Introduction
Lower back pain and neck pain are two common
symptoms caused by intervertebral disc degeneration (IDD) (1). The global lower back and neck pain
disability rates increased by 59.5% from 1990 to 2015, and will
likely increase further with an aging population (2). Currently, therapies for cervical IDD
(CIDD) are intended to relieve a multitude of symptoms, offering
only temporary benefits rather than a permanent recovery (3). This is mainly a result of poor
understanding of the exact etiology and pathogenesis. Xia et
al (4) previously reported that
transplantation of gelatin microspheres loaded with nucleus
pulposus-like cells (NP-1Cs) and growth and differentiation
factor-5 (GDF-5) into the intervertebral discs of the acupuncture
rat tail vertebrae partially regenerated damaged intervertebral
discs in a rat model. However, cartilage endplate (CE) degeneration
(CED) is an obstacle for effective stem cell therapy (5). Therefore, it is necessary to determine
the mechanisms underlying CIDD and CED to develop novel effective
treatment methods for these diseases.
The outer layer of intervertebral discs is annulus
fibrosus tissue, which surrounds the nucleus pulposus tissue and
connects the superior and inferior vertebral bodies with CE. The
supply of nutrition to intervertebral discs depends on the pores of
the superior and inferior CE of the vertebral body (6,7).
Previous studies have reported that CE serves a significant role in
maintaining the basic function of the intervertebral disc (8). Therefore, CED is the primary factor
leading to IDD, and maintaining the physiological function of CE is
essential to prevent and treat IDD. Previous studies have
identified factors associated with cartilage (9). For example, one study reported that
cartilage degeneration is associated with the downregulation of
Cbp/p300 interacting transactivator with Glu/Asp rich
carboxy-terminal domain 2 (CITED2) expression (9).
A recent study revealed that long non-coding RNAs
(lncRNAs), a subset of non-coding transcripts with a length of
>200 nucleotides that have a low protein-coding potential, are
involved in a series of biological processes such as glucose and
lipid metabolism (10). Some
lncRNAs can serve as competitive endogenous RNAs (ceRNAs) of
microRNA (miRNA/miR) to modulate the downregulation of miRNA
targets (11). The functions of
lncRNAs in cancer and several other diseases have been investigated
(12-15).
For instance, Chen et al (12) reported that LINC00173.v1 upregulated
the expression of VEGFA via sponging miR-511-5p to promote the
proliferation and migration of vascular endothelial cells. Wang
et al (13) also revealed
that CARL lncRNA inhibited mitochondrial fission and apoptosis by
upregulating prohibitin 2 expression via decreasing the expression
of miR-539. However, to the best of our knowledge, the expression
profiles and potential functions of lncRNAs in CED of cervical
vertebra remain unknown.
In the present study, lncRNA and mRNA microarrays
were used to identify differentially expressed lncRNAs (DELs) and
differentially expressed genes (DEGs) between degenerate and
healthy CE. Furthermore, a ceRNA regulatory network was constructed
based on DELs and DEGs. The expression levels of selected DELs and
DEGs were further assessed using reverse transcription-quantitative
PCR (RT-qPCR). The present study aimed to identify novel lncRNAs
and genes that are relevant to CED, which may further guide
investigations and contribute to the development of novel
therapeutic strategies for the treatment of CIDD.
Materials and methods
Tissue samples
A total of 38 CE specimens from 19 patients with
CIDD and 19 healthy subjects from February 2017 to February 2020
were enrolled in the study. The CIDD and healthy samples were
collected from The Second Affiliated Hospital of Nanchang
University (Nanchang, China). Details of the samples are presented
in Table SI. Degenerated cervical
vertebral CE specimens were collected from patients with CIDD that
suffered from cervical spondylosis myelopathy, who had received
anterior cervical discectomy and fusion (ACDF). CE specimens of
healthy subjects were obtained from patients with a cervical
fracture who received ACDF. None of the enrolled subjects had
undergone radiotherapy or chemotherapy, and none had a history of
surgery. Sample weight was evaluated before the study was
conducted. Of the lesions collected, six legions weighing >50 mg
were used for microarray detection. The remaining 32 lesions, which
weighed <50 mg, were used for RT-qPCR and western blot analyses.
The present study was approved by the Ethics Committee of The
Second Affiliated Hospital of Nanchang University. Written informed
consent was obtained from all participants. All tissue samples were
preserved in liquid nitrogen until RNA and protein extraction.
lncRNA and mRNA microarray
analysis
Total RNAs from three degenerative CE and three
healthy CE (HCE) tissues were extracted and purified using the
RNeasy micro kit (Qiagen GmBH) and the RNase-Free DNase set (Qiagen
GmBH). The RNA integrity coefficient number, as a measure of RNA
integrity, was measured using an Agilent Bioanalyzer 2100 (Agilent
Technologies, Inc.). The purity and quantity of initial total RNA
chip samples were determined using a NanoDrop ND-2000
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.) and an Agilent Bioanalyzer 2100 to exclude genomic DNA
contamination. RNA integrity was also determined on an ethidium
bromide-stained 2% agarose gel. Qualified RNAs, of which the
absorbance ratio at 260 and 280 nm is 2.0 was used for the
following chip experiments.
RNA amplification and labeling
Total RNA was amplified and labeled using the Low
Input Quick Amp Labeling kit, One-Color (Agilent Technologies,
Inc.) according to the manufacturer's instructions. Cy3-labeled
cRNA was purified using the RNeasy mini kit according to the
manufacturer's instructions (Qiagen GmBH).
Microarray hybridization
The SBC Human (4x180 K) ceRNA array (Shanghai Shibei
Biotechnology Co., Ltd.) was used to detect lncRNA and mRNA
expression profiles. Each slide was hybridized with 1.65 µg
Cy3-labeled cRNA using a Gene Expression Hybridization kit (Agilent
Technologies, Inc.) in a Hybridization Oven (Agilent Technologies,
Inc.) at 65˚C, according to the manufacturer's protocols. After 17
h of hybridization, the slides were washed in staining dishes
(Thermo Fisher Scientific, Inc.) using a Gene Expression Wash
Buffer kit (Agilent Technologies, Inc.), according to the
manufacturer's protocol. All raw data have been uploaded to Gene
Expression Omnibus (GSE153761).
Data acquisition
Slides were scanned using an Agilent Microarray
scanner (Agilent Technologies, Inc.) with the default settings: Dye
channel=Green, Scan resolution=3 µm, photomultiplier tube 100% and
20 bit. Data were extracted using Feature Extraction 12.0 software
(Agilent Technologies, Inc.). Raw data were normalized using the
Quantile algorithm and 3.11 version of limma packages (https://www.bioconductor.org/packages/release/bioc/html/limma.html)
in R 3.6.0 software (https://www.R-project.org/). Statistically significant
DELs and DEGs between the two groups were defined as fold-change ≥2
and P<0.05. Furthermore, heat maps were constructed to present
expression profiles of DELs and DEGs using hierarchical clustering,
which was performed using the 1.0.12 version of pheatmap package
(https://cran.r-project.org/web/packages/pheatmap/index.html)
in R. In addition to these, the repeatability and accuracy of ceRNA
array in the current study was also assessed using the Coefficient
of Variation (CV). The value of CV was calculated according to the
10 repeated probe signals. If the CV is <15%, the outcomes of
ceRNA array can be considered to be of excellent stability.
Statistical analyses were performed using R. A two-tailed P<0.05
was considered indicate a statistically significant difference for
all tests.
Function and pathway analysis of
DEGs
Gene Ontology (GO; www.geneontology.org) is a widely used ontology
resource in the bioinformatics field. The present study analyzed
the association of DEGs in the Biological Processes (BP), Molecular
Function (MF) and Cellular Component (CC) sets in the GO database.
The P-value denotes the significance of the enriched GO terms among
DEGs.
Kyoto Encyclopedia of Genes and Genomes (KEGG;
https://www.genome.jp/kegg/) pathway
analysis is a systematic analysis tool and database of gene
function and genome information, which helps researchers to study
and express information in the context of entire gene networks
(16). KEGG enrichment analysis of
DEGs can identify differentially enriched pathways, which is useful
for identifying biological regulation pathways that are
significantly altered under experimental conditions (16). The enrichment analysis was performed
using Fisher's exact test in the PANTHER classification system
(http://www.pantherdb.org) and the 3.11 version of
Cluster Profiler package from R/Bioconductor (https://www.bioconductor.org/) (17,18).
The standard of selection was the number of genes attaining a
P<0.05 threshold.
Construction of ceRNA regulatory
network
To determine the interaction between lncRNAs and
mRNAs, data regarding lncRNAs and mRNAs were combined with miRNA
data to construct a lncRNA-miRNA-mRNA ceRNA regulatory network.
Using the miRanda tool (version 3.3a; http://www.microrna.org/), the present study predicted
the lncRNA-miRNA regulatory combinations. miRNAs that had
regulatory associations with mRNAs in the ceRNA network were
queried in three databases, miRDB (version 6.0; http://www.mirdb.org), TargetScan (version 7.2;
http://www.ta-rgetscan.org/vert_72/)
and miRTarBase (version 8.0; http://mirtarbase.mbc.nctu.edu.t-w), which included
experimentally validated miRNA-target interactions (19-21).
The overlapping genes between targets of the identified miRNAs and
the total of DEGs were retained for the ceRNA network construction,
with associated DELs and miRNAs. Based on the identified miRNAs and
miRNA targets in DELs and DEGs, a ceRNA regulatory network was
constructed and visualized using Cytoscape software version
3.7.2(22).
Functional analysis of genes in the
ceRNA regulatory network
In the ceRNA regulatory network, a GO enrichment
analysis was performed using Fisher's exact test in the PANTHER
classification system to assess the potential functions of DEGs in
the categories of BP, MF and CC. Over-represented enriched KEGG
pathways were searched for using the 3.11 version of
ClusterProfiler package of R/Bioconductor to identify pathways that
were strongly associated with DEGs in the ceRNA network. P<0.05
was set as the threshold for significance.
RT-qPCR
Total RNA was extracted from 32 samples (16 patients
and 16 healthy control) of CE using TRIzol® reagent
(Thermo Fisher Scientific, Inc.). Expression levels of one DEG
[integrin subunit β8 (ITGB8)], miR-20a-5p and five DELs
(ENST00000548900, lnc-MYBPC1-1:1, lnc-ARL13A-1:1, lnc-C2orf67-1:1
and lnc-DNAJB6-3:1) were detected using a PrimeScript™ RT reagent
kit (Perfect Real-Time) and SYBR® Fast qPCR Mix (both
from Takara Bio, Inc.). The temperature protocol for reverse
transcription was: 16˚C for 30 min, 42˚C for 30 min and 85˚C for 5
min. cDNA was subjected to initial denaturation at 94˚C for 3 min,
followed by 40 cycles at 94˚C for 10 sec and 60˚C for 40 sec,
followed by extension at 72˚C for 10 min, using the specific
primers. All experiments were repeated three times. β-actin was
used as an internal reference for these lncRNAs and ITGB8 whereas
U6 was used as an internal reference for miR-20a-5p. In the present
study, 2-ΔΔCq method was used for
relative quantification (23).
Primer sequences are presented in Table SII.
Western blotting
Western blot analyses were performed on the proteins
extracted from 22 samples (11 patients and 11 healthy control) of
CE tissue samples by using the RIPA lysis buffer (cat. no. P0013B;
Beyotime Institute of Biotechnology). Protein concentrations were
measured using a BCA protein assay reagent (Beyotime Institute of
Biotechnology). Each lane was loaded with 15 µg protein. Samples
were then separated via 10% SDS-PAGE and separated proteins were
transferred to PVDF membranes. After blocking in 5% non-fat dry
milk in TBS-T (0.1% Tween 20) for 2 h at 37˚C, the membranes were
incubated with anti-ITGB8 (4˚C; 8 h; 1:500; cat. no. ab80673) and
anti-GAPDH (4˚C; 8 h; 1:5,000; cat. no. ab9485) primary antibodies
(both from Abcam), and then with the secondary goat anti-rabbit IgG
antibodies (37˚C; 2 h; 1:5,000; cat. no. ab205718; Abcam),
detection was performed using chemiluminescence (Beyotime Institute
of Biotechnology). ImageJ software (1.52v; National Institutes of
Health) was used to quantify protein band intensity.
Statistical analysis
Correlation was assessed by using Pearson's
correlation coefficient. Except for the Microarray, each experiment
was repeated ≥ three times. Relative expression levels of ITGB8,
miR-20a-5p and the five DELs were calculated using the 1.1.2
version of pcr package (https://www.
rdocumentation.org/packages/pcr/versions/1.1.2). Statistical
significance was determined using an unpaired Student's t-test
between two groups. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS (version 20.0; IBM, Corp.) and GraphPad Prism
(version 8.0; GraphPad Software, Inc.) software.
Results
Identifying DELs and DEGs via
microarray
In order to investigate the expression pattern of
lncRNAs in degenerative CE and HCE tissues, the present study used
ceRNA microarray to identify DELs and DEGs. A box plot was used to
demonstrate the distribution of the hybridization data and the
degree of dispersion. After log2 normalization, no abnormal
distributions of data were observed in the six healthy and CED
samples (Fig. 1A). Analysis of
correlations between expression and CE disease status showed that
the expression level of genes was highly similar among Dg
(>0.98) and less so for Hg (0.93-0.96; Fig. 1B). The variation between CED and HCE
was demonstrated in a scatter plot of the lncRNA expression profile
(Fig. 1C). A volcano plot
identified the DELs at different P-values and fold-changes between
the two groups (Fig. 1D). CED
samples were compared with healthy samples, where a total of 369
DELs, including 316 upregulated and 53 downregulated lncRNAs
(Fig. 1C), and a total of 246 DEGs,
including 171 upregulated and 75 downregulated mRNAs (Fig. 1D), were identified from the
microarray results.
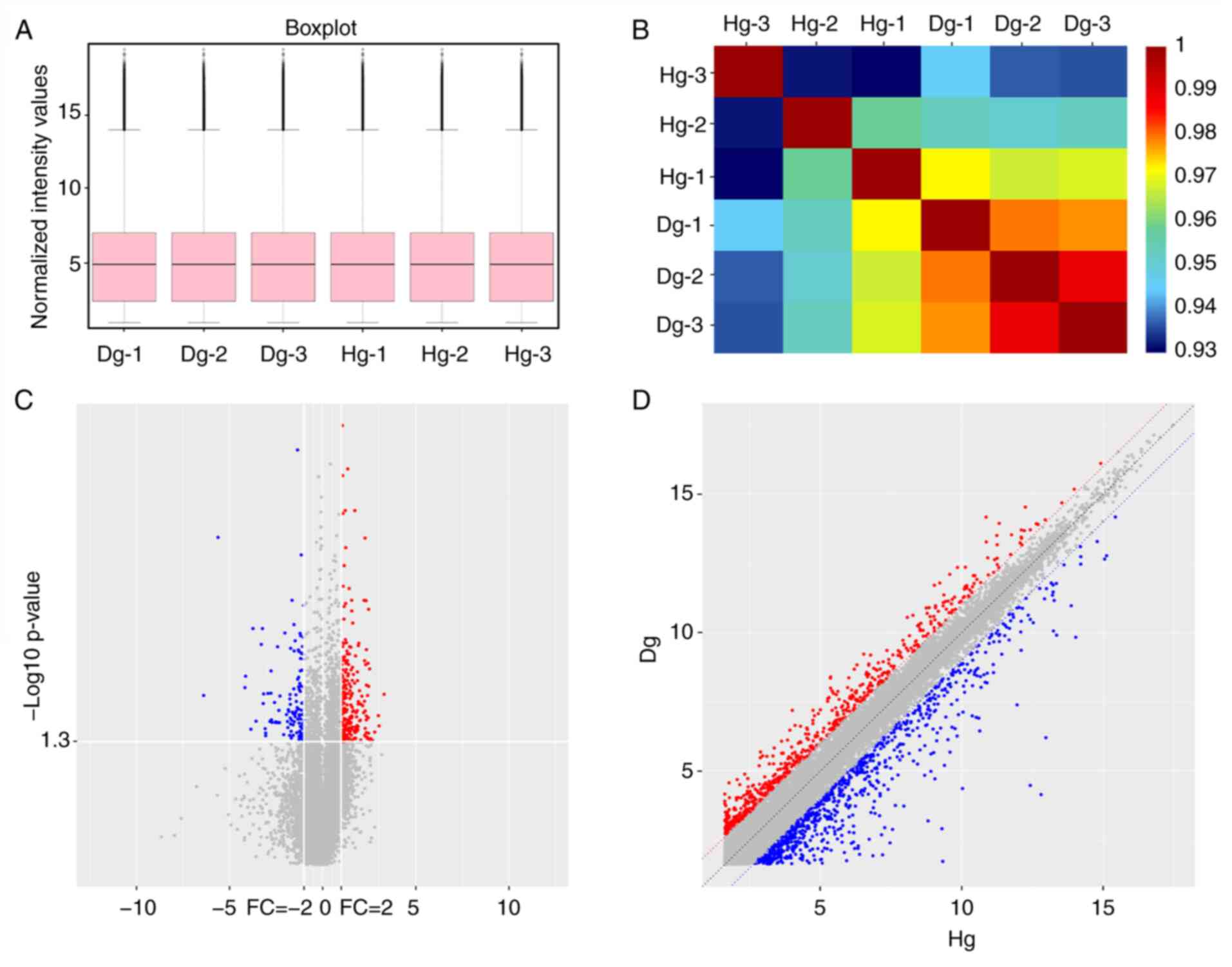 | Figure 1Expression profile of lncRNAs
detected using a microarray in degeneration CE compared with
healthy CE. (A) Box plots were used to visualize the distributions
of lncRNAs for the two groups. After normalization, the
distributions of the log2 ratios among six samples were nearly the
same. (B) A block diagram for the correlation coefficient r. The
blue or red of the color represents the degree of correlation
between samples, and the deeper the red, the higher the correlation
between the two samples. (C) Volcano plot visualizing differential
lncRNA expression between the two groups. The vertical lines
correspond to a 2.0-FC (log2 scaled), showing upregulation and
downregulation. The horizontal line represents a P<0.05 (log10
scaled). The red and blue points in the plot present the
statistically significantly upregulated and downregulated lncRNAs,
respectively. Red points mark upregulated expression of lncRNAs in
CED vs. healthy CE (P<0.05, FC ≥2). Blue points mark
downregulated expression of lncRNAs in CED vs. healthy CE
(P<0.05, FC ≤0.5). (D) Scatter plot demonstrating the variation
of lncRNAs expression in Dg (y-axis) vs. Hg (x-axis). The values of
the x- and y-axes are the averaged normalized signal values of each
group (log2 scaled). The middle line represents no difference
between the two groups. In the figure, representing the probe point
in the two groups, the signal difference was FC ≥2; red represents
upregulation and blue represents downregulation. FC, fold-change;
Dg, degeneration group; Hg, healthy group; lnc, long non-coding
RNA. |
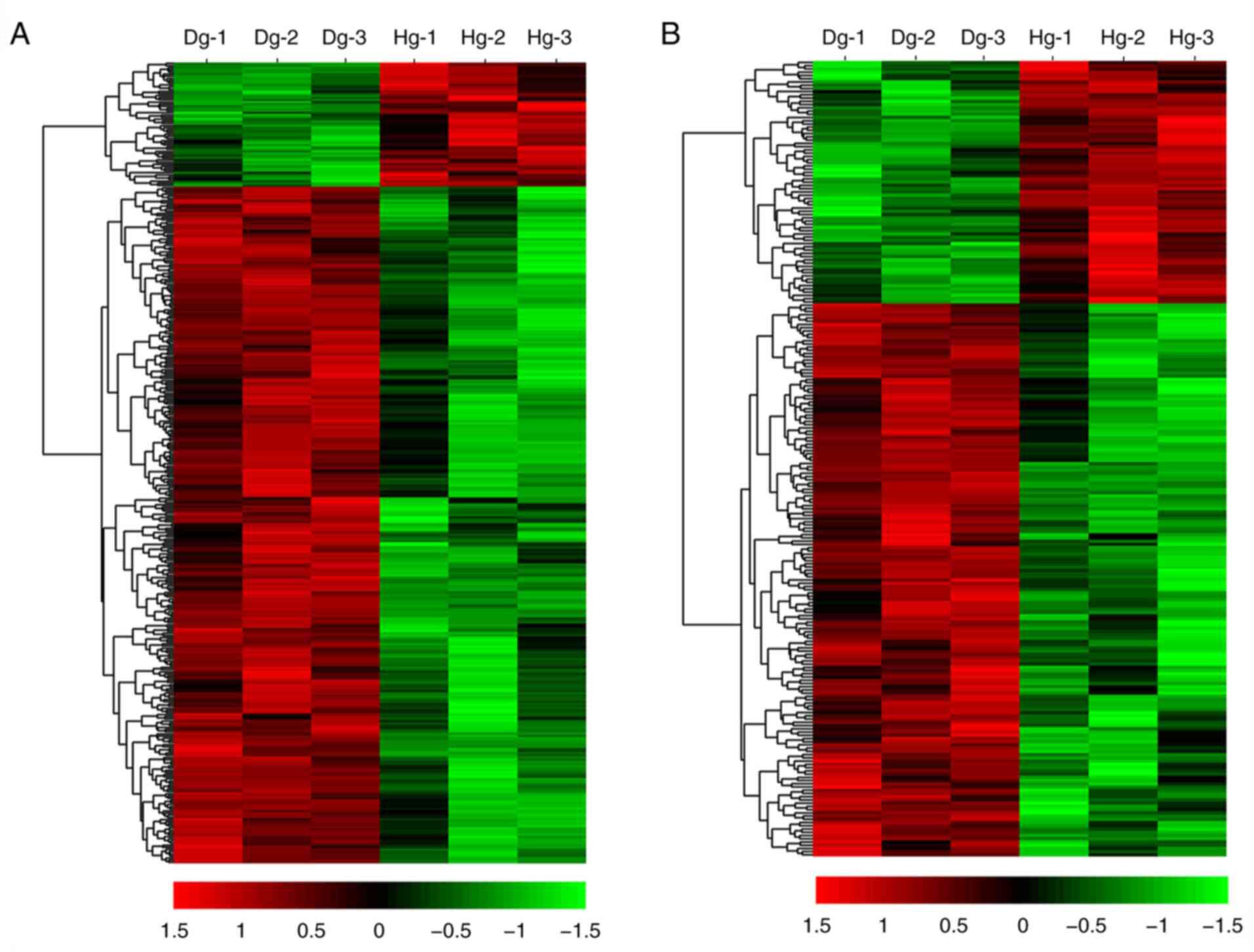
Hierarchical clustering identified that lncRNA and
gene expression levels were distinguishable between CED and healthy
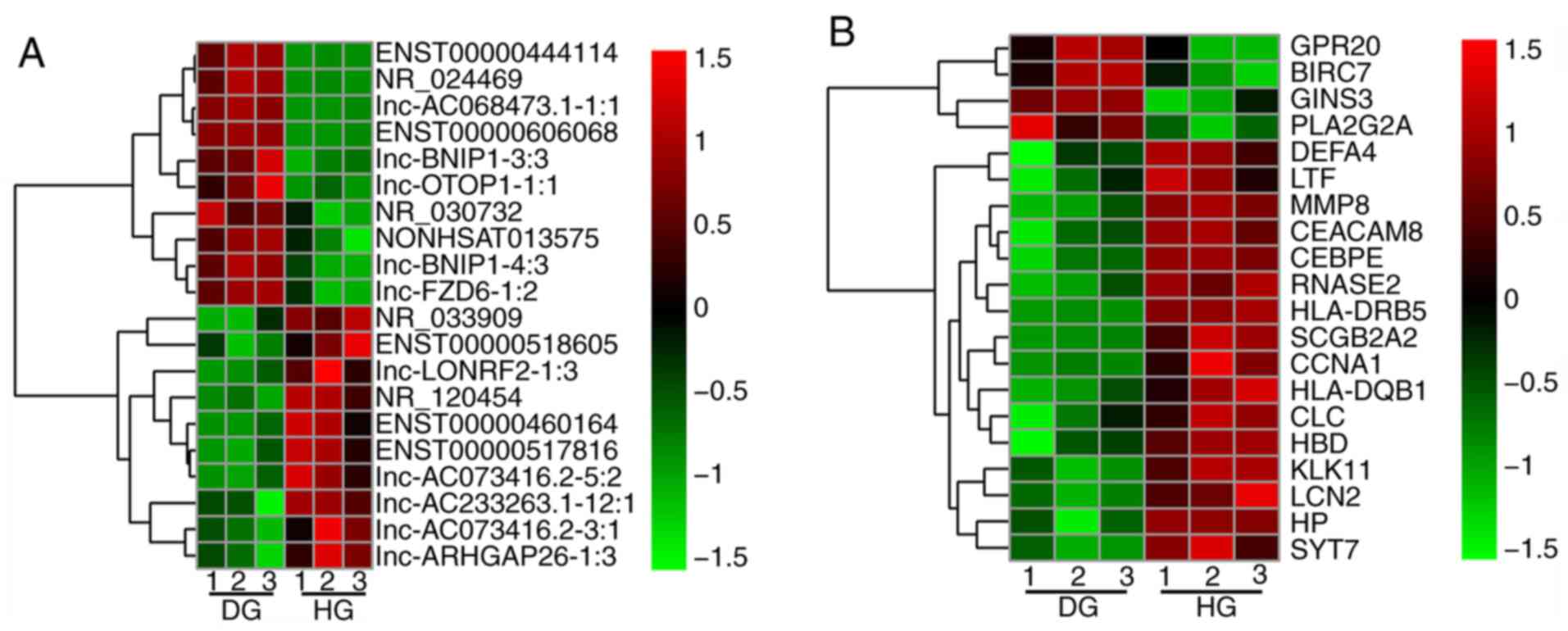
samples, as presented by the associated heat map (Fig. 2). The top 20 fold-change upregulated
or downregulated DELs are presented in Fig. 3A and Table I. The top 20 fold-change upregulated
and downregulated DEGs are presented in Fig. 3B and Table II.
 | Table ITop 20 upregulated and downregulated
lncRNAs in degenerative CE vs. healthy CE were sorted according to
FC. |
Table I
Top 20 upregulated and downregulated
lncRNAs in degenerative CE vs. healthy CE were sorted according to
FC.
| lncRNA-ID |
Log2FC | P-value | Regulation |
|---|
|
ENST00000460164 |
-4.0654447321111 | 0.010925989 | Down |
|
ENST00000444114 |
3.15320239767769 | 0.005654941 | Up |
|
lnc-ARHGAP26-1:3 |
-2.97917502630228 | 0.035859204 | Down |
|
ENST00000518605 |
-2.80565656425262 | 0.02692457 | Down |
|
lnc-AC068473.1-1:1 |
2.78060506466701 | 0.026317694 | Up |
| NR_033909 |
-2.77339818775925 | 0.01209491 | Down |
| NONHSAT013575 |
2.72406891252417 | 0.006270757 | Up |
|
ENST00000606068 |
2.71093666603456 | 0.007273469 | Up |
| lnc-OTOP1-1:1 |
2.6826499083849 | 0.004281053 | Up |
|
lnc-AC073416.2-3:1 |
-2.55407843103034 | 0.022005983 | Down |
|
lnc-AC233263.1-12:1 |
-2.54967640076035 | 0.03761393 | Down |
| NR_024469 |
2.52303431159003 | 0.000477517 | Up |
|
lnc-AC073416.2-5:2 |
-2.51306945648041 | 0.017447387 | Down |
| lnc-FZD6-1:2 |
2.50613392195141 | 0.014306711 | Up |
| lnc-BNIP1-3:3 |
-2.47300950984058 | 0.041505299 | Down |
| lnc-BNIP1-4:3 |
-2.4537777630073 | 0.017295824 | Down |
| lnc-LONRF2-1:3 |
2.30742206138951 | 1.99172E-06 | Up |
|
ENST00000517816 |
-2.30653135357461 | 0.048332914 | Down |
| NR_120454 |
2.30548698577468 | 0.030404793 | Up |
| NR_030732 |
2.29451834582489 | 0.019540145 | Up |
 | Table IITop 20 upregulated and downregulated
genes in degenerative CE vs. healthy CE were sorted according to
FC. |
Table II
Top 20 upregulated and downregulated
genes in degenerative CE vs. healthy CE were sorted according to
FC.
| Gene symbol |
log2FC | P-value | Regulation |
|---|
| SCGB2A2 |
-6.38069654020843 | 0.016239341 | Down |
| HLA-DRB5 |
-5.60888034944606 | 0.000344626 | Down |
| SYT7 |
-4.12512677137271 | 0.010160235 | Down |
| DEFA4 |
-3.84312421870298 | 0.03700143 | Down |
| KLK11 |
-3.73246320843984 | 0.003181221 | Down |
| HBD |
-3.56787972602964 | 0.030431781 | Down |
| PLA2G2A |
3.30813047877424 | 0.015774342 | Up |
| MMP8 |
-3.288405759072 | 0.004677465 | Down |
| RNASE2 |
-3.22555107120361 | 0.00318142 | Down |
| HLA-DQB1 |
-3.18445779878179 | 0.019791381 | Down |
| CEACAM8 |
-3.16284892181939 | 0.015625014 | Down |
| CCNA1 |
-3.05409089387395 | 0.040614958 | Down |
| GINS3 |
3.03459748011711 | 0.034104828 | Up |
| BIRC7 |
2.99886168508531 | 0.026568151 | Up |
| CLC |
-2.8225612164875 | 0.030603049 | Down |
| GPR20 |
2.8001431232719 | 0.039633617 | Up |
| LCN2 |
-2.7633057628854 | 0.015383988 | Down |
| CEBPE |
-2.76040800943709 | 0.008924767 | Down |
| HP |
-2.73937831942498 | 0.029404319 | Down |
| LTF |
-2.7084261374062 | 0.031266564 | Down |
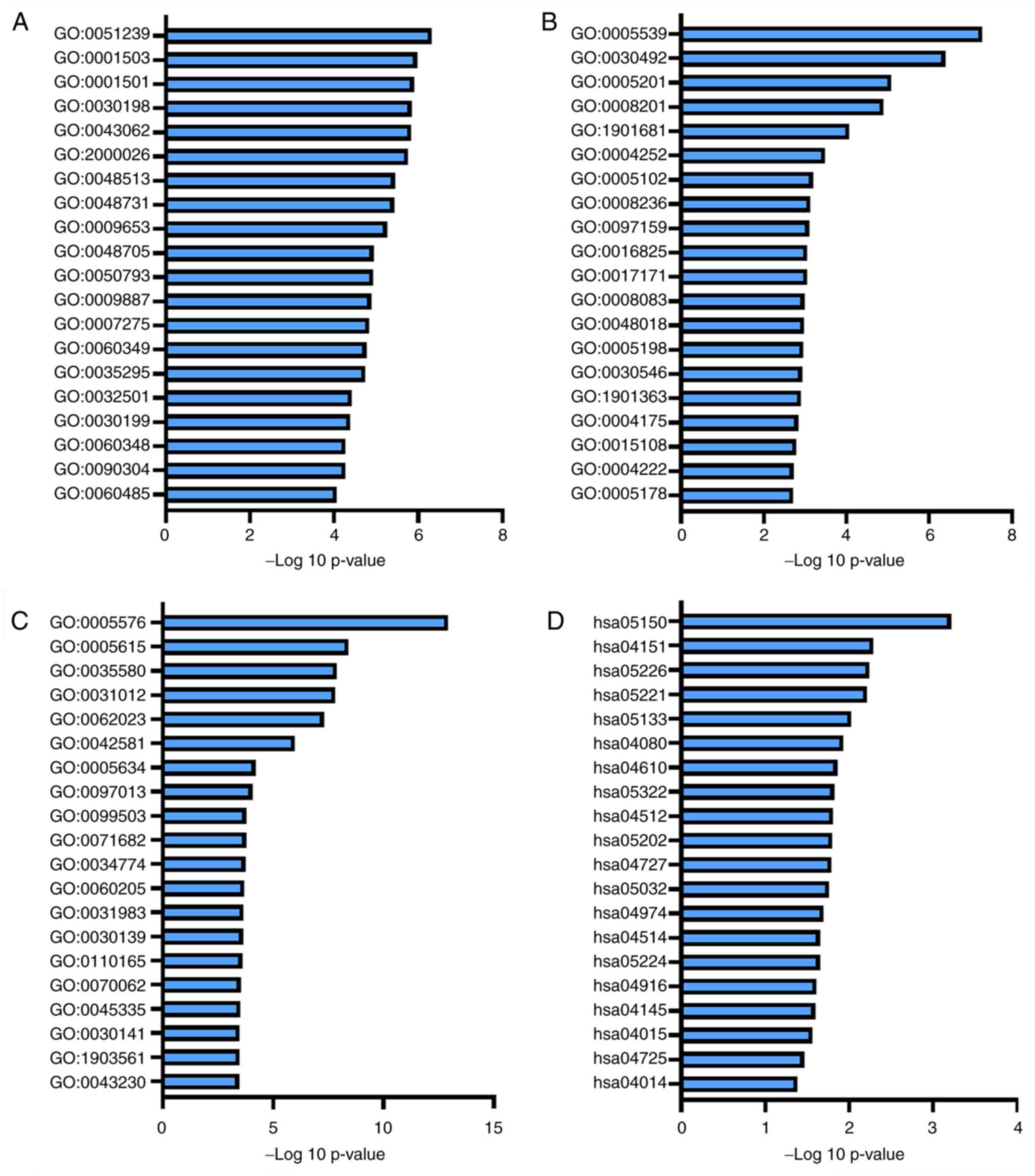
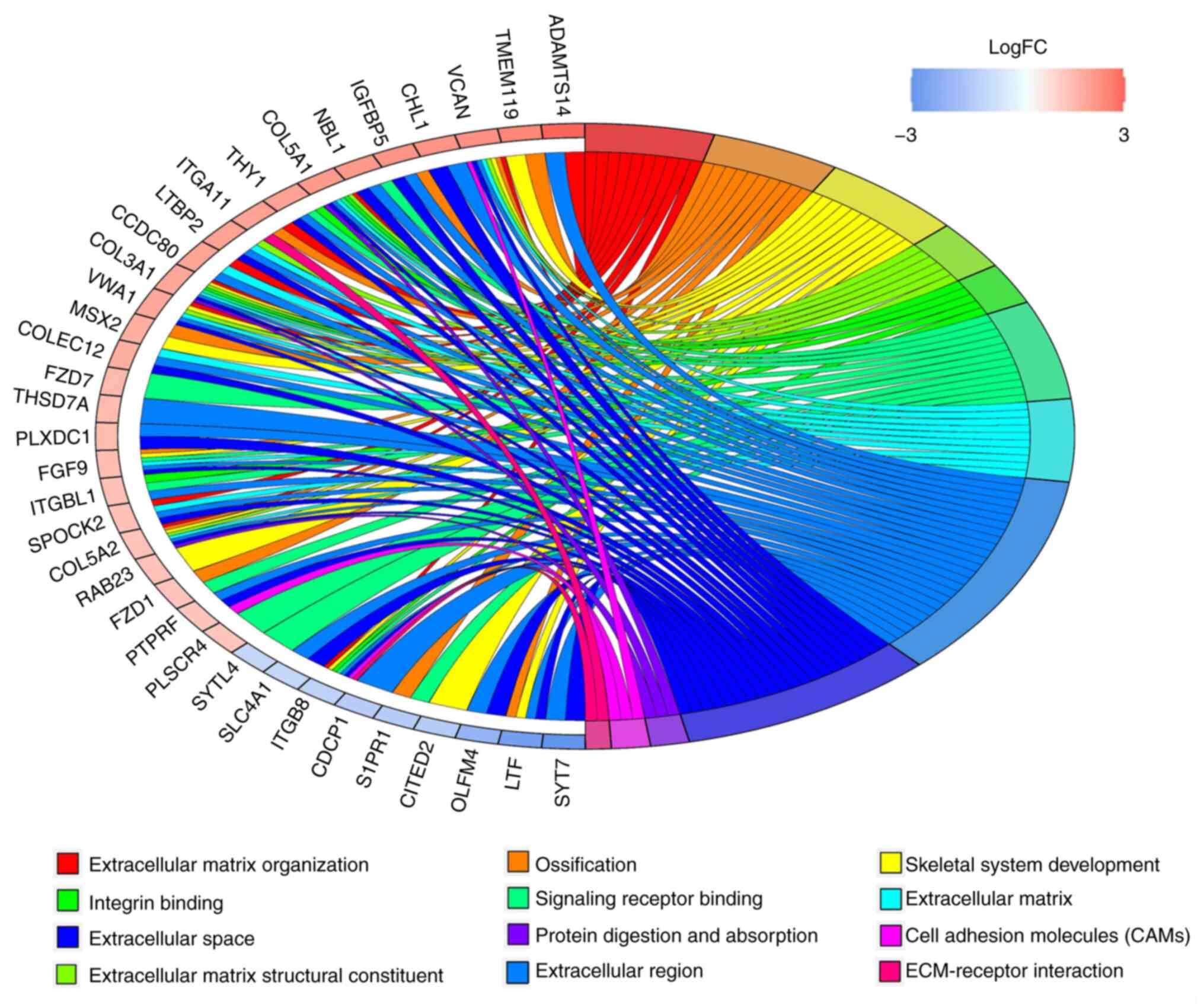
GO and KEGG pathway enrichment
analysis on basis of total DEGs
In order to identify the potential etiological
factors and key genes associated with the pathogenesis of CIDD, BP,
MF and CC analyses in GO and KEGG enrichment pathways were
performed for DEGs. The top 20 results are presented in Fig. 4 and
Tables SIII-VI. BP, MF, CC and KEGG terms that were
significantly associated with DEGs included ‘integrin binding (GO:
0005178)’ in Fig. 4B, ‘ossification
(GO: 0001503)’ in Fig. 4A,
‘extracellular matrix (ECM)-receptor interaction (hsa04512)’ in the
Fig. 4D and ‘skeletal system
development (GO: 0001501)’ in the Fig.
4A.
Construction of a ceRNA network
To evaluate the potential functions of DELs in CIDD,
the same putative miRNA response element (MREs) in DELs and DEGs
were identified. Using the miRanda tool, the present study
determined the regulatory associations between miRNAs and lncRNAs.
Data from the miRDB, TargetScan and miRTarBase databases were
integrated to determine the regulatory associations between miRNAs
and mRNAs (Table III). A ceRNA
regulatory network was then constructed by combining the identified
miRNA-mRNA and lncRNA-miRNA associations (Fig. S1). The ceRNA network was composed
of 451 nodes [168 miRNAs, 226 DELs (including 189 upregulated and
37 downregulated DELs) and 57 DEGs (including 47 upregulated and 10
downregulated DEGs)] and 1,087 lines (including 852 lncRNA-miRNA
connections and 235 mRNA-miRNA connections).
 | Table IIIRelationship between mRNAs and miRNA
predicted by all three databases (miRTarBase, TargetScan and miRDB)
in competitive endogenous RNA network. |
Table III
Relationship between mRNAs and miRNA
predicted by all three databases (miRTarBase, TargetScan and miRDB)
in competitive endogenous RNA network.
| Gene | miR |
|---|
| ABHD13 | miR-6507-5p |
| ADAMTS14 | miR-4667-5p,
miR-423-5p, miR-6762-5p, miR-8089, miR-6845-5p, miR-4700-5p |
| ANKRD50 | miR-93-5p,
miR-20b-5p, miR-20a-5p, miR-3934-3p, miR-548t-5p, miR-153-5p,
miR-6736-3p, miR-106b-5p, miR-17-5p, miR-6884-3p, miR-106a-5p |
| BNC2 | miR-567,
miR-513c-5p, miR-514b-5p, miR-8485, miR-6867-5p |
| CCDC80 | miR-3916 |
| CDCP1 | miR-1233-5p,
miR-6778-5p, miR-665, miR-6878-5p, miR-1827 |
| CHL1 | miR-182-5p |
| CITED2 | miR-3121-3p,
miR-548t-3p, miR-548t-5p, miR-548aa, miR-182-5p |
| COL3A1 | let-7b-5p,
miR-29b-3p, miR-767-5p |
| COL5A1 | miR-6785-5p,
miR-4728-5p, miR-6878-5p, miR-4516, miR-6883-5p, miR-149-3p |
| COL5A2 | miR-29b-3p,
miR-143-3p, miR-767-5p |
| COLEC12 | miR-6770-5p,
miR-7-5p |
| COMMD3-BMI1 | miR-195-3p,
miR-3120-3p |
| DYRK2 | miR-6767-3p,
miR-193a-3p, miR-484, miR-193b-3p, miR-8077, miR-24-3p,
miR-3613-3p, miR-8485, miR-4447, miR-1275 |
| EBF1 | miR-4776-3p |
| FGF9 | miR-942-5p,
miR-182-5p, miR-622, miR-140-5p, miR-8485 |
| FOXN2 | miR-6124,
miR-6809-3p, miR-367-3p, miR-25-3p, miR-363-3p, miR-3613-3p,
miR-92b-3p, miR-5692a, miR-92a-3p, miR-7109-3p, miR-188-5p,
miR-4724-5p |
| FZD1 | miR-7109-3p |
| FZD7 | miR-145-5p |
| GPR173 | miR-8485,
miR-6785-5p, miR-4524a-3p, miR-4516, miR-6883-5p, miR-92a-2-5p,
miR-1249-5p, miR-4728-5p, miR-6825-5p, miR-6797-5p, miR-149-3p,
miR-3202, miR-6867-5p |
| HES6 | miR-4739, miR-6129,
miR-6127, miR-4510, miR-6133, miR-6130 |
| IGFBP5 | miR-548aa,
miR-185-5p, miR-3613-3p, miR-3925-5p, miR-143-3p, miR-548t-3p,
miR-4755-5p, miR-4446-5p |
| ITGA11 | miR-1827,
miR-6867-5p, miR-650, miR-3612 |
| ITGB8 | miR-3613-3p,
miR-93-5p, miR-20a-5p, miR-145-5p, miR-6507-5p, miR-17-5p |
| ITGBL1 | miR-8485 |
| KCNJ12 | miR-1915-3p,
miR-6764-5p, miR-8485 |
| KCNQ5 | miR-543,
miR-6507-5p, miR-6867-5p |
| LTBP2 | miR-6880-5p,
miR-4708-3p, miR-3165 |
| LTF | miR-214-3p |
| MSX2 | miR-5196-5p,
miR-4747-5p |
| NBL1 | miR-4270,
miR-6754-5p, miR-4441 |
| NKX2-5 | miR-1538,
miR-4745-3p |
| NOVA1 | miR-338-3p |
| NUAK2 | miR-589-5p,
miR-3925-5p |
| OLFM4 | miR-486-5p |
| PDZRN4 | miR-7844-5p,
miR-3613-3p |
| PHYHIP | miR-6785-5p,
miR-1296-3p, miR-6883-5p, miR-4728-5p, miR-6825-5p, miR-3616-3p,
miR-149-3p, miR-6081, miR-6731-5p, miR-8085 |
| PLSCR4 | miR-15b-5p |
| PLXDC1 | miR-4732-3p,
miR-670-5p, miR-150-5p |
| PTPRF | miR-24-3p,
miR-298 |
| RAB23 | miR-545-3p,
miR-1468-3p, miR-518a-5p, miR-424-5p, miR-527, miR-195-5p,
miR-497-5p, miR-548p |
| S1PR1 | miR-363-3p |
| SLC4A1 | miR-504-3p,
miR-4430, miR-5698, miR-3652 |
| SNTB2 | miR-20a-5p,
miR-17-5p, miR-20b-5p, miR-5582-5p, miR-106b-5p, miR-93-5p |
| SPATS2L | miR-103a-3p |
| SPOCK2 | miR-8485 |
| SPTLC3 | miR-4534 |
| SVOP | miR-532-3p,
miR-3612, miR-4667-3p, miR-4727-5p, miR-3972, miR-6852-5p,
miR-1202, miR- 6749-3p, miR-7162-5p, miR-650, miR-1827,
hsa-miR-4768-3p |
| SYT7 | miR-4632-5p,
miR-3202, miR-4436b-3p, miR-6735-5p, miR-6760-5p, miR-4270,
miR-6879-5p, miR-1207-5p, miR-7843-5p, miR-4516, miR-4441,
hsa-miR-6887-3p, hsa-miR-4763-3p |
| SYTL4 | miR-8485,
miR-4789-3p |
| TBXA2R | miR-31-5p,
miR-1275, miR-6779-5p, miR-149-3p, miR-6785-5p, miR-508-5p,
miR-1273h-5p, miR-6883-5p, miR-7160-5p, miR-4478, miR-7106-5p,
hsa-miR-4728-5p |
| THSD7A | miR-3617-5p,
miR-153-5p, miR-5581-5p, miR-4297, miR-6867-5p, miR-641 |
| THY1 | miR-6778-3p,
miR-6825-5p |
| TMEM119 | miR-2114-3p |
| TSPAN12 | miR-140-5p,
miR-196a-5p |
| VCAN | miR-103a-3p,
miR-107, miR-578 |
| VWA1 | miR-765,
miR-6825-5p |
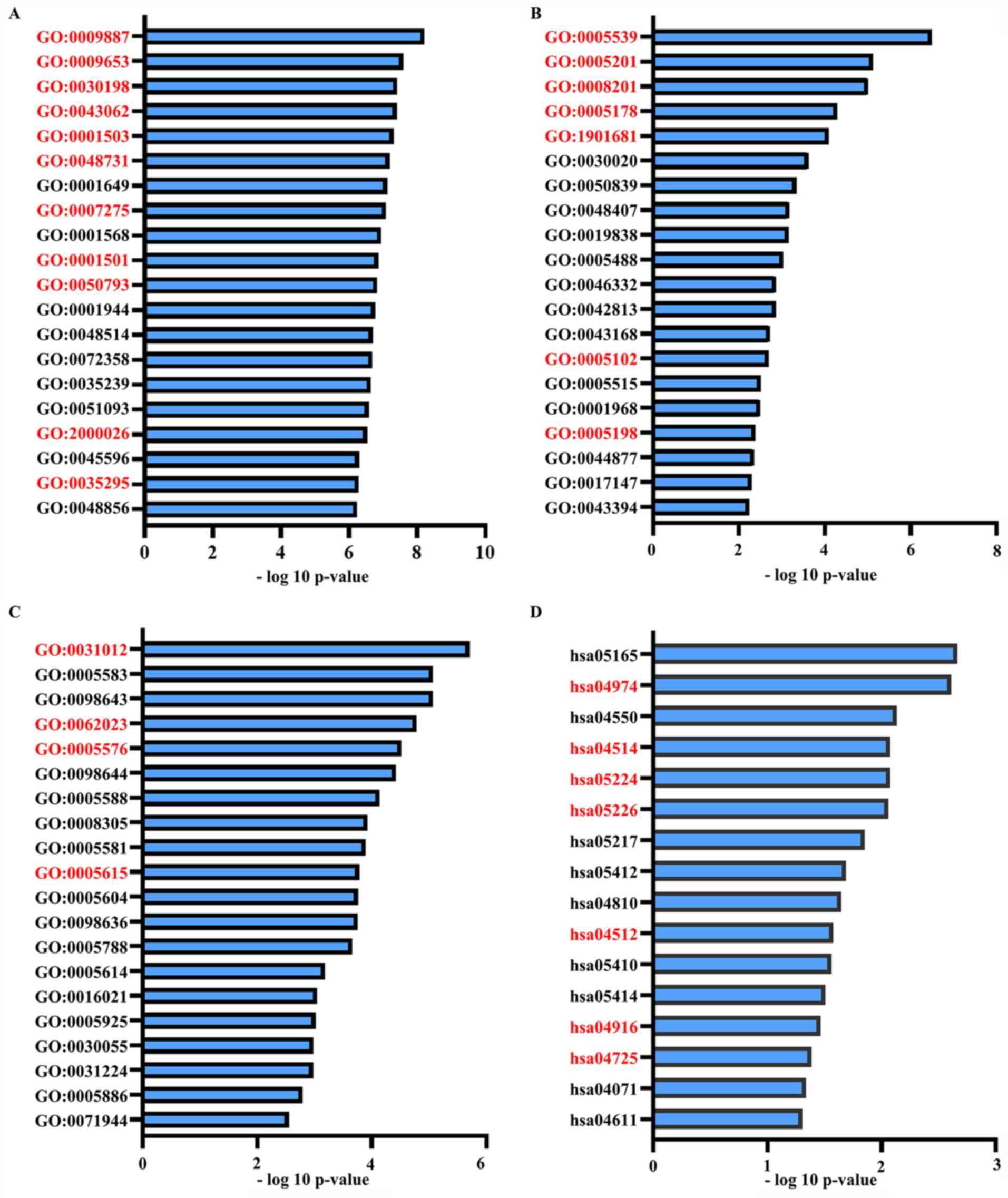
GO and KEGG pathway enrichment
analysis on basis of DEGs in the ceRNA network
To determine the potential functions of identified
DEGs and DELs in the ceRNA network, GO and KEGG pathway enrichment
analyses were performed, and the results are presented in Fig. 5 and Tables
SVII-X, which included ‘Ossification’, ‘Blood vessel
development’, ‘Integrin binding’ and ‘SMAD binding’. The
overlapping items in both of the functional analysis results of the
ceRNA network and functional analysis results of all DEGs were
considered to represent significant enrichments from BP and MF
analyses in GO and KEGG. The results are presented in Fig. 6 and Table IV, which included ‘Ossification’,
‘Integrin binding’, ‘Skeletal system development’ and ECM.
 | Table IVMajority of significant enrichments
and genes of Gene Ontology and Kyoto Encyclopedia of Genes and
Genomes pathway enrichments. |
Table IV
Majority of significant enrichments
and genes of Gene Ontology and Kyoto Encyclopedia of Genes and
Genomes pathway enrichments.
| Enrichment
terms | Gene |
|---|
| Extracellular
matrix organization | ADAMTS14, CCDC80,
COL3A1, COL5A1, COL5A2, ITGA11, ITGB8, SPOCK2, VCAN, VWA1 |
| Ossification | COL5A2, FGF9, FZD1,
IGFBP5, ITGA11, LTF, MSX2, S1PR1, TMEM119, VCAN |
| Skeletal system
development | FGF9, COL3A1, VWA1,
VCAN, COL5A2, TMEM119, MSX2, RAB23, CITED2, ITGB8, LTF |
| Extracellular
matrix structural constituent | COL3A1, COL5A1,
COL5A2, LTBP2, VCAN, VWA1 |
| Integrin
binding | COL5A1, COL3A1,
THY1, ITGBL1, ITGB8 |
| Signaling receptor
binding | FZD7, NBL1, COL5A1,
FGF9, FZD1, COL3A1, THY1, ITGBL1, PLSCR4, ITGB8, SYTL4, S1PR1 |
| Extracellular
matrix | VCAN, FGF9, CCDC80,
SPOCK2, LTBP2, VWA1, COLEC12, COL3A1, COL5A2, COL5A1 |
| Extracellular
region | VCAN, SLC4A1, FGF9,
CDCP1, CHL1, PLXDC1, OLFM4, CCDC80, SPOCK2, PTPRF, ITGB8, LTBP2,
NBL1, VWA1, THSD7A, LTF, ADAMTS14, COLEC12, SYT7, COL3A1, ITGBL1,
THY1, COL5A2, IGFBP5, COL5A1, CHL1 |
| Extracellular
space | VCAN, SLC4A1, FGF9,
CHL1, PLXDC1, OLFM4, SPOCK2, PTPRF, ITGB8, LTBP2, NBL1, VWA1, LTF,
COLEC12, SYT7, COL3A1, THY1, COL5A2, IGFBP5, COL5A1, CHL1 |
| Protein digestion
and absorption | COL3A1, COL5A1,
COL5A2 |
| Cell adhesion
molecules | ITGB8, PTPRF,
VCAN |
| Extracellular
matrix-receptor interaction | ITGA11, ITGB8 |
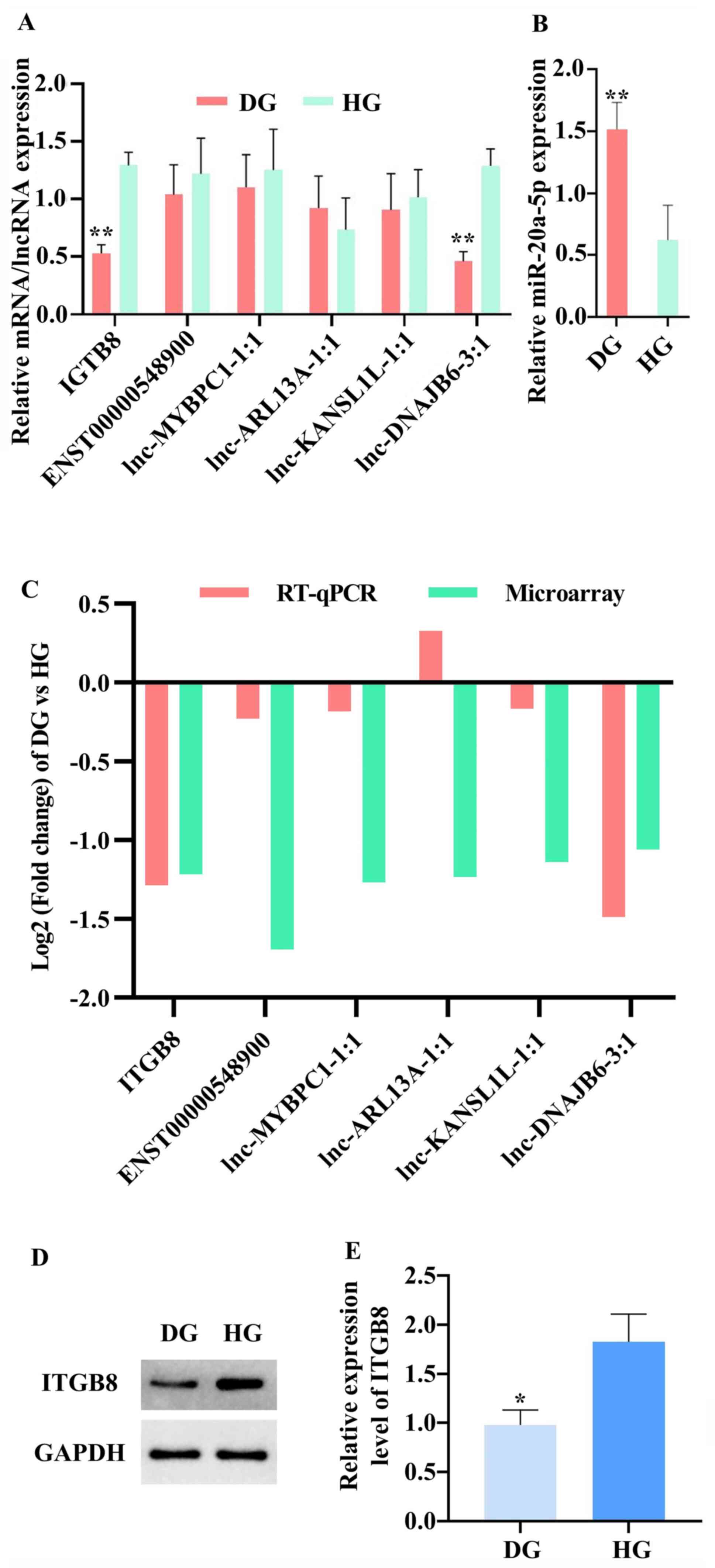
Validation of lncRNAs, miRNA and gene
expression in tissue samples using RT-qPCR and western blot
analyses
To assess the results from the ceRNA microarray, the
present study selected one DEG, ITGB8(24), miR-20a-5p (25) and its related five DELs
(ENST00000548900, lnc-MYBPC1-1:1, lnc-ARL13A-1:1, lnc-C2orf67-1:1
and lnc-DNAJB6-3:1), based on a literature review of the miRNAs and
genes identified in the functional analysis in the ceRNA network by
searching ‘geneID’ and ‘cartilage’ on PubMed (https://pubmed.ncbi.nlm.nih.gov/) and the
selected five DELs were associated with miR-20a-5p in the ceRNA
network. The expression patterns of seven RNAs from the RT-qPCR
results demonstrated that ITGB8 (0.45-fold change) and
lnc-DNAJB6-3:1 (0.34-fold change) expression levels were
significantly downregulated in CED samples compared with HCE
samples. The expression levels of miR-20a-5p (2.10-fold change) was
upregulated in CED samples compared with those in HCE samples
(Fig. 7A). The RT-qPCR results of
ITGB8 and lnc-DNAJB6-3:1 expression levels are consistent with the
microarray data (Fig. 7B).
Moreover, the western blotting results shown that ITGB8 is
downregulated in CED samples compared with that in HCE samples
(Fig. 7C and D).
Discussion
CED is the main cause of IDD (26). Although the etiology of CED remains
unknown, it is a complex, multifactorial disorder that is
influenced by cartilage degeneration due to angiogenesis,
inflammation, ECM degeneration, oxidative stress, cell
hyperproliferation, chondrocyte apoptosis and autophagy (27,28).
The study into the pathogenesis of CED is ongoing.
lncRNAs have been reported to serve essential roles
in angiogenesis, inflammation and ECM degeneration (29). However, to the best of our
knowledge, the expression profile of lncRNAs and their potential
biological functions in CED have not yet been reported. The present
study utilized a lncRNA and mRNA microarray to provide
comprehensive lncRNA and mRNA expression profiles in CED and HCE,
with the aim of investigating the potential involvement of
dysregulated lncRNAs in the development of CED. The present study
identified a large number of DELs and DEGs from diverse genomic
locations, with 246 DEGs (171 upregulated and 75 downregulated) and
369 lncRNAs (316 upregulated and 53 downregulated) found to be
differentially expressed between cervical vertebra CED samples and
healthy controls. A ceRNA network was established, which included
168 miRNAs, 226 DELs (including 189 upregulated and 37
downregulated DELs) and 57 DEGs (including 47 upregulated and 10
downregulated DEGs). To the best of our knowledge, the present
study was the first to report the comprehensive lncRNA expression
profile in CED. Therefore, the results of the present study provide
a novel theoretical basis for further investigations into the
function of lncRNA in CED.
lncRNAs are rich in miRNA binding sites and can act
as ceRNAs in cells, which leads to relief of the inhibitory effect
of miRNAs on their target genes and, ultimately, increased
expression of the target genes (30). To determine the potential function
of lncRNAs as miRNAs sponges in cervical CED, GO BP, GO MF, GO CC
and KEGG pathway enrichment analyses were performed on total DEGs
and ceRNA network DEGs in the present study. The common significant
enrichments between DEGs in the ceRNA network and all DEGs from GO
BP, GO MF, GO CC and KEGG pathway analysis were associated with
cartilage development, degeneration and regeneration.
Integrins are well-known as cell adhesion molecules
and act via ECM-receptor interaction (31). Moreover, integrins are suggested to
control intracellular signaling pathways both physically and
chemically as mechanoreceptors (32). Previous studies have reported that
if the fibrillar network of the ECM is dysfunctional or assembled
from fibers that cannot bind and activate α5β1 integrins,
chondrocytes increase the MMP expression, which inhibits cartilage
regeneration (33). The increased
expression of α2β1 and activation of integrin signaling may also
induce a pathological increase in MMP expression following exposure
to static compression (33,34).
The significant items in the results of enrichment
analyses in the present study included ‘integrin binding’,
‘ossification’, ‘ECM’ and ‘skeletal system development’ Li et
al (35) previously reported
that the loss of OPG leads to the occurrence of IDD by promoting
the ‘ossification’ biological process of the cartilage endplate in
the osteoprotegerin (OPG)-knockout mice. Qu et al (36) reported that DEGs were primarily
involved in ‘skeletal system development’ in a co-expression
network in IDD and serve an important role the pathobiology of the
disease.
Signaling pathways and the term ‘signal receptor
binding’ are similar. Xue et al (37) previously reported that the decreased
expression of NF-κB interacting lncRNA, a miR-145 sponge, inhibited
SP1 expression and regulated the NF-κB signaling pathway to inhibit
proliferation and promote apoptosis of chondrocytes. Furthermore,
Gu et al (38) revealed that
the majority of hub genes associated with osteoarthritic cartilage
screened from GSE51588 were primarily enriched in ‘protein
digestion and absorption’.
The present study identified a group of DEGs that
serve important roles in cartilage development and degeneration
from a literature review (Table V).
Genes whose microarray results were consistent with those reported
in the literature include ITGB8, lactotransferrin (LTF), msh
homeobox 2 (MSX2), latent transforming growth factor β binding
protein 2 (LTBP2), neuroblastoma suppressor of tumorigenicity 1
(NBL1) and CITED2. Genes whose microarray results are inconsistent
with those reported in the literature include insulin like growth
factor binding protein 5 (IGFBP5), collagen type III α 1 chain
(COL3A1), COL5A1, COL5A2, fibroblast growth factor 9 (FGF9),
ITGBL1, versican (VCAN), RAB23, frizzled class receptor 7 (FZD7)
and ITGA11. LaPointe et al (24) reported that the downregulation of
ITGB8 increased the expression of ITGA11 and caused ablation of
COL2A1 expression, as detected in the chondrogenic differentiation
of mesenchymal stem cells. Xue et al (39) observed that LTF can inhibit
IL-1β-induced apoptosis via AKT1-induced cAMP responsive element
binding protein 1 phosphorylation in human articular chondrocytes.
Furthermore, Nishimura et al (40) suggested that MSX2, a homeobox Runx2
family member, may promote endochondral ossification via
upregulation of Osterix (Sp7) expression. He et al (9) also reported that the transcriptional
regulator CITED2 promoted chondroprotection by inhibiting the
expression of MMP13, and that the decreased expression of CITED2
induced cartilage degradation via increased expression of MMP13.
Moreover, Sideek et al (41)
reported that exogenous LTBP2 inhibits elastinogenesis in ear
cartilage chondrocytes in culture, while Goessler et al
(42) reported that LTBP2 was
activated during chondrogenic dedifferentiation to decrease the
repairability of cartilage defects in vitro. In addition,
Wei et al (43) used
microarray technology to demonstrate that the attenuation of
cartilage anabolism by increased expression of NBL1, a secreted BMP
antagonist, is a molecular mechanism underlying cartilage
degeneration.
 | Table VIdentifying crucial genes using a
literature review. |
Table V
Identifying crucial genes using a
literature review.
| Gene group | Upregulated genes
in cartilage degeneration | Downregulated genes
in cartilage degeneration |
|---|
| A | MSX2, LTBP2,
NBL1 | ITGB8, LTF,
CITED2 |
| B | IGFBP5, COL3A1,
COL5A1, COL5A2, FGF9, ITGBL1, VCAN, RAB23, FZD7, ITGA11 | - |
Genes from the present study that were not
consistent with those in the literature may decrease CED. For
instance, Weimer et al (44)
revealed that IGFBP5, a potentiator of IGF-I, could increase the
expression of IGF-1 to maintain the proliferation and survival of
chondrocytes. Type III collagen, encoded by COL3A1, together with
type I collagen, provides a structural framework for the synovium
surrounding the synovial joint to protect articular cartilage
(45). Type V collagen, encoded by
COL5A1 and COL5A2, is present in tissues containing type I collagen
and appears to regulate the assembly of shaped fibers composed of
type I and type V collagen, with the type V collagen being
associated with articular cartilage maturity (46,47).
Exogenous FGF9 attenuates cartilage degradation in mice (48), and its increased expression may
represent a protective mechanism that remains intact during
cartilage degeneration. Song et al (49) reported that overexpression of ITGBL1
inhibits integrin signaling in developing chondrocytes to promote
cartilage formation. Moreover, Choocheep et al (50) observed that VCAN is required for
chondrocyte differentiation during cartilage formation. Yang et
al (51) also demonstrated that
too much or too little RAB23 protein induced the expression of SOX9
and caused the failure of chondrogenic differentiation. In
addition, this differential expression profile of RAB23 may not
have been specific to chondrocyte differentiation in their study by
using gain-of-function and loss-of-function experiments (51). Randall et al (52) reported that activation of FZD7, a
component of the Wnt planar cell polarity pathway, was able to
promote growth plate chondrocyte column formation.
Of the genes identified in the present study that
were consistent with those in the literature, only four miRNAs with
MREs within ITGB8 were associated with cartilage degeneration and
calcification, including miR-20a-5p, miR-145-5p, miR-93-5p and
miR-17-5p. Guo et al (25)
reported that miR-20a-5p in exosomes from breast cancer cells
promoted the proliferation and differentiation of osteoclasts.
Furthermore, Chen et al (53) revealed that decreased miR-145-5p
expression promoted the proliferation of osteoclasts to aggravate
cartilage erosion by targeting OPG. Xiao et al (54) also found that increased osteoclasts
in the CE of ovariectomized mice resulted in cartilage remodeling
to stimulate IDD. It has been demonstrated that increased HOX
transcript antisense RNA expression, decreased the expression of
miR-17-5p to promote cartilage injury by mediating the
Wnt/β-catenin pathway via fucosyltransferase 2(55). Moreover, Xue et al (56) suggested that increased expression of
miR-93-5p promoted chondrocyte viability and suppressed chondrocyte
apoptosis in vitro.
Previous studies have reported that the ceRNA
mechanism can be used for the analysis of multiple diseases
(36,57). Qu et al (36) constructed a miRNA-lncRNA-mRNA ceRNA
regulatory network on the basis of DEGs and lncRNAs in patients
with IDD. In addition, Wang et al (57) identified a novel ceRNA network that
provides a novel insight for investigating the underlying molecular
mechanism of diabetic peripheral neuropathy. In the present study,
ITGB8 was inhibited by increased expression of MREs resulting from
decreased expression of lncRNAs in the ceRNA network. After
screening, the expression levels of IGTB8, miR-20a-5p and its five
regulated lncRNAs within ceRNA network were validated in 16 CIDD
and 16 healthy subjects using RT-qPCR. It was suggested that
downregulated lnc-DNAJB6-3:1 may have decreased the expression of
ITGB8 to promote cartilage degeneration via upregulation of
miR-20a-5p expression. To the best of our knowledge, the present
study was the first to report that lnc-DNAJB6-3:1 is downregulated
in CED, as well as demonstrating that lnc-DNAJB6-3:1 regulated
miR-20a-5p/ITGB8 axis in the CED.
In the present research, the box plots (Fig. 1A) were used to visualize the
distributions of lncRNAs for two groups. Although the normalized
data were skewed, the distributions of the log2 ratios among the
six samples presented an excellent similarity after normalization,
which indicated that the data could be further analyzed. The
phenomenon that the normalized data were skewed (long tail above
mean/median) was also demonstrated in previously published studies
(58-60).
It was suggested that the sampling error and selective bias are
responsible for this phenomenon, and enlarging the sample sizes is
an effective strategy to decrease the sampling error and selective
bias. In the present study, gene expression was highly similar
among Dg (>0.98) and less so for Hg (0.93-0.96), which can also
be due to sampling error and selective bias.
Technical replicates were performed in order to
further determine the reliability of detection platform for
identifying DEGs using microarray. The SBC Human ceRNA array
(Agilent-078298 human ceRNA array V1.0 4X180K) has demonstrated
excellent repeatability in previously published papers (61-63).
Moreover, the Shanghai Shibei Biotechnology Co., Ltd. has obtained
official certification of Microarray repeatability and accuracy
from Agilent Technologies, Inc. (R2>0.95, MAQC Plan)
(61-63).
The CV of the all samples in present study were <15% [Dg1,
4.23209%; Dg2, 4.88349%; Dg3, 5.57233%; Hg1, 4.19178%; Hg2,
3.85391%; Hg3, 5.49377%). Therefore, technical replicates were not
conducted in the current study.
Previous studies (61-63)
have improved the reliability of microarray results using
biological repetition. Biological repetition for six different
individuals (three samples from healthy control and three samples
from patients with CIDD) was performed in the present study.
Subsequently, the authors further verified the outcomes of ceRNA
array using RT-qPCR in 32 samples (16 patients and 16 control
individuals), and demonstrated that the ceRNA array and RT-qPCR
results had an excellent reliability.
Therefore, a total of 32 samples (16 patients and 16
normal control) were used in the current study to further assess
the expression levels of lnc-DNAJB6-3:1, ITGB8 and miR-20a-5p using
RT-qPCR or western blot analysis. Previous reports have aimed at
identifying the association between disease and potential lncRNA
based on the semi-supervised learning framework and database
(64-66).
In future research, with the accumulation of cases and the
collection of follow-up information, it will be possible to
construct a clinical prognosis model of lnc-DNAJB6-3:1 in CED.
In conclusion, to the best of our knowledge, the
present study was the first to use a microarray to investigate the
lncRNA expression profile in CED. lnc-DNAJB6-3:1 was significantly
downregulated in CED, indicating that lnc-DNAJB6-3:1 may be a
candidate biomarker for CED. Collectively, the present results
provide a new perspective toward an improved understanding of
ceRNA-mediated gene regulation in CED and suggest a novel
theoretical basis for further studies on the function of lncRNA in
CED-associated IDD.
Supplementary Material
Figure S1. Construction of ceRNA
network. Triangular green nodes denote miRNAs. Diamond-shaped red
nodes indicate lncRNAs and oval blue nodes indicate mRNAs. ceRNA,
competing endogenous RNA; miRNA/miR, microRNA; lnc, long non.coding
RNA.
Table SI. Details of the Dg and
Hg.
Table SII. Specific primer sequences
for reverse transcription-quantiative PCR.
Table SIII. Top 20 of GO biological
process enrichment analysis for differentially expressed genes
(P<0.05).
Table SIV. Top 20 of GO molecular
function enrichment analysis for differentially expressed genes
(P<0.05).
Table SV. Top 20 of GO cellular
component enrichment analysis for differentially expressed genes
(P<0.05).
Table SVI. Top 20 of Kyoto
Encyclopedia of Genes and Genomes pathway enrichment analysis for
differentially expressed genes (P<0.05).
Table SVII. Top 20 of GO biological
process enrichment analysis for differentially expressed genes in
competing endogenous RNA network (P<0.05).
Table SVIII. Top 20 of GO molecular
function enrichment analysis for differentially expressed genes in
competing endogenous RNA network (P<0.05).
Table SIX. Top 20 of GO cellular
component enrichment analysis for differentially expressed genes in
competing endogenous RNA network (P<0.05).
Acknowledgements
The authors would like to thank Dr Wenzhou Huang and
Dr Xinxin Miao (Department of Orthopedics, Second Affiliated
Hospital of Nanchang University, Nanchang, China) for providing
technical help, including assistance with the follow-up and advice
regarding manuscript preparation.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 8166090137 and
8186090165).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY conceived the study and XC designed the study.
JY, RD, JZ and CP performed the experiments. JJ, TW, SH and XL
collected the clinical samples and analyzed the data. JY wrote the
study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Nanchang University.
Written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Livshits G, Popham M, Malkin I, Sambrook
PN, Macgregor AJ, Spector T and Williams FM: Lumbar disc
degeneration and genetic factors are the main risk factors for low
back pain in women: The UK Twin Spine Study. Ann Rheum Dis.
70:1740–1745. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hurwitz EL, Randhawa K, Yu H, Cote P and
Haldeman S: The Global Spine care initiative: A summary of the
global burden of low back and neck pain studies. Eur Spine J.
27:796–801. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gantenbein B, Illien-Jünger S, Chan SC,
Walser J, Haglund L, Ferguson SJ, Iatridis JC and Grad S: Organ
culture bioreactors-platforms to study human intervertebral disc
degeneration and regenerative therapy. Curr Stem Cell Res Ther.
10:339–352. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xia K, Zhu J, Hua J, Gong Z, Yu C, Zhou X,
Wang J, Huang X, Yu W, Li L, et al: Intradiscal injection of
induced pluripotent stem cell-derived nucleus pulposus-like
cell-seeded polymeric microspheres promotes rat disc regeneration.
Stem Cells Int. 2019(6806540)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bendtsen M, Bünger CE, Zou X, Foldager C
and Jørgensen HS: Autologous stem cell therapy maintains vertebral
blood flow and contrast diffusion through the endplate in
experimental intervertebral disc degeneration. Spine (Phila Pa
1976). 36:E373–E379. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rodriguez AG, Slichter CK, Acosta FL,
Rodriguez-Soto AE, Burghardt AJ, Majumdar S and Lotz JC: Human disc
nucleus properties and vertebral endplate permeability. Spine
(Phila Pa 1976). 36:512–520. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yuan W, Che W, Jiang YQ, Yuan FL, Wang HR,
Zheng GL, Li XL and Dong J: Establishment of intervertebral disc
degeneration model induced by ischemic sub-endplate in rat tail.
Spine J. 15:1050–1059. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Colombier P, Clouet J, Hamel O, Lescaudron
L and Guicheux J: The lumbar intervertebral disc: From embryonic
development to degeneration. Joint Bone Spine. 81:125–129.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
He Z, Leong DJ, Xu L, Hardin JA, Majeska
RJ, Schaffler MB, Thi MM, Yang L, Goldring MB, Cobelli NJ and Sun
HB: CITED2 mediates the cross-talk between mechanical loading and
IL-4 to promote chondroprotection. Ann N Y Acad Sci. 1442:128–137.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lu W, Cao F, Wang S, Sheng X and Ma J:
LncRNAs: The regulator of glucose and lipid metabolism in tumor
cells. Front Oncol. 9(1099)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang H, Huo X, Yang XR, He J, Cheng L,
Wang N, Deng X, Jin H, Wang N, Wang C, et al: STAT3-mediated
upregulation of lncRNA HOXD-AS1 as a ceRNA facilitates liver cancer
metastasis by regulating SOX4. Mol Cancer. 16(136)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen J, Liu A, Wang Z, Wang B, Chai X, Lu
W, Cao T, Li R, Wu M, Lu Z, et al: LINC00173.v1 promotes
angiogenesis and progression of lung squamous cell carcinoma by
sponging miR-511-5p to regulate VEGFA expression. Mol Cancer.
19(98)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang K, Long B, Zhou LY, Liu F, Zhou QY,
Liu CY, Fan YY and Li PF: CARL lncRNA inhibits anoxia-induced
mitochondrial fission and apoptosis in cardiomyocytes by impairing
miR-539-dependent PHB2 downregulation. Nat Commun.
5(3596)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen X, Yan CC, Zhang X and You ZH: Long
non-coding RNAs and complex diseases: From experimental results to
computational models. Brief Bionform. 18:558–576. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen X, Sun YZ, Guan NN, Qu J, Huang ZA,
Zhu ZX and Li JQ: Computational models for lncRNA function
prediction and functional similarity calculation. Brief Funct
Genomics. 18:58–82. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res. 45:D353–D361.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mi H, Muruganujan A, Huang X, Ebert D,
Mills C, Guo X and Thomas PD: Protocol update for large-scale
genome and gene function analysis with the PANTHER classification
system (v.14.0). Nat Protoc. 14:703–721. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen Y and Wang X: miRDB: An online
database for prediction of functional microRNA targets. Nucleic
Acids Res. 48:D127–D131. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chou CH, Shrestha S, Yang CD, Chang NW,
Lin YL, Liao KW, Huang WC, Sun TH, Tu SJ, Lee WH, et al: miRTarBase
update 2018: A resource for experimentally validated
microRNA-target interactions. Nucleic Acids Res. 46:D296–D302.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4(e05005)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
LaPointe VL, Verpoorte A and Stevens MM:
The changing integrin expression and a role for integrin β8 in the
chondrogenic differentiation of mesenchymal stem cells. PLoS One.
8(e82035)2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guo L, Zhu Y, Li L, Zhou S, Yin G, Yu G
and Cui H: Breast cancer cell-derived exosomal miR-20a-5p promotes
the proliferation and differentiation of osteoclasts by targeting
SRCIN1. Cancer Med. 8:5687–5701. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Berg-Johansen B, Han M, Fields AJ,
Liebenberg EC, Lim BJ, Larson PE, Gunduz-Demir C, Kazakia GJ, Krug
R and Lotz JC: Cartilage endplate thickness variation measured by
ultrashort Echo-time MRI is associated with adjacent disc
degeneration. Spine (Phila Pa 1976). 43:E592–E600. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang JF, Wang GL, Zhou ZJ, Fang XQ, Chen
S and Fan SW: Expression of matrix metalloproteinases, tissue
inhibitors of metalloproteinases, and interleukins in vertebral
cartilage endplate. Orthop Surg. 10:306–311. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang Y, He F, Chen Z, Su Q, Yan M, Zhang
Q, Tan J, Qian L and Han Y: Melatonin modulates IL-1β-induced
extracellular matrix remodeling in human nucleus pulposus cells and
attenuates rat intervertebral disc degeneration and inflammation.
Aging (Albany NY). 11:10499–10512. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xu T, Wu K and Zhang L, Zheng S, Wang X,
Zuo H, Wu X, Tao G, Jiang B and Zhang L: Long non-coding RNA
LINC00858 exerts a tumor-promoting role in colon cancer via
HNF4alpha and WNK2 regulation. Cell Oncol (Dordr). 43:297–310.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liao C, Long Z, Zhang X, Cheng J, Qi F, Wu
S and Huang T: LncARSR sponges miR-129-5p to promote proliferation
and metastasis of bladder cancer cells through increasing SOX4
expression. Int J Biol Sci. 16:1–11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Alanko J and Ivaska J: Endosomes: Emerging
platforms for integrin-mediated FAK signalling. Trends Cell Biol.
26:391–398. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hirose N, Okamoto Y, Yanoshita M, Asakawa
Y, Sumi C, Takano M, Nishiyama S, Su SC, Mitsuyoshi T, Kunimatsu R,
et al: Protective effects of cilengitide on inflammation in
chondrocytes under excessive mechanical stress. Cell Biol Int.
44:966–974. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yan Z, Pan Y, Wang S, Cheng M, Kong H, Sun
C, Hu K, Chen T, Dong Q and Chen J: Static Compression induces ECM
remodeling and integrin α2β1 expression and signaling in a rat tail
caudal intervertebral disc degeneration model. Spine (Phila Pa
1976). 42:E448–E458. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Almonte-Becerril M, Gimeno LI, Villarroya
O, Benito-Jardon M, Kouri JB and Costell M: Genetic abrogation of
the fibronectin-α5β1 integrin interaction in articular cartilage
aggravates osteoarthritis in mice. PLoS One.
13(e0198559)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li XF, Xue CC, Zhao YJ, Cheng SD, Zhao DF,
Liang QQ, Chen L, Wang Q, Lu S, Shi Q, et al: Deletion of Opg leads
to increased neovascularization and expression of inflammatory
cytokines in the lumbar intervertebral disc of mice. Spine (Phila
Pa 1976). 42:E8–E14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Qu Z, Quan Z, Zhang Q, Wang Z, Song Q,
Zhuang X, Fu C, Xu F, Liu Y, Wang Y, et al: Comprehensive
evaluation of differential lncRNA and gene expression in patients
with intervertebral disc degeneration. Mol Med Rep. 18:1504–1512.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xue H, Yu P, Wang WZ, Niu YY and Li X: The
reduced lncRNA NKILA inhibited proliferation and promoted apoptosis
of chondrocytes via miR-145/SP1/NF-κB signaling in human
osteoarthritis. Eur Rev Med Pharmacol Sci. 24:535–548.
2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gu HY, Yang M, Guo J, Zhang C, Lin LL, Liu
Y and Wei RX: Identification of the biomarkers and pathological
process of osteoarthritis: Weighted Gene Co-expression network
analysis. Front Physiol. 10(275)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xue H, Tu Y, Ma T, Liu X, Wen T, Cai M,
Xia Z and Mei J: Lactoferrin inhibits IL-1β-induced chondrocyte
apoptosis through AKT1-induced CREB1 activation. Cell Physiol
Biochem. 36:2456–2465. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nishimura R, Hata K, Matsubara T,
Wakabayashi M and Yoneda T: Regulation of bone and cartilage
development by network between BMP signalling and transcription
factors. J Biochem. 151:247–254. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sideek MA, Menz C, Parsi MK and Gibson MA:
LTBP-2 competes with tropoelastin for binding to fibulin-5 and
heparin, and is a negative modulator of elastinogenesis. Matrix.
34:114–123. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Goessler UR, Bugert P, Bieback K, Deml M,
Sadick H, Hormann K and Riedel F: In-vitro analysis of the
expression of TGFbeta-superfamily-members during chondrogenic
differentiation of mesenchymal stem cells and chondrocytes during
dedifferentiation in cell culture. Cell Mol Biol Lett. 10:345–362.
2005.PubMed/NCBI
|
|
43
|
Wei T, Kulkarni NH, Zeng QQ, Helvering LM,
Lin X, Lawrence F, Hale L, Chambers MG, Lin C, Harvey A, et al:
Analysis of early changes in the articular cartilage
transcriptisome in the rat meniscal tear model of osteoarthritis:
Pathway comparisons with the rat anterior cruciate transection
model and with human osteoarthritic cartilage. Osteoarthr Cartil.
18:992–1000. 2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Weimer A, Madry H, Venkatesan JK, Schmitt
G, Frisch J, Wezel A, Jung J, Kohn D, Terwilliger EF, Trippel SB
and Cucchiarini M: Benefits of recombinant adeno-associated virus
(rAAV)-mediated insulinlike growth factor I (IGF-I) overexpression
for the long-term reconstruction of human osteoarthritic cartilage
by modulation of the IGF-I axis. Mol Med. 18:346–358.
2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hu Y, Wu R, Li H, Gu Y and Wei W:
Expression and significance of metalloproteinase and collagen in
vaginal wall tissues of patients with pelvic organ prolapse. Ann
Clin Lab Sci. 47:698–705. 2017.PubMed/NCBI
|
|
46
|
Willard K, Mannion S, Saunders CJ, Collins
M and September AV: The interaction of polymorphisms in
extracellular matrix genes and underlying miRNA motifs that
modulate susceptibility to anterior cruciate ligament rupture. J
Sci Med Sport. 21:22–28. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wu JJ, Weis MA, Kim LS, Carter BG and Eyre
DR: Differences in chain usage and cross-linking specificities of
cartilage type V/XI collagen isoforms with age and tissue. J Biol
Chem. 284:5539–5545. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhou S, Wang Z, Tang J, Li W, Huang J, Xu
W, Luo F, Xu M, Wang J, Wen X, et al: Exogenous fibroblast growth
factor 9 attenuates cartilage degradation and aggravates osteophyte
formation in post-traumatic osteoarthritis. Osteoarthritis
Cartilage. 24:2181–2192. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Song EK, Jeon J, Jang DG, Kim HE, Sim HJ,
Kwon KY, Medina-Ruiz S, Jang HJ, Lee AR, Rho JG, et al: ITGBL1
modulates integrin activity to promote cartilage formation and
protect against arthritis. Sci Transl Med.
10(eaam7486)2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Choocheep K, Hatano S, Takagi H and
Watanabe H, Kimata K, Kongtawelert P and Watanabe H: Versican
facilitates chondrocyte differentiation and regulates joint
morphogenesis. J Biol Chem. 285:21114–21125. 2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yang L, Clinton JM, Blackburn ML, Zhang Q,
Zou J, Zielinska-Kwiatkowska A, Tang BL and Chansky HA: Rab23
regulates differentiation of ATDC5 chondroprogenitor cells. J Biol
Chem. 283:10649–10657. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Randall RM, Shao YY, Wang L and Ballock
RT: Activation of Wnt planar cell polarity (PCP) signaling promotes
growth plate column formation in vitro. J Orthop Res. 30:1906–1914.
2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chen Y, Wang X, Yang M, Ruan W, Wei W, Gu
D, Wang J, Guo X, Guo L and Yuan Y: miR-145-5p Increases osteoclast
numbers in vitro and aggravates bone erosion in collagen-induced
arthritis by targeting osteoprotegerin. Med Sci Monit.
24:5292–5300. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Xiao ZF, He JB, Su GY, Chen MH, Hou Y,
Chen SD and Lin DK: Osteoporosis of the vertebra and osteochondral
remodeling of the endplate causes intervertebral disc degeneration
in ovariectomized mice. Arthritis Res. 20(207)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hu J, Wang Z, Shan Y, Pan Y, Ma J and Jia
L: Long non-coding RNA HOTAIR promotes osteoarthritis progression
via miR-17-5p/FUT2/β-catenin axis. Cell Death Dis.
9(711)2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Xue H, Tu Y, Ma T, Wen T, Yang T, Xue L,
Cai M, Wang F and Guan M: miR-93-5p attenuates IL-1β-induced
chondrocyte apoptosis and cartilage degradation in osteoarthritis
partially by targeting TCF4. Bone. 123:129–136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wang C, Xu X, Chen J, Kang Y, Guo J,
Duscher D, Yang X, Guo G, Ren S, Xiong H, et al: The construction
and analysis of lncRNA-miRNA-mRNA competing endogenous RNA network
of Schwann cells in diabetic peripheral neuropathy. Front Bioeng
Biotechnol. 8(490)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zhang S, Chang YY, Gong YW, Gao YJ, Guo Q,
Wang YH, Zhao YL and Wang ZP: Comprehensive analysis of
microRNA-messenger RNA regulatory network in gemcitabine-resistant
bladder cancer cells. J Cell Biochem. 120:6347–6360.
2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Cao M, Zhang L, Wang JH, Zeng H, Peng Y,
Zou J, Shi J, Zhang L, Li Y, Yoshida S, et al: Identifying
circRNA-associated-ceRNA networks in retinal neovascularization in
mice. Int J Med Sci. 16:1356–1365. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wang S, Zhan J, Lin X, Wang Y, Wang Y and
Liu Y: CircRNA-0077930 from hyperglycaemia-stimulated vascular
endothelial cell exosomes regulates senescence in vascular smooth
muscle cells. Cell Biochem Funct: Apr 19 doi: 10.1002/cbf.3543.2020
(Epub ahead of print).
|
|
61
|
Wang R, Zhang S, Chen X, Li N, Li J, Jia
R, Pan Y and Liang H: EIF4A3-induced circular RNA MMP9 (circMMP9)
acts as a sponge of miR-124 and promotes glioblastoma multiforme
cell tumorigenesis. Mol Cancer. 17(166)2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Gao A, Gong Y, Zhu C, Yang W, Li Q, Zhao
M, Ma S, Li J, Hao S, Cheng H and Cheng T: Bone marrow endothelial
cell-derived interleukin-4 contributes to thrombocytopenia in acute
myeloid leukemia. Haematologica. 104:1950–1961. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zhao X, Li D, Yang F, Lian H, Wang J, Wang
X, Fang E, Song H, Hu A, Guo Y, et al: Long noncoding RNA NHEG1
drives β-catenin transactivation and neuroblastoma progression
through interacting with DDX5. Mol Ther. 28:946–962.
2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Chen X and Yan G: Novel human
lncRNA-disease association inference based on lncRNA expression
profiles. Bioinformatics. 29:2617–2624. 2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Herszage J and Censor N: Modulation of
learning and memory: A shared framework for interference and
generalization. Neuroscience. 392:270–280. 2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Gershman SJ and Daw ND: Reinforcement
learning and episodic memory in humans and animals: An integrative
framework. Annu Rev Psychol. 68:101–128. 2017.PubMed/NCBI View Article : Google Scholar
|