transfected with plasmids (2 µg) and miRNA-124 mimics (50 nM) or
miRNA-124 negative control (50 nM) using TurboFect transfection
reagent (R0531, Thermo Fisher Scientific). The following cell
groups were generated: i) WT plasmid + miRNA-124 negative control
group (WT-STAT3 + NC); ii) WT plasmid + miRNA-124 mimic group
(WT-STAT3 + miRNA-124); iii) MT plasmid + miRNA-124 negative
control group (MT-STAT3 + NC); and iv) MT plasmid + miRNA-124 mimic
group (MT-STAT3 + miRNA-124). At 48 h post-transfection, a
dual-luciferase reporter assay system kit (cat. no. E1910; Promega
Corporation) was used to determine the relative luciferase
activity. Relative luciferase activity=Firefly
luciferase/Renilla luciferase.
Immunohistochemistry
Animals were anesthetized with an intraperitoneal
injection of 2% sodium pentobarbital (30 mg/kg) and the ascending
aorta was immediately perfused with 4% paraformaldehyde.
Subsequently, the brain tissue was fixed in 4% paraformaldehyde for
24 h at 4˚C and the infarct sections were embedded in paraffin and
cut into 3 µm slices. Sections were blocked in 10% normal goat
serum (cat. no. SP-9000; OriGene Technologies) for 2 h at room
temperature and then incubated at 4˚C overnight with the following
primary antibodies: Anti-gasdermin D (1:50; cat. no. sc-81868;
Santa Cruz Biotechnology, Inc.), anti-caspase-1 (1:100; 2225, Cell
Signaling Technology, Inc.), anti-IL-1β (1:100; cat. no. 12242;
Cell Signaling Technology, Inc.) and anti-IL-18 (1:100; cat. no.
ab52914; Abcam). Following primary incubation, the sections were
incubated with Cy3-conjugated and fluorescein
isothiocyanate-conjugated secondary antibodies (1:50; cat. no.
ZF-0311; OriGene Technologies) for 1 h at room temperature. Stained
sections were visualized using a DP-80 fluorescence microscope
(Olympus Corporation) in ten randomly selected fields of view and
the positive cell rate was calculated as follows: (Positive
cells/total cells) x100%.
TTC staining
Brain tissues were obtained following 12 h
reperfusion and immediately stored at -20˚C for 30 min. Frozen
brain tissue was cut into five coronal 2-mm thick sections. The
sections were placed in TTC staining solution (cat. no. DK003;
Beijing Leagene Biotech Co., Ltd.) to incubation at 37˚C for 30 min
in the dark, and turned over at 15 min. TTC staining solution
reacted with the dehydrogenase in the healthy brain tissue to stain
the healthy brain tissue red. By contrast, decreased dehydrogenase
activity in the ischemic brain tissue was indicated by a pale
color, as the lack of dehydrogenase did not react with the TTC
staining solution. Subsequently, TTC stained brain tissue sections
were fixed in 4% paraformaldehyde at 4˚C for 24 h. ImageJ software
(version 1.6.0; National Institutes of Health) was used to
calculate the infarct area, which was expressed as a percentage (%)
of the infarct area. The ischemic area ratio (%)=(sum of white
ischemic areas)/(sum of brain slice areas) x100%.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 5.0; GraphPad Software, Inc.). Data are
presented as the mean ± SD with at least three experimental
repeats. Statistical differences among multiple groups were
determined using one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Dynamic expression levels of
pyroptosis-associated proteins and proinflammatory cytokines
following CIRI
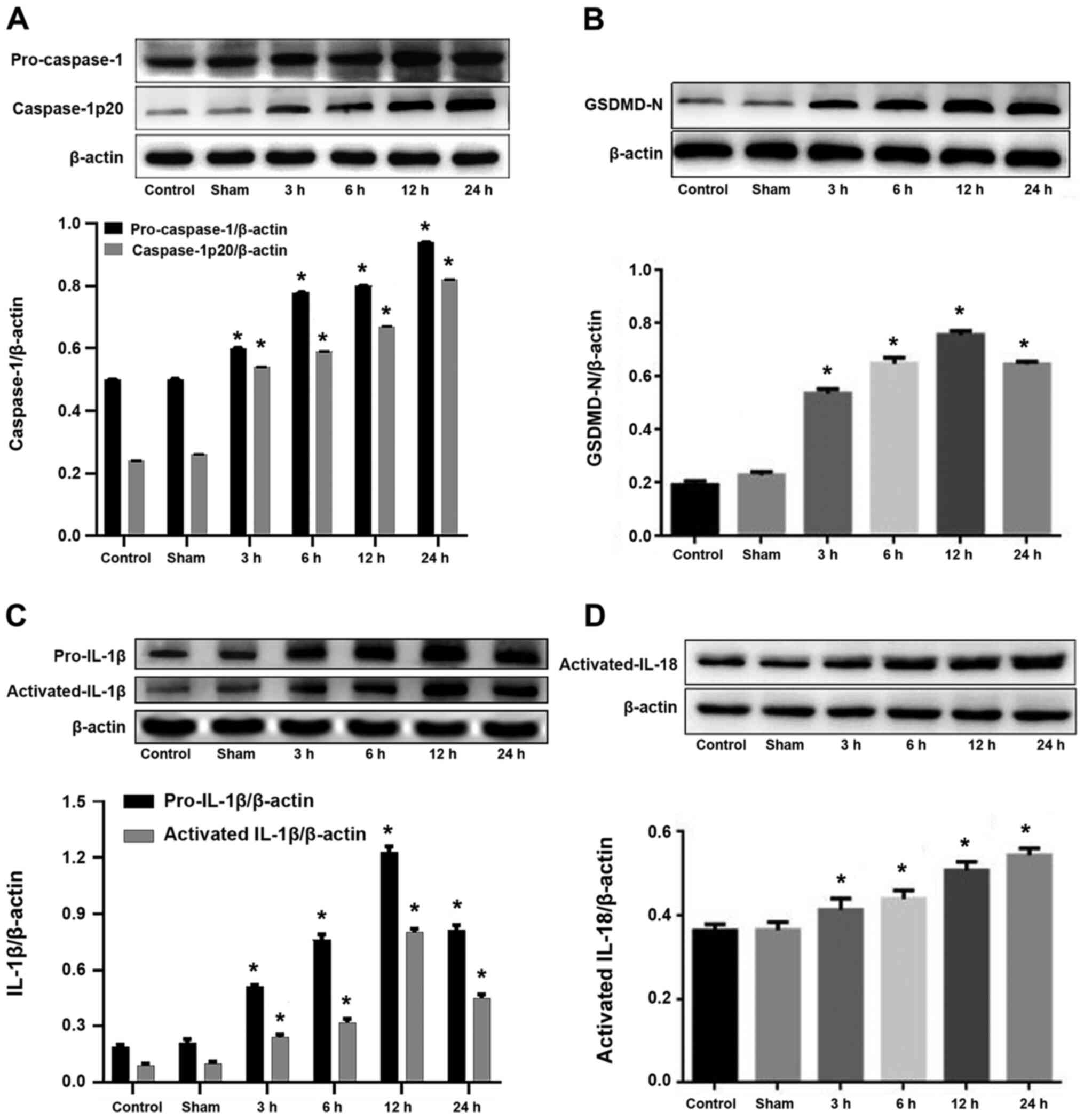
The western blotting results indicated that the
expression levels of caspase-1 (Fig.
1A) and IL-18 (Fig. 1D) were
significantly increased in the I/R groups compared with the control
group, reaching a peak at 24 h following CIRI (P<0.05). In
addition, the expression levels of gasdermin D (Fig. 1B) and IL-1β (Fig. 1C) were significantly increased in
the I/R groups compared with the control group, peaking at 12 h but
decreasing by 24 h following CIRI (P<0.05). There was no
significant difference in expression levels of
pyroptosis-associated proteins and proinflammatory cytokines
observed between the sham operation and control groups (P>0.05;
Fig. 1).
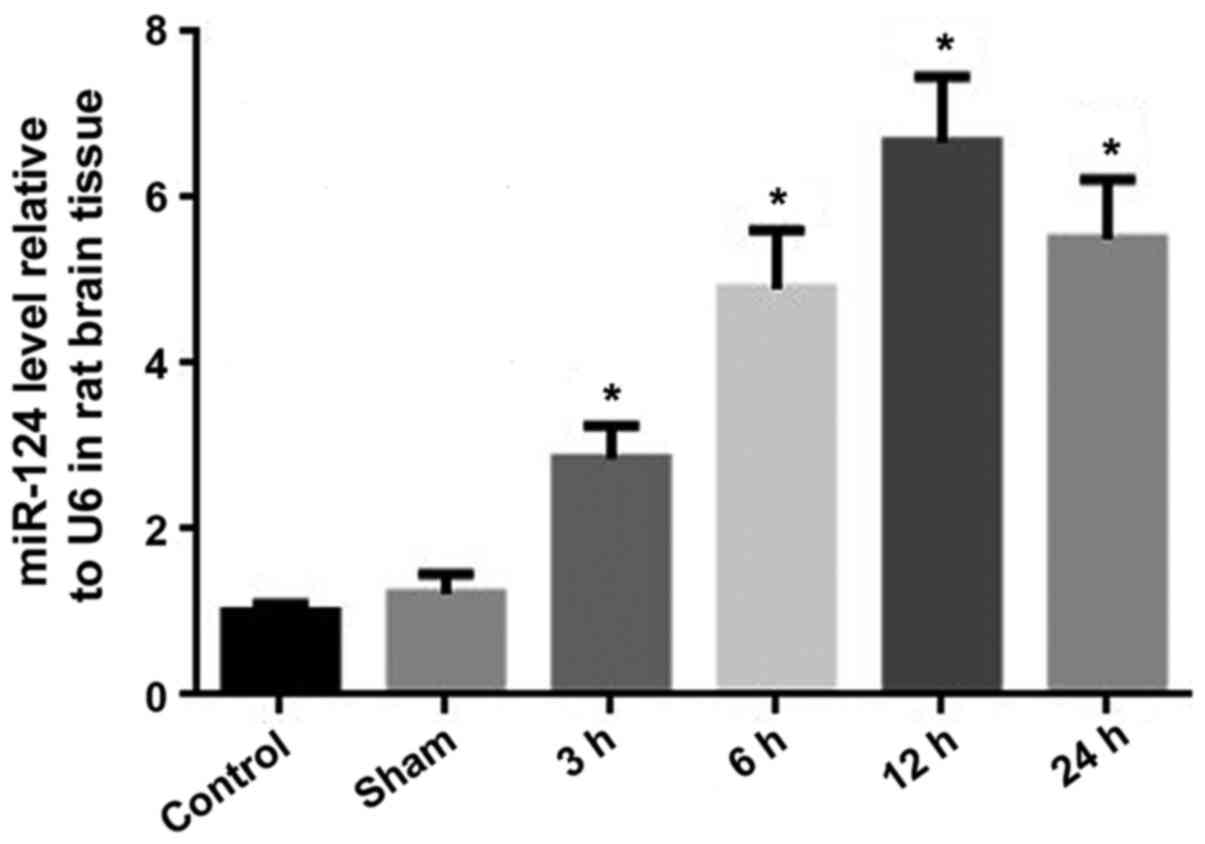
Dynamic expression levels of miRNA-124
following CIRI
The RT-qPCR results revealed that there was no
significant difference in miRNA-124 expression levels between the
sham operation and control groups (P>0.05). The expression
levels of miRNA-124 in the I/R groups were significantly increased
compared with the control group (P<0.05). At 12 h following
CIRI, the relative expression levels of miRNA-124 were ~7 times
higher compared with the control group. Between 12 and 24 h
following CIRI, the expression levels of miRNA-124 decreased;
however, the expression levels of miRNA-124 at 24 h were higher
compared with the expression levels at 6 h (Fig. 2).
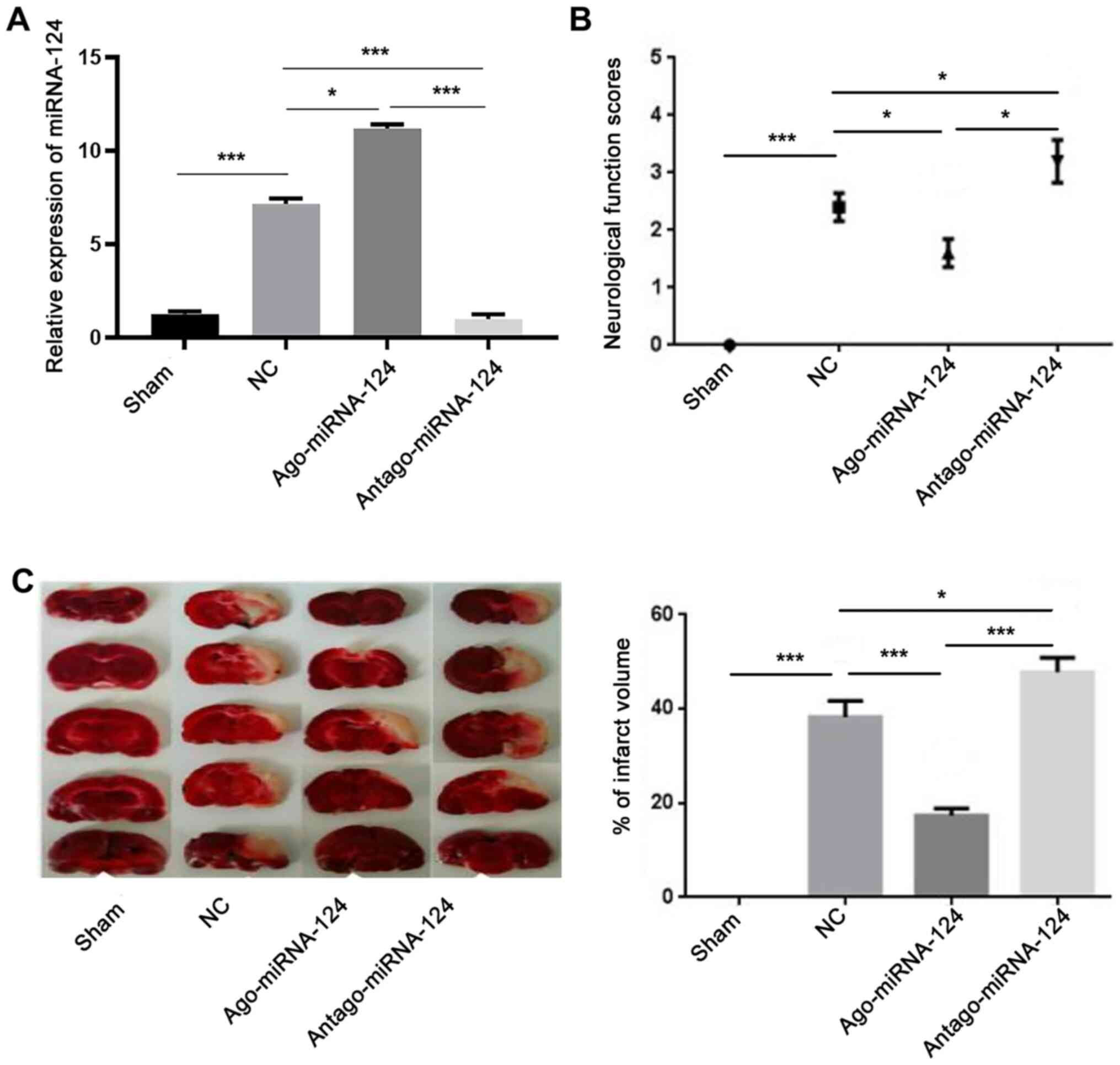
miR-124 serves a neuroprotective role
in CIRI
The RT-qPCR results suggested that the expression
levels of miR-124 in the NC group were significantly increased
compared with the sham group (P<0.001; Fig. 3A). Furthermore, the expression
levels of miRNA-124 in the agomiRNA-124 and antagomiRNA-124 groups
were significantly increased (P<0.05; Fig. 3A) and decreased (P<0.001;
Fig. 3A) compared with the NC
group, respectively, which indicated that the drugs were effective.
The neurological function scores revealed that there was no obvious
neurological deficit in the sham group. The neurological function
score of the agomiRNA-124 group was significantly decreased
compared with the NC group (P<0.05; Fig. 3B), whereas the neurological function
score of the antagomiRNA-124 group was significantly increased
compared with the NC and agomiRNA-124 groups (P<0.05; Fig. 3B). TTC staining also indicated that
the infarct size in the agomiR-124 group was significantly
decreased compared with the NC group (P<0.001; Fig. 3C), whereas the infarct size in the
antagomiRNA-124 group was significantly increased compared with the
NC group (P<0.05; Fig. 3B). The
results suggested that increased expression levels of miRNA-124 may
significantly reduce CIRI.
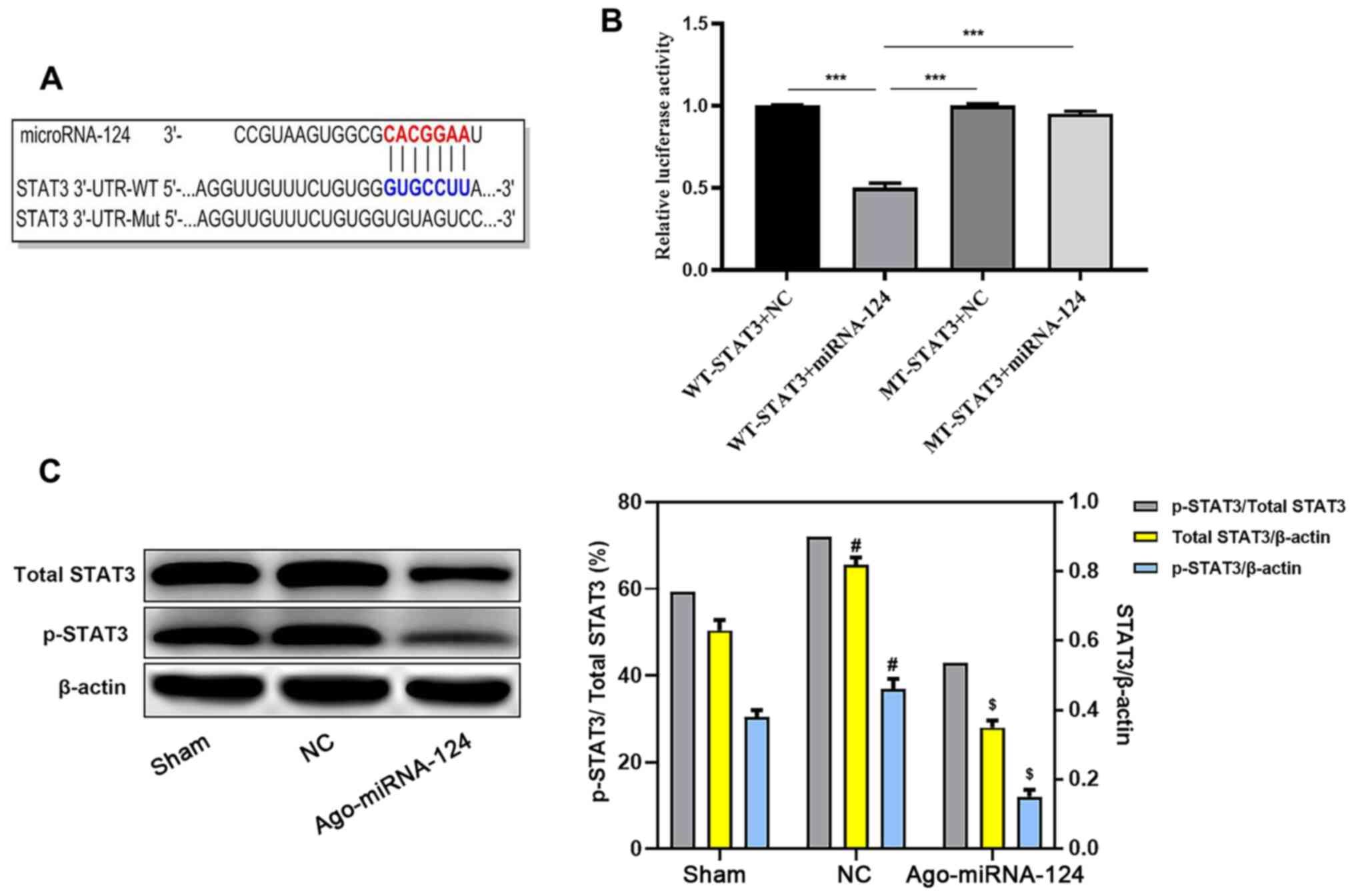
miR-124 inhibits STAT3 activation
TargetScan software identified a complementary
binding site between miRNA-124 and the 3'-UTR of STAT3 (Fig. 4A). Subsequently, the dual-luciferase
reporter assay demonstrated that the 3'-UTR of STAT3 bound to
miRNA-124. Compared with the WT-STAT3 + NC group, the WT-STAT3 +
miRNA-124 group displayed significantly reduced relative luciferase
activity (P<0.05; Fig. 4B). The
western blotting results also suggested that the expression levels
of STAT3 and p-STAT3 were significantly decreased in the
agomiRNA-124 group compared with the NC group (P<0.05; Fig. 4C).
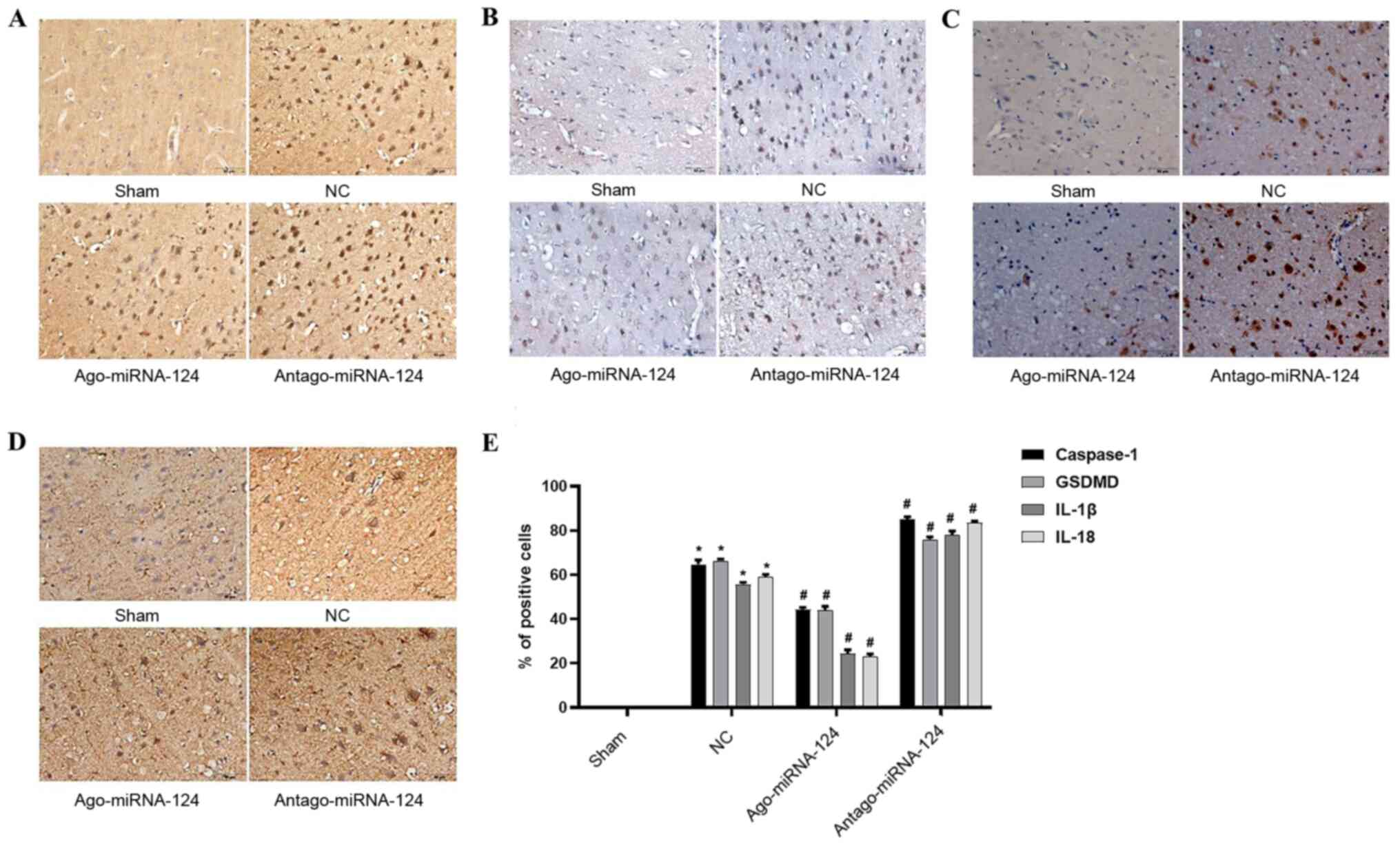
miRNA-124 inhibits pyroptosis in
CIRI
Immunohistochemical staining was conducted to
investigate the role of important proteins and proinflammatory
cytokines involved in pyroptosis. No positive cells were observed
in the sham operation group (Fig.
5), whereas the number of caspase-1- (P<0.05; Fig. 5A), gasdermin D- (P<0.05; Fig. 5B), IL-1β- (P<0.05; Fig. 5C) and IL-18-positive cells
(P<0.05; Fig. 5D) were
significantly increased in the NC group compared with the sham
operation group. miRNA-124 agonist significantly decreased the
expression levels of caspase-1, gasdermin D, IL-1β and IL-18
compared with the NC group, whereas antagomiRNA-124 displayed the
opposite effect (P<0.05; Fig.
5E).
Discussion
CIRI is a major cause of death and disability in
adults worldwide (23). Previous
studies have reported the occurrence of apoptosis (24) and necroptosis (25) in CIRI, and that inhibition of
caspase-1, an important mediator protein of pyroptosis,
significantly reduced CIRI (26,27).
Caspase-1 normally exists in the cytoplasm in the form of an
inactive proenzyme, pro-caspase-1. Upon receiving danger signals,
cells assemble into inflammatory bodies and recruit pro-caspase-1,
resulting in a relatively high local concentration of
pro-caspase-1. Subsequently, the autologous hydrolysis of the
zymogen produces the activated form of caspase-1, caspase-1 p20,
which then activates IL-1β and IL-18 (28,29).
Concurrently, activated caspase-1 has also been found to cleave
gasdermin D, releasing the active N-terminal residue fragment,
which binds to the cell membrane and forms a 10-15 nm pore.
Collectively, the aforementioned events lead to the osmotic death
of neurons and the release of intracellular mature proinflammatory
cytokines (30). IL-1β and IL-18
belong to the IL superfamily; both cytokines activate multiple
inflammatory signaling pathways, resulting in the release of
inflammatory cytokines. Activation of the inflammatory cascade can
lead to the production of increased endogenous danger signals and
can induce periphery pyroptosis (31). In the present study, the expression
levels of caspase-1, caspase-1, gasdermin D, IL-1β and IL-18 in the
brain tissue were significantly increased following CIRI and
following aggravation of CIRI compared with the control group. The
results suggested that pyroptosis may be an important factor that
aggravates CIRI, thus inhibiting pyroptosis may serve as an
effective treatment strategy for CIRI.
It was recently discovered that the regulation of
pyroptosis in I/R injury was mediated by miRNAs (32-34).
miRNAs can affect pyroptosis by directly or indirectly regulating
the expression of caspase-1. For example, miRNA-133 improves
myocardial I/R injury by regulating the assembly of inflammatory
bodies, inhibiting the activation of caspase-1 and reducing
pyroptosis (32,33). miRNA-124 is a highly specific miRNA
expressed in the central nervous system, which was observed to
serve neuroprotective roles in a variety of nervous system
diseases, such as ischemic stroke (17) and CIRI (34). The present study indicated that the
expression levels of miRNA-124 increased following CIRI compared
with the control group; however, the compensatory effect was
weakened following aggravation of CIRI. In addition, an exogenous
miRNA-124 injection significantly reduced the neuronal damage
observed in the I/R group and significantly decreased the
expression levels of pyroptosis-related proteins compared with the
NC group. miRNA-124-induced effects were reversed by inhibiting the
expression levels of miRNA-124, suggesting that miRNA-124 may serve
a neuroprotective role in CIRI by inhibiting pyroptosis.
STAT3, an important member of the STAT family, is
responsible for transmitting signals from activated plasma membrane
receptors to the nucleus (35).
Previous studies have reported that STAT3 affected the activation
of caspase-1 by regulating the expression levels of inflammatory
corpuscles (36,37). Previous bioinformatics analysis
discovered that miRNA-124 targeted STAT3(38). Therefore, it was hypothesized that
miRNA-124 may affect the degree of pyroptosis by regulating STAT3.
The findings obtained in the present study further supported the
hypothesis. The dual-luciferase reporter assay revealed that
miRNA-124 bound to STAT3, and the experimental results and analysis
demonstrated that the expression levels of STAT3 and p-STAT3 were
decreased. Furthermore, the expression levels of caspase-1,
gasdermin D, IL-1β and IL-18 were decreased in the agomiRNA-124
group compared with the NC group. The results indicated that
miRNA-124 may prevent the phosphorylation and subsequent activation
of STAT3 into p-STAT3, thereby blocking pyroptosis and reducing
neuronal damage following CIRI.
Previous studies have also revealed that miRNA-124
regulated neuronal injury following CIRI (14,18),
with one study reporting that miRNA124 regulated proliferation and
apoptosis via STAT3(39). However,
whether miR-124 regulates pyroptosis via STAT3 is not completely
understood. The results of the present study revealed a potential
mechanism underlying miRNA-124-mediated regulation of pyroptosis in
CIRI, which may provide a novel therapeutic target. In view of the
various injury mechanisms in CIRI, the present study indicated that
miRNA-125 may serve a neuroprotective role in CIRI, but cannot
completely cure the condition. Therefore, a strategy to cure CIRI
required further investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS performed the immunohistochemistry, TTC staining
and reverse transcription-quantitative PCR experiments as well as
modified the manuscript. JL constructed the middle cerebral artery
occlusion model, performed the western blotting experiments and
analyzed the data. ZF and HL constructed the luciferase reporter
plasmid technology, administered drugs and conducted neurological
deficit scoring. AM designed the study and drafted the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of North China University of Technology (Tangshan, China;
approval no. LX201901).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Murray CJ and Lopez AD: Measuring the
global burden of disease. N Engl J Med. 369:448–457.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ahmad M, Dar NJ, Bhat ZS, Hussain A, Shah
A, Liu H and Graham SH: Inflammation in ischemic stroke:
Mechanisms, consequences and possible drug targets. CNS Neurol
Disord Drug Targets. 13:1378–1396. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Neuhaus AA, Couch Y, Hadley G and Buchan
AM: Neuroprotection in stroke: The importance of collaboration and
reproducibility. Brain. 140:2079–2092. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Huang J, Wang T, Yu D, Fang X, Fan H and
Liu Q, Yi G, Yi X and Liu Q: l-Homocarnosine attenuates
inflammation in cerebral ischemia-reperfusion injury through
inhibition of nod-like receptor protein 3 inflammasome. Int J Biol
Macromol. 118:357–364. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Han M, Hu L and Chen Y: Rutaecarpine may
improve neuronal injury, inhibits apoptosis, inflammation and
oxidative stress by regulating the expression of ERK1/2 and
Nrf2/HO-1 pathway in rats with cerebral ischemia-reperfusion
injury. Drug Des Devel Ther. 13:2923–2931. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu Q and Zhang Y: PRDX1 enhances cerebral
ischemia-reperfusion injury through activation of TLR4-regulated
inflammation and apoptosis. Biochem Biophys Res Commun.
519:453–461. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li Z, Zhao F, Cao Y, Zhang J, Shi P, Sun
X, Zhang F and Tong L: DHA attenuates hepatic ischemia reperfusion
injury by inhibiting pyroptosis and activating PI3K/Akt pathway.
Eur J Pharmacol. 835:1–10. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sborgi L, Ruhl S, Mulvihill E, Pipercevic
J, Heilig R, Stahlberg H, Farady CJ, Muller DJ, Broz P and Hiller
S: GSDMD membrane pore formation constitutes the mechanism of
pyroptotic cell death. EMBO J. 35:1766–1778. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cookson BT and Brennan MA:
Pro-inflammatory programmed cell death. Trends Microbiol.
9:113–114. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang JR, Yao FH, Zhang JG..Ji ZY, Li KL,
Zhan J, Tong YN, Lin LR and He YN: Ischemia-reperfusion induces
renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am J
Physiol Renal Physiol. 306:F75–F84. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kawaguchi M, Takahashi M, Hata T, Kashima
Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J,
et al: Inflammasome activation of cardiac fibroblasts is essential
for myocardial ischemia/reperfusion injury. Circulation.
123:594–604. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu P, Duan L, Chen J, Xiong A, Xu Q,
Zhang H, Zheng F, Tan Z, Gong F and Fang M: Gene silencing of NALP3
protects against liver ischemia-reperfusion injury in mice. Hum
Gene Ther. 22:853–864. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ponomarev ED, Veremeyko T and Weiner HL:
MicroRNAs are universal regulators of differentiation, activation,
and polarization of microglia and macrophages in normal and
diseased CNS. Glia. 61:91–103. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chang KP, Lee HC, Huang SH, Lee SS, Lai CS
and Lin SD: MicroRNA signatures in ischemia-reperfusion injury. Ann
Plast Surg. 69:668–671. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ge Y, Yan X, Jin Y, Yang X, Yu X, Zhou L,
Han S, Yuan Q and Yang M: MiRNA-192 [corrected] and miRNA-204
directly suppress lncRNA HOTTIP and interrupt GLS1-mediated
glutaminolysis in hepatocellular carcinoma. PLoS Genet.
11(e1005726)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kynast KL, Russe OQ, Moser CV, Geisslinger
G and Niederberger E: Modulation of central nervous system-specific
microRNA-124a alters the inflammatory response in the formalin test
in mice. Pain. 154:368–376. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Taj SH, Kho W, Riou A, Wiedermann D and
Hoehn M: MiRNA-124 induces neuroprotection and functional
improvement after focal cerebral ischemia. Biomaterials.
91:151–165. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ponomarev ED, Veremeyko T, Barteneva N,
Krichevsky AM and Weiner HL: MicroRNA-124 promotes microglia
quiescence and suppresses EAE by deactivating macrophages via the
C/EBP-α-PU.1 pathway. Nat Med. 17:64–70. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yu ZH, Cai M, Xiang J, Zhang ZN, Zhang JS,
Song XL, Zhang W, Bao J, Li WW and Cai DF: PI3K/Akt pathway
contributes to neuroprotective effect of Tongxinluo against focal
cerebral ischemia and reperfusion injury in rats. J Ethnopharmacol.
181:8–19. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Song X, Zhou B, Zhang P, Lei D, Wang Y,
Yao G, Hayashi T, Xia M, Tashiro S, Onodera S and Ikejima T:
Protective effect of silibinin on learning and memory impairment in
LPS-treated rats via ROS-BDNF-TrkB pathway. Neurochem Res.
41:1662–1672. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ramos E, Patiño P, Reiter RJ, Gil-Martín
E, Marco-Contelles J, Parada E, Rios C, Romero A and Egea J:
Ischemic brain injury: New insights on the protective role of
melatonin. Free Radic Biol Med. 104:32–53. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li PF, Shen MH, Gao F, Wu JP, Zhang JH,
Teng FM and Zhang CB: An Antagomir to MicroRNA-106b-5p ameliorates
cerebral ischemia and reperfusion injury in rats via inhibiting
apoptosis and oxidative stress. Mol Neurobiol. 54:2901–2921.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nikseresht S, Khodagholi F and Ahmadiani
A: Protective effects of ex-527 on cerebral ischemia-reperfusion
injury through necroptosis signaling pathway attenuation. J Cell
Physiol. 234:1816–1826. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fann DY, Lee SY, Manzanero S, Tang SC,
Gelderblom M, Chunduri P, Bernreuther C, Glatzel M, Cheng YL,
Thundyil J, et al: Intravenous immunoglobulin suppresses NLRP1 and
NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell
Death Dis. 4(e790)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fann DY, Santro T, Manzanero S,
Widiapradja A, Cheng YL, Lee SY, Chunduri P, Jo DG, Stranahan AM,
Mattson MP and Arumugam TV: Intermittent fasting attenuates
inflammasome activity in ischemic stroke. Exp Neurol. 257:114–119.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lu A and Wu H: Structural mechanisms of
inflammasome assembly. FEBS J. 282:435–444. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu X and Lieberman J: A mechanistic
understanding of pyroptosis: The fiery death triggered by invasive
infection. Adv Immunol. 135:81–117. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wei R, Wang J, Xu Y, Yin B, He F, Du Y,
Peng G and Luo B: Probenecid protects against cerebral
ischemia/reperfusion injury by inhibiting lysosomal and
inflammatory damage in rats. Neurosci. 301:168–177. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xiao L, Jiang L, Hu Q and Li Y:
MicroRNA-133b ameliorates allergic inflammation and symptom in
murine model of allergic rhinitis by targeting Nlrp3. Cell Physiol
Biochem. 42:901–912. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yu SY, Dong B, Tang L and Zhou SH: LncRNA
MALAT1 sponges miR-133 to promote NLRP3 inflammasome expression in
ischemia-reperfusion injured heart. Int J Cardiol. 254(50)2017.
|
|
34
|
Yang Q, Shi Q and Fu J: Applications of
cerebrospinal miRNA in the detection and treatment of acute CNS
injury. Front Lab Med. 2:83–88. 2018.
|
|
35
|
Ray JP, Marshall HD, Laidlaw BJ, Staron
MM, Kaech SM and Craft J: Transcription factor STAT3 and type I
interferons are corepressive insulators for differentiation of
follicular helper and T helper 1 cells. Immunity. 40:367–377.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu CC, Huang ZX, Li X, Shen KF, Liu M,
Ouyang HD, Zhang SB, Ruan YT, Zhang XL, Wu SL, et al: Upregulation
of NLRP3 via STAT3-dependent histone acetylation contributes to
painful neuropathy induced by bortezomib. Exp Neurol. 302:104–111.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cuesta N, Nhu QM, Zudaire E, Polumuri S,
Cuttitta F and Vogel SN: IFN regulatory factor-2 regulates
macrophage apoptosis through a STAT1/3- and caspase-1-dependent
mechanism. J Immunol. 178:3602–3611. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wei J, Wang F, Kong LY, Xu S, Doucette T,
Ferguson SD, Yang YH, McEnery K, Jethwa K, Gjyshi O, et al: miR-124
inhibits STAT3 signaling to enhance T cell-mediated immune
clearance of glioma. Cancer Res. 73:3913–3926. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Nagata K, Hama I, Kiryu-Seo S and Kiyama
H: microRNA-124 is down regulated in nerve-injured motor neurons
and it potentially targets mRNAs for KLF6 and STAT3. Neurosci.
256:426–432. 2013.PubMed/NCBI View Article : Google Scholar
|