Introduction
Atherosclerosis is an important pathological
manifestation of cardiovascular diseases and chronic inflammatory
disease (1), which is the result of
a build-up of fatty materials, such as cholesterol and lipids
(2-4).
Atherosclerosis affects arterial blood vessels, resulting in
thickening of the vascular wall and a narrowing of the lumen
(5). Previous studies have
indicated that cells of the arterial wall, including T cells,
monocyte-derived macrophages, endothelial cells and vascular smooth
muscle cells (VSMCs), are associated with the development of
atherosclerosis (1,6). Although the mechanism is not fully
understood, it is widely accepted that the abnormal proliferation
of VSMCs located in the arterial intima leads to intimal thickening
of the aorta, serving an important role in the pathogenesis and
progression of atherosclerosis (7,8).
Therefore, inhibiting VSMC proliferation may serve as a useful
therapeutic approach for atherosclerosis.
Pueraria lobata (Wild.) Ohwi, is widely used
in traditional Chinese medicine as a treatment for cardiovascular
diseases, diabetes and liver diseases (9-11).
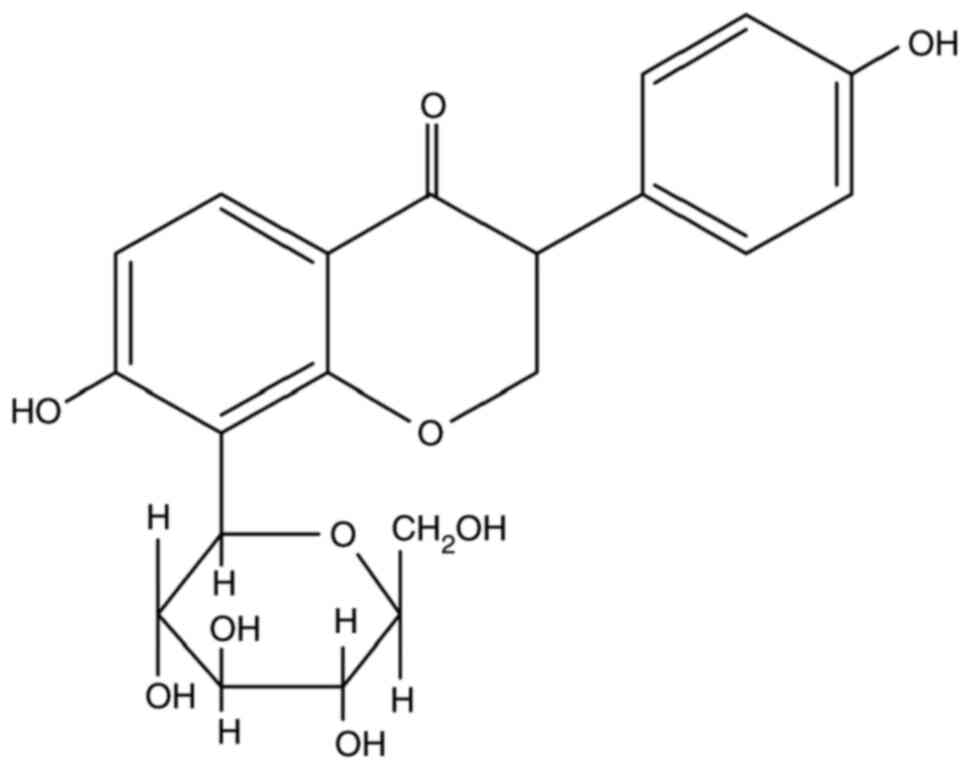
Puerarin (4'-7'-dihydroxy-8-β-D-glucosylisoflavone,
C21H20O10; Fig. 1), one of the major isoflavonoid
compounds isolated from the root of Pueraria lobata, is
considered as one of the main pharmacologically active constituents
of this treatment (12). Moreover,
puerarin has been reported to display various pharmacological
properties, including anti-oxidative (13), anti-inflammatory (14), antitumor (15), anti-hypercholesterolemic (9), anti-hyperglycemic (16) and anti-hypertensive activities
(13). Furthermore, puerarin has
been reported to have beneficial effects in the treatment of
atherosclerosis (12,17,18).
However, the role and mechanism underlying puerarin activity on
oxidized low-density lipoprotein (ox-LDL)-stimulated VSMCs has not
been previously reported. Ox-LDL-induced VSMC proliferation in the
intima of the arterial wall serves a critical role in the progress
of atherosclerosis (19), therefore
inhibiting ox-LDL-induced proliferation may serve as a potential
therapeutic strategy for atherosclerosis. The present study aimed
to evaluate the potential protective effect of puerarin on
ox-LDL-stimulated VSMCs and to further identify the underlying
mechanisms of its action.
Materials and methods
Cell culture and treatments
Human VSMCs were obtained from The Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences. VSMCs were
seeded (1x104 cells/well) into 96-well microplates and
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% heat-inactivated FBS (Hangzhou Sijiqing
Biological Engineering Materials Co., Ltd.) and 1%
penicillin/streptomycin (Sigma-Aldrich; Merck KGaA) at 37˚C with 5%
CO2. Following pretreatment with 0, 20, 40 or 80 µM
puerarin (purity, ≥98%; Sichuan Weikeqi Biological Technology Co.,
Ltd.) for 24 h at 37˚C, cells were treated with 50 µg/ml ox-LDL
(Guangzhou Yiyuan Biological Technology Co., Ltd.) for 24 h at 37˚C
(20).
Cell viability assay
Cell viability was evaluated by performing an MTT
assay (Beijing Solarbio Science & Technology Co., Ltd.). Cells
were seeded (1x104 cells/well) into 96-well microplates
and cultured in DMEM overnight at 37˚C. Subsequently, cells were
incubated with DMEM containing 0.01% DMSO or puerarin (20, 40 or 80
µM) at 37˚C for 24 h. Cells were then stimulated with ox-LDL (50
µg/ml) for 24 h at 37˚C. Subsequently, 5 mg/ml MTT solution was
added to each well for 4 h at 37˚C. DMSO (150 µl) was added to each
well for 15 min to dissolve the purple formazan. Absorbance was
measured at a wavelength of 490 nm using a microplate reader
(Thermo Fisher Scientific, Inc.).
The Cell Counting Kit-8 (CCK-8) assay
(Sigma-Aldrich; Merck KGaA) was conducted to determine cell
viability according to the manufacturer's protocol. Briefly,
following treatment, 10 µl CCK-8 reagent was added into each well
for 2 h at 37˚C. Absorbance was measured at a wavelength of 450 nm
using a microplate reader (Thermo Fisher Scientific, Inc.). Cell
viability was calculated according to the following formula: Cell
viability (%)=absorbance of test sample/absorbance of control
x100.
Detection of oxidative stress
biomarkers, superoxide dismutase (SOD) and malondialdehyde
(MDA)
Cells were seeded (1x104 cells/well) into
6-well microplates and cultured in DMEM overnight at 37˚C.
Subsequently, cells were incubated with DMEM containing 0.01% DMSO
or puerarin (20, 40 and 80 µM) for 24 h at 37˚C. Cells were then
stimulated with 50 µg/ml ox-LDL for 24 h at 37˚C. Cells were
collected and lysed on ice using RIPA lysis buffer (Beyotime
Institute of Biotechnology). Following centrifugation at 13,000 x g
for 5 min at 4˚C, the supernatant was collected to determine SOD
(cat. no. 20190602) activity and MDA (cat. no. 20190508) content
using commercial kits (Nanjing Jiancheng Institute of Biological
Engineering Co., Ltd.) according to the manufacturer's
protocol.
Western blotting
Cells were seeded (1x104 cells/well) into
6-well microplates and cultured in DMEM overnight at 37˚C. Cells
were incubated with DMEM containing 0.01% DMSO or puerarin (20, 40
and 80 µM) for 24 h at 37˚C. Cells were then stimulated with 50
µg/ml ox-LDL for 24 h at 37˚C. Cells were collected and lysed on
ice with RIPA lysis buffer (Beyotime Institute of Biotechnology).
Total protein was quantified using a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology). Equal amounts of
protein (50 µg) were separated via 10% SDS-PAGE (120 V for 1.5 h)
and transferred onto PVDF membranes (100 V for 1 h), which were
blocked with 5% skimmed milk in TBST (0.05% Tween-20) for 1 h at
room temperature. After blocking, the membranes were incubated
overnight at 4˚C with the following primary antibodies: Anti-p38
(1:1,000; cat. no. sc-7281; Santa Cruz Biotechnology, Inc.),
anti-p-p38 (1:1,000; cat. no. sc-7937; Santa Cruz Biotechnology,
Inc.), JNK (1:1,000; cat. no. sc-6531; Santa Cruz Biotechnology,
Inc.), p-JNK (1:1,000; cat. no. sc-3824; Santa Cruz Biotechnology,
Inc.), anti-GAPDH (1:1,000; cat. no. sc-6341; Santa Cruz
Biotechnology, Inc.). Following incubation, the membranes were
washed three times in TBST, before the membranes were incubated
with an appropriate horseradish peroxidase-conjugated secondary
antibody for 1 h at room temperature (1:5,000; cat. no. sc-5203;
Santa Cruz Biotechnology, Inc.). Protein bands were visualized
using enhanced chemiluminescence reagent (Amersham; Cytiva).
Protein expression levels were semi-quantified using ImageJ
software (version 1.46; National Institutes of Health) with GAPDH
as the loading control.
Reverse transcription-quantitative PCR
(RT-qPCR)
Cells were seeded (1x104 cells/well) into
6-well microplates and cultured in DMEM overnight at 37˚C. Cells
were incubated with DMEM containing 0.01% DMSO or puerarin (20, 40
and 80 µM) for 24 h at 37˚C. Cells were then exposed to 50 µg/ml
ox-LDL for 24 h at 37˚C. Subsequently, total RNA was extracted from
the cells using TRIzol® (Takara Biomedical Technology
Co., Ltd., China) according to the manufacturer's protocol. Total
RNA was reverse transcribed using the PrimeScript RT Master Mix kit
(Takara Biotechnology Co., Ltd.) at 37˚C for 15 min and 85˚C for 5
min. Subsequently, qPCR was performed using SYBR® Premix
Ex Taq (TransGen Biotech Co., Ltd.) with the following
thermocycling conditions: Initial denaturation at 95˚C for 5 min;
followed by 40 cycles of denaturation at 95˚C for 10 sec, annealing
at 60˚C for 15 sec and extension at 72˚C for 30 sec; followed by a
final extension at 72˚C for 10 min. The following primers were used:
IL-6 forward, 5'-CTCTCCGCAAGAGACTTCCA-3' and reverse,
5'-TGGTCTTCTGGAGTTCCGTT-3'; TNF-α forward,
5'-TCTCATCAGTTCTATGGCCC-3' and reverse, 5'-GGGAGTAGACAAGGTACAAC-3';
GAPDH forward, 5'-GTTACCAGGGCTGCCTTCTC-3' and reverse,
5'-GATGGTGATGGGTTTCCCGT-3'. mRNA expression levels were quantified
using the 2-ΔΔCq method (21) and normalized to the internal
reference gene GAPDH.
Statistical analysis
Statistical analyses were performed using SPSS
(version 19.0; IBM Corp.). All experiments were performed in
triplicate. Comparisons among multiple groups were analyzed using
one-way ANOVA followed by Tukey's post hoc test. Data are presented
as the mean ± SEM. P<0.05 was considered to indicate a
statistically significant difference.
Results
Puerarin inhibits cell viability in
ox-LDL-stimulated VSMCs
To evaluate the cytotoxicity of puerarin on VSMCs,
cells were treated with puerarin (0, 20, 40 and 80 µM) for 24 h.
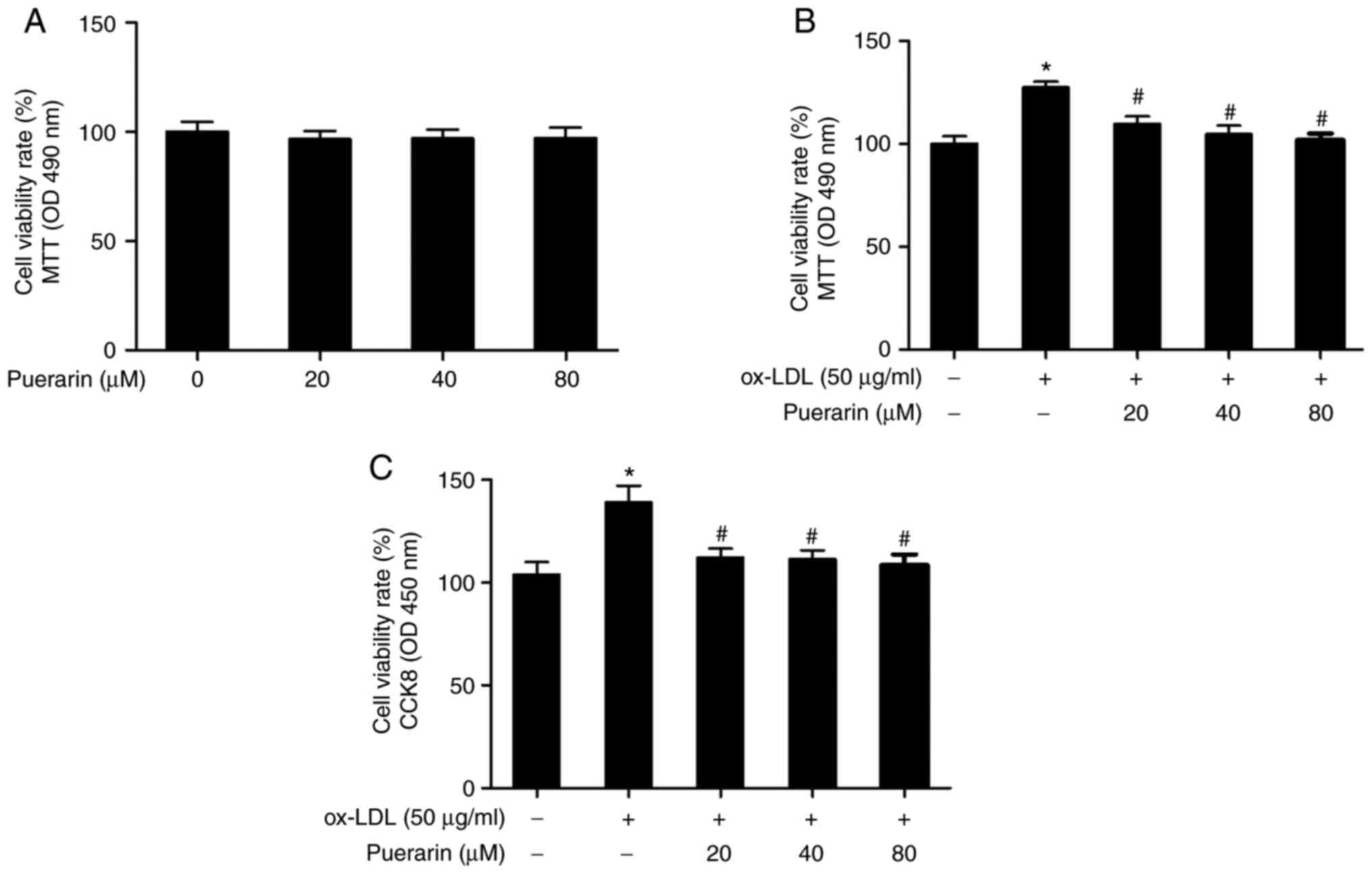
The MTT assay results suggested that puerarin did not significantly
alter VSMC viability compared with the control group (Fig. 2A). Following incubation with
different concentrations of puerarin for 24 h, cells were
stimulated with 50 µg/ml ox-LDL for 24 h to evaluate the effect of
puerarin on cell viability in ox-LDL-induced VSMCs. Cell viability
was significantly increased in ox-LDL-induced VSMCs compared with
the control group (Fig. 2B).
However, pretreatment with puerarin significantly inhibited
ox-LDL-induced cell viability. Similar results were observed in the
CCK-8 assay (Fig. 2C).
Puerarin regulates the production of
oxidative stress-related markers in ox-LDL-stimulated VSMCs
To investigate the mechanism underlying the
inhibitory effect of puerarin on ox-LDL-induced cell proliferation,
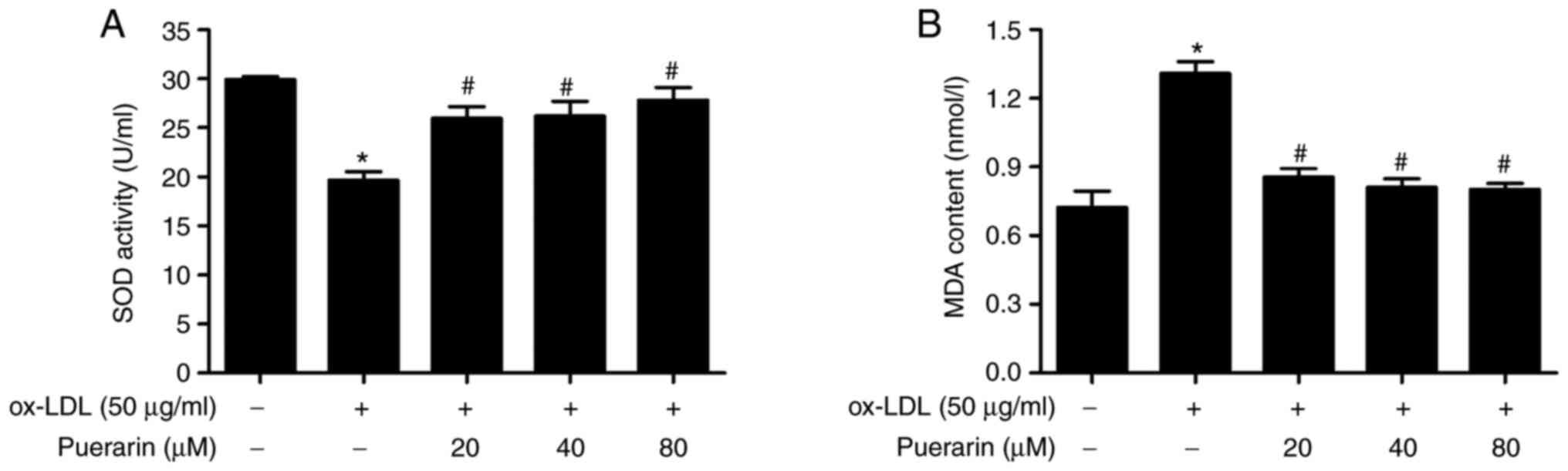
the effect of puerarin on SOD activity and MDA content was
determined. ox-LDL significantly increased the content of MDA in
VSMCs compared with the control group (Fig. 3B). Puerarin pretreatment
significantly attenuated ox-LDL-induced MDA levels. In addition,
compared with the control group, SOD activity was significantly
decreased by ox-LDL in VSMCs, but pretreatment with puerarin
diminished ox-LDL-mediated effects on SOD activity (Fig. 3B). The results suggested that
puerarin significantly inhibited ox-LDL-induced oxidative stress in
VSMCs.
Puerarin inhibits proinflammatory
cytokines in ox-LDL-stimulated VSMCs
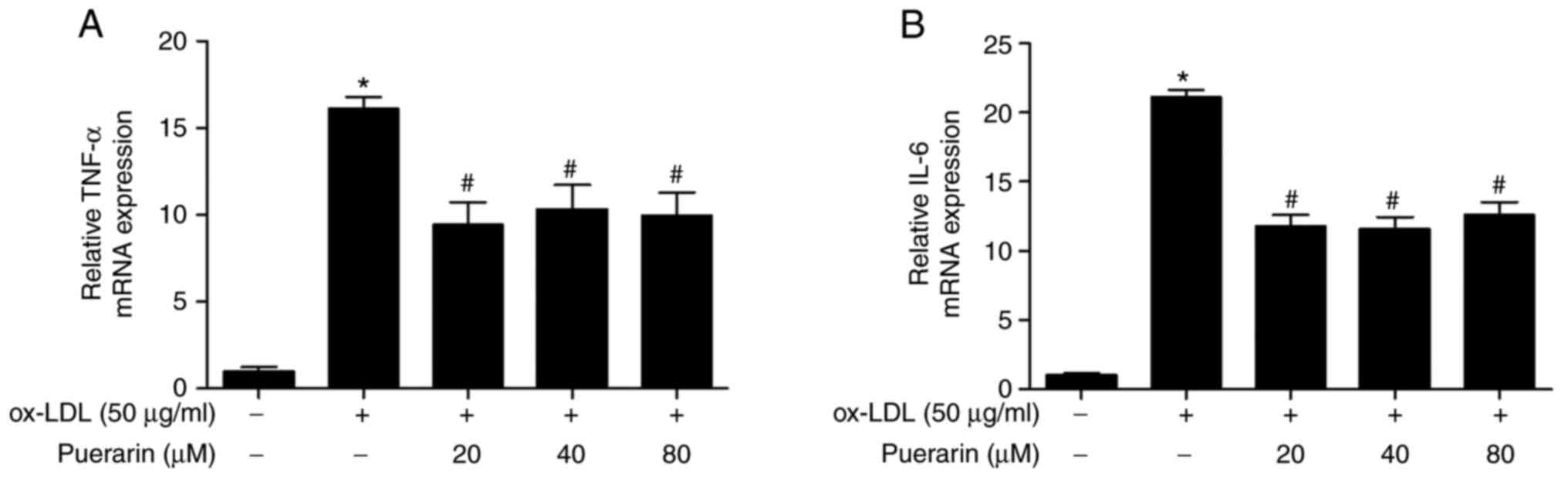
The effect of puerarin on IL-6 and TNF-α mRNA
expression levels in ox-LDL-stimulated VSMCs was examined. ox-LDL
significantly increased the mRNA expression levels of IL-6 and
TNF-α in VSMCs compared with the control group (Fig. 4A). However, puerarin pretreatment
significantly decreased the mRNA expression levels of IL-6 and
TNF-α in ox-LDL-stimulated VSMCs (Fig.
4B). The results suggested that puerarin inhibited
proinflammatory cytokines in ox-LDL-stimulated VSMCs.
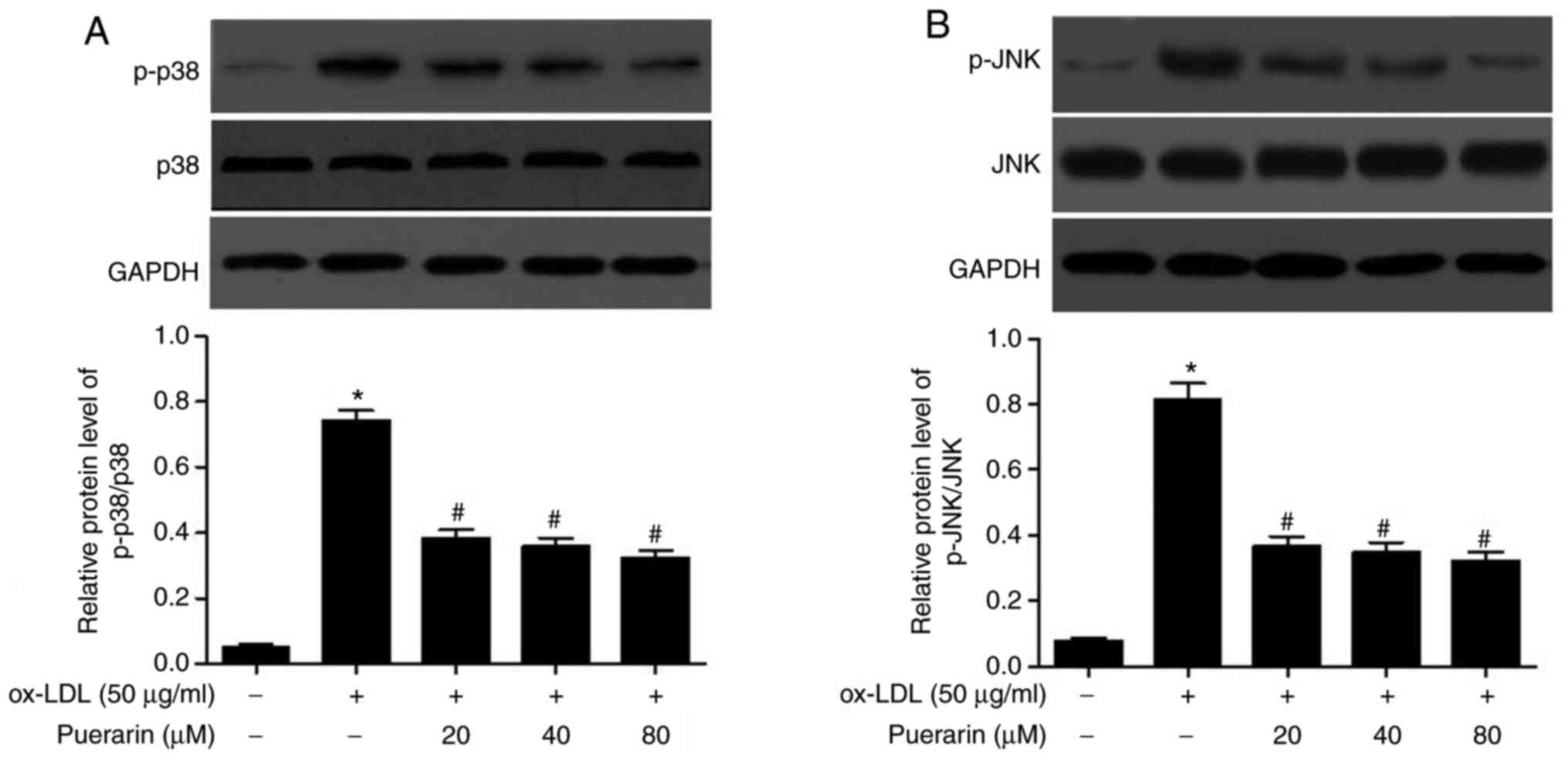
Puerarin prevents the activation of
p38 MAPK and JNK signaling pathways in ox-LDL-stimulated VSMCs
To further elucidate whether the p38 MAPK and JNK
signaling pathways were involved in the effect of puerarin in
ox-LDL-stimulated VSMCs, the phosphorylation levels of p38 and JNK
were analyzed via western blotting. The expression levels of p-p38
and p-JNK were significantly increased by ox-LDL in VSMCs compared
with the control group (Fig. 5A and
B). Puerarin pretreatment
significantly decreased the expression levels of p-p38 and p-JNK in
ox-LDL-stimulated VSMCs. The results indicated that puerarin
inhibited the activation of p38 MAPK and JNK signaling pathways in
ox-LDL-induced VSMCs.
Discussion
During the progression of atherosclerosis, abnormal
VSMC proliferation and migration serve key roles in causing
stenosis and intimal thickening (22). A number of factors affect VSMC
proliferation and migration, including hypertension, dyslipidemia
and oxidative stress (4). As a
well-established index of oxidative stress, ox-LDL is involved in
the generation of atherosclerotic lesions (4). Numerous studies have indicated that
ox-LDL can induce VSMC proliferation and migration, which are
pathological events that are crucial for neointima formation
(23) and stimulate activation of
the MAPK signaling pathway (24,25).
Therefore, if a substance can effectively prevent ox-LDL-induced
VSMC proliferation, it should inhibit the development of
atherosclerosis.
Puerarin, a major isoflavone isolated from the root
of Pueraria lobata, has been reported to treat various
cardiovascular diseases, including arterial hypertension, angina,
myocardial infarction and arrhythmia (26-28).
Puerarin has anti-atherosclerotic activity in diet-induced
atherosclerosis in rabbits and rats (17,18).
Puerarin prevents the pathogenesis of atherosclerosis, which may be
mediated by its anti-inflammatory and antioxidative stress actions
(28,29). A key finding of the present study
was that puerarin significantly inhibited ox-LDL-induced VSMC
viability. In the present study, whether cell viability was
inhibited by puerarin when VSMCs were exposed to ox-LDL was
investigated. The results demonstrated that ox-LDL enhanced VSMC
viability compared with the control group, but pretreatment with
puerarin significantly reversed ox-LDL-mediated effects in VSMCs.
Moreover, compared with the control group, ox-LDL significantly
decreased SOD activity and significantly increased MDA content,
suggesting that the level of oxidative stress in VSMCs induced by
ox-LDL was higher compared with control conditions. The antioxidant
activity of puerarin was further indicated by its ability to
reverse ox-LDL-mediated alterations in SOD activity and MDA
content.
For the clinical development of puerarin as a
treatment, understanding the mechanism underlying how it inhibits
VSMC proliferation is important. The present study focused on the
MAPK signaling pathway in VSMCs. Previous studies demonstrated that
ox-LDL activated the MAPK signaling pathway, including p38, ERK and
JNK in VSMCs, which promoted cell proliferation (30,31).
Puerarin prevents ox-LDL-induced proliferation and inhibits the
phosphorylation of ERK1/2 in VSMCs (32). Puerarin inhibits VSMC proliferation
induced by fine particulate matter via reducing the elevated
expression levels of p-p38 MAPK (33). Moreover, puerarin markedly
stimulates bone marrow stromal cell differentiation towards an
osteogenic phenotype via the ERK1/2 and p38-MAPK signaling pathways
(34). The aforementioned study
results indicated that puerarin displays protective effects in
different diseases by regulating MAPK signaling pathways. In the
present study, puerarin inhibited the activation of the p38 MAPK
and JNK signaling pathways in ox-LDL-induced VSMCs.
Certain studies have speculated that the p38 MAPK
signaling pathway is associated with inflammasome activation
(5,35,36).
Activation of the p38 MAPK signaling pathway increases the
expression of TNF-α and IL-6 (36,37).
In the present study, similar results were obtained. Although the
present study did not further investigate the mechanism of MAPK
signaling pathway and inflammasome activation, relative indices
were observed and it was suggested that the MAPK signaling pathway
was involved in the activation of inflammation in ox-LDL-induced
VSMCs. The results of the present study indicated that the
phosphorylation levels of p38 and JNK were significantly increased
in ox-LDL-induced VSMCs compared with the control group. Similarly,
the mRNA expression levels of IL-6 and TNF-α were significantly
increased in ox-LDL-induced VSMCs compared with control VSMCs.
However, puerarin significantly downregulated the expression levels
of p-p38 and p-JNK and reduced the mRNA expression levels of IL-6
and TNF-α in ox-LDL-stimulated VSMCs. The effect of puerarin on
IL-6 and TNF-α did not display a concentration gradient trend,
which indicated that the effect of puerarin was not closely related
to the concentration. The effect of puerarin on other
proinflammatory cytokines requires further investigation. The
results of the present study suggested that alterations in
proinflammatory cytokines were closely related to alterations in
the expression levels of p-p38 MAPK and p-JNK, indicating that
puerarin altered the expression of TNF-α and IL-6 by regulating the
p38 MAPK and JNK signaling pathways in ox-LDL-induced VSMCs.
However, cell networks are complex, thus, whether puerarin exerts
its function via other signaling pathways in ox-LDL-induced VSMCs
requires further investigation.
In conclusion, the results of the present study
suggest that puerarin inhibited ox-LDL-induced VSMC viability, and
that the antiproliferative effects of puerarin were partly
associated with inactivation of the p-p38 MAPK and p-JNK signaling
pathways, which was mediated via suppression of the expression
levels of TNF-α and IL-6. Therefore, the results of the present
study suggest that puerarin may inhibit the development of
atherosclerosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Research Project of the Education Department of Jilin
Province (grant no. JJKH20191098KJ) and the Science And Technology
Project of Traditional Chinese Medicine of Jilin Administration of
Traditional Chinese Medicine (grant no. 2020135).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH performed the experiments and wrote the
manuscript. RL, ZW and WY performed the cell experiments. HL
conceived the study and revised the manuscript. WQ designed the
study and analysed the data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu J, Ren Y, Kang L and Zhang L: Oxidized
low-density lipoprotein increases the proliferation and migration
of human coronary artery smooth muscle cells through the
upregulation of osteopontin. Int J Mol Med. 33:1341–1347.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dell'omo G, Penno G, Pucci L, Lucchesi D,
Fotino C, Del Prato S and Pedrinelli R: ACE gene insertion/deletion
polymorphism modulates capillary permeability in hypertension. Clin
Sci (Lond). 111:357–364. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhu F, Li C, Jin XP, Weng SX, Fan LL,
Zheng Z, Li WL, Wang F, Wang WF, Hu XF, et al: Celastrol may have
an anti-atherosclerosis effect in a rabbit experimental carotid
atherosclerosis model. Int J Clin Exp Med. 7:1684–1691.
2014.PubMed/NCBI
|
|
4
|
Liu Z, Ren X, Yang Z, Zhao Y, Ji L, Li J
and Yue W: Effects and mechanisms of indol-2,3-dione on
atherosclerosis. Int J Clin Exp Med. 7:2087–2091. 2014.PubMed/NCBI
|
|
5
|
Hu Y, Sun B, Liu K, Yan M, Zhang Y, Miao C
and Ren L: Icariin attenuates high-cholesterol diet induced
atherosclerosis in rats by inhibition of inflammatory response and
p38 MAPK signaling pathway. Inflammation. 39:228–236.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
González-Navarro H, Abu Nabah YN, Vinué A,
Andrés-Manzano MJ, Collado M, Serrano M and Andrés V: p19(ARF)
deficiency reduces macrophage and vascular smooth muscle cell
apoptosis and aggravates atherosclerosis. J Am Coll Cardiol.
55:2258–2268. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Daemen MJ, Lombardi DM, Bosman FT and
Schwartz SM: Angiotensin II induces smooth muscle cell
proliferation in the normal and injured rat arterial wall. Circ
Res. 68:450–456. 1991.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ross R: The pathogenesis of
atherosclerosis: A perspective for the 1990s. Nature. 362:801–809.
1993.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Yan LP, Chan SW, Chan AS, Chen SL, Ma XJ
and Xu HX: Puerarin decreases serum total cholesterol and enhances
thoracic aorta endothelial nitric oxide synthase expression in
diet-induced hypercholesterolemic rats. Life Sci. 79:324–330.
2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fu J, Jing W, Wang W, Chen S, Zhang J and
Liu A: A novel and effective chromatographic approach to the
separation of isoflavone derivatives from Pueraria lobata.
Molecules. 20:4238–4253. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhu X, Xie M, Wang K, Zhang K, Gao Y, Zhu
L and Zhou F: The effect of puerarin against IL-1β-mediated
leukostasis and apoptosis in retinal capillary endothelial cells
(TR-iBRB2). Mol Vis. 20:1815–1823. 2014.PubMed/NCBI
|
|
12
|
Bao MH, Zhang YW, Lou XY, Xiao Y, Cheng Y
and Zhou HH: Puerarin protects endothelial cells from oxidized low
density lipoprotein induced injuries via the suppression of LOX-1
and induction of eNOS. Can J Physiol Pharmacol. 92:299–306.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Meng XH, Ni C, Zhu L, Shen YL, Wang LL and
Chen YY: Puerarin protects against high glucose-induced acute
vascular dysfunction: Role of heme oxygenase-1 in rat thoracic
aorta. Vascul Pharmacol. 50:110–115. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang X, Hu W, Zhang Q, Wang Y and Sun L:
Puerarin inhibits C-reactive protein expression via suppression of
nuclear factor kappaB activation in lipopolysaccharide-induced
peripheral blood mononuclear cells of patients with stable angina
pectoris. Basic Clin Pharmacol Toxicol. 107:637–642.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hien TT, Kim HG, Han EH, Kang KW and Jeong
HG: Molecular mechanism of suppression of MDR1 by puerarin from
Pueraria lobata via NF-kappaB pathway and cAMP-responsive element
transcriptional activity-dependent up-regulation of AMP-activated
protein kinase in breast cancer MCF-7/adr cells. Mol Nutr Food Res.
54:918–928. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC and
Cheng JT: Antihyperglycemic effect of puerarin in
streptozotocin-induced diabetic rats. J Nat Prod. 66:788–792.
2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bao L, Zhang Y, Wei G, Wang Y, Ma R, Cheng
R, Ren X and Agula B: The anti-atherosclerotic effects of puerarin
on induced-atherosclerosis in rabbits. Biomed Pap Med Fac Univ
Palacky Olomouc Czech Repub. 159:53–59. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fu R, Zhang Y, Guo Y, Xu Y and Chen F:
Digital gene expression analysis of the pathogenesis and
therapeutic mechanisms of ligustrazine and puerarin in rat
atherosclerosis. Gene. 552:75–80. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chang WC, Yu YM, Chiang SY and Tseng CY:
Ellagic acid suppresses oxidised low-density lipoprotein-induced
aortic smooth muscle cell proliferation: Studies on the activation
of extracellular signal-regulated kinase 1/2 and proliferating cell
nuclear antigen expression. Br J Nutr. 99:709–714. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hu Y, Liu K, Yan M, Zhang Y, Wang Y and
Ren L: Icariin inhibits oxidized low-density lipoprotein-induced
proliferation of vascular smooth muscle cells by suppressing
activation of extracellular signal-regulated kinase 1/2 and
expression of proliferating cell nuclear antigen. Mol Med Rep.
13:2899–2903. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yasunari K, Kohno M, Kano H, Hanehira T,
Minami M and Yoshikawa J: Anti-atherosclerotic action of vascular
D1 receptors. Clin Exp Pharmacol Physiol Suppl. 26:S36–S40.
1999.PubMed/NCBI
|
|
23
|
Witztum JL and Steinberg D: The oxidative
modification hypothesis of atherosclerosis: Does it hold for
humans? Trends Cardiovasc Med. 11:93–102. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guo LL, Chen YJ, Wang T, An J, Wang CN,
Shen YC, Yang T, Zhao L, Zuo QN, Zhang XH, et al: Ox-LDL-induced
TGF-β1 production in human alveolar epithelial cells: Involvement
of the Ras/ERK/PLTP pathway. J Cell Physiol. 227:3185–3191.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liao L, Zhou Q, Song Y, Wu W, Yu H, Wang
S, Chen Y, Ye M and Lu L: Ceramide mediates Ox-LDL-induced human
vascular smooth muscle cell calcification via p38 mitogen-activated
protein kinase signaling. PLoS One. 8(e82379)2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Prasain JK, Peng N, Rajbhandari R and Wyss
JM: The Chinese Pueraria root extract (Pueraria lobata) ameliorates
impaired glucose and lipid metabolism in obese mice. Phytomedicine.
20:17–23. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fu C, Chen B, Jin X, Liu X, Wang F, Guo R,
Chen Z, Zheng H, Wang L and Zhang Y: Puerarin protects endothelial
progenitor cells from damage of angiotensin II via activation of
ERK1/2Nrf2 signaling pathway. Mol Med Rep. 17:3877–3883.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gao Y, Wang X and He C: An
isoflavonoid-enriched extract from Pueraria lobata (kudzu) root
protects human umbilical vein endothelial cells against oxidative
stress induced apoptosis. J Ethnopharmacol. 193:524–530.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ji L, Du Q, Li Y and Hu W: Puerarin
inhibits the inflammatory response in atherosclerosis via
modulation of the NF-kappaB pathway in a rabbit model. Pharmacol
Rep. 68:1054–1059. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hu YW, Li HT, Liu K, Yan MT, Zhang Y and
Ren LQ: Effects of icariin on proliferation of vascular smooth
muscle cell induced by ox-LDL via impacting MAPK signaling pathway.
Zhongguo Zhong Yao Za Zhi. 41:3655–3660. 2016.(In Chinese).
PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li W, Zhi W, Zhao J, Yao Q, Liu F and Niu
X: Cinnamaldehyde protects VSMCs against ox-LDL-induced
proliferation and migration through S arrest and inhibition of p38,
JNK/MAPKs and NF-κB. Vascul Pharmacol. 108:57–66. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hu Y, Liu K, Bo S, Yan M, Zhang Y, Miao C
and Ren L: Inhibitory effect of puerarin on vascular smooth muscle
cells proliferation induced by oxidised low-density lipoprotein via
suppressing ERK 1/2 phosphorylation and PCNA expression. Pharmazie.
71:89–93. 2016.PubMed/NCBI
|
|
33
|
Wan Q, Liu Z and Yang Y: Puerarin inhibits
vascular smooth muscle cells proliferation induced by fine
particulate matter via suppressing of the p38 MAPK signaling
pathway. BMC Complement Altern Med. 18(146)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang X, Yang Y, Zhou S, Gong X, Dai Q,
Zhang P and Jiang L: Puerarin stimulates osteogenic differentiation
and bone formation through the ERK1/2 and p38-MAPK signaling
pathways. Curr Mol Med. 17:488–496. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang M, Li Z, Zhang X, Xie X, Zhang Y,
Wang X and Hou Y: Rosuvastatin attenuates atrial structural
remodelling in rats with myocardial infarction through the
inhibition of the p38 MAPK signalling pathway. Heart Lung Circ.
24:386–394. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Craig R, Larkin A, Mingo AM, Thuerauf DJ,
Andrews C, McDonough PM and Glembotski CC: p38 MAPK and NF-kappa B
collaborate to induce interleukin-6 gene expression and release.
Evidence for a cytoprotective autocrine signaling pathway in a
cardiac myocyte model system. J Biol Chem. 275:23814–23824.
2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang C, Liu W, Shan H, Yu X, Zhang X, Zeng
B and Qian Y: Naringin inhibits titanium particles-induced
up-regulation of TNF-α and IL-6 via the p38 MAPK pathway in
fibroblasts from hip periprosthetic membrane. Connect Tissue Res.
1–10. 2020.PubMed/NCBI View Article : Google Scholar
|