Introduction
Coronary artery disease (CAD) is a major
cardiovascular disease that affects the global population. CAD is a
major cause of death in developed and developing countries
(1). With advances in medical
technology, effective medication and interventional therapy,
off-pump coronary artery bypass grafting (OPCABG) has become
increasingly available for patients with coronary heart disease in
Asia. OPCABG can be achieved with a low mortality and a good medium
to long term survival. OPCABG is associated with fewer
post-operative complications and comparable late coronary
interventions (2). However, in the
majority of patients who suffer from severe myocardial ischemia,
surgery can activate the sympathetic nerves, triggering the release
of catecholamine. This subsequently causes adverse cardiovascular
reactions, such as elevated blood pressure and increased heart
rate, directly affecting the prognosis of patients (3,4).
Therefore, anesthesiologists should select appropriate anesthetic
drugs to create safe and comfortable conditions for surgery, reduce
the post-operative inflammatory response and have little impact on
the hemodynamic indices.
Dexmedetomidine is a novel and highly selective α-2
adrenergic receptor agonist, with an α2:α1 receptor affinity of
1620:1(5). It has analgesic,
sedative and anxiolytic properties, with a mild inhibitory effect
on respiratory function (6-8).
Dexmedetomidine is used as a sedative in critical illness and may
serve anti-inflammatory effects (9). Hence the present study investigated
the effects of dexmedetomidine on the hemodynamics and inflammatory
response of 239 patients undergoing OPCABG surgery.
Materials and methods
Patient selection
A total number of 300 patients undergoing OPCABG,
aged 55-75 years old, weighing 55-85 kg and with American Society
of Anesthesiology II-III (10),
were included in the present study regardless of their sex and
education level (Table I). Patients
with neurological/mental health conditions or organs (including
brain, lung, liver and kidney) dysfunction were excluded. All
subjects were well-informed and provided written informed consent
before accepting anesthesia. The present study was approved by The
Ethics Committee of Tianjin Chest Hospital (approval no.
2012KY-001-01) and was registered at the Chinese Clinical Trial
Registry, under trial registration number ChiCTR-OOC-15005978
(2015).
 | Table IBaseline characteristics. |
Table I
Baseline characteristics.
| | Group | |
|---|
| Variable | C, n=116 | D, n=123 | P-value |
|---|
| Mean age ± SD,
years | 66±4.5 | 67±3.6 | 0.352 |
| Mean education
duration ± SD, years | 10.6±3.1 | 10.6±3.2 | 0.961 |
| Sex, female, n
(%) | 53(47) | 56(46) | 0.498 |
| Sex, male, n (%) | 63(53) | 67(54) | 0.924 |
| Mean height ± SD,
cm | 171±8.3 | 170±8.5 | 0.723 |
| Mean weight ± SD,
kg | 73±5.2 | 73±5.9 | 0.998 |
| Hypertension, n
(%) | 103(78) | 105(85) | 0.721 |
| Diabetes mellitus, n
(%) | 86(74) | 91(74) | 0.145 |
| Previous myocardial
infarction, n (%) | 58(50) | 50(41) | 0.235 |
| Meanpreoperative EF ±
SD | 55±4.6 | 54±5.1 | 0.535 |
Randomization and study blinding
Patients were randomly assigned into the control
group (group C), in which no intraoperative intravenous pump
infusion of dexmedetomidine was used. In the dexmedetomidine group
(group D), dexmedetomidine was infused at a rate of 0.4
µg.kg-1.h-1, starting from endotracheal
intubation to the end of the surgery. The dosage and ratio of
dexmedetomidine were calculated according to the recommended dosage
in combination with the body weight of the patient. Based on the
findings from retrospective studies (11,12), a
total of 127 patients were required in each group with an α-level
of 0.05 and power of 0.8. Assuming a 20% withdrawal rate during the
course of the clinical trial, including study design deviation and
consent withdrawal, 150 patients were enrolled into each group.
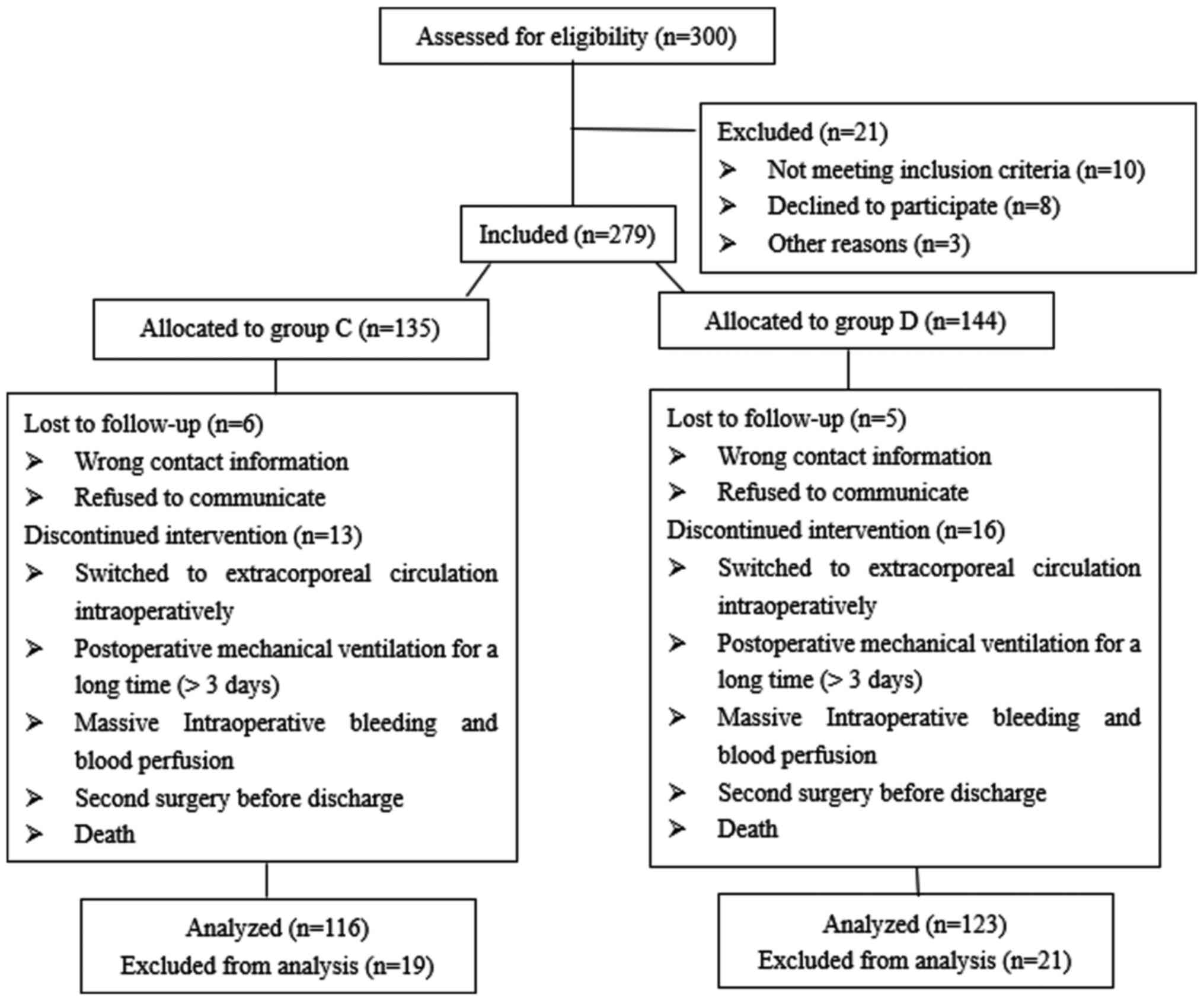
During the study period, 300 patients were screened,
and 279 of these met the inclusion criteria (including undergoing
OPCABG, an age of 55-75 years old, weighing 55-85 kg and with an
American Society of Anesthesiology score of II-III), leaving 135
patients in group C and 144 patients in group D. Among the enrolled
patients, 40 were excluded due to the following reasons: i)
Intraoperative switch to extracorporeal circulation; ii)
intraoperative massive bleeding and blood perfusion; iii) second
surgery within a short time following initial surgery; iv) lengthy
postoperative mechanical ventilation support; v) lost to follow-up
post-operatively; and vi) death. Thus, 239 patients were enrolled
in the present study, 116 and 123 in groups C and D, respectively
(Fig. 1).
The results of randomization were sealed in
envelopes with serial numbers and placed in the study center under
the supervision of the clinical research coordinator (GW).
Randomization was performed 30-60 min prior to surgery, which was
30-60 min following the provision of informed consent from the
patients. The clinical research coordinator was not blinded to the
grouping results. The responsibilities of the clinical research
coordinator included assigning the patients to randomized groups,
distributing medicine, providing instructions to the
anesthesiologists regarding the intraoperative measures, conducting
the study protocol under strict guidelines, collecting the data and
coordinating the entire study. The anesthesiologists responsible
for intraoperative management were blinded to the grouping results.
Strict blinding during the follow-up was ensured. In addition, the
medical team providing postoperative care in the intensive care
unit (ICU) and the wards, the investigators, patients,
statisticians, and data and safety oversight committees were all
blinded to the grouping results.
Anesthesia management
Identical anesthesia induction with 0.03-0.05 mg/kg
midazolam, 0.15-0.3 mg/kg etomidate, 0.5-1 µg/kg sufentanil and 0.6
mg/kg rocuronium was adopted for all the enrolled patients. After
anesthesia induction, oral endotracheal intubation (tidal volume,
6-8 ml/kg; respiratory rate, 13/min) was performed, and a radial
artery puncture for central venous catheter placement or Swan-Ganz
catheter placement was performed. The parameters, including the
heart rate, mean arterial pressure, oxygen saturation, end-tidal
CO2, brain spectral index (BIS), central venous
pressure, cardiac output and pulmonary artery pressure were
monitored. During anesthesia maintenance, supplementation with
identical general anesthetics, analgesics and muscle relaxants was
performed, namely propofol and sevoflurane, sufentanil and
cis-atracurium. The vasoactive drugs utilized included
norepinephrine, nicardipine, milrinone, and esmolol. Following
surgery, the patient was transferred to the ICU.
Outcome measures
Venous blood was collected at pre-incision (t1),
immediately following sternal closure (t2), then 4, 12 and 24 h
postoperatively (t3, t4 and t5, respectively). ELISA kits (all,
Abcam) were used to detect interleukin (IL)-6 (cat. no. ab46027),
tumor necrosis factor-α (cat. no. ab181421), C-reactive protein
(CRP; cat. no. ab260058) and IL-10 (cat. no. ab185986) levels. BIS
values were recorded a intraoperatively at the t1 and t2 time
points. Hemodynamic parameters (HR and MAP) at each time point,
both intraoperatively and postoperatively were also recorded.
Statistical analysis
The SPSS v21.0 software (IBM Corp.) was used for
data analysis. Continuous data are presented as the mean ± SD and
were analyzed using a t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Comparison of clinical data and
surgical records
No significant difference in the general condition,
complications, cardiac function and surgical records was observed
between the two groups (Tables I
and II). No significant difference
was also detected in the intraoperative doses of the vasoactive
drugs between the two groups (Table
III).
 | Table IIClinical record. |
Table II
Clinical record.
| | Group | |
|---|
| Variable | C, n=116 | D, n=123 | P-value |
|---|
| Number of grafts ±
SD | 3±0.5 | 3±0.6 | 0.984 |
| Mean duration of
anesthesia ± SD, h | 3.8±0.6 | 4.1±0.7 | 0.845 |
| Mean time spent in
ICU ± SD, days | 2.2±0.2 | 2.0±0.1 | 0.624 |
| Mean time spent in
hospital ± SD, days | 8.3±1.2 | 7.2±1.3 | 0.397 |
 | Table IIIVasoactive drugs. |
Table III
Vasoactive drugs.
| | Group | |
|---|
| Variable | C, n=116 | D, n=123 | P-value |
|---|
| Premedication |
|
Dose of
μorphine ± SD, mg | 5±0.9 | 5±1.0 | 0.922 |
|
Dose of
σcopolamine ± SD, mg | 0.3±0.05 | 0.3±0.06 | 0.987 |
| Positive inotropic
drugs |
|
Mean level
of dopamine ± SD, mg/kg/min | 3.82±0.71 | 4.03±0.55 | 0.367 |
|
Mean level
of milrinone ± SD, mg/kg/min | 0.35±0.02 | 0.39±0.03 | 0.201 |
|
Mean level
of norepinephrine ± SD, µg/kg/min | 0.41±0.06 | 0.37±0.06 | 0.145 |
Comparison of hemodynamic parameters
and BIS values at each time point
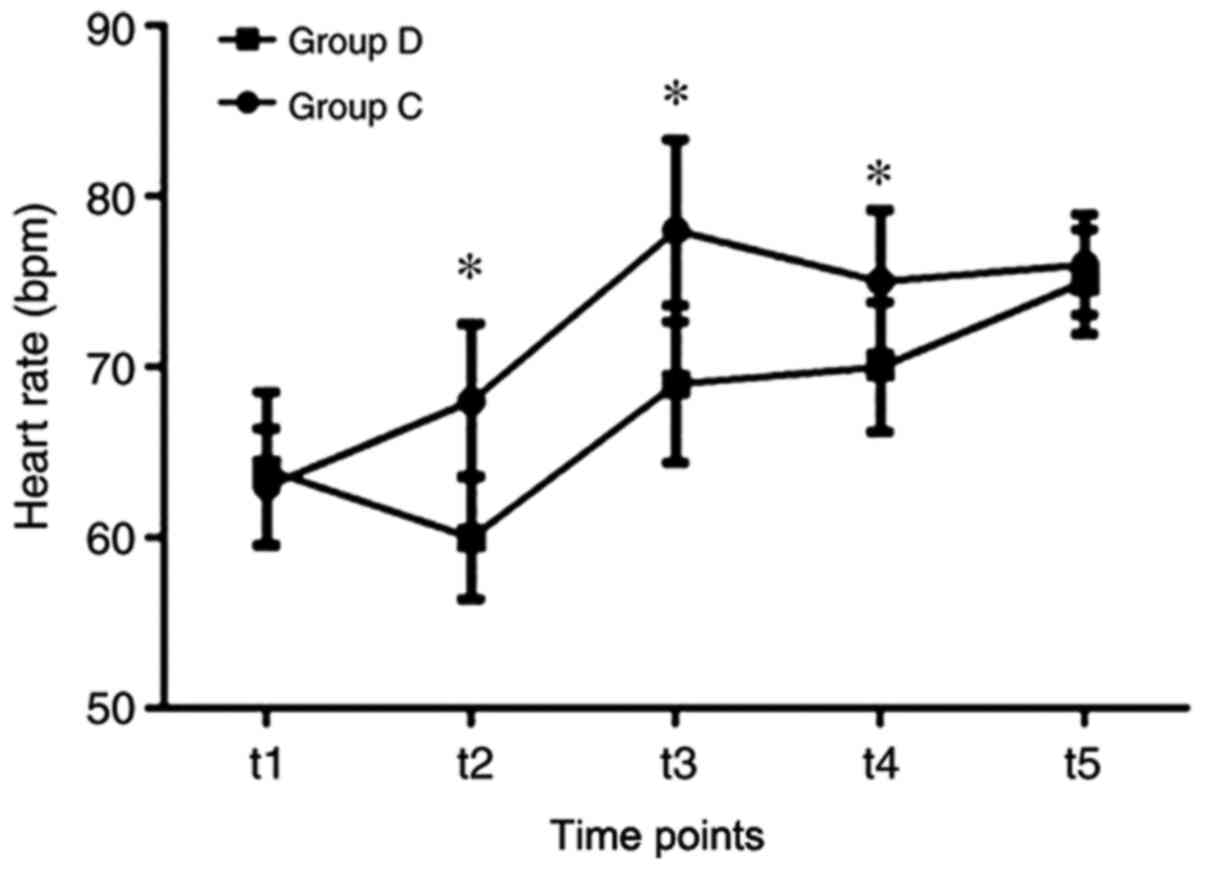
Patients in the group D had a significantly slower
heart rate at t2, t3, and t4, compared with that in group C
(Fig. 2). A significantly lower
mean arterial pressure was also observed in patients in group D at
the t2 and t3 time points (Fig. 3),
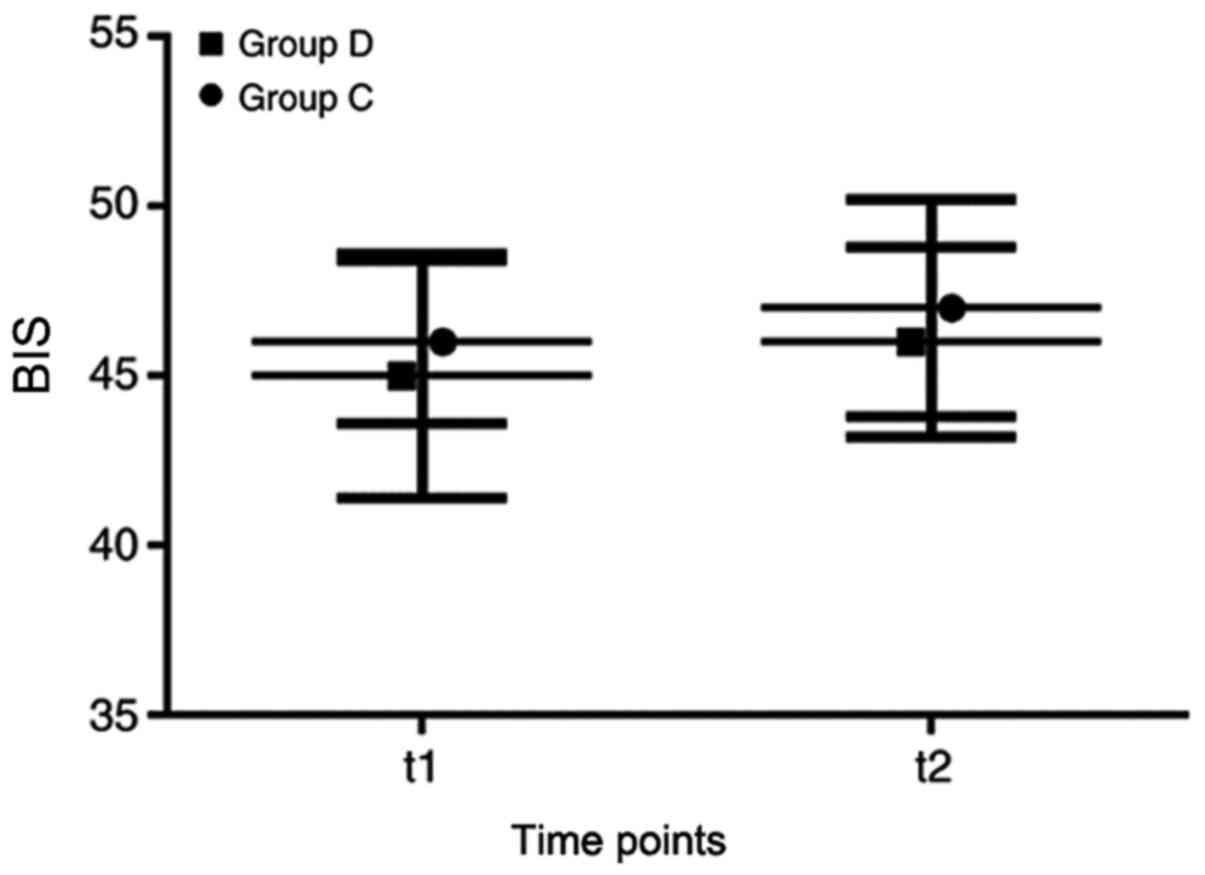
while BIS values at t1 and t2 in both groups ranged from 40-55
indicating that the depth of anesthesia in both the patients was
moderate. However, no significant difference was observed in BIS
values between the two groups at these time points (Fig. 4).
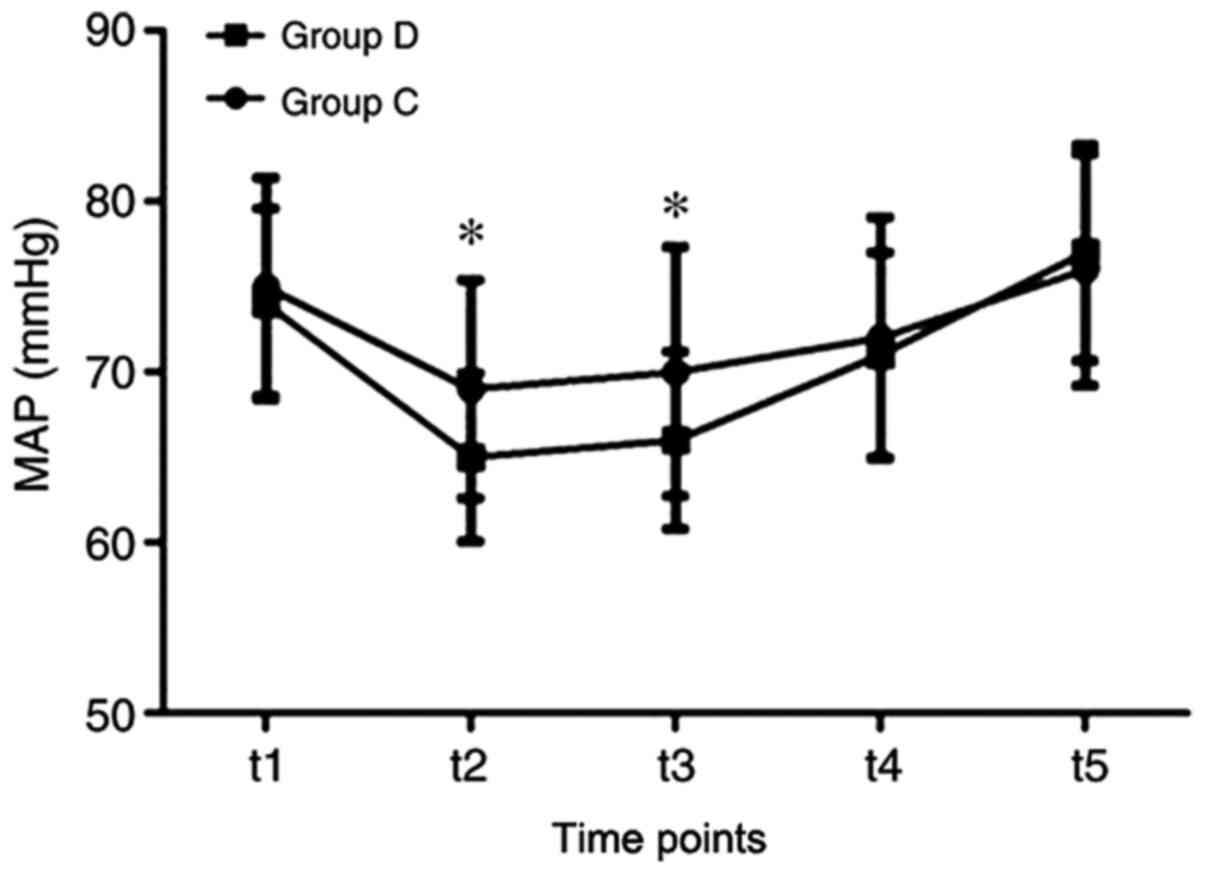 | Figure 3Comparisons of MAP between the two
groups at each time point. MAP was measured at pre-incision (t1),
immediately following sternal closure (t2), then 4, 12 and 24 h
post-operatively (t3, t4 and t5, respectively), and compared
between each group at the same time points. Data are presented as
the mean ± SD. *P<0.05 vs. control. D,
dexmedetomidine; C, control; MAP, mean arterial pressure; t,
time. |
Comparison of biochemical indices
Differences were observed in the levels of IL-6,
TNF-α, CRP and IL-10 between the two groups. TNF-α levels were
significantly lower in group D compared with that in group C at the
t2 and t3 time points (Fig. 5).
IL-6 levels were also significantly reduced at the t2 time point
compared with that in group C (Fig.
6), while the levels of CRP were significantly reduced in group
D compared with that in group C, at the t3, t4 and t5 time points
(Fig. 7). Furthermore, the amount
of IL-10 in group C was marginally reduced, compared with that on
group D, at the t3, t4 and t5 time points; however, this difference
was not statistically significant (Fig.
8).
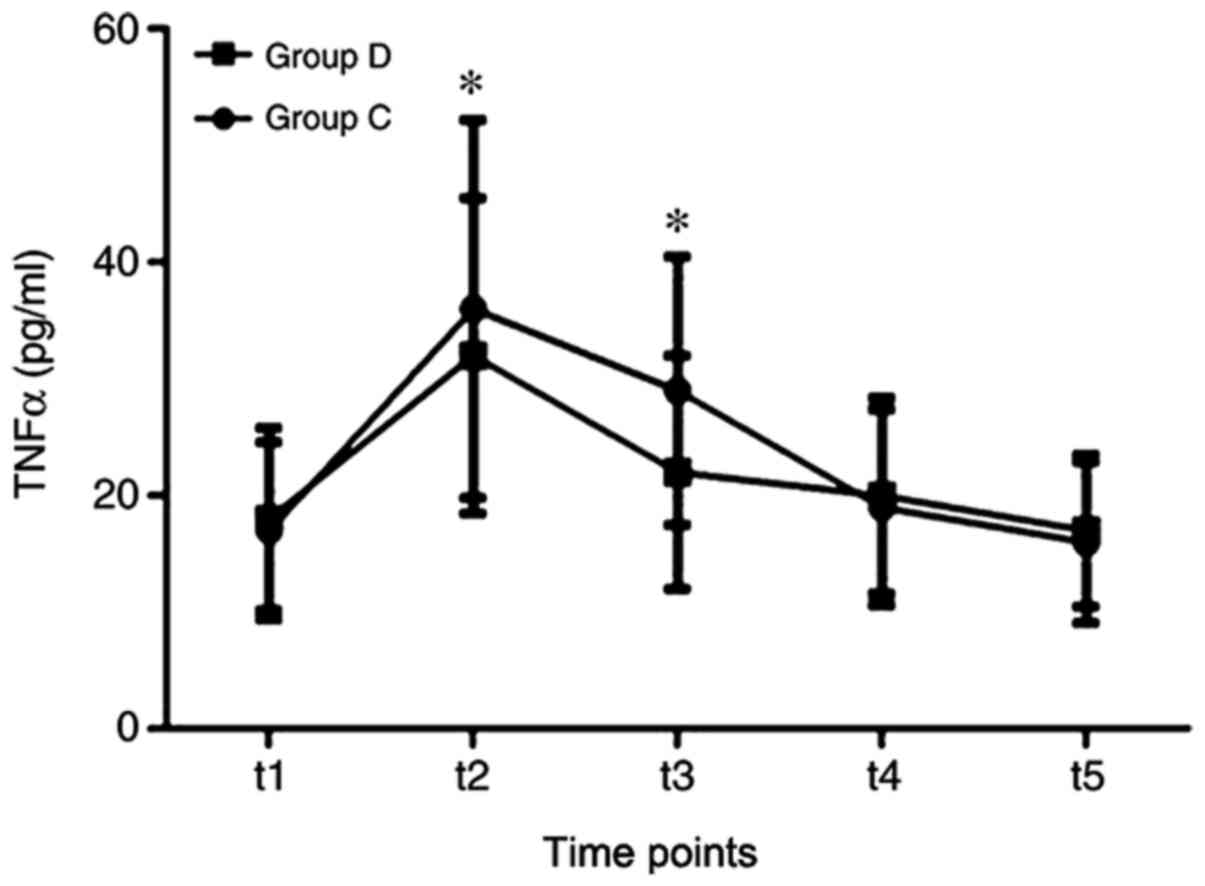 | Figure 5Comparisons of TNF-α between the two
groups at each time point. TNF-α levels were measured at
pre-incision (t1), immediately following sternal closure (t2), then
4, 12 and 24 h postoperatively (t3, t4 and t5, respectively), and
compared between each group at the same time points. Data are
presented as the mean ± SD. *P<0.05 vs. control. D,
dexmedetomidine; C, control; TNF-α, tumor necrosis factor-α; t,
time. |
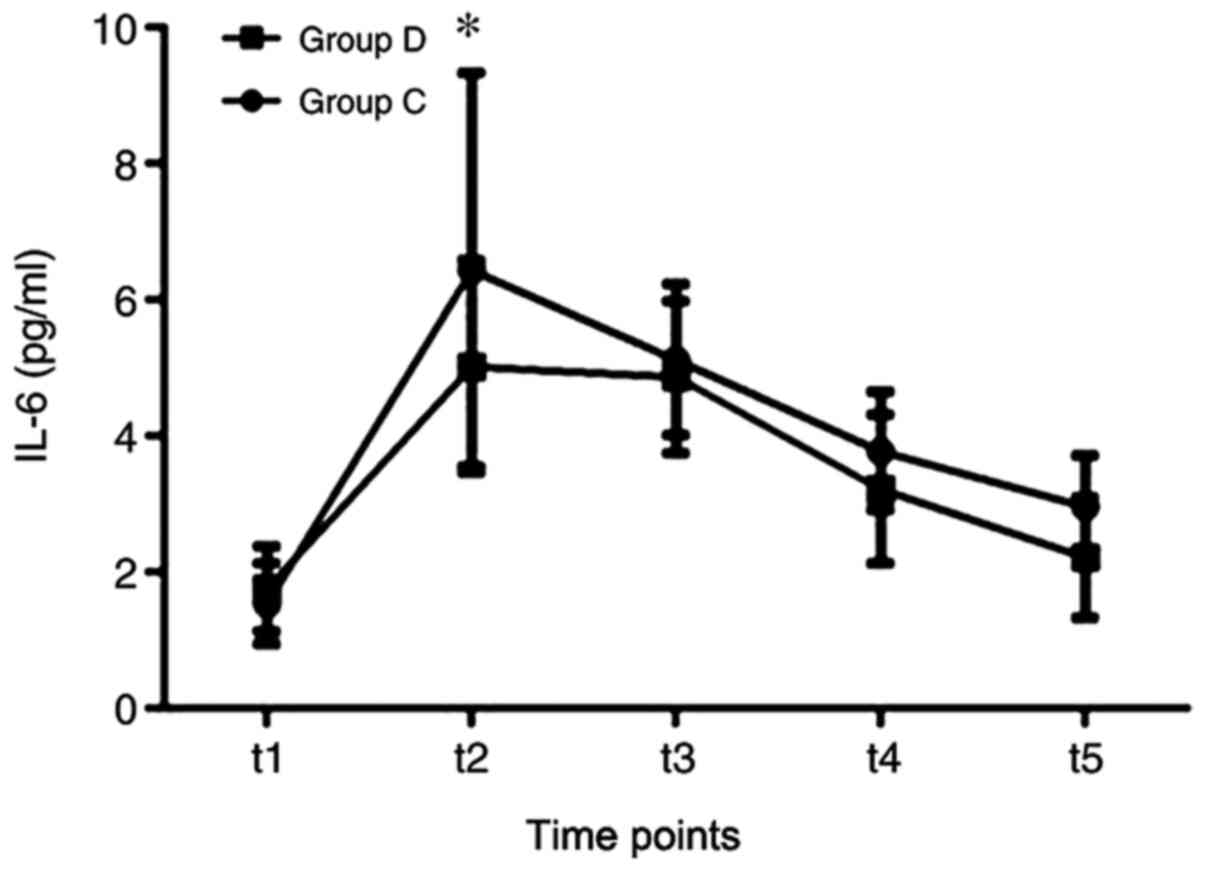 | Figure 6Comparisons of IL-6 between the two
groups at each time point. IL-6 levels were measured at
pre-incision (t1), immediately following sternal closure (t2), then
4, 12 and 24 h postoperatively (t3, t4 and t5, respectively), and
compared between each group at the same time points. Data are
presented as the mean ± SD. *P<0.05 vs. control. D,
dexmedetomidine; C, control; IL, interleukin; t, time. |
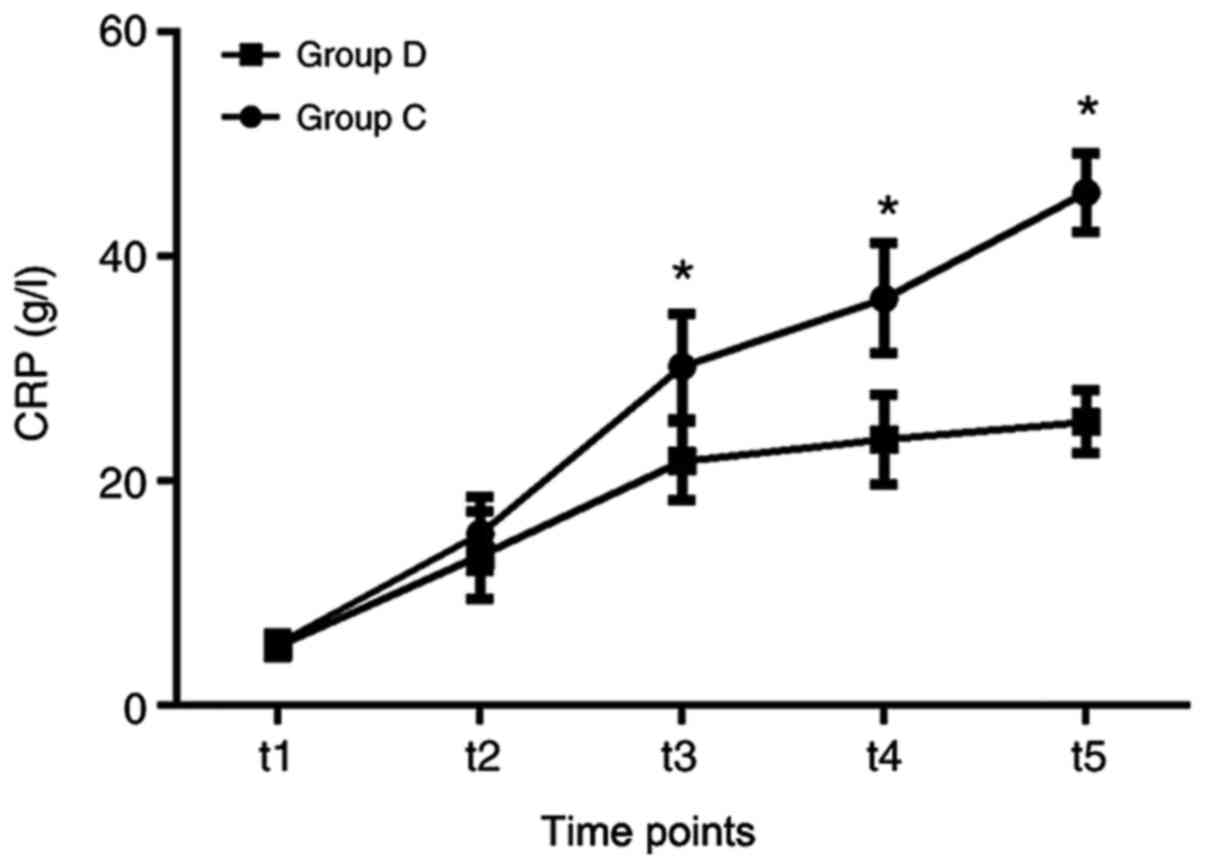 | Figure 7Comparisons of CRP between the two
groups at each time point. CRP levels were measured at pre-incision
(t1), immediately after sternal closure (t2), then 4, 12 and 24 h
postoperatively (t3, t4 and t5, respectively), and compared between
each group at the same time points. Data are presented as the mean
± SD. *P<0.05 vs. control. D, dexmedetomidine; C,
control; CRP, C-reactive protein; t, time. |
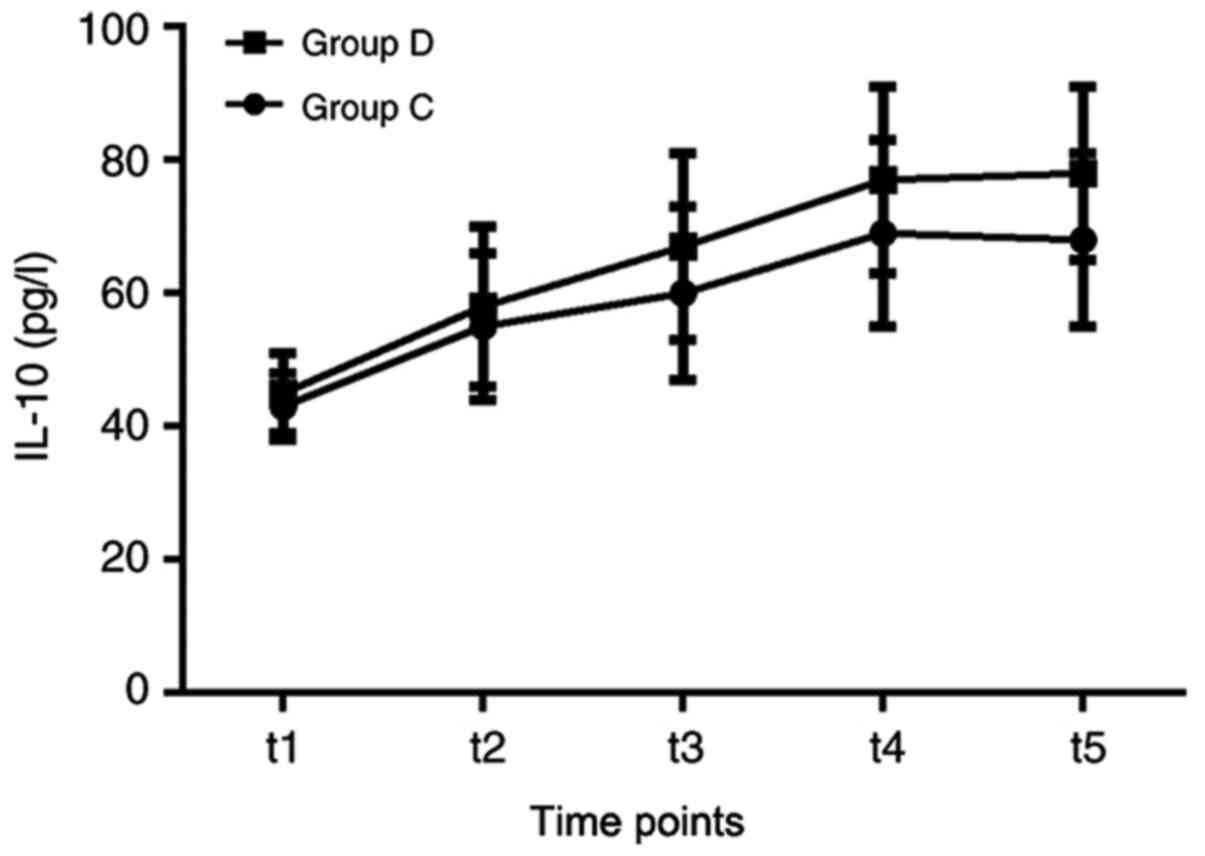 | Figure 8Comparisons of IL-10 between the two
groups at each time point. IL-10 levels were measured at
pre-incision (t1), immediately after sternal closure (t2), then 4,
12 and 24 h postoperatively (t3, t4 and t5, respectively), and
compared between each group at the same time points. Data are
presented as the mean ± SD. D, dexmedetomidine; C, control; IL,
interleukin; t, time. |
Discussion
In the USA, the Food and Drug Administration (FDA)
has approved the use of dexmedetomidine for short-term sedation (24
h) in ICUs for patients undergoing mechanical ventilation and
endotracheal intubation. In October 2008, the FDA extended the use
of this drug for sedation of non-intubated patients prior to and
during surgical and non-surgical procedures (3).
In the present study, the effects of intraoperative
use of dexmedetomidine on hemodynamics and inflammatory responses
were evaluated during OPCABG surgery. Previous studies have
demonstrated the anti-inflammatory effect of dexmedetomidine in
clinical practice, yet few have documented this effect in OPCABG
(13-16).
Furthermore, unlike previous studies (11,12),
the clinical trial described in the present study combined clinical
indicators and measurement of inflammatory factors to jointly
describe the anti-inflammatory effect of dexmedetomidine and its
potential clinical benefit.
Dexmedetomidine was found to reduce the mean
arterial pressure and heart rate, suggesting a stabilizing effect
on hemodynamics (17-20).
This could be attributed to the ability of dexmedetomidine to
reduce sympathetic nerve activity, inhibit the release of
sympathetic impulses, and relieve nerve tension primarily according
to the highly selective nature of the drug (21). In previous studies, dexmedetomidine
did not demonstrate any myocardial inhibition and even exhibited
cardioprotection, neuroprotection, and renoprotection by reducing
inflammation (22-24).
A previous study suggested that the use of dexmedetomidine reduced
the requirement for propofol and improved the hemodynamic stability
in patients with BIS-guided sedation (25). Consistent with this previous study,
the present findings also confirmed that administration of
dexmedetomidine did not decrease the BIS values and demonstrated
effective stability during anesthetic maintenance at the sedation
level.
Trauma and postoperative pain following cardiac
surgery contribute to the inflammatory response in patients
(26). Inflammatory responses occur
intraoperatively, but can also persist postoperatively.
Hyperinflammatory responses damage particular organs, including the
lung, heart and kidney, and in turn, trigger unfavorable
pathological changes, including tissue and cell degeneration,
necrosis, changes in hemodynamics (inflammatory hyperemia),
increased vascular permeability (inflammatory exudation), fluid
exudation and cellular exudation (inflammatory infiltration)
resulting in systemic inflammatory response syndrome. This leads to
secondary multiple organ injury and dysfunction, affecting
postoperative outcomes (including prolonged hospitalization and
increased medical costs) and quality of life (27). The postoperative use of
dexmedetomidine in patients in ICU effectively inhibits the release
of inflammatory mediators, reducing the incidence rate of systemic
inflammatory response syndrome (28-31).
Dexmedetomidine also significantly suppresses the production of
lipopolysaccharide-induced proinflammatory mediators, including
TNF-α, IL-6, and CRP, both in vivo and in vitro
(31-34).
IL-10 is an anti-inflammatory cytokine that inhibits the production
of IL-6 and TNF-α (35). The
present findings also suggested that the use of dexmedetomidine
during surgery significantly reduced the intraoperative and
short-term post-operative levels TNF-α and IL-6. Moreover,
dexmedetomidine also reduced the postoperative levels of CRP. Thus,
it may be hypothesized that dexmedetomidine activates the central
nervous system, as well as the peripheral α2 adrenergic receptor,
to reduce sympathetic nerve activity and plasma catecholamine
concentration, which then reduces perioperative inflammatory
responses (36,37). Indeed, dexmedetomidine has
previously been demonstrated to reduce sympathetic tone and
indirectly improve parasympathetic tone, which may inhibit systemic
inflammatory responses (38). Yang
and Hong (39) also suggested that
dexmedetomidine could inhibit nuclear factor-κB activity and
activate cholinergic anti-inflammatory pathways. Chen et al
(40) investigated whether
dexmedetomidine served an anti-inflammatory effect on myocardial
ischemia-reperfusion injury in patients who underwent coronary
artery bypass graft surgery (CABG) with CPB. The study demonstrated
that dexmedetomidine inhibited the increase in cardiac troponin I
and creatine kinase isoenzymes, attenuated the production of
pro-inflammatory cytokines TNF-α, IL-6 and IL-8, and promoted
anti-inflammatory cytokine IL-10 production. The results
demonstrated that dexmedetomidine regulated the anti-inflammatory
response and provided myocardial protection in CABG with CPB
(40). Thus, dexmedetomidine could
maintain the perioperative hemodynamic stability in patients
receiving OPCABG, alleviate the perioperative inflammatory
response, and might be beneficial to patients during postoperative
recovery.
However, only a single-dose intravenous pump
infusion of dexmedetomidine was evaluated in the present study. The
study confirmed that dexmedetomidine stabilized hemodynamic
parameters in patients undergoing OPCABG surgery; however, the
study design could not establish whether the dose used produced an
optimal result on coronary indices. Furthermore, follow-up
information regarding the short and long-term postoperative
outcomes, including the mortality rate and complications, were
unavailable. In addition, the use of dexmedetomidine during time
spent in the ICU and whether this could also improve patient
outcomes was not assessed. The present study also lacked a positive
control group, as anti-inflammatory drugs used are not approved for
surgery in China, with the exception of ulinastatin. Thus, the
findings of the present study should be validated in future studies
and the limitations addressed.
In conclusion, anesthesia with dexmedetomidine
during OPCABG surgery effectively alleviated stress-induced
responses, promoted hemodynamic stability and produced an effective
sedative action. Thus, dexmedetomidine may be a potentially
suitable anesthetic for clinical use.
Acknowledgements
Not applicable.
Funding
This work was supported by the program of ‘131’
Innovative Talent Team from the Tianjin Talent Working Group of
China.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ analyzed the data and wrote the manuscript. LY
assisted with cardiac surgery and performed the post-operative
follow-up of patients. PS and JH implemented anesthesia and
recorded intraoperative data. YL collected patient blood samples
and conducted ELISA. GW designed and supervised the trial. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Tianjin Chest Hospital (approval no. 2012KY-001-01) and was
registered at the Chinese Clinical Trial Registry with the trial
registration number ChiCTR-OOC-15005978 (2015). All subjects were
well informed and provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Malakar AK, Choudhury D, Halder B, Paul P,
Uddin A and Chakraborty S: A review on coronary artery disease, its
risk factors, and therapeutics. J Cell Physiol. 234:16812–16823.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nwaejike N, Mansha M, Bonde P and
Campalani G: Myocardial revascularization by off pump coronary
bypass surgery (OPCABG): A ten year review. Ulster Med J.
77:106–109. 2008.PubMed/NCBI
|
|
3
|
Wang R: Effect of dexmedetomidine
continuous infusion on off-pump coronary artery bypass surgery. J
Cardiothorac Vasc Anesth. 17:24–26. 2013.
|
|
4
|
Zhang Q, Lu C and Liu G: Effects of
dexmedetomidine hydrochloride on postoperative cognitive function
in patients undergoing off-pump coronary-artery bypass grafting.
Chin J Lab Diagn. 16:346–348. 2012.
|
|
5
|
Weerink MAS, Struys MMRF, Hannivoort LN,
Barends CRM, Absalom AR and Colin P: Clinical pharmacokinetics and
pharmacodynamics of dexmedetomidine. Clin Pharmacokinet.
56:893–913. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou H, Xiao W and Zhao H: The effect of
dexmedetomidine on hemodynamics and stress reaction in patients
undergoing cardiac valve replacement surgery. Chin J Exp Surg.
28:2230–2233. 2011.
|
|
7
|
Ling Y, Sun Y and Liang Q: Effects of
dexmedetomidine on hemodynamics and stress reaction in pediatric
patients undergoing cardiac surgery. Shanghai Medical J. 35:96–100.
2012.
|
|
8
|
Wang X and Chen H: Effect of remifentanil
combined with dexmedetomidine on hemodynamics in the anesthetic of
cardiac surgery. Pract Pharm Clin Rem. 16:799–801. 2013.
|
|
9
|
Flanders CA, Rocke AS, Edwardson SA,
Baillie JK and Walsh TS: The effect of dexmedetomidine and
clonidine on the inflammatory response in critical illness: A
systematic review of animal and human studies. Crit Care.
23(402)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Portier K and Ida KK: The ASA physical
status classification: What is the evidence for recommending its
use in veterinary anesthesia?-A systematic review. Front Vet Sci.
5(204)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dong W, Chen MH, Yang YH, Zhang X, Huang
MJ, Yang XJ and Wang HZ: The effect of dexmedetomidine on
expressions of inflammatory factors in patients with radical
resection of gastric cancer. Eur Rev Med Pharmacol Sci.
21:3510–3515. 2017.PubMed/NCBI
|
|
12
|
Zhang Y, Jia S, Gao T, Zhang R, Liu Z and
Wang Y: Dexmedetomidine mitigate acute lung injury by inhibiting
IL-17-induced inflammatory reaction. Immunobiology. 223:32–37.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gallego-Ligorit L, Vives M, Vallés-Torres
J, Sanjuán-Villarreal TA, Pajares A and Iglesias M: Use of
dexmedetomidine in cardiothoracic and vascular anesthesia. J
Cardiothorac Vasc Anesth. 32:1426–1438. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rong H, Zhao Z, Feng J, Lei Y, Wu H, Sun
R, Zhang Z, Hou B, Zhang W, Sun Y, et al: The effects of
dexmedetomidine pretreatment on the pro- and anti-inflammation
systems after spinal cord injury in rats. Brain Behav Immun.
64:195–207. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li H, Zhang X, Chen M, Chen J, Gao T and
Yao S: Dexmedetomidine inhibits inflammation in microglia cells
under stimulation of LPS and ATP by c-Fos/NLRP3/caspase-1 cascades.
EXCLI J. 17:302–311. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cheng M, Gao T, Xi F, Cao C, Chen Y, Zhao
C, Li Q and Yu W: Dexmedetomidine ameliorates muscle wasting and
attenuates the alteration of hypothalamic neuropeptides and
inflammation in endotoxemic rats. PLoS One.
12(e0174894)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ihmsen H and Saari TI: Dexmedetomidine.
Pharmacokinetics and pharmacodynamics. Anaesthesist. 61:1059–1066.
2012.(In German). PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mo W, Li B and Tan W: Effect of
dexmdetomidine on hemodynamics during induction of anesthesia in
cardiac surgery. Chin J Misdiagn. 11:1604–1605. 2011.
|
|
19
|
Jiao F, Chen H and Huang C: Effects of
dexmedetomidine on hemodynamics and stress response in the
perioperative period of infant cardiac surgery. Modern Hospital.
14:45–47. 2014.
|
|
20
|
Xue Z, Wang S and Liu A: Effects of
dexmedetomidine on hemodynamics during induction of anesthesia in
patients with atrial fibrillation with rapid ventricular rate
undergoing noncardiac surgery. Chinese J Anesthesiol. 34:1452–1454.
2014.
|
|
21
|
Kawazoe Y, Miyamoto K, Morimoto T,
Yamamoto T, Fuke A, Hashimoto A, Koami H, Beppu S, Katayama Y, Itoh
M, et al: Effect of dexmedetomidine on mortality and
ventilator-free days in patients requiring mechanical ventilation
with sepsis: A randomized clinical trial. JAMA. 317:1321–1328.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mato M, Pérez A, Otero J and Torres LM:
Dexmedetomidine, a promising drug. Rev Esp Anestesiol Reanim.
49:407–420. 2002.(In Spanish). PubMed/NCBI
|
|
23
|
Afonso J and Reis F: Dexmedetomidine:
Current role in anesthesia and intensive care. Rev Bras Anestesiol.
62:118–133. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Arcangeli A, D'Alo C and Gaspari R:
Dexmedetomidine use in general anaesthesia. Curr Drug Targets.
10:687–695. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Triltsch AE, Welte M, von Homeyer P,
Grosse J, Genähr A, Moshirzadeh M, Sidiropoulos A, Konertz W, Kox
WJ and Spies CD: Bispectral index-guided sedation with
dexmedetomidine in intensive care: A prospective, randomized,
double blind, placebo-controlled phase II study. Crit Care Med.
30:1007–1014. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sommer C, Leinders M and Üçeyler N:
Inflammation in the pathophysiology of neuropathic pain. Pain.
159:595–602. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Marshall JC: SIRS and MODS: What is their
relevance to the science and practice of intensive care? Shock.
14:586–589. 2000.PubMed/NCBI
|
|
28
|
Deng Y, Tan F, Gan X, Li X, Ge M, Gong C,
Hei Z, Zhu Q and Zhou S: Perioperative application of
dexmedetomidine for postoperative systemic inflammatory response
syndrome in patients undergoing percutaneous nephrolithotomy
lithotripsy: Results of a randomised controlled trial. BMJ Open.
8(e019008)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Seyrek M, Binbay M, Yuruk E, Akman T,
Aslan R, Yazici O, Berberoglu Y and Muslumanoglu AY: Perioperative
prophylaxis for percutaneous nephrolithotomy: Randomized study
concerning the drug and dosage. J Endourol. 26:1431–1436.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ueki M, Kawasaki T, Habe K, Hamada K,
Kawasaki C and Sata T: The effects of dexmedetomidine on
inflammatory mediators after cardiopulmonary bypass. Anaesthesia.
69:693–700. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li B, Li Y, Tian S, Wang H, Wu H, Zhang A
and Gao C: Anti-inflammatory effects of perioperative
dexmedetomidine administered as an adjunct to general anesthesia: A
Meta-analysis. Sci Rep. 5(12342)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sukegawa S, Higuchi H, Inoue M, Nagatsuka
H, Maeda S and Miyawaki T: Locally injected dexmedetomidine
inhibits carrageenin-induced inflammatory responses in the injected
region. Anesth Analg. 118:473–480. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Taniguchi T, Kidani Y, Kanakura H,
Takemoto Y and Yamamoto K: Effects of dexmedetomidine on mortality
rate and inflammatory responses to endotoxin-induced shock in rats.
Crit Care Med. 32:1322–1326. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kawasaki T, Kawasaki C, Ueki M, Hamada K,
Habe K and Sata T: Dexmedetomidine suppresses proinflammatory
mediator production in human whole blood in vitro. J Trauma Acute
Care Surg. 74:1370–1375. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yuan X: Effect of laparoscopic radical
resection of colorectal cancer on serum CRP, IL-6 and IL-10
expression. J Pract Clin Med. 18:47–48. 2014.
|
|
36
|
Xu F, Yang CX and Deng SZ: Progress of
clinical application of dexmedetomidine in perioperative period.
Int J Anesthesiol Resuscit. 32:336–340. 2011.
|
|
37
|
Tanskanen PE, Kyttä JV, Randell TT and
Aantaa RE: Dexmedetomidineas an anesthetic adjuvant in patients
undergoing intracranialtumor surgery: A double-blind, randomized
and placebo-controlled study. Br J Anaesth. 97:658–665.
2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Geng F, Yu L and Long D: The effect of
dexmedetomidine on postoperative cognitive function and
inflammatory response of elderly patients undergoing laparoscopic
radical gastrectomy. J Prev Med PLA. 5:146–147, 149. 2019.
|
|
39
|
Yang D and Hong JH: Dexmedetomidine
modulateshistamine-induced Ca2+ signaling and
pro-inflammatorycytokine expression. Korean J Physiol Pharmacol.
19:413–420. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen S, Hua F, Lu J, Jiang Y, Tang Y, Tao
L, Zou B and Wu Q: Effect of dexmedetomidine on myocardial
ischemia-reperfusion injury. Int J Clin Exp Med. 8:21166–21172.
2015.PubMed/NCBI
|