Introduction
Superficial mycosis, commonly known as ringworm, is
a common infectious disease in dermatology caused by pathogenic
fungi parasitic on keratin tissue (1,2). Its
incidence rate is increasing year by year (3). In recent years, with the wide
application of broad-spectrum antibiotics, glucocorticoids,
immunosuppressants and anti-tumor drugs, the possibility of
secondary fungal infection in patients has increased. Therefore,
fungal infection has received extensive attention (4-6).
There are 1-1.5 million kinds of fungi in nature, most of which are
beneficial to human beings. However, a few of them could infect
human beings (or animals) and cause mycosis (7-9).
According to reports, under normal circumstances, pathogenic fungi
of superficial mycosis are always in a dynamic state. The
pathogenic fungi could change with time and region (10,11).
Northeast China has high population density, a large number of
ethnic groups, a large area, distinct seasons, high forest
coverage, rich species, unique geographical conditions and natural
and human environment. There are few studies on pathogenic fungi of
superficial mycosis in this region. Therefore, this study explored
the pathogenic fungi of 5,374 patients with superficial mycosis
received by our hospital from December 2008 to December 2018. We
analyzed the relationship between disease infection, distribution
of pathogenic fungi in China and age, time, and gender, in order to
provide a certain theoretical basis for the diagnosis, treatment
and prevention of related diseases.
Materials and methods
Study design
From December 2008 to December 2018, 5,374 patients
with superficial mycosis from northeast China were selected as
research subjects. The inclusion criteria were: Patients with
typical clinical symptoms; Patients diagnosed with superficial
mycosis according to the medical history, physical signs and
laboratory tests; The diagnosis were confirmed by more than two
doctors according to the diagnostic criteria of superficial mycosis
(12,13). Exclusion criteria: Patients with
tinea versicolor; patients took anti-fungal drugs orally within 3
months or externally within 1 month; patients with skin diseases
with severe local suppurative infection; patients suffered from
severe generalized non-infectious skin diseases; pregnant or
lactating women; patients with severe systemic diseases. This study
was approved by the Ethics Committee of the First Affiliated
Hospital of Qiqihar Medical University. All patients signed an
informed consent form.
Method Microscopic examination of
fungi
Before sampling, skin lesions were routinely
disinfected with 75% alcohol. Parts that cannot be treated with
alcohol could be cleaned several times with sterile physiological
saline. Samples were collected from diseased nails, nail clippings,
interphalangeal dandruff, hair, etc. by sterile operation. Then
samples were placed on glass slides in small quantities, 2 drops of
10% potassium hydroxide solution were added on the samples. The
slides were covered and placed on an alcohol lamp for 1-2 sec, and
the cover glass was lightly pressed to remove bubbles. Finally, the
hyphae, spores and their morphological characteristics were
observed under low-power microscope (10x10) and high-power
microscope (10x40) (Jiangnan BM2000). Fluorescent staining was
performed if necessary.
Fungal culture and identification
Unified sabouraud medium (25 mg chloramphenicol, 15
g agar, 40 g glucose and 10 g peptone were thermally dissolved in
1,000 ml distilled water at 115˚C, autoclaving for 10 min) was used
for basic culture. Plate multi-point culture method was used for
identification. Positive microscopic examination specimens were
taken, inoculated into the culture medium with an inoculation
needle, 7 points at each dish. Then the specimens were placed in a
25˚C constant temperature incubator (Shanghai Yuejin Medical
Instrument Factory No. 1, China) for 2-4 weeks. Deep fungi were
additionally cultured in a 37˚C constant temperature incubator, and
checked twice a week to observe the growth speed, size, morphology,
color, microscopic size and conidial morphological characteristics
of colonies. Suspected cases of Malassezia folliculitis were
cultured on Sandcastle agar medium containing olive oil at 32˚C. If
there were ≥3 points of the same pathogen in 2 culture dishes, it
was identified as pathogenic fungi. Sabouraud agar medium
containing olive oil was used to culture Malassezia
folliculitis specimens at 37˚C. Candida species were
identified by the French Meria Fungus Identification Card.
Dermatophyte and non-dermatophyte were mainly identified according
to the characteristics of colony growth speed, morphology, size,
pigment produced, colony structure, morphology of hyphae and spores
under microscope.
Molecular biological
identification
In order to further determine the microscopic
examination and culture results, positive specimens were taken for
molecular biological identification. Fungal total DNA was extracted
with DNA extraction kit (Qiagen). Fungal rDNA gene conserved region
(rDNA ITS) was taken as the target. Fungal universal primers ITS1
and ITS4 were used to amplify ITS region. Polymerase Chain Reaction
amplification products were sequenced. Gene Bank was registered for
blast comparison, and strain identification was carried out.
Statistical methods
SPSS 20.0 was used to make statistical analysis of
the data. The measurement data were expressed as mean ± SD, the
counting data were expressed as rate, and the comparison of rates
was performed by Chi-square test. The difference was statistically
significant at P<0.05.
Explanation
In the process of fungi identification, some
non-superficial pathogenic fungus are often identified, such as
Candida, Mold and other deep infection pathogenic fungus. However,
they can also cause superficial mycosis such as tinea pedis and
onychomycosis. In the clinical diagnosis and treatment process, due
to the diverse morphology of skin lesions caused by fungal
infections, multi-species co-infection often occurs during the
infection process. Damage to the surface of the skin also increases
the infection rate of non-superficial fungal pathogens and may
aggravate the progression of the disease (such as sporotrichosis).
Therefore, considering the clinical significance of these fungus,
they were not deleted in the result analysis. Although they are not
superficial mycosis, they are still included in the result
data.
Results
Study population characteristics
There were 2,863 males and 2,511 females. The
patients were aged 6-70 years, and the average age was 36.39±17.38
years. The duration of the disease ranged from (0.25-50) years,
with an average course of 8.81±10.56 years. Among them, 3,875
patients had a history of mycosis in their families or relatives,
and 1,891 patients had the experience of feeding pet cats and dogs
in their families.
Fungus and Genbank accession
numbers
The obtained DNA was compared with blast sequence,
and the relative Genbank accession numbers are shown in Table I.
 | Table IFungus and Genbank accession
numbers. |
Table I
Fungus and Genbank accession
numbers.
| Fungus | Genbank accession
numbers |
|---|
| Trichophyton
rubrum | KT155686 |
| Trichophyton
mentagrophytes | KT156074 |
| Trichophyton
schoenleinii | KT272016 |
| Epidermophyton
floccosum | KT155569 |
| Microsporum
canis | KT156124 |
| Microsporum
gypseum | KT261753 |
|
Malassezia | NR_126122 |
| Candida | BD014669 |
| Aspergillus
fumigatus | FJ406497 |
| Aspergillus
niger | DQ374430 |
|
Sporotrichum | AF135797 |
Clinical types of superficial mycosis
and distribution of pathogenic fungi
A total of 5,374 patients suspected of superficial
mycosis were included in this study. Among them, there were 1,538
cases of tinea pedis (28.62%), 1,018 cases of tinea cruris
(18.94%), 938 cases of tinea corporis (17.45%), 773 cases of
onychomycosis (14.38%), 496 cases of tinea capitis (9.23%), 394
cases of tinea manuum (7.33%), 164 cases of Malassezia
folliculitis (3.05%), and 53 cases of sporotrichosis (0.99%).
Fungal culture was carried out on the specimen, and a total of
5,867 strains of 11 species were isolated. The distribution of
pathogenic fungi is shown in Table
II.
 | Table IIClinical types of superficial
dermatomycosis and distribution of pathogenic bacteria. |
Table II
Clinical types of superficial
dermatomycosis and distribution of pathogenic bacteria.
| Type of
bacteria | Tinea pedis | Tinea cruris | Tinea corporis | Onycho-
mycosis | Tinea capitis | Tinea manuum | Malassezia
folliculitis |
Sporotri-chosis | Total [strains
(%)] |
|---|
| Dermatophyte | 1,502 | 895 | 824 | 472 | 570 | 423 | 0 | 0 | 4,686 (79.87) |
| Trichophyton
rubrum | 1,012 | 523 | 605 | 377 | 47 | 285 | 0 | 0 | 2,849 (48.56) |
| Trichophyton
mentagrophytes | 309 | 212 | 83 | 51 | 205 | 87 | 0 | 0 | 947 (16.14) |
| Trichophyton
schoenleinii | 17 | 0 | 0 | 12 | 73 | 5 | 0 | 0 | 107 (1.82) |
|
Epidermophyton
floccosum | 58 | 21 | 2 | 4 | 0 | 16 | 0 | 0 | 101 (1.72) |
| Microsporum
canis | 67 | 98 | 121 | 8 | 184 | 19 | 0 | 0 | 497 (8.47) |
| Microsporum
gypseum | 39 | 41 | 13 | 20 | 61 | 11 | 0 | 0 | 185 (3.15) |
|
Saccharomyces | 178 | 93 | 87 | 383 | 13 | 50 | 0 | 0 | 804 (13.70) |
| Candida | 178 | 93 | 87 | 383 | 13 | 50 | 0 | 0 | 804 (13.70) |
|
Aspergillus | 19 | 30 | 27 | 77 | 0 | 7 | 0 | 0 | 160 (2.73) |
| Aspergillus
fumigatus | 11 | 23 | 22 | 53 | 0 | 5 | 0 | 0 | 114 (1.94) |
| Aspergillus
niger | 8 | 7 | 5 | 24 | 0 | 2 | 0 | 0 | 46 (0.78) |
|
Malassezia | 0 | 0 | 0 | 0 | 0 | 0 | 164 | 0 | 164 (2.80) |
| Dimorphic
fungi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 53 | 53 (0.90) |
| Sporotrichum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 53 | 53 (0.90) |
Distribution of superficial mycosis,
pathogenic fungi and age
In this study, the patients with superficial mycosis
aged 6-70 years, with an average age of 36.39±17.38. Patients were
grouped according to the age: Child group, 0-15 years old (516
cases, 9.60%); young people group, 15-30 years old (1,229 cases,
22.87%); adults group, 31-60 years old (2,656 cases, 49.42%); the
elderly group, over 60 years old (973 cases, 18.11%).
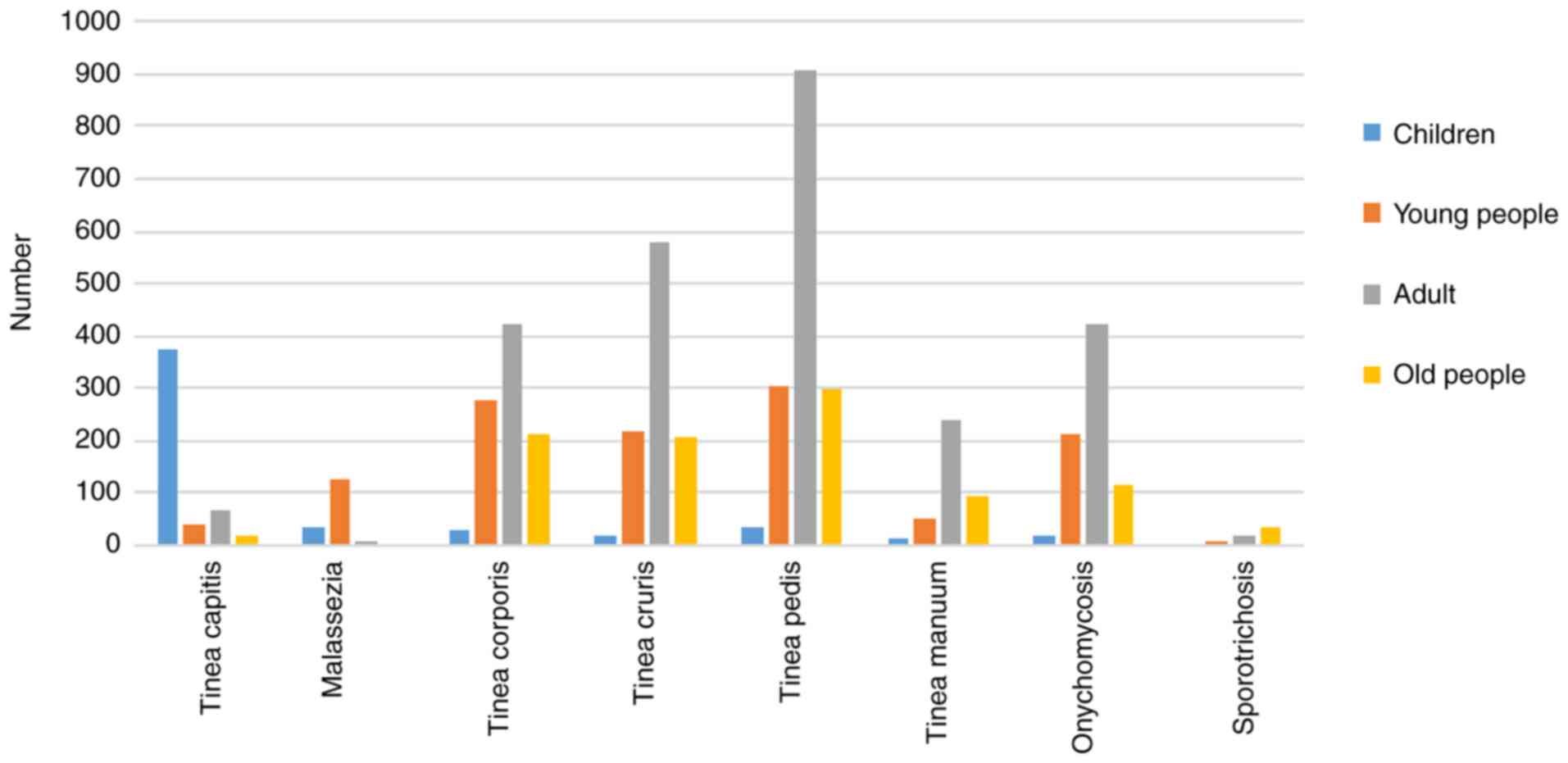
The number of patients of all ages and diseases is
shown in Fig. 1. The group with the
highest incidence of tinea capitis was the children (n=372, 6.92%).
The group with the highest incidence of tinea pedis was the adults
(n=905, 16.84%), and the incidence of tinea pedis was higher than
any other mycoses in all age groups. Onychomycosis was concentrated
in the young people group and the adults group.
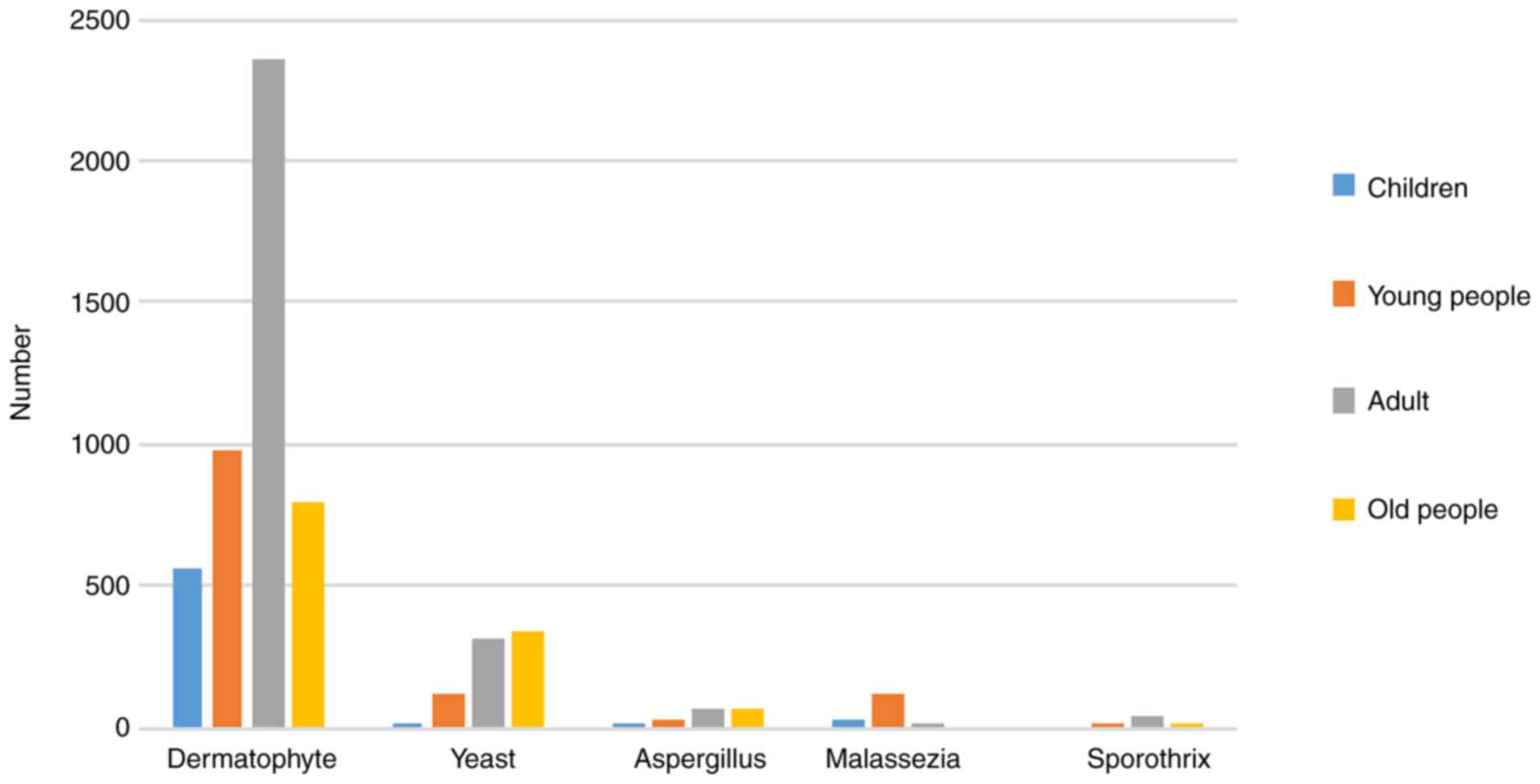
The age of the patients and the distribution of
pathogenic fungi are shown in Fig.
2, in which Malassezia mainly invades young people,
yeast and mold mainly invades elderly patients aged over 60
years.
Distribution of superficial mycosis
and sex
The prevalence rate of tinea pedis, tinea cruris,
tinea corporis and Malassezia folliculitis in men was higher
than that in women, and the prevalence rate of onychomycosis in
women was higher than that in men (P<0.05). The specific data
are shown in Table III.
 | Table IIIComparison of main clinical types of
superficial dermatomycosis in patients of different sex [cases
(%)]. |
Table III
Comparison of main clinical types of
superficial dermatomycosis in patients of different sex [cases
(%)].
| Gender | Number of
cases | Tinea pedis | Tinea cruris | Tinea corporis | Onycho-
mycosis | Tinea capitis | Tinea manuum | Malassezia
folliculitis | Sporotri-
chosis |
|---|
| Male | 2,863 | 1,059 (36.99) | 683 (23.86) | 659 (23.02) | 237 (8.28) | 308 (10.76) | 269 (9.40) | 125 (4.37) | 30 (1.05) |
| Female | 2,511 | 479 (19.08) | 335 (13.34) | 279 (11.11) | 695 (27.68) | 275 (10.95) | 211 (8.40) | 39 (1.55) | 23 (0.92) |
| χ2 | | 210.123 | 96.323 | 131.630 | 351.223 | 0.052 | 1.621 | 35.776 | 0.238 |
| P
valuea | | <0.05 | <0.05 | <0.05 | <0.05 | 0.820 | 0.203 | <0.05 | 0.625 |
Distribution of superficial mycosis,
pathogenic fungi and time
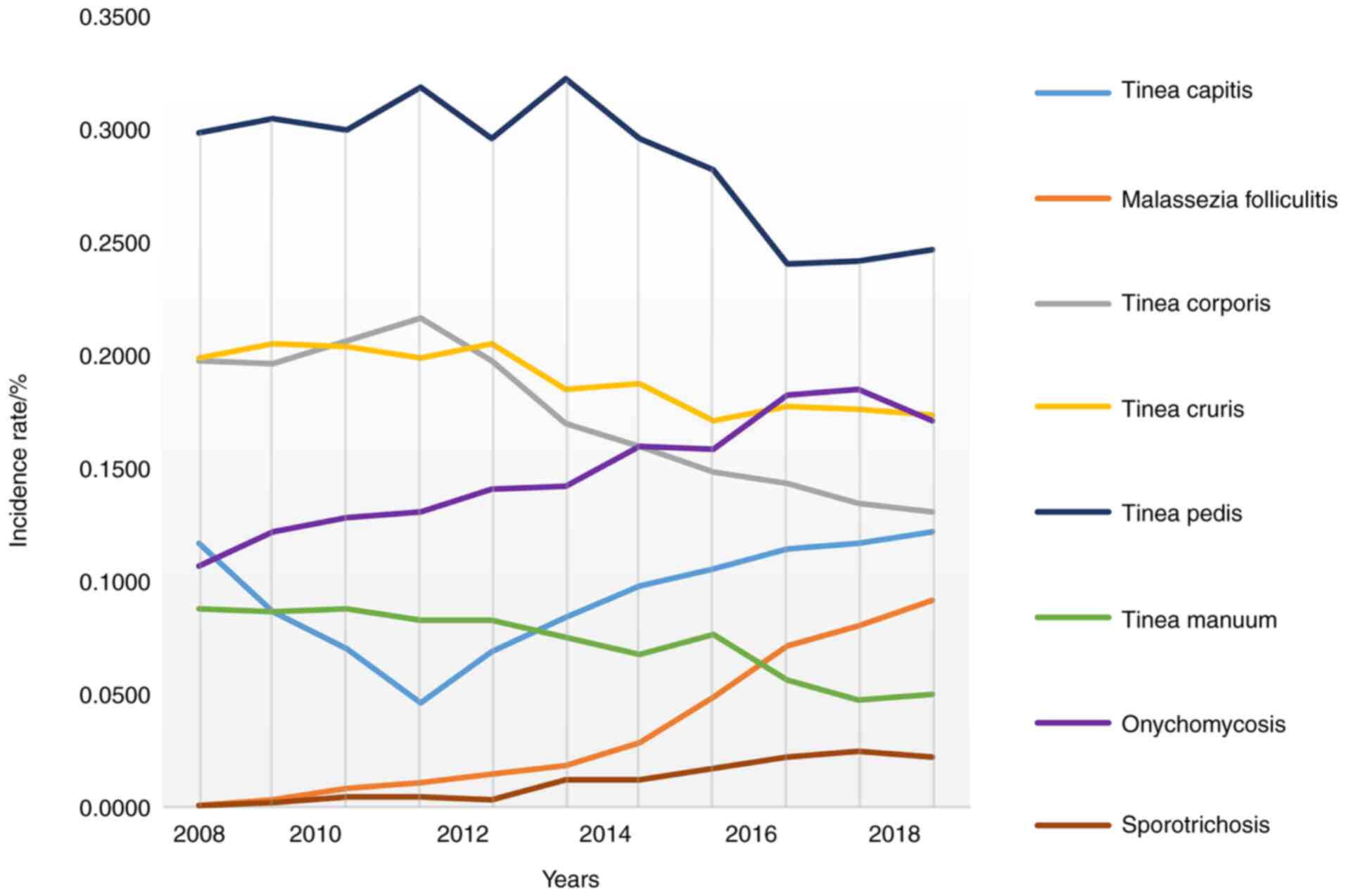
As shown in Fig. 3,
from 2008 to 2018, the incidence of tinea pedis, tinea corporis,
tinea manuum, and tinea cruris showed a downward trend among 5,374
patients with superficial mycosis admitted to the dermatological
department of our hospital from the northeast region. The incidence
of Malassezia folliculitis, onychomycosis, and
sporotrichosis showed an upward trend. The incidence of tinea
capitis first decreased and then increased.
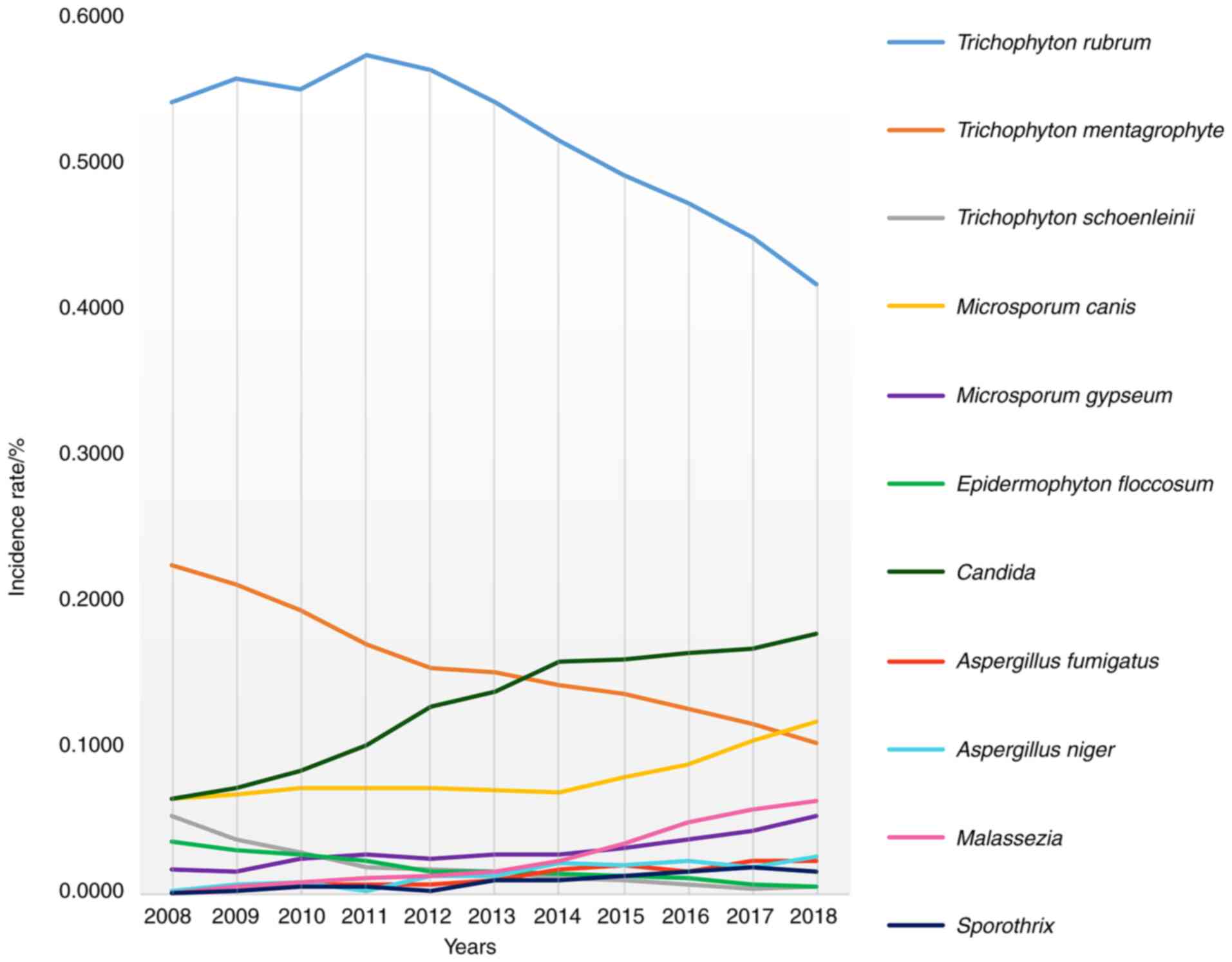
As shown in Fig. 4,
in terms of the isolation rate of pathogenic fungi, Candida,
Mold, Microsporum canis, Malassezia and
sporothrix significantly increased, while Trichophyton
rubrum, Trichophyton mentagrophytes, Trichophyton
schoenleinii and Epidermophyton floccosum showed a
downward trend.
Discussion
Superficial mycosis is a common disease in
dermatology, which is characterized by common occurrence, multiple
occurrence and recurrence, with a prevalence rate of about 20%
worldwide (14,15). In recent years, the incidence rate
of individual superficial mycosis has increased year by year, and
the types of pathogenic fungi are also diversified (16). In 5,743 cases of superficial mycosis
collected in this subject, tinea pedis was the most common disease,
followed by tinea cruris and onychomycosis. The top three
pathogenic fungi of superficial mycosis isolated in the 10 years
from 2008 to 2018 were Trichophyton rubrum, Trichophyton
mentagrophytes and Candida. The results were different
from the previous research results in Jilin (17). However, Trichophyton rubrum
is still the first pathogen species, and most studies in China and
worldwide also show that Trichophyton rubrum is the primary
pathogen of superficial mycosis (18-21).
The difference is that in northern China, Trichophyton
mentagrophytes and Microsporum canis are often the
second and third pathogenic fungi, while in southern China,
Candida is often the second pathogenic fungi (22). The distribution difference of
strains is affected by climate, population composition, lifestyle
and socio-economic conditions, resulting in different
epidemiological profiles of dermatophytes in different geographical
regions (23,24). According to the existing diagnostic
criteria, tinea versicolor is a superficial fungal disease.
However, when the study was designed, the diagnosis of tinea
versicolor was controversial. Some scholars believed that tinea
versicolor was not a fungal infection. Therefore, to avoid
controversial conclusions, tinea versicolor was included in this
study.
In this study, tinea pedis infection was mainly in
adults. Patients of this age were the main labor force. They have
more opportunities to contact pathogenic fungi due to work or
housework, which may lead to an increase in the incidence rate.
Tinea manuum is similar to tinea pedis, and the composition of
pathogenic fungi is basically the same. The reason may be that
tinea manuum can be secondary to tinea pedis and is often
manifested as the two feet-one hand syndrome. Superficial fungal
infection can also spread among different parts of patients. For
example, tinea pedis can cause tinea manuum, tinea corporis, tinea
cruris and onychomycosis. Approximately 1/3 of tinea pedis patients
are accompanied by onychomycosis (25). There are more male patients with
tinea pedis than female patients; tinea cruris and tinea corporis
also have similar rules. After analyzing the causes, we found that
it may be related to male physiological structure, more active
habits, and exuberant secretion of sweat glands and sebaceous
glands, which is more conducive to the growth of dermatophytes.
Female patients with onychomycosis are more than male patients,
which may be due to the subjective consciousness, occupation and
living habits of females. This is basically consistent with the
research results in other regions (26-28).
Therefore, in daily life, we should strengthen the related
propaganda of mycosis and improve the awareness of visiting a
doctor.
Tinea capitis is mainly concentrated in children,
and its pathogenic fungi are Trichophyton mentagrophytes and
Microsporum canis. Microsporum canis is a kind of
animal-friendly fungus. According to the results of this survey,
the isolation rate in Microsporum canis has gradually
increased in recent years, which may be caused by the increasing
popularity of pet raising. However, the incidence of tinea capitis
in children is the highest, which may be related to children's poor
resistance and close contact with pets such as cats and dogs.
Before 1985, the main pathogen of tinea capitis in northeast China
was Trichophyton schoenleinii, which is a kind of family
fungus. However, in this study, the isolation rate of
Trichophyton schoenleinii decreased year by year, which may
be related to the economic development in northeast China. Relevant
studies also showed that with the development of China's economy in
the past 60 years, pets have become the most likely source of tinea
capitis in modern society instead of the early human-to-human
transmission model (29). The
difference with this result is that the isolation rate of
Microsporum canis in Jiangxi, Anhui and Xinjiang is
relatively low. Trichophyton violaceum is the dominant species in
these regions, accounting for 53.38, 46.17 and 48.55% respectively
(30), which may be related to the
differences in lifestyle in different regions. Therefore, in
northeast China, pet health publicity and education should be
strengthened. If skin fungal infection from pets is found, we
should treated both humans and animals.
Among the changes of pathogenic fungi, the changes
of Candida and Mold should be observed by doctors.
This study showed that the isolation rate of Candida and
Mold is increasing year by year. Candida and
Mold were common conditioned pathogen, which could cause
severe invasive fungal infection, such as invasive candidiasis,
invasive aspergillosis. It mainly affected patients with low immune
function, and the mortality rate was extremely high (31). Fungal infection is not uncommon in
systemic diseases with hypoimmunity. Over 90% of AIDS patients have
at least one skin disease, especially infectious diseases involving
skin and mucosa, with a prevalence rate of 60% (32). In recent years, the decrease of
immunity and dysbacteriosis of organisms have increased the
possibility of infection caused by these fungi due to the increase
of various chronic consumptive diseases, the extensive application
of corticosteroids and immunosuppressants, and the long-term
application of broad-spectrum antibiotics. In clinical work,
medical workers should pay more attention to fungal infection,
apply antibiotics, corticosteroids and anti-tumor drugs more
reasonably, and improve the diagnosis rate and treatment effect of
superficial mycosis of skin (33,34).
In superficial mycosis, Candida and Mold mainly cause
onychomycosis, and the increasing isolation rate year by year also
suggests that doctors should consider broad-spectrum antifungal
drugs with consideration of dermatophyte, yeast and mould.
In addition, the changes of Malassezia
deserve attention. Malassezia caused by this strain is often
clinically misdiagnosed, and the misdiagnosis rate is relatively
high. Malassezia folliculitis has similar clinical symptoms
to acne vulgaris and seborrheic dermatitis. It is often
misdiagnosed with antibiotics and corticosteroid preparations
during treatment, resulting in lingering and even aggravating
diseases. This study and many other domestic studies show that the
incidence rate of Malassezia folliculitis has increased each
year recently (35-37).
It mostly invades young people, who are at an immature stage of
psychology and physiology, and pay more attention to their
appearance and image. Repeated occurrence of the disease is easy to
burden young people's psychology and has a great impact on their
growth.
Strictly speaking, sporotrichosis does not belong to
superficial mycosis, but its incidence rate is gradually increasing
and it also has the manifestation of invading superficial skin. So
it is also included in the discussion here. Sporothrix is prevalent
in northeast China and its incidence rate is on the rise. During
1991-2007, Jilin, China reported >2,000 confirmed cases of
sporotrichosis, making northeast China one of the most serious
epidemic areas of sporotrichosis in China and even in the world
(38). Jiangsu and Guangdong are
the main areas in southern China, but the incidence rate is far
lower than that in northeast China. Due to its various skin
lesions, it is often misdiagnosed as inflammatory granuloma and
skin tuberculosis. Although most sporotrichosis can be cured, it
takes a long time, and if proper drugs are not selected, it is easy
to aggravate infection. Therefore, in clinical work, we should
strengthen the understanding of related diseases and rationally
choose drugs. With the increase in the number of immunocompromised
patients, the epidemiological monitoring of sporotrichosis is
increasingly needed, and additional protective measures should be
taken to avoid infection during agricultural activities.
This study showed that the distribution of
superficial mycosis and its pathogenic fungi in northeast China has
certain regularity and is affected by age, sex and time. With the
passage of time, the distribution of various pathogenic fungi has
changed correspondingly, and targeted treatment programs should be
implemented according to its trend. In our daily life, we should
also strengthen the general public's awareness of fungal infection
and persist in carrying out epidemiological monitoring of
superficial mycosis. It is of great significance to the prevention
and treatment of superficial mycosis. This study also has
limitations. The inclusion of tinea versicolor was not included
(the diagnosis of tinea versicolor was controversial at the
beginning of the study, but it is now clear that it is a
superficial fungal disease caused by pityrosporum infection). The
negative samples were abandoned, which makes the results biased. In
the future, more rigorous research design should be carried out to
better serve clinical diagnosis and treatment.
Acknowledgements
Not applicable.
Funding
Project of Qiqihar Science and Technology Plan
(SFZD-2013118).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW, CD, YX and HY conceived and designed the study,
and drafted the manuscript. XW, CD, SZ and CY collected, analyzed
and interpreted the experimental data. XW revised the manuscript
for important intellectual content. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Affiliated Hospital of Qiqihar Medical University. Signed
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feng W, Chen C, Mo S, Qi C, Gong J, Li XN,
Zhou Q, Zhou Y, Li D, Lai Y, et al: Highly oxygenated
meroterpenoids from the Antarctic fungus Aspergillus
terreus. Phytochemistry. 164:184–191. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bezshapochny SB, Zachepilo SV, Polyanskaya
VP, Bobrova NA and Fedorchenko VI: Opportunistic mycoses of ENT
organs. Part 1. Vestn Otorinolaringol. 83:67–71. 2018.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
3
|
Lao M, Wang X, Ding M, Yang Z, Chen H,
Liang L, Zhan Z and Chen D: Invasive fungal disease in patients
with systemic lupus erythematosus from Southern China: A
retrospective study. Lupus. 28:77–85. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Upadhyay V, Kumar A, Singh AK and Pandey
J: Epidemiological characterization of dermatophytes at a tertiary
care hospital in Eastern Uttar Pradesh, India. Curr Med Mycol.
5:1–6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moriello KA: Decontamination of 70 foster
family homes exposed to Microsporum canis infected cats: A
retrospective study. Vet Dermatol. 30:178–e55. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hilmioğlu-Polat S, Seyedmousavi S, Ilkit
M, Hedayati MT, Inci R, Tumbay E and Denning DW: Estimated burden
of serious human fungal diseases in Turkey. Mycoses. 62:22–31.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Alvarez-Moreno CA and Combariza JF: Risk
of invasive fungal infections during hospital construction: How to
minimize its impact in immunocompromised patients. Curr Opin Infect
Dis. 32:322–329. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rouzaud C, Chosidow O, Brocard A, Fraitag
S, Scemla A, Anglicheau D, Bouaziz JD, Dupin N, Bougnoux ME, Hay R,
et al: Severe dermatophytosis in solid organ transplant recipients:
A French retrospective series and literature review. Transpl Infect
Dis: Feb 20, 2018 (Epub ahead of print). doi:
10.1111/tid.12799.
|
|
9
|
Deptuła A, Trejnowska E, Dubiel G,
Żukowski M, Misiewska-Kaczur A, Ozorowski T and Hryniewicz W:
Prevalence of healthcare-associated infections in Polish adult
intensive care units: Summary data from the ECDC European Point
Prevalence Survey of hospital-associated infections and
antimicrobial use in Poland 2012-2014. J Hosp Infect. 96:145–150.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nenoff P, Verma SB, Vasani R, Burmester A,
Hipler UC, Wittig F, Krüger C, Nenoff K, Wiegand C, Saraswat A, et
al: The current Indian epidemic of superficial dermatophytosis due
to Trichophyton mentagrophytes - A molecular study. Mycoses.
62:336–356. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Otašević S, Momčilović S, Golubović M,
Ignjatović A, Rančić N, Đorđević M, Ranđelović M, Hay R and
Arsić-Arsenijević V: Species distribution and epidemiological
characteristics of superficial fungal infections in Southeastern
Serbia. Mycoses. 62:458–465. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dainton C and Chu CH: A narrative review
of dermatologic protocols for primary care medical service trips in
Latin America and the Caribbean. Int J Dermatol. 56:1425–143.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Singh S, Ehsani-Chimeh N, Kornmehl H and
Armstrong AW: Quality of life among dermatology patients: A
systematic review of investigations using qualitative methods. G
Ital Dermatol Venereol. 154:72–78. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nilsson K, Friberg M, Rollman O and Tano
E: Impact of prolonged storage of clinical samples at 4˚C on the
recovery of dermatophytes by culture or PCR analysis. J Mycol Med.
29:1–6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Leung AKC, Leong KF and Lam JM: Tinea
imbricata: An overview. Curr Pediatr Rev. 15:170–174.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Farag AGA, Hammam MA, Ibrahem RA, Mahfouz
RZ, Elnaidany NF, Qutubuddin M and Tolba RRE: Epidemiology of
dermatophyte infections among school children in Menoufia
Governorate, Egypt. Mycoses. 61:321–325. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gawdzik A, Nowogrodzka K,
Hryncewicz-Gwóźdź A, Maj J, Szepietowski J and Jankowska-Konsur A:
Epidemiology of dermatomycoses in southwest Poland. Postepy
Dermatol Alergol. 36:604–608. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
de Albuquerque Maranhão FC,
Oliveira-Júnior JB, Dos Santos Araújo MA and Silva DMW: Mycoses in
northeastern Brazil: Epidemiology and prevalence of fungal species
in 8 years of retrospective analysis in Alagoas. Braz J Microbiol.
50:969–978. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Antuori A, Fernández G, Fernández A,
Alcaide M, Boada A, Bielsa MI, Romaní N and Matas L: Epidemiology
of dermatophytic infections between 2008 and 2017 in Barcelona,
Spain. Enferm Infecc Microbiol Clin. 37:642–647. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sen S, Borah SN, Kandimalla R, Bora A and
Deka S: Efficacy of a rhamnolipid biosurfactant to inhibit
Trichophyton rubrum in vitro and in a mice model of
dermatophytosis. Exp Dermatol. 28:601–608. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bitencourt TA, Oliveira FB, Sanches PR,
Rossi A and Martinez-Rossi NM: The prp4 kinase gene and related
spliceosome factor genes in Trichophyton rubrum respond to
nutrients and antifungals. J Med Microbiol. 68:591–599.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cai W, Lu C, Li X, Zhang J, Zhan P, Xi L,
Sun J and Yu X: Epidemiology of superficial fungal infections in
Guangdong, Southern China: A retrospective study from 2004 to 2014.
Mycopathologia. 181:387–395. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Havlickova B, Czaika VA and Friedrich M:
Epidemiological trends in skin mycoses worldwide. Mycoses. 51
(Suppl 4):S2–S15. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ginter-Hanselmayer G, Weger W, Ilkit M and
Smolle J: Epidemiology of tinea capitis in Europe: Current state
and changing patterns. Mycoses. 50 (Suppl 2):S6–S13.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nasr A, Vyzantiadis TA, Patsatsi A, Louka
A, Ioakimidou A, Zachrou E, Chavale A, Kalabalikis D, Malissiovas N
and Sotiriadis D: Epidemiology of superficial mycoses in Northern
Greece: A 4-year study. J Eur Acad Dermatol Venereol. 30:837–839.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gawaz A and Weisel G: Mixed infections are
a critical factor in the treatment of superficial mycoses. Mycoses.
61:731–735. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
de Hoog S, Monod M, Dawson T, Boekhout T,
Mayser P and Gräser Y: Skin fungi from colonization to infection.
Microbiol Spectr Jul 5, 2017 (Epub ahead of print). doi:
0.1128/microbiolspec.FUNK-0049-2016.
|
|
28
|
Bond R: Superficial veterinary mycoses.
Clin Dermatol. 28:226–236. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhan P, Geng C, Li Z, Jin Y, Jiang Q, Tao
L, Luo Y, Xiong Z, Wu S, Li D, et al: Evolution of tinea capitis in
the Nanchang area, Southern China: A 50-year survey (1965-2014).
Mycoses. 58:261–266. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhan P, Li D, Wang C, Sun J, Geng C, Xiong
Z, Seyedmousavi S, Liu W and de Hoog GS: Epidemiological changes in
tinea capitis over the sixty years of economic growth in China. Med
Mycol. 53:691–698. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen M, Xu Y, Hong N, Yang Y, Lei W, Du L,
Zhao J, Lei X, Xiong L, Cai L, et al: Epidemiology of fungal
infections in China. Front Med. 12:58–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tschachler E: The dermatologist and the
HIV/AIDS pandemic. Clin Dermatol. 32:286–289. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fay VDS, Rodrigues DMG, Gonçalves SMB,
Gregianini TS and Bonamigo RR: Drug susceptibility in emerging
fungal infections: Tests with fluconazole, itraconazole, and
amphotericin B. An Bras Dermatol. 93:462–464. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wypij M, Czarnecka J, Dahm H, Rai M and
Golinska P: Silver nanoparticles from Pilimelia
columellifera subsp. pallida SL19 strain demonstrated
antifungal activity against fungi causing superficial mycoses. J
Basic Microbiol. 57:793–800. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Durdu M, Güran M and Ilkit M:
Epidemiological characteristics of Malassezia folliculitis and use
of the May-Grünwald-Giemsa stain to diagnose the infection. Diagn
Microbiol Infect Dis. 76:450–457. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tsai YC, Wang JY, Wu YH and Wang YJ:
Clinical differences in pediatric and adult Malassezia
folliculitis: Retrospective analysis of 321 cases over 9 years. J
Am Acad Dermatol. 81:278–280. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Harada K, Saito M, Sugita T and Tsuboi R:
Malassezia species and their associated skin diseases. J Dermatol.
42:250–257. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chakrabarti A, Bonifaz A,
Gutierrez-Galhardo MC, Mochizuki T and Li S: Global epidemiology of
sporotrichosis. Med Mycol. 53:3–14. 2015.PubMed/NCBI View Article : Google Scholar
|