Introduction
Kawasaki disease (KD), alternatively known as
cutaneous mucosal lymph node syndrome (MCLS), is a self-limited
systemic immune vasculitis (1). A
total of 20-25% of untreated children with KD develop coronary
artery damage (CAL), which has become a major cause of acquired
heart disease in children (2).
Currently, the exact cause of the disease remains unknown. In terms
of treatment, the use of intravenous immunoglobulin (IVIG) in the
acute phase can reduce the incidence of CAL to 2-4%, and, to some
extent, restore CAL (3). CAL is the
most serious complication of KD, mainly including coronary artery
disease (CAD) and coronary artery aneurysm (CAA) (2,4-6).
KD is characterized by endothelial function damage, coagulation and
vascular morphology abnormality at the early stage; injecting IVIG
in the acute phase can improve endothelial function and reduce CAD.
A single dose of 2 g/kg per day IVIG combined with aspirin has been
used as the standard treatment of KD in the USA and Japan (7). However, 10-20% of children exhibit
IVIG resistance, presenting a high risk of developing CAL (8). After initial treatment failure, 3-4%
of children with KD need to receive further IVIG treatment, but
still exhibit a limited response. Infliximab (IFX) is a monoclonal
antibody that specifically blocks tumor necrosis factor (TNF)-α,
which is an important pro-inflammatory factor significantly
elevated in the blood circulation of children with KD. The
effectiveness of IFX in combination with IVIG treatment of
non-reactive type KD is controversial; studies have reported that
IFX combined with IVIG therapy does not reduce IVIG resistance, but
IFX is effective in treating KD (9). In addition, IFX is safe and tolerable
for children with KD who are resistant to IVIG (2,10-14).
The aim of the present study was to perform a
meta-analysis of the available literature to obtain updated
evidence about the efficacy of IFX in the treatment of KD.
Materials and methods
Search strategy
To identify studies conducted on the efficacy of IFX
in the treatment of KD, relevant articles published before July
2019 in the Cochrane (https://www.cochranelibrary.com/), Pubmed (https://pubmed.ncbi.nlm.nih.gov/) and Embase
(https://www.embase.com/) databases were reviewed.
The references of all identified articles were also reviewed to
identify additional studies. The search terms were as follows:
‘Infliximab’, ‘IFX’, ‘Kawasaki disease’, ‘KD’ and ‘Kawasaki’. These
terms were used in combination with ‘and’ or ‘or’. The literature
review was performed independently by two researchers, with a third
researcher resolving any disputes when needed.
Following the Participants, Interventions,
Comparisons, Outcomes and Study design principle (15), the key criteria included:
Participants, patients with KD; interventions, the patients of the
single-arm study were treated by IFX, in the case-control study,
patients in the experiment group were treated by IFX or IFX
combined with intravenous immunoglobulin (IVIG), patients in the
control group were treated by placebo or IVIG or polyethylene
glycol-treated human immunoglobulin (VGIH); comparisons and
outcomes, compared IFX with IVIG/VGIH in the treatment of KD and
the outcomes included clinical efficacy indexes; and study design,
case-control study or single-arm study.
Study selection criteria
Studies included in the current meta-analysis met
the following criteria: i) Case-control study or single-arm study;
ii) the inventions of the treatment group and single-arm study were
IFX or IFX combined with IVIG, and the control group were treated
by placebo or IVIG or VGIH; iii) the research subjects were
patients with KD; and iv) studies published in English or
Chinese.
Studies were excluded if they met the following
criteria: i) Repeat articles or results; ii) clear data errors;
iii) case reports case-control studies, theoretical research,
conference reports, systematic reviews, meta-analyses, or other
forms of research or comment that were not designed in a randomized
controlled manner; and iv) studies without clinical outcomes.
Two researchers independently determined whether
studies met the inclusion criteria, with a third resolving any
disputes when needed.
Data extraction and quality
assessment
For each included study, two categories of
information (basic information and primary study outcomes) were
extracted. Basic information relevant to the present meta-analysis
was as follows: Author names, year of publication, sample size,
mean age, sex and intervention. Primary clinical outcomes were as
follows: CAL, CAA, non-response rate, length of hospital stay,
adverse events, fever, conjunctival injection, changes in lip and
oral cavity, cervical lymphadenopathy, polymorphous exanthema,
combined coronary artery Z-score, white blood cell (WBC),
neutrophil, platelet, aspartate aminotransferase (AST), alanine
transaminase (ALT), and C-reactive protein (CRP) levels. The data
extraction was performed independently by two researchers, with a
third resolving any disputes when needed.
Statistical analysis
STATA v10.0 (StataCorp LP) was used for the data
analysis. Heterogeneity in study results was assessed by the
χ2 and I2 tests, and the appropriate
analytical models (fixed-effect or random-effect) were determined.
A χ2 P≤0.05 and an I2>50% indicated high
heterogeneity, and a random-effects model was used, whereas a
χ2 P>0.05 and an I2≤50% indicated
acceptable heterogeneity, and a fixed-effects model was used.
Continuous variables are reported as the mean ± standard deviation
and compared by the mean difference (MD). Categorical data are
reported as percentages and compared based on relative risk
(RR)/odds ratios (ORs). MD and 95% confidence intervals (95% CIs)
were used to analyze the levels of WBC, neutrophils, platelets,
AST, ALT, CRP and Z-scores. Other values were analyzed by RR and
95% CI.
Results
Overview of the included studies
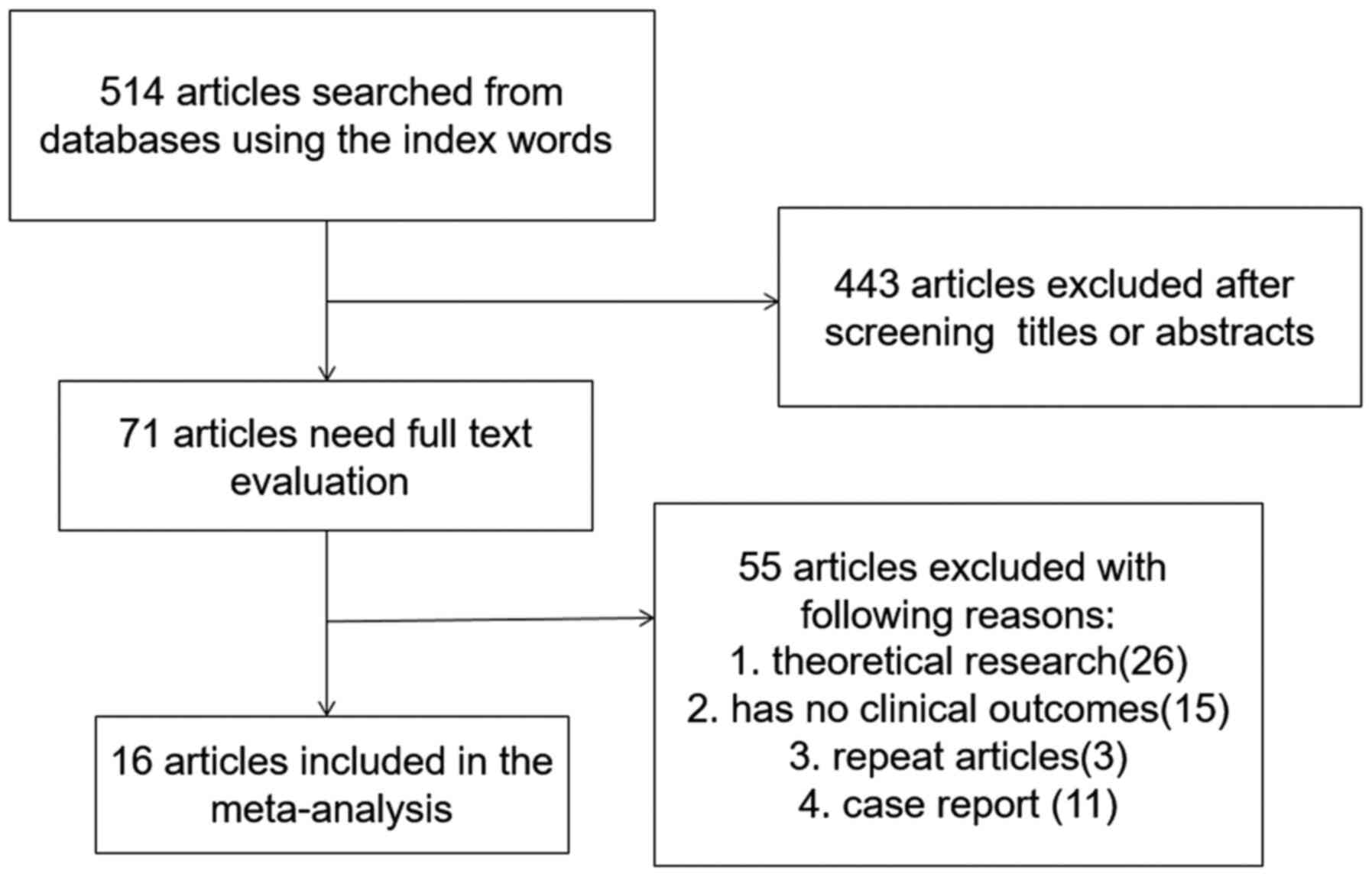
A total of 514 articles were identified by the
initial keyword search, but 443 of them were excluded following
title and abstract review. The remaining 71 articles were subjected
to a complete full-text assessment, and 55 articles were excluded
for failing to meet study inclusion criteria. The reasons for
exclusion were: i) Theoretical research (n=26); ii) no clinical
outcomes (n=15); iii) repeated articles (n=3); and iv) case reports
(n=11). Ultimately, a total of 16 studies (4,13,16-29)
incorporating 429 patients met the inclusion criteria for the
current meta-analysis. The study selection is outlined in Fig. 1.
Table I summarizes
the basic information of each study, including author names, year
of publication, sample size, mean age, sex and intervention.
According to the study design and interventions, the 16 studies
were divided into groups for the subgroup analysis: i) IFX or IFX
plus IVIG vs. IVIG or VGIH group (IFX vs. IVIG or VGIH; IFX plus
IVIG vs. IVIG or VGIH); and ii) single-arm study (IFX, IFX plus
IVIG or IVMP (intravenous methyl prednisolone).
 | Table IBasic characteristics of included
studies. |
Table I
Basic characteristics of included
studies.
| A, IFX or IFX plus
IVIG vs. IVIG or VGIH groups |
|---|
| | Sample | Age (mean) | Sex | Intervention | |
|---|
| Author, year
(4,13,16-29) | T | C | T | C | T | C | T | C | (Refs.) |
|---|
| Dionne et al,
2019 | 58 | 33 | 1.1 | 2 | 44M | 19M | Infliximab 5 mg/kg or
10 mg/kg, and IVIG 2 g/kg | IVIG, 2 g/kg | (25) |
| Han and Zhao et
al, 2018 | 77 | 77 | 2.1 | 2.3 | 34M | 28M | Infliximab 5 mg/kg,
and IVIG 1 g/kg | IVIG, 1 g/kg | (27) |
| Jone et al,
2018 | 35 | 34 | 2.1 | 3.5 | 26M | 28M | Infliximab 5 mg/kg
and IVIG 2 g/kg | IVIG, 2 g/kg | (17) |
| Mori et al,
2018 | 16 | 15 | 2.5 | 3 | 10M | 11M | Infliximab 5
mg/kg | VGIH, 2 g/kg | (18) |
| Youn et al,
2016 | 11 | 32 | - | - | - | - | Infliximab 5
mg/kg | IVIG 2 g/kg | (23) |
| Tremoulet et
al, 2014 | 98 | 98 | 3 | 2.8 | 60M | 61M | Infliximab 5
mg/kg | Placebo | (22) |
| Son et al,
2011 | 20 | 86 | 23 | 29 | 14M | 55M | Infliximab 5
mg/kg | IVIG 2 g/kg | (20) |
| Hirono et
al, 2009a | 11 | 18 | 4 | 2.6 | 6M | 9M | Infliximab 5
mg/kg | IVIG 2 g/kg | (16) |
| Hirono et
al, 2009b | 11 | 14 | 4 | 2.8 | 6M | 10M | Infliximab 5
mg/kg | IVIG 2 g/kg | (16) |
| Zhang et al,
2018 | 22 | 66 | 2.5 | 2.8 | 14M | 45M | Infliximab 5
mg/kg | IVIG 2 g/kg | (19) |
| B, Single-arm
study |
| Authors, year | Sample size | Mean age | Sex | Intervention | (Refs.) (4,13,21,24,26,30,29) |
| Hur et al,
2019a | 27 | 41.49 | 19M | IVIG → IVIG →
infliximab | (24) |
| Hur et al,
2019b | 15 | 36.53 | 10M | IVIG →
infliximab | (24) |
| Hur et al,
2019c | 47 | 27.11 | 33M | IVIG → IVIG → IVMP
→ infliximab | (24) |
| Hur et al,
2019d | 11 | 30.27 | 8M | IVIG → IVIG + IVMP
→ infliximab | (24) |
| Hur et al,
2019e | 2 | 43 | 2M | IVIG → IVMP →
infliximab | (24) |
| Koizumi et
al, 2018 | 13 | - | - | Infliximab 5
mg/kg | (4) |
| Masuda et
al, 2018 | 443 | 33 | 284M | Infliximab 5
mg/kg | (28) |
| Ogihara et
al, 2014a | 8 | 4.5 | 5M | Infliximab | (13) |
| Ogihara et
al, 2014b | 9 | 4.2 | 6M | Infliximab | (13) |
| Sonoda et
al, 2014 | 76 | 3.4 | 51M | Infliximab 5
mg/kg | (21) |
| Mori et al,
2012 | 20 | 4.6 | 10M | Infliximab 5
mg/kg | (26) |
| Song et al,
2010 | 16 | 41.49 | 19M | Infliximab 5
mg/kg | (29) |
IFX or IFX plus IVIG vs. IVIG or VGIH
group
A total of nine studies containing 170 patients in
the IFX or IFX plus IVIG group and 144 patients in the IVIG or VGIH
group were included. No significant differences were observed in
CAL (RR: 0.410; 95% CI: 0.124, 1.353), CAA (RR: 0.687; 95% CI:
0.286, 1.652), non-response rate (RR: 0.466; 95% CI: 0.165, 1.315),
length of hospital stay (weighted mean difference, WMD): -1.135;
95% CI: -2.436, -0.167), conjunctival injection (RR: 1.054; 95% CI:
0.765, 1.452) or polymorphous exanthema (RR: 1.040; 95% CI: 0.664,
1.629) between the two groups. However, the incidence of adverse
events (RR: 0.811; 95% CI: 0.674, 0.977), and the WBC (WMD: -0.060;
95% CI: -0.07, -0.049), neutrophil (WMD: -1.160; 95% CI: -1.171,
-1.149) and CRP (WMD: -3.00; 95% CI: -3.017, -2.983) levels were
significantly reduced in the IFX or IFX plus IVIG group compared
with those in the IVIG or VGIH group. The platelet count (WMD:
10.040; 95% CI: 9.803, 10.277), ALT level (WMD: 1.200; 95% CI:
1.162, 1.238) and Z-score (WMD: 0.165; 95% CI: 0.038, 0.292) were
increased in the IFX or IFX plus IVIG group compared with those in
the IVIG or VGIH group. The number of patients with fever (RR:
1.706; 95% CI: 1.287, 2.261), changes in lip and oral cavity (RR:
1.452; 95% CI: 1.043, 2.021) or cervical lymphadenopathy (RR:
1.586; 95% CI: 1.128, 2.23) was reduced in the IFX or IFX plus IVIG
group compared with those in the IVIG or VGIH group.
In the subgroup analysis, no significant differences
were observed between length of hospital stay, the incidence of
CAL, CAA, non-response rate or adverse events between the IFX and
the IVIG or VGIH groups. The WBC, neutrophil and CRP levels were
significantly reduced in the IFX group compared with those in the
IVIG or VGIH groups. In addition, the platelet and ALT levels were
increased in the IFX group compared with those in the IVIG or VGIH
groups. No significant differences were observed in CAA, adverse
events, conjunctival injection or polymorphous exanthema between
the IFX plus IVIG group and the IVIG or VGIH groups. The incidence
of non-response rate and length of hospital stay were all
significantly reduced in the IFX plus IVIG group compared with the
IVIG or VGIH groups. However, the incidence of fever, changes in
lip and oral cavity and cervical lymphadenopathy were noticeably
increased in the IFX plus IVIG group compared with the IVIG or VGIH
groups. The Z-score was also significantly increased in the IFX
plus IVIG group compared with that of the IVIG or VGIH groups. All
the above results are presented in Figs.
2-4 and Table II.
 | Table IIResults of case-control studies. |
Table II
Results of case-control studies.
| | | | | | | | P-value |
|---|
| Index | N
(case/control) | Intervention | RR (95% CI) | Pa | I2 | Pb | Begg's | Egger's |
|---|
| Coronary artery
lesion | 49/113 | IFX vs. IVIG or
VGIH | 0.410 (0.124,
1.353) | 0.822 | 0.0% | 0.143 | >0.009 | 0.990 |
| Coronary artery
aneurysm | 156/131 | overall | 0.687 (0.286,
1.652) | 0.942 | 0.0% | 0.402 | >0.009 | - |
| | 98/98 | IFX vs. IVIG or
VGIH | 0.667 (0.194,
2.289) | - | - | 0.520 | - | - |
| | 58/53 | IFX plus IVIG vs.
IVIG or VGIH | 0.711 (0.205,
2.466) | - | - | 0.591 | - | - |
| Non-response
rate | 144/164 | overall | 0.466 (0.165,
1.315) | 0.082 | 60.1% | 0.149 | >0.009 | 0.577 |
| | 109/130 | IFX vs. IVIG or
VGIH | 0.688 (0.208,
2.277) | 0.201 | 38.7% | 0.540 | >0.009 | - |
| | 35/34 | IFX plus IVIG vs.
IVIG or VGIH | 0.259 (0.096,
0.702) | - | - | 0.008 | - | - |
| Length of | 164/250 | overall | -1.135 (-2.436,
0.167)c | 0.000 | 99.9% | 0.087 | >0.009 | 0.578 |
| Hospital stay | 129/216 | IFX vs. IVIG or
VGIH | -0.852 (-2.492,
0.787)c | 0.000 | 99.8% | 0.308 | >0.009 | 0.661 |
| | 35/34 | IFX plus IVIG vs.
IVIG or VGIH | -1.920 (-1.986,
-1.854)c | - | - | <0.001 | - | - |
| Adverse event | 180/265 | overall | 0.811 (0.674,
0.977) | 0.243 | 26.8% | 0.027 | 0.806 | 0.193 |
| | 145/231 | IFX vs. IVIG or
VGIH | 0.858 (0.715,
1.029) | 0.736 | 0.0% | 0.099 | >0.009 | 0.493 |
| | 35/34 | IFX plus IVIG vs.
IVIG or VGIH | 0.162 (0.021,
1.275) | - | - | 0.084 | - | - |
| Fever | 77/77 | IFX plus IVIG vs.
IVIG or VGIH | 1.706 (1.287,
2.261) | - | - | <0.001 | - | - |
| Conjunctival
injection | 77/77 | IFX plus IVIG vs.
IVIG or VGIH | 1.054 (0.765,
1.452) | - | - | 0.747 | - | - |
| Changes in lip and
oral cavity | 77/77 | IFX plus IVIG vs.
IVIG or VGIH | 1.452 (1.043,
2.021) | - | - | 0.027 | - | - |
| Cervical
lymphadenopathy | 77/77 | IFX plus IVIG vs.
IVIG or VGIH | 1.586 (1.128,
2.23) | - | - | 0.008 | - | - |
| Polymorphous
exanthema | 77/77 | IFX plus IVIG vs.
IVIG or VGIH | 1.040 (0.664,
1.629) | - | - | 0.864 | - | - |
| Z-score | 93/67 | IFX plus IVIG vs.
IVIG or VGIH | 0.165 (0.038,
0.292)c | 0.000 | 93.2% | 0.011 | >0.009 | - |
| WBC count,
103/mm3 | 98/98 | IFX vs. IVIG or
VGIH | -0.060 (-0.071,
-0.049)c | - | - | <0.001 | - | - |
| Neutrophil count,
103/mm3 | 98/98 | IFX vs. IVIG or
VGIH | -1.160 (-1.171,
-1.149)c | - | - | <0.001 | - | - |
| Platelet count,
104/l | 98/98 | IFX vs. IVIG or
VGIH | 10.040 (9.803,
10.277)c | - | - | <0.001 | - | - |
| ALT, IU/l | 98/98 | IFX vs. IVIG or
VGIH | 1.200 (1.162,
1.238)c | - | - | <0.001 | - | - |
| CRP, mg/dl | 120/130 | IFX vs. IVIG or
VGIH | -3.000 (-3.017,
-2.983)c | 0.592 | 0.0% | <0.001 | >0.009 | 0.992 |
Single-arm study
A total of seven studies containing 115 patients
were included. The incidence of non-response rate (0.083; 95% CI:
0.028, 0.138), adverse events (0.156; 95% CI: 0.122, 0.190), fever
(ES: 0.842; 95% CI: 0.760, 0.924), conjunctival injection (ES:
0.618; 95% CI: 0.509, 0.728), changes in lip and oral cavity (ES:
0.434; 95% CI: 0.323, 0.546), cervical lymphadenopathy (ES: 0.303;
95% CI: 0.199, 0.406) and polymorphous exanthema (ES: 0.329; 95%
CI: 0.223, 0.435) was significantly reduced after the IFX therapy.
In addition, the WBC, neutrophil, ALT and CRP levels were reduced
after the IFX therapy. The platelet count was significantly
increased after the IFX therapy. No significant changes were
observed in AST or ALT after the IFX therapy. After the IFX plus
IVIG or IVMP therapy, the incidence of CAA was 0.150 (95% CI:
0.024, 0.277), and the non-response rate was 0.114 (95% CI: 0.053,
0.175). All the above results are presented in Figs.
5-7 and Table III.
 | Table IIIResults of single-arm studies. |
Table III
Results of single-arm studies.
| | | | | | | | P-value |
|---|
| Index | N | Intervention | ES (95% CI) | Pa | I2 | Pb | Begg's | Egger's |
|---|
| Coronary artery
aneurysm | 102 | IFX plus IVIG or
IVMP | 0.150 (0.024,
0.277) | 0.003 | 75.6% | 0.020 | >0.009 | - |
| Non-response
rate | 198 | overall | 0.097 (0.056,
0.138) | 0.762 | 0.0% | <0.001 | >0.009 | 0.854 |
| | 96 | IFX | 0.083 (0.028,
0.138) | 0.776 | 0.0% | 0.003 | - | - |
| | 102 | IFX plus IVIG or
IVMP | 0.114 (0.053,
0.175) | 0.604 | 0.0% | <0.001 | 0.117 | 0.031 |
| Adverse event | 443 | IFX | 0.156 (0.122,
0.190) | - | - | <0.001 | - | - |
| Fever | 76 | IFX | 0.842 (0.760,
0.924) | - | - | <0.001 | - | - |
| Conjunctival
injection | 76 | IFX | 0.618 (0.509,
0.728) | - | - | <0.001 | - | - |
| Changes in lip and
oral cavity | 76 | IFX | 0.434 (0.323,
0.546) | - | - | <0.001 | - | - |
| Cervical
lymphadenopathy | 76 | IFX | 0.303 (0.199,
0.406) | - | - | <0.001 | - | - |
| Polymorphous
exanthema | 76 | IFX | 0.329 (0.223,
0.435) | - | - | <0.001 | - | - |
| WBC count,
103/mm3 | 473 | IFX | -4.011 (-5.485,
-2.536)c | 0.000 | 87.2% | <0.001 | >0.009 | 0.629 |
| Neutrophil count,
103/mm3 | 30 | IFX | -4.771 (-5.675,
-3.867)c | 0.001 | 84.8% | <0.001 | >0.009 | 0.914 |
| Platelet count,
104/µl | 460 | IFX | 11.568 (10.440,
12.697)c | 0.768 | 0.0% | <0.001 | >0.009 | 0.479 |
| AST, IU/l | 460 | IFX | 1.271 (-1.729,
4.271)c | 0.000 | 87.8% | 0.406 | >0.009 | 0.780 |
| ALT, IU/l | 460 | IFX | -1.416 (-5.339,
2.507)c | 0.005 | 81.2% | 0.479 | 0.296 | 0.370 |
| CRP, mg/dl | 489 | IFX | -5.029 (-5.341,
-4.718)c | 0.596 | 0.0% | <0.001 | >0.009 | 0.703 |
Quality and bias assessment
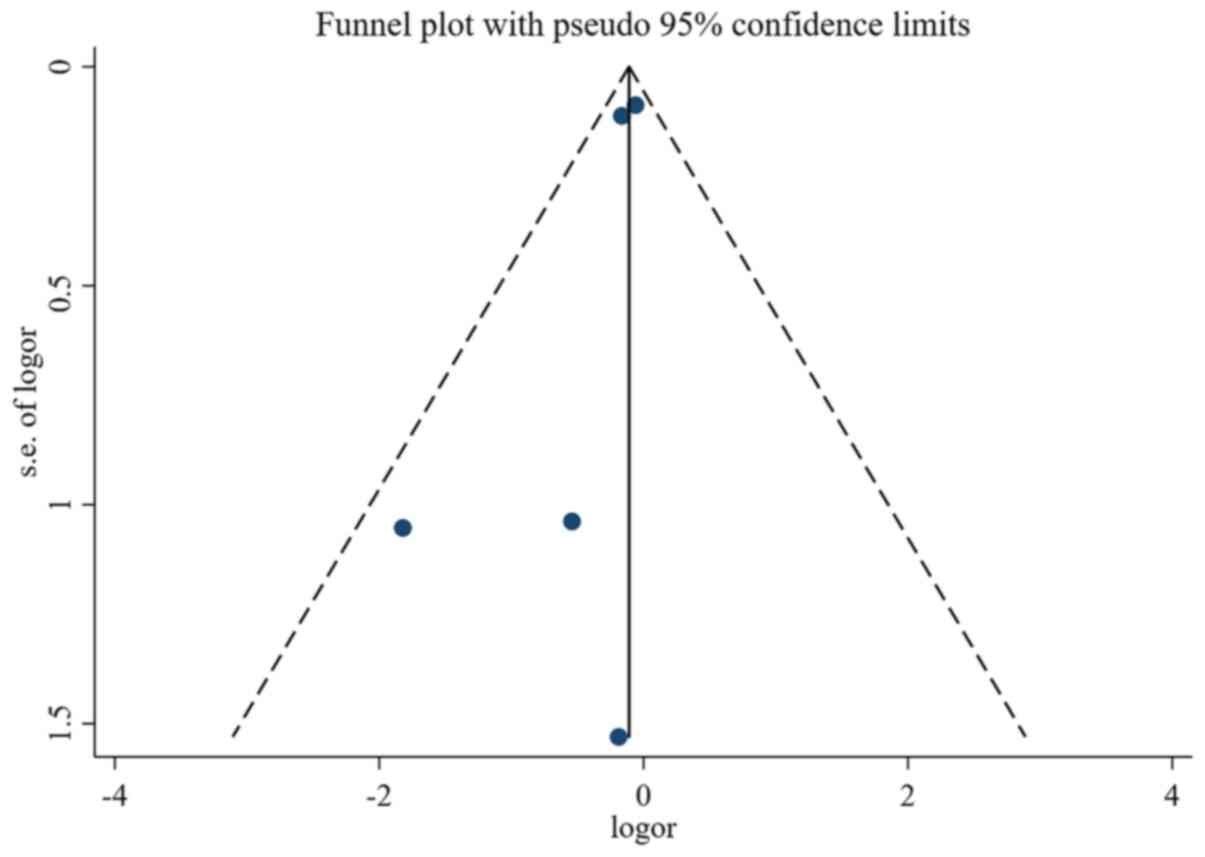
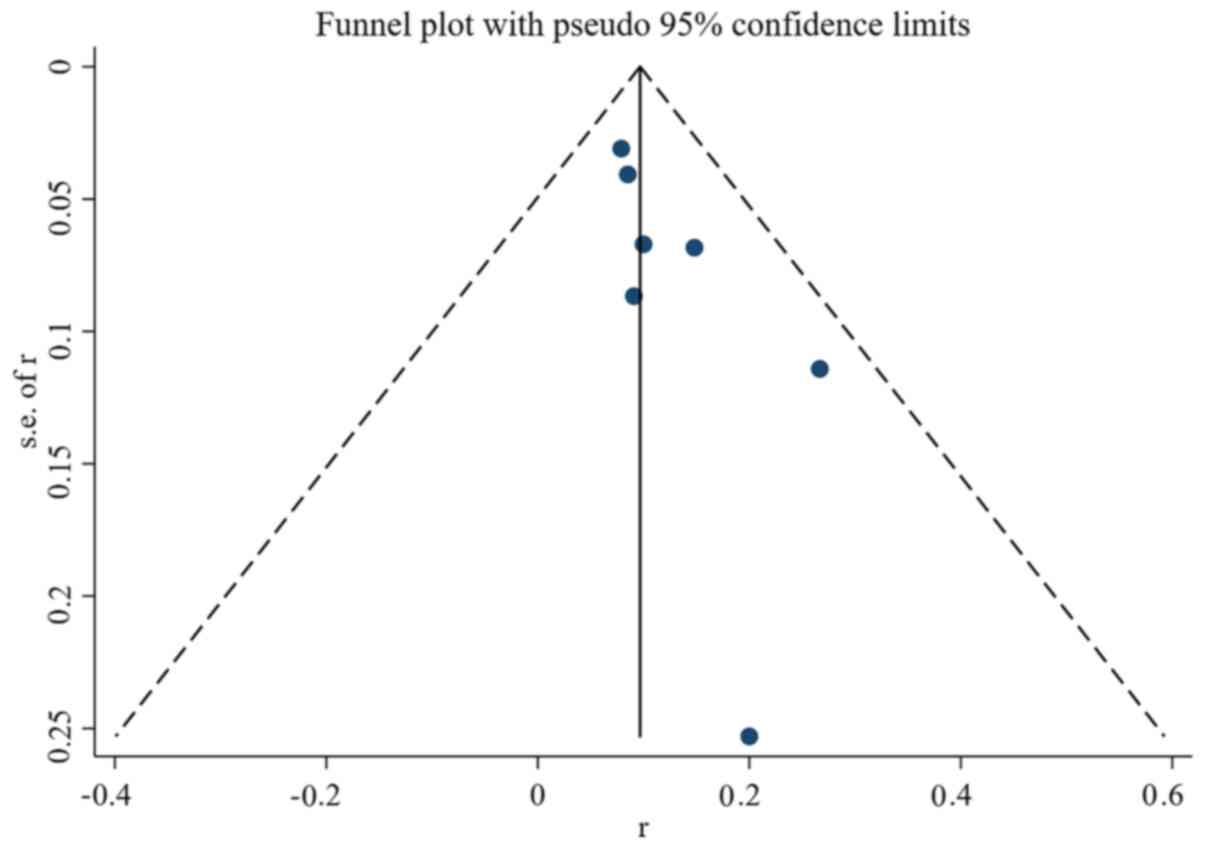
Assessment of study quality and risk of bias was
performed using multiple complementary methods including funnel
plots, Begg's and Mazumdar's rank test, and Egger's test. Clear
symmetry was observed in the log RR funnel plots for adverse events
(Fig. 8) and non-response rate
(Fig. 9) among the studies,
suggesting a low publication bias risk. The results of Begg's and
Mazumdar's rank test and Egger's test indicated no significant risk
of bias among the study results (Tables II and III).
Discussion
The main pathological change of KD is systemic
vasculitis, and its pathogenesis is associated with autoimmune
abnormalities. The main worst complication of KD is CAL, and a
number of patients also develop giant tubular aneurysms, which may
lead to thrombosis and rupture bleeding (30,31).
IVIG is isolated and purified from healthy human plasma and has
been initially used as a replacement therapy for treating children
with primary and secondary immunodeficiency or autoimmune and
inflammatory diseases (32-34).
IVIG therapy may benefit children with KD through the following
potential mechanisms (35): i)
Inhibiting the activation of innate immune cells such as dendritic
cells, macrophages, monocytes, neutrophils, and secretion of
inflammatory mediators; ii) regulating B cell responses and
reducing the production of autoantibodies; iii) inhibiting
pathogenic T helper (Th)1 and Th17 cells and endothelial cell
activation; and iv) enhancing regulatory T lymphocyte levels.
Although none of these mechanisms could fully explain the
pathogenesis of KD, some have been demonstrated to be feasible in
children with KD (36). The
efficacy of IVIG in preventing CAL can be confirmed (37). As a blood product, IVIG has a number
of limitations such as limited sources, a high price and the
potential risk of infection. In addition, the incidence rate of
non-responders to IVIG can reach as high as 10% (38). Thus, scientists are actively looking
for other treatments to achieve better and faster clinical symptom
relief and reduce the incidence of CAL.
In the acute phase of KD, the plasma TNF-α level is
significantly increased, and the plasma TNF-α level in children
with CAD is higher compared with those without CAD. TNF-α can
directly induce vascular endothelial cells to express intercellular
adhesion molecule-1 and monocyte chemokine-1, thus promoting the
infiltration of neutrophils, monocytes and other inflammatory cells
to aggravate the inflammatory injury of blood vessels. IFX is a
human and mouse chimeric monoclonal antibody that specifically
blocks TNF-α with high affinity and inhibits the binding of TNF-α
to its receptor. Currently, IFX is mainly used for the treatment of
rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis
and Crohn's disease. Specific blocking of TNF-α has been
demonstrated to prevent coronary inflammation and the formation of
CAA in mouse KD models induced by Lactobacillus casei cell
wall extracts. In a previous study of a KD model in mice, it was
indicated that a TNF-α anticoagulant can reduce the adhesion of
neutrophils to vascular endothelial cells and the inflammation of
arterial vascular endothelial cells (24,39-42).
In the present meta-analysis, no significant differences in CAL,
CAA, non-response rate, length of hospital stay, conjunctival
injection or polymorphous exanthema between the two groups were
observed; however, IFX or IFX plus IVIG significantly reduced the
incidence of adverse events, the number of patients with fever,
changes in lip and oral cavity and cervical lymphadenopathy. The
WBC, neutrophil and CRP levels were also reduced in the IFX or IFX
plus IVIG groups compared with those in the IVIG or VGIH groups. In
addition, the platelet and ALT levels, as well as the Z-score were
increased in the IFX or IFX plus IVIG groups compared with those in
the IVIG or VGIH groups. Thus, IFX or IFX plus IVIG exhibited
improved clinical efficacy in the treatment of KD compared with
that of IVIG or VGIH.
In the single-arm studies, IFX would decrease the
incidence of CAA, the non-response rate and the incidence of
adverse events. IFX demonstrated a high efficacy in reducing the
incidence of fever, conjunctival injection, changes in lip and oral
cavity, cervical lymphadenopathy, polymorphous exanthema, as well
as WBC, neutrophil, ALT and CRP levels. In addition, the platelet
count was significantly increased after the IFX therapy. However,
as only a limited number of studies were included in the current
meta-analysis, the clinical efficacy of IFX in the treatment of KD
should be further verified.
In a similar meta-analysis, Yamaji et al
(42) evaluated the efficacy and
safety of using TNF-α blockers (i.e. IFX and etanercept) to treat
children with KD. Low-certainty evidence from five trials indicated
that TNF-α blockers exhibited positive effects on treatment
resistance and the infusion reaction after treatment initiation for
KD compared with non-treatment or additional treatment with IVIG.
However, further large-scaled high-quality trials including the
timing and type of TNF-α blockers are needed to determine the
effects of TNF-α blockers for KD. In the study of Yamaji et
al (42), TNF-α blockers
reduced the incidence of treatment resistance and infusion
reaction, but no clear difference was observed between groups in
the incidence of CAAs, rash or contact dermatitis. The conclusions
about CAA is consistent with the present analysis.
The systematic nature of the present meta-analysis
allowed the results to be more convincing compared with those of
individual studies, since these results relied upon a large pooled
sample size, which was one of the strengths of the current
meta-analysis. In addition, strict inclusion and exclusion criteria
were used to select the qualified studies, and all the data were
analyzed by standard statistical analyses to ensure accuracy, thus
allowing the conclusion to be clinically significant.
However, there were certain limitations to the
present analysis. For example, the therapy course and the
combination of drugs, as well as the severity of KD and combined
disease lacked conformity, and each study had variations in the
exclusion/inclusion criteria. Furthermore, a limited number of
studies was included, and as individual patient data were not
available, only pooled data were analyzed, thus precluding more
in-depth analyses.
In conclusion, IFX or IFX plus IVIG exhibited
improved clinical efficacy in the treatment of KD compared with
that of IVIG or VGIH.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL and FW conceived and designed the study. HL, ZL
and FW acquired, analyzed and interpreted the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kawasaki T: Acute febrile mucocutaneous
syndrome with lymphoid involvement with specific desquamation of
the fingers and toes in children. Arerugi. 16:178–222. 1967.(In
Japanese). PubMed/NCBI
|
|
2
|
Miura M, Tamame T, Naganuma T, Chinen S,
Matsuoka M and Ohki H: Steroid pulse therapy for Kawasaki disease
unresponsive to additional immunoglobulin therapy. Paediatr Child
Health. 16:479–484. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Soriano-Ramos M, Martínez-Del Val E,
Negreira Cepeda S, González-Tomé MI, Cedena Romero P,
Fernández-Cooke E, Albert de la Torre L and Blázquez-Gamero D: Risk
of coronary artery involvement in Kawasaki disease. Arch Argent
Pediatr. 114:107–113. 2016.(In English, Spanish). PubMed/NCBI View Article : Google Scholar
|
|
4
|
Koizumi K, Hoshiai M, Katsumata N, Toda T,
Kise H, Hasebe Y, Kono Y, Sunaga Y, Yoshizawa M, Watanabe A, et al:
Infliximab regulates monocytes and regulatory T cells in Kawasaki
disease. Pediatr Int. 60:796–802. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lin Y, Xiao-hui L and Shi L:
Interpretation of the 2017 edition of diagnosis, treatment, and
long-term management of Kawasaki disease: A scientific statement
for health professionals from the American heart association. Chin
J Pract Pediatr, 2017 (In Chinese).
|
|
6
|
Makino N, Nakamura Y, Yashiro M, Sano T,
Ae R, Kosami K, Kojo T, Aoyama Y, Kotani K and Yanagawa H:
Epidemiological observations of Kawasaki disease in Japan,
2013-2014. Pediatr Int. 60:581–587. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Newburger JW, Takahashi M, Gerber MA,
Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P,
Baltimore RS, et al: Diagnosis, treatment, and long-term management
of Kawasaki disease: A statement for health professionals from the
committee on rheumatic fever, endocarditis, and Kawasaki disease,
council on cardiovascular disease in the young, American heart
association. Pediatrics. 114:1708–1733. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee Y, Schulte DJ, Shimada K, Chen S,
Crother TR, Chiba N, Fishbein MC, Lehman TJ and Arditi M:
Interleukin-1β is crucial for the induction of coronary artery
inflammation in a mouse model of Kawasaki disease. Circulation.
125:1542–1550. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Newburger JW, Takahashi M, Beiser AS,
Burns JC, Bastian J, Chung KJ, Colan SD, Duffy CE, Fulton DR, Glode
MP, et al: A single intravenous infusion of gamma globulin as
compared with four infusions in the treatment of acute Kawasaki
syndrome. N Engl J Med. 324:1633–1639. 1991.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Furukawa T, Kishiro M, Akimoto K, Nagata
S, Shimizu T and Yamashiro Y: Effects of steroid pulse therapy on
immunoglobulin-resistant Kawasaki disease. Arch Dis Child.
93:142–146. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Matsubara T, Ichiyama T and Furukawa S:
Immunological profile of peripheral blood lymphocytes and
monocytes/macrophages in Kawasaki disease. Clin Exp Immunol.
141:381–387. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Research Committee of the Japanese Society
of Pediatric Cardiology; Cardiac Surgery Committee for Development
of Guidelines for Medical Treatment of Acute Kawasaki Disease:
Guidelines for medical treatment of acute Kawasaki disease: Report
of the research committee of the Japanese society of pediatric
cardiology and cardiac surgery (2012 revised version). Pediatr Int
56: 135-158, 2014.
|
|
13
|
Ogihara Y, Ogata S, Nomoto K, Ebato T,
Sato K, Kokubo K, Kobayashi H and Ishii M: Transcriptional
regulation by infliximab therapy in Kawasaki disease patients with
immunoglobulin resistance. Pediatr Res. 76:287–293. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Saji BT and Kobayashi T: Overview of the
new Japanese guideline2012 for the medical treatment of acute stage
of Kawasaki disease. In: Kawasaki Disease. Saji B, Newburger J,
Burns J, and Takahashi M (eds). Springer, Tokyo, pp103-167,
2017.
|
|
15
|
Xiantao Zeng and Zhu Sun: Tang. H. No. 10
of the Meta Analysis Series: Formulation of Eligibility Criteria.
Chin J Evid Based Cardiovasc Med. 5:e927–e999. 2013.
|
|
16
|
Hirono K, Kemmotsu Y, Wittkowski H, Foell
D, Saito K, Ibuki K, Watanabe K, Watanabe S, Uese K, Kanegane H, et
al: Infliximab reduces the cytokine-mediated inflammation but does
not suppress cellular infiltration of the vessel wall in refractory
Kawasaki disease. Pediatr Res. 65:696–701. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jone PN, Anderson MS, Mulvahill MJ, Heizer
H, Glodé MP and Dominguez SR: Infliximab plus intravenous
immunoglobulin (IVIG) versus IVIG alone as initial therapy in
children with kawasaki disease presenting with coronary artery
lesions: Is dual therapy more effective? Pediatr Infect Dis J.
37:976–980. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mori M, Hara T, Kikuchi M, Shimizu H,
Miyamoto T, Iwashima S, Oonishi T, Hashimoto K, Kobayashi N, Waki
K, et al: Infliximab versus intravenous immunoglobulin for
refractory Kawasaki disease: A phase 3, randomized, open-label,
active-controlled, parallel-group, multicenter trial. Sci Rep.
8(1994)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang P, Tang C, Pan X, Chi H, Sun W and
Qu S: Comparison of the effects of infliximab and IVIG in the
treatment of ineffective IVIG. China Med Front Magazine (electronic
version). 10:66–69. 2018.
|
|
20
|
Son MB, Gauvreau K, Burns JC, Corinaldesi
E, Tremoulet AH, Watson VE, Baker A, Fulton DR, Sundel RP and
Newburger JW: Infliximab for intravenous immunoglobulin resistance
in kawasaki disease: A retrospective study. J Pediatr.
158:644–649.e1. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sonoda K, Mori M, Hokosaki T and Yokota S:
Infliximab plus plasma exchange rescue therapy in Kawasaki disease.
J Pediatr. 164:1128–1132.e1. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tremoulet AH, Jain S, Jaggi P,
Jimenez-Fernandez S, Pancheri JM, Sun X, Kanegaye JT, Kovalchin JP,
Printz BF, Ramilo O and Burns JC: Infliximab for intensification of
primary therapy for Kawasaki disease: A phase 3 randomised,
double-blind, placebo-controlled trial. Lancet. 383:1731–1738.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Youn Y, Kim J, Hong YM and Sohn S:
Infliximab as the first retreatment in patients with Kawasaki
disease resistant to initial intravenous immunoglobulin. Pediatr
Infect Dis J. 35:457–459. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hur G, Song MS, Sohn S, Lee HD, Kim GB,
Cho HJ, Yoon KL, Joo CU, Hyun MC and Kim CH: Infliximab treatment
for intravenous immunoglobulin-resistant Kawasaki disease: A
multicenter study in Korea. Korean Circ J. 49:183–191.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dionne A, Burns JC, Dahdah N, Tremoulet
AH, Gauvreau K, de Ferranti SD, Baker AL, Son MB, Gould P, Fournier
A, et al: Treatment intensification in patients with Kawasaki
disease and coronary aneurysm at diagnosis. Pediatrics. 143(pii:
e20183341)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mori M, Imagawa T, Hara R, Kikuchi M, Hara
T, Nozawa T, Miyamae T and Yokota S: Efficacy and limitation of
infliximab treatment for children with Kawasaki disease intractable
to intravenous immunoglobulin therapy: Report of an open-label case
series. J Rheumatol. 39:864–867. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Han CL and Zhao SL: Intravenous
immunoglobulin gamma (IVIG) versus IVIG plus infliximab in young
children with Kawasaki disease. Med Sci Monit. 24:7264–7270.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Masuda H, Kobayashi T, Hachiya A,
Nakashima Y, Shimizu H, Nozawa T, Ogihara Y, Ito S, Takatsuki S,
Katsumata N, et al: Infliximab for the treatment of refractory
Kawasaki disease: A nationwide survey in Japan. J Pediatr.
195:115–120.e3. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Song MS, Lee SB, Sohn S, Oh JH, Yoon KL,
Han JW and Kim CH: Infliximab treatment for refractory kawasaki
disease in korean children. Korean Circ J. 40:334–338.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Burns JC, Song Y, Bujold M, Shimizu C,
Kanegaye JT, Tremoulet AH and Franco A: Immune-monitoring in
Kawasaki disease patients treated with infliximab and intravenous
immunoglobulin. Clin Exp Immunol. 174:337–344. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Galeotti C, Kaveri SV, Cimaz R, Koné-Paut
I and Bayry J: Predisposing factors, pathogenesis and therapeutic
intervention of Kawasaki disease. Drug Discov Today. 21:1850–1857.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Moffett BS, Syblik D, Denfield S, Altman C
and Tejtel-Sexson K: Epidemiology of immunoglobulin resistant
Kawasaki disease: Results from a large, national database. Pediatr
Cardiol. 36:374–378. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Furusho K, Kamiya T, Nakano H, Kiyosawa N,
Shinomiya K, Hayashidera T, Tamura T, Hirose O, Manabe Y, Yokoyama
T, et al: High-dose intravenous gammaglobulin for Kawasaki disease.
Lancet. 2:1055–1058. 1984.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rasouli M, Heidari B and Kalani M:
Downregulation of Th17 cells and the related cytokines with
treatment in Kawasaki disease. Immunol Lett. 162:269–275.
2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Guo MH, Tseng WN, Ko CH, Pan HM, Hsieh KS
and Kuo HC: Th17- and Treg-related cytokine and mRNA expression are
associated with acute and resolving Kawasaki disease. Allergy.
70:310–318. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bai X, Zhang R and Zhong J: Prediction of
unresponsiveness to intravenous immunoglobulin retreatmentin
patients with Kawasaki disease. Chin J Evid Based Pediatr.
11:16–20. 2016.(In Cbinese).
|
|
37
|
Burns JC, Capparelli EV, Brown JA,
Newburger JW and Glode MP: Intravenous gamma-globulin treatment and
retreatment in Kawasaki disease. US/Canadian Kawasaki syndrome
study group. Pediatr Infect Dis J. 17:1144–1148. 1998.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Newburger JW, Takahashi M, Gerber MA,
Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P,
Baltimore RS, et al: Diagnosis, treatment, and long-term management
of Kawasaki disease: A statement for health professionals from the
committee on rheumatic fever, endocarditis and Kawasaki disease,
council on cardiovascular disease in the young, American heart
association. Circulation. 110:2747–2771. 2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Matsubara T, Furukawa S and Yabuta K:
Serum levels of tumor necrosis factor, interleukin 2 receptor, and
interferon-gamma in Kawasaki disease involved coronary-artery
lesions. Clin Immunol Immunopathol. 56:29–36. 1990.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ahn SY, Jang GC, Shin JS, Shin KM and Kim
DS: Tumor necrosis factor-alpha levels and promoter polymorphism in
patients with Kawasaki disease in Korea. Yonsei Med J.
44:1021–1026. 2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hui-Yuen JS, Duong TT and Yeung RS:
TNF-alpha is necessary for induction of coronary artery
inflammation and aneurysm formation in an animal model of Kawasaki
disease. J Immunol. 176:6294–6301. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yamaji N, da Silva Lopes K, Shoda T,
Ishitsuka K, Kobayashi T, Ota E and Mori R: TNF-α blockers for the
treatment of Kawasaki disease in children. Cochrane Database Syst
Rev. 8(CD012448)2019.PubMed/NCBI View Article : Google Scholar
|