Introduction
Diabetes mellitus is a lifelong metabolic disease
with a variety of causes, and is characterized by chronic
hyperglycemia (1). Diabetic
angiopathy is a common complication in patients with diabetes
mellitus and is one of the main causes of disability and mortality
(1). Vascular endothelial cells are
single-layer cells of the vascular intima that serve important
roles in the maintenance of vascular structure and function
(2). It has been demonstrated that
vascular endothelial dysfunction may represent the
pathophysiological basis for diabetic angiopathy (2). Hyperglycemia can damage vascular
endothelial cell function through a variety of mechanisms,
including inflammation, oxidative stress and apoptosis (2). High rates of apoptosis in endothelial
cells can lead to a series of complex chain reactions, including
the release of interleukin-1, cytochrome C and other active
substances, which promote the inflammatory response and aggravate
vascular damage (2-4).
MicroRNAs (miRNAs/miRs) are a class of non-coding
RNAs between 18 and 22 base pairs in length. These molecules bind
to the 3'-untranslated region (UTR) of target genes to regulate
their expression, which subsequently affects various biological
processes within the cell (5). It
has been demonstrated that miRNA molecules are associated with
endothelial cell injury under high-glucose conditions. For
instance, miR-503 can inhibit endothelial cell proliferation and
promote apoptosis under high glucose conditions via the insulin
like growth factor 1 receptor (6).
Additionally, miR-145 has been revealed to inhibit the oxidative
stress and inflammation induced by high glucose in retinal
endothelial cells (7). miR-329-3p
is a member of the 14q32 miRNA gene cluster (8). A variety of 14q32 miRNA molecules have
been demonstrated to serve a role in cardiovascular disease,
regulate the interaction between focal adhesion plaques and the
extracellular matrix, and serve roles in vascular remodeling
(9). Welten et al (10) demonstrated that downregulation of
miR-329-3p promotes vascular endothelial cell proliferation and
restores blood flow in the ischemic lower limbs of mice. In
addition, Wang et al (11)
revealed that miR-329-3p inhibited angiogenesis by targeting
cluster of differentiation 146. However, to the best of the
authors' knowledge, the expression of miR-329-3p in diabetes
mellitus, and whether miR-329-3p may be involved in the regulation
of high glucose-induced vascular endothelial cell injury, has not
yet been investigated.
Toll like receptor (TLR) 4 is a transmembrane
protein that belongs to the pattern recognition receptor family.
TLR4 activation induces the intracellular signaling pathway
responsible for activating the innate immune system via nuclear
factor (NF)-κB (12). A number of
studies have demonstrated that the TLR4/NF-κB signaling pathway
serves a critical role in high glucose-induced inflammation and
apoptosis in retinal ganglion cells, retinal microvascular
endothelial cells and human retinal endothelial cells (13-15).
Welten et al (10) revealed
that downregulation of miR-329-3p leads to reduced TLR4 mRNA in a
lower limb ischemic mouse model (10). However, the association between
miR-329-3p and the TLR4/NF-κB signaling pathway in endothelial
cells under high-glucose condition is unclear. Therefore,
miR-329-3p expression in patients with type 2 diabetes mellitus
(T2DM) and the effect of miR-329-3p on vascular endothelial cell
function under high glucose conditions was investigated in the
present study.
Materials and methods
Patients
A total of 31 patients (sex, 16 males and 15
females; mean age, 58±7.1 years) who were diagnosed with T2DM at
The First People's Hospital of Jinan (Shandong, China) between
January 2016 and January 2018 were included in the present study.
In addition, a total of 33 healthy subjects with similar sex ratio
and mean age (16 males and 17 females; mean age, 56±6.4 years old)
who underwent a physical examination at The First People's Hospital
of Jinan in the same time period were included as the control
group. The inclusion criteria for patients with T2DM were: A
fasting blood glucose level of ≥7.0 mmol/l or a 2 h postprandial
blood glucose level of ≥11.1 mmol/l. The inclusion criteria for the
control group were as follows: i) Contemporaneous healthy subjects
undertaking physical examinations; ii) without diabetes; iii)
without dyslipidemia and; iv) no history of hypertension or
vascular disease. The exclusion criteria were: i) Subjects
exhibited signs or symptoms of infection; ii) had received drugs
that affected glucose metabolism; iii) presented with liver or
kidney failure; or iv) had a history of malignant tumors.
Peripheral blood (3 ml) was collected and transferred to
anticoagulant tubes prior to plasma separation via centrifugation
at 3,000 x g and 4˚C for 10 min. Separated plasma samples were
stored in aliquots of 500 µl at -80˚C prior to subsequent use. All
procedures performed in the current study were approved by the
Ethics Committee of The First People's Hospital of Jinan. Written
informed consent was obtained from all patients or their
families.
Cells and transfection
Human umbilical vein endothelial cells (HUVECs) were
cultured in endothelial cell medium supplemented with 5% FBS and 1%
endothelial cell growth factor (all purchased from ScienCell
Research Laboratories, Inc.) at 37˚C and 5% CO2. Cells
in the high-glucose group were treated with 25 mmol/l glucose for
24, 48 or 72 h to simulate a high-glucose environment in the body.
Control group cells were treated with 25 mmol/l D-mannose for 24,
48 or 72 h (Sigma-Aldrich; Merck KGaA) (6,14).
HUVECs in the miR-329-3p mimic group, miR-329-3p
inhibitor and miRNA negative control (NC) groups were transfected
with 50 nmol/l miR-329-3p mimic, 100 nmol/l miR-329-3p inhibitor
(Guangzhou RiboBio Co., Ltd.) and 50 nmol/l miR-NC (Merck KGaA),
respectively, using the riboFECT™ CP kit (Guangzhou RiboBio Co.,
Ltd.) according to the manufacturer's protocol. Cells were
collected for further study after 48 h transfection.
The TLR4 overexpression Ad5 adenovirus and
adenovirus with empty vector (negative control) were purchased from
Hanbio Biotechnology Co., Ltd. and transfection was performed
according to the manufacturer's protocol. For co-transfection with
miR-329-3p mimics/miR-NC and TLR4 overexpression vectors, HUVECs
were first transfected with TLR4 overexpression adenovirus or
adenovirus with empty vector (multiplicity of transfection, 20) for
24 h, following which cells were transfected with miR-329-3p mimics
(50 nmol) or miR-NC (50 nmol). Cells were collected for further
study 48 h following transfection.
Cell Counting Kit (CCK)-8 assay
HUVECs were seeded at a density of 1,000 cells/well
in 96-well plates. At 0, 24, 48 and 72 h, 10 µl CCK-8 reagent (5
g/l; Beyotime Institute of Biotechnology) was mixed with 100 µl
complete medium and then added to the cells following which the
cells were incubated at 37˚C for 1 h. The absorbance of each well
was subsequently measured at 490 nm using a plate reader (Thermo
Fisher Scientific, Inc.) and used to chart cell viability. Each
group was tested in three replicate wells and the values were
averaged.
Flow cytometry
Following transfection and high-glucose treatment,
HUVECs (1x106) in each group were washed with pre-cooled
PBS twice and subjected to flow cytometry analysis using the
Annexin V/FITC Apoptosis Detection kit (BD Biosciences) according
to the manufacturer's protocol. Annexin V-positive cells were
considered to be early apoptotic cells, while propidium
iodide-positive cells were considered to be necrotic cells and
double-positive cells were considered to be late apoptotic cells.
The data were analyzed using the NovoExpress® software
(version 1.2.1; ACEA Biosciences; Agilent Technologies, Inc.).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from 200 µl plasma using a
miRNeasy Serum/Plasma kit (Qiagen GmbH). A total of 3.5 µl spike-in
Caenorhabditis elegans miRNA-39 (cel-miR-39;
1.6x108 copies) was used as an external reference to
evaluate miRNA extraction from the plasma. RNA was extracted from
HUVECs using miRNeasy Mini Kit (Qiagen GmbH) according to the
manufacturer's instructions. Extracted total RNA (6 µl) was
subjected to RT using the miScript II RT kit (Qiagen GmbH),
according to the manufacturer's protocol. The RT reaction
conditions were as follows: 37˚C for 60 min, followed by 95˚C for 5
min. A total of 10 µl cDNA solution was subsequently mixed with 100
µl ddH2O prior to RT-qPCR being performed using a ABI
StepOne Plus instrument (Thermo Fisher Scientific, Inc.). The
reaction system (25 µl) comprised 12.5 µl SYBR Green Master Mix
(Qiagen GmbH), 2.5 µl 10X miR-329-3p/cel-miR-39 primers (Qiagen
GmbH), 2.5 µl 10X universal primer, 2 µl diluted cDNA and 5.5 µl
RNase-free ddH2O. Each sample was tested in triplicate.
The thermal cycling parameters for qPCR were as follows: 95˚C for
15 min, followed by 40 cycles of 95˚C for 15 sec, 55˚C for 30 sec
and 70˚C for 30 sec.
Total RNA was extracted from cells using TRIzol
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Extracted RNA (500 ng) was subjected to RT
and qPCR using a ReverTra Ace® qPCR RT kit (Toyobo Life
Science), according to the manufacturer's protocol. The primer
sequences used were as follows: Tumor necrosis factor receptor
associated factor 6 (TRAF6) forward, 5'-TCTACACTGGCAAACCCG-3', and
reverse, 3'-AGGGAGGTGGCTGTCATA-5'; TLR4 forward,
5'-GACCTGTCCCTGAACCCTA-3', and reverse, 3'-TCTCCCAGAACCAAACGA-5';
GAPDH forward, 5'-ATGCTGGCGCTGAGTACGTC-3', and reverse,
3'-GGTCATGAGTCCTTCCACGATA-5'. The 2-ΔΔCq method
(16) was used to calculate
miR-329-3p expression relative to cel-miR-39, as well as the
expression of TRAF6 or TLR4 relative to GAPDH. Each sample was
tested in triplicate.
Total protein and nuclear protein
isolation
Total protein was extracted using RIPA Lysis Buffer
(Beyotime Institute of Biotechnology). To isolate the nuclear
protein, 3x106 cells were collected and treated with a
Minute Plasma Membrane Protein Isolation kit (Invent
Biotechnologies, Inc.) according to the manufacturer's protocol.
Cells were lysed using buffer A on ice for 5 min prior to transfer
into filtration columns. Centrifugation was performed at 16,000 x g
for 30 sec and again at 700 x g for 1 min, both at 4˚C. The
sediment contained complete nuclei and was subsequently lysed using
RIPA buffer (Beyotime Institute of Biotechnology) on ice for 10
min. Following the addition of 1/4 volumes of 5X SDS-PAGE loading
buffer, samples were boiled at 100˚C for 10 min. The protein
concentration of total and nuclear protein was using Bicinchoninic
Acid Protein Assay Kit (Beyotime Institute of Biotechnology).
Western blot analysis
Protein samples (20 µg) were subjected to 10%
SDS-PAGE at 100 V. Proteins were subsequently transferred to PVDF
membranes on ice for 1 h (250 mA) and blocked with 5% skimmed milk
at room temperature for 1 h. Membranes were subsequently incubated
with rabbit anti-human TLR4 (dilution, 1:1,000; cat. no. ab13556;
Abcam), rabbit anti-human TRAF6 (dilution, 1:4,000; cat. no.
ab181622; Abcam), rabbit anti-human NF-κB (dilution, 1:1,000; cat.
no. AF1234; Beyotime Institute of Biotechnology), and rabbit
anti-human cleaved caspase-3 (dilution, 1:1,000; cat. no. 9664;
Cell Signaling Technology, Inc.) polyclonal primary antibodies, and
mouse anti-human GAPDH (dilution, 1:2,000; cat. no. AF0006;
Beyotime Institute of Biotechnology), mouse anti-human β-actin
(dilution, 1:2,000; cat. no. AF0003; Beyotime Institute of
Biotechnology) and mouse anti-human H3 (dilution, 1:1,000; cat. no.
AF0009; Beyotime Institute of Biotechnology) monoclonal primary
antibodies overnight at 4˚C. After washing with PBS containing
Tween-20 in triplicate (15 min each time), membranes were incubated
with goat anti-mouse horseradish peroxidase-conjugated IgG (H+L;
dilution, 1:3,000; cat. no. A0216; Beyotime Institute of
Biotechnology) or goat anti-rabbit horseradish
peroxidase-conjugated IgG (H+L; dilution, 1:3,000; cat. no. A0208;
Beyotime Institute of Biotechnology) for 1 h at room temperature.
The membrane was then developed with an enhanced chemiluminescence
detection kit (Sigma-Aldrich; Merck KGaA). Quantity One software
(version 4.4.0.36; Bio-Rad Laboratories, Inc.) was used to acquire
and analyze imaging signals. The relative target proteins were
expressed relative to their respective internal reference
proteins.
Laser confocal microscopy
HUVECs were seeded onto polylysine-coated cover
slips (diameter, 14 mm). When 40-60% confluence was achieved, the
cells were transfected using the aforementioned methods. At 24 h
following transfection, cells were treated with high glucose for 24
h. The cells were then fixed with 4% paraformaldehyde for 10 min
and treated with 0.2% Triton X-100 for 10 min, both at room
temperature. After blocking with 10% goat serum (Sigma-Aldrich;
Merck KGaA) for 30 min at room temperature, 50 µl rabbit anti-NF-κB
primary antibody was added (dilution, 1:100; cat. no. AF1234;
Beyotime Institute of Biotechnology) and the cells were incubated
at 4˚C overnight. Samples were then incubated with Cy3-labeled Goat
anti-mouse IgG secondary antibody (dilution, 1:500; cat. no. A0521;
Beyotime Institute of Biotechnology) at room temperature for 1 h
prior to observation using laser confocal microscopy.
Bioinformatics
Bioinformatics-based predictions are a powerful tool
for the assessment of miRNA function. In the current study,
TargetScan (version no. 7.2; http://www.targetscan.org) was used to predict target
genes that may be regulated by miR-329-3p. The targeted genes with
at least 7 binding sites with miR-329-3p and those previously
reported to play serve roles in HG-induced endothelial dysfunction
were given priority.
Dual luciferase reporter assay
According to the bioinformatics analysis, the
wild-type (WT) and mutant seed regions (designed to break the
binding) of miR-329-3p in the 3'-UTR of the TLR4 gene were
chemically synthesized in vitro. To achieve this, the two
ends were attached using SpeI and HindIII restriction
sites and cloned into pMIR-REPORT luciferase reporter plasmids
(Takara Biotechnology Co., Ltd.). Plasmids (0.8 µg) encoding the WT
or mutant 3'-UTR sequences were co-transfected with 100 nM
agomiR-329-3p (Guangzhou RiboBio Co., Ltd.) into 293T cells (Type
Culture Collection of the Chinese Academy of Sciences) using
Lipofectamine 3000 (Thermo Fisher Scientific, Inc.). 293T cells
were transfected with an agomiR-NC as a control. At 48 h following
transfection, the cells were lysed using a dual luciferase reporter
assay kit (Promega Corporation) according to the manufacturer's
protocol, and the luminescence intensity was measured using a
GloMax 20/20 luminometer (Promega Corporation). Using
Renilla luminescence activity as an internal reference, the
luminescence values of each group of cells were measured.
Statistical analysis
The results were analyzed using SPSS 20.0
statistical software (IBM Corp.). The data were expressed as the
mean ± standard deviation. Data were tested for normality.
Differences among multiple groups were analyzed by one-way ANOVA.
In cases of homogeneity of variance, a least significant difference
and Student-Newman-Keuls test were used. In cases of heterogeneity
of variance, a Tamhane's T2 or a Dunnett's T3 test was used.
Comparisons between two groups were performed using a Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
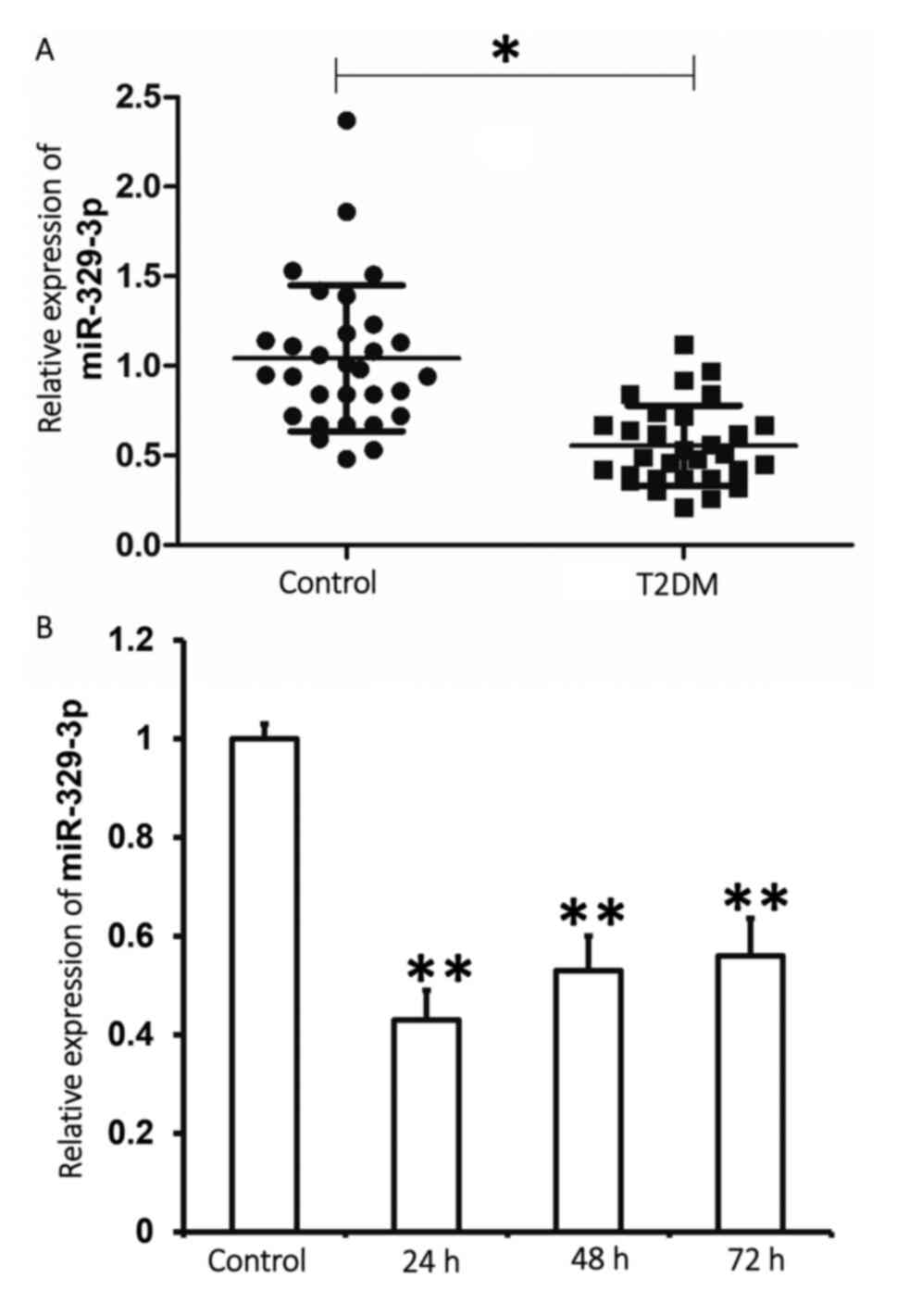
Plasma miR-329-3p expression is
decreased in patients with T2DM and following high-glucose
treatment of HUVECs
To determine miR-329-3p expression in human samples
and cell lines, RT-qPCR analysis was performed. The results
indicated that miR-329-3p expression in the plasma of patients with
T2DM was significantly lower when compared with the control group
(P<0.01; Fig. 1A). In addition,
miR-329-3p expression in HUVECs treated with 25 mmol/l glucose for
24, 48 or 72 h was significantly lower when compared with the
control group (P<0.01; Fig. 1B).
The results indicate that miR-329-3p expression is lower in the
plasma of patients with T2DM and in HUVECs treated with a high
glucose concentration.
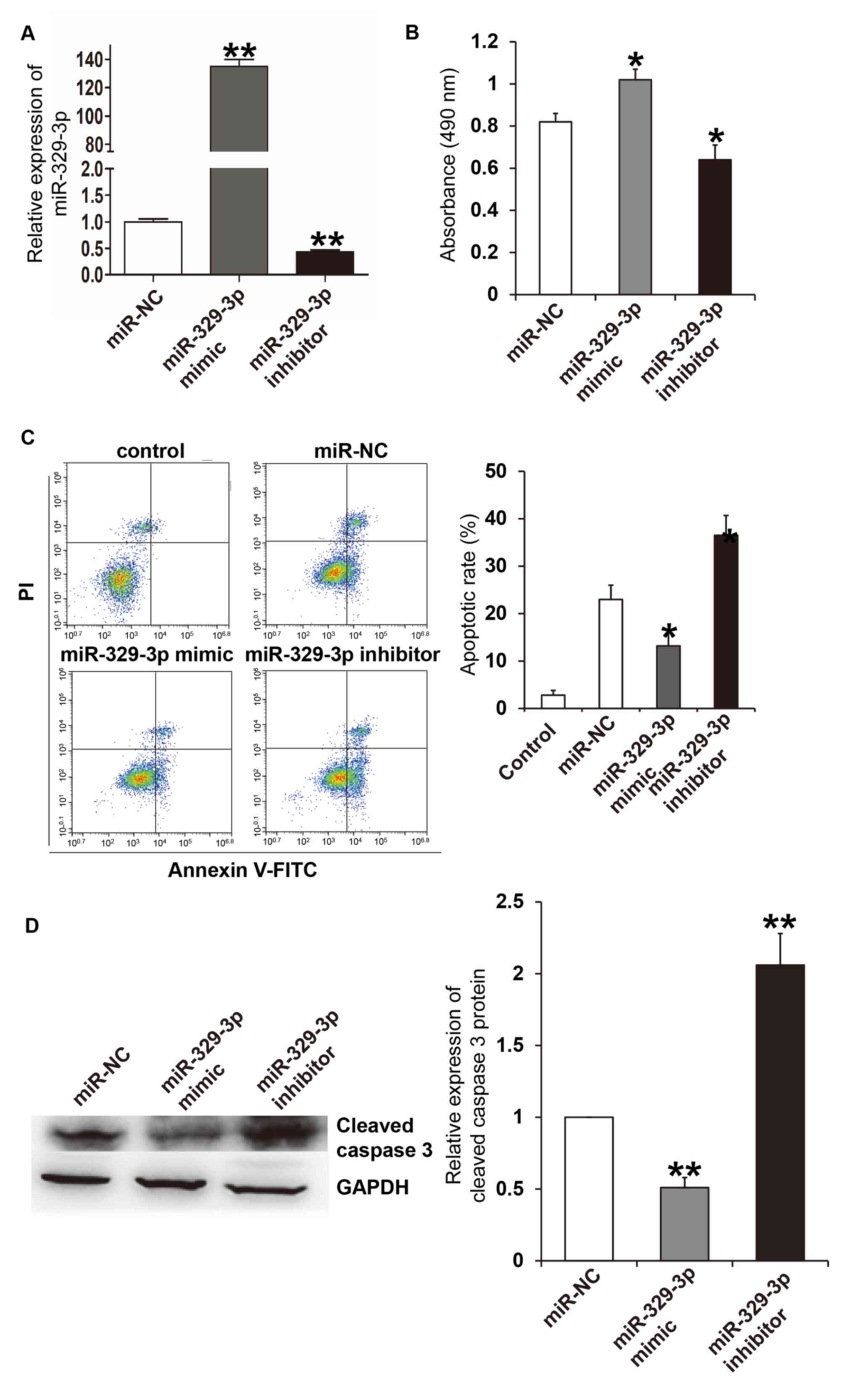
miR-329-3p reduces high
glucose-induced damage to HUVECs
RT-qPCR analysis was performed to assess the
efficiency of the miR-329-3p mimic and inhibitor. The results
demonstrated that transfection with the miR-329-3p mimic and
inhibitor significantly increased and decreased miR-329-3p
expression levels compared with the miR-NC group, respectively
(P<0.01; Fig. 2A). A CCK-8
assay, flow cytometry and western blot analysis were then performed
to assess the effect of miR-329-3p overexpression and inhibition on
cell viability and apoptosis in HUVEC cells. The results of the
CCK-8 assay indicated that, following treatment with high glucose
for 24 h, the absorbance of HUVECs transfected with the miR-329-3p
mimic was significantly higher when compared with the NC group
(P<0.05), and the absorbance of HUVECs transfected with the
miR-329-3p inhibitor was significantly lower compared with the NC
group (P<0.05; Fig. 2B). The
level of apoptosis was then detected using flow cytometry. The
results indicated that for HUVECs cultured under D-mannose
conditions (control group), the apoptotic rate was ~2.9±1.2%
(Fig. 2C). Following treatment with
high glucose concentrations for 24 h, the apoptotic rate of HUVECs
transfected with the miR-329-3p mimic was significantly lower
compared with the NC group (P<0.05), whereas HUVECs transfected
with miR-329-3p inhibitor exhibited a significantly higher
apoptotic rate compared with the NC group (P<0.05; Fig. 2C). Consistent with the flow
cytometry results, western blot analysis demonstrated that cleaved
caspase-3 (an apoptotic marker) expression in HUVECs treated with a
high glucose concentration for 24 h and transfected with miR-329-3p
mimic, was significantly lower when compared with the NC group
(P<0.05). However, HUVECs transfected with the miR-329-3p
inhibitor had significantly higher cleaved caspase-3 expression
compared with the NC group (P<0.05; Fig. 2D). These results suggest that
miR-329-3p reduces high glucose-induced HUVEC cell damage.
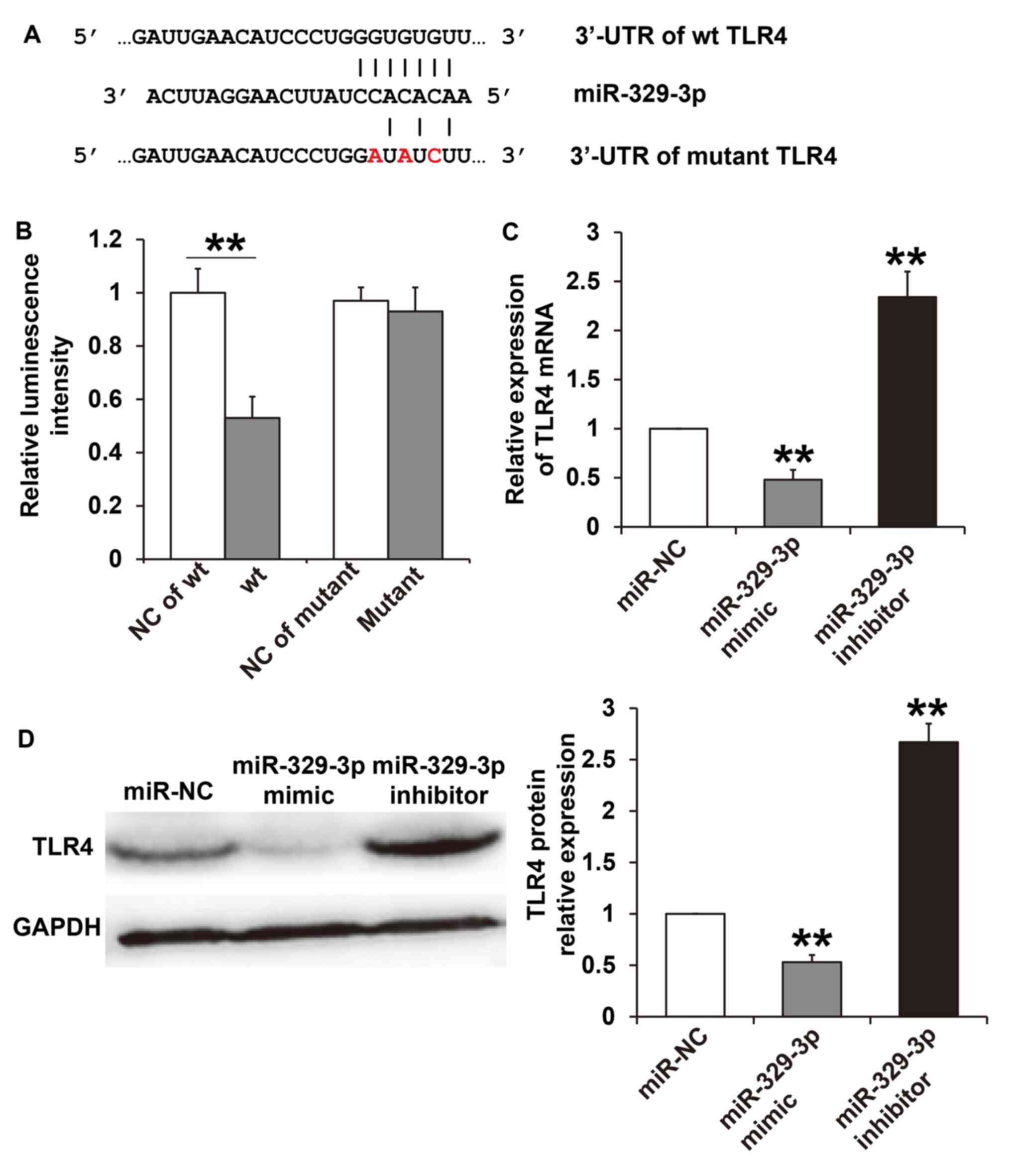
miR-329-3p binds directly to TLR4 and
regulates its expression at the transcriptional and
post-transcriptional level
To understand the mechanisms of miR-329-3p,
TargetScan was used to predict target genes of miR-329-3p. The
results demonstrated that miR-329-3p has seven complementary
binding sites with the 3'-UTR of TLR4 (Fig. 3A). To confirm direct binding of
miR-329-3p with the TLR4 3'-UTR, a dual luciferase reporter assay
was performed. The results demonstrated that miR-329-3p
significantly reduced the luminescence of the WT when compared with
the NC group (P<0.05), but had no effect on luminescence in the
mutant group (P>0.05; Fig. 3B).
RT-qPCR and western blot analyses were performed to assess the
effect of miR-329-3p on TLR4 mRNA and protein expression. The
results revealed that TLR4 mRNA and protein expression in HUVECs
transfected with the miR-329-3p mimic were significantly lower when
compared with the miR-NC group (P<0.05). By contrast, TLR4 mRNA
and protein expression in HUVECs transfected with the miR-329-3p
inhibitor were significantly higher compared with the miR-NC group
(P<0.05; Fig. 3C and D). These results indicate that miR-329-3p
may directly bind with TLR4 and regulate its expression at the
transcriptional and post-transcriptional level.
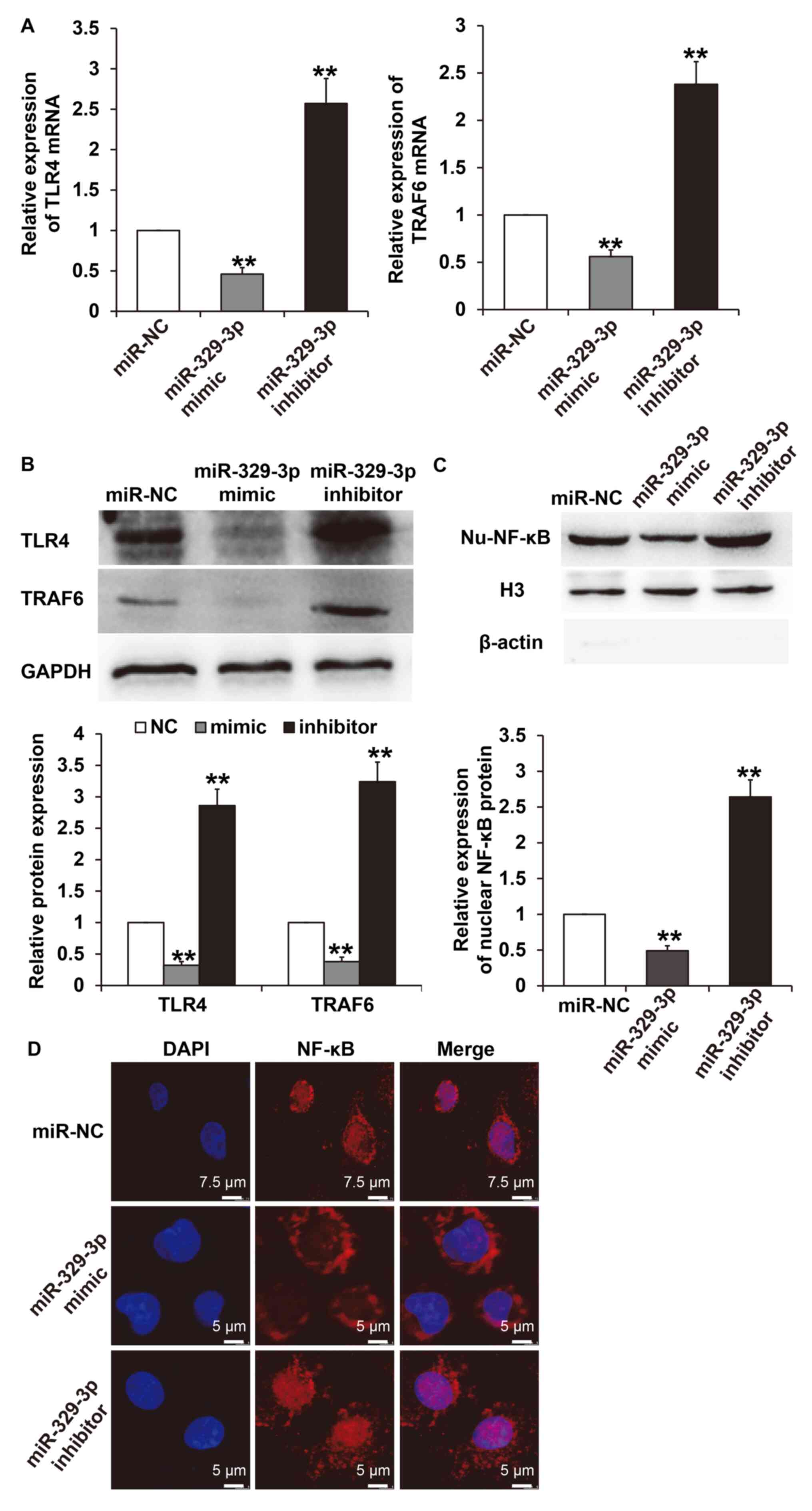
miR-329-3p regulates HUVEC cell injury
under high glucose conditions via the TLR4/TRAF6/NF-κB signaling
pathway
To further elucidate the molecular mechanisms by
which miR-329-3p regulates endothelial cell injury induced by high
glucose, the expression of TLR4 and its downstream signaling
molecule, TRAF6, were examined. The results demonstrated that
transfection with the miR-329-3p mimic significantly decreased the
expression of TLR4 and TRAF6 mRNA in HUVECs when compared with
cells transfected with the miR-NC (P<0.05). However,
transfection with the miR-329-3p inhibitor increased TLR4 and TRAF6
mRNA expression in HUVECs compared with cells transfected with
miR-NC (P<0.05; Fig. 4A).
Similarly, western blot analysis indicated that miR-329-3p
upregulation inhibited TLR4 and TRAF6 protein expression
(P<0.05), while downregulation of miR-329-3p promoted the
expression of TLR4 and TRAF6 (P<0.05; Fig. 4B). In addition, nuclear NF-κB
protein expression in HUVEC cells transfected with the miR-329-3p
mimic was significantly lower compared with the miR-NC group
(P<0.05), while expression in HUVEC cells transfected with the
miR-329-3p inhibitor was significantly higher compared with the
miR-NC group (P<0.05; Fig. 4C).
Immunofluorescence results indicated that the upregulation of
miR-329-3p markedly decreased the nuclear translocation of NF-κB,
while downregulation of miR-329-3p markedly increased NF-κB nuclear
translocation (Fig. 4D). These
results suggest that miR-329-3p may be involved in regulating the
TLR4/TRAF6/NF-κB signaling pathway and NF-κB nuclear translocation
under high glucose conditions.
miR-329-3p protects HUVEC cells from
high glucose-induced apoptosis via inhibition of the
TLR4/TRAF6/NF-κB signaling pathway
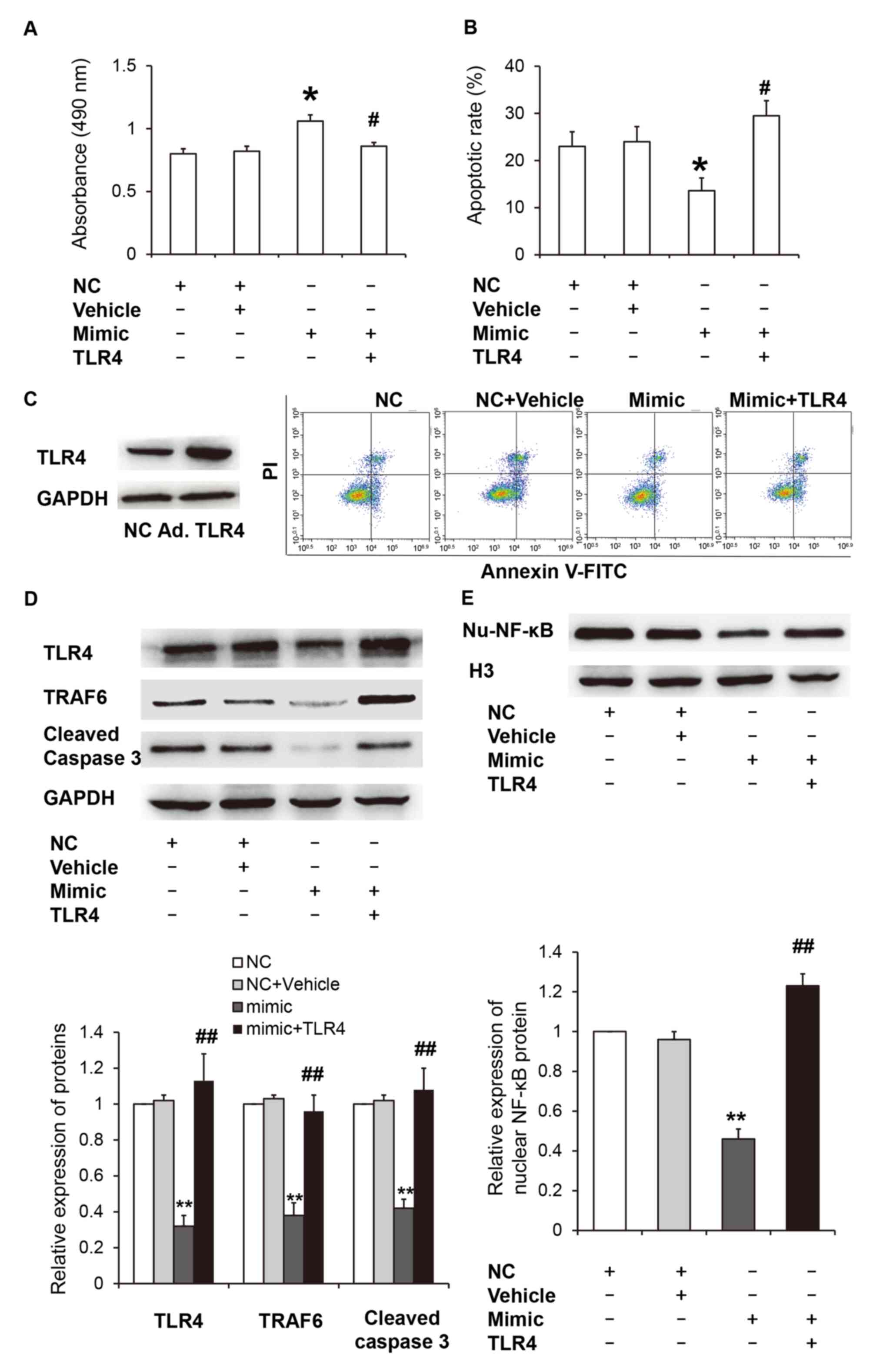
Next, TLR4 was overexpressed to determine the effect
of miR-329-3p on the TLR4/TRAF6/NF-κB signaling pathway during
HUVECs apoptosis under high glucose conditions. CCK-8 assay
demonstrated that transfection with the miR-329-3p mimic
significantly increased the absorbance of HUVECs compared with the
miR-NC group and the empty vector control (vehicle group)
(P<0.05), and overexpression of TLR4 significantly reduced the
absorbance of HUVECs transfected with the miR-329-3p mimic
(P<0.05; Fig. 5A). Flow
cytometry analysis revealed that transfection with the miR-329-3p
mimic significantly reduced the HUVEC apoptotic rate when compared
with the miR-NC and vehicle groups (P<0.05), and overexpression
of TLR4 significantly increased the apoptotic rate of HUVECs
transfected with the miR-329-3p mimic (P<0.05; Fig. 5B). A TLR4 overexpression adenovirus
was used to further elucidate the role of TLR4 in HUVECs under high
glucose conditions. The results demonstrated that TLR4
overexpression markedly increased TLR4 protein expression when
compared with the negative control (Fig. 5C). Western blot analysis revealed
that TLR4, TRAF6 and cleaved caspase-3 protein expression in HUVECs
transfected with the miR-329-3p mimic were significantly reduced
when compared with the miR-NC group (P<0.05). However, TLR4
overexpression significantly increased TLR4, TRAF6 and cleaved
caspase-3 protein expression in HUVECs transfected with the
miR-329-3p mimic (P<0.05; Fig.
5D). In addition, the expression of nuclear NF-κB protein in
HUVECs transfected with the miR-329-3p mimic was significantly
reduced when compared with the miR-NC group (P<0.05), while
overexpression of TLR4 significantly increased the expression of
nuclear NF-κB protein in HUVECs transfected with the miR-329-3p
mimic (P<0.05; Fig. 5E). These
results indicate that miR-329-3p may protect endothelial cells from
high glucose-induced apoptosis via inhibition of the
TLR4/TRAF6/NF-κB signaling pathway.
Discussion
Previous studies have demonstrated that the abnormal
expression of miRNA is frequently associated with diseases such as
diabetes (5,6). miR-329-3p exhibits abnormal expression
in a variety of tumors and is associated with tumor stage and
prognosis (17-19).
Wang et al (17)
demonstrated that miR-329-3p expression in pancreatic cancer was
reduced and survival rates were decreased in patients with low
miR-329-3p expression (17). Jiang
et al (18) revealed that
miR-329-3p expression is decreased in patients with osteosarcoma,
and its expression is correlated with tumor stage (18). Li et al (19) demonstrated that low miR-329-3p
expression is exhibited in gastric cancer and glioma, and that
upregulation of miR-329-3p can inhibit the proliferation and
migration of gastric cancer and glioma cells (20). However, to the best of our
knowledge, miR-329-3p expression in patients with diabetes has not
yet been reported. In the present study, the results demonstrated
that plasma miR-329-3p expression in patients with T2DM, or in
HUVECs treated with a high glucose concentration, is decreased when
compared with normal controls. This suggests that miR-329-3p may
affect T2DM progression by modulating the damaging effects of high
glucose on endothelial cells. Future studies will assess whether
miR-329-3p expression in patients with T2DM is associated with sex,
age or additional factors, using a larger sample size.
TLRs are key transmembrane proteins that transmit
extracellular antigen recognition information intracellularly and
activate inflammatory responses (21). TLR4 serves an important role in
mediating signal transduction and inflammation in a variety of cell
types (13). Elevated expression of
TLR4 has been observed in peripheral blood mononuclear cells
obtained from patients with type 1 and type 2 diabetes (22,23).
In clinical and experimental studies (23,24),
TLR4 expression in a variety of cell types was observed to be
significantly increased under high glucose conditions, which was
associated with the occurrence of complications associated with
diabetes (24). Welten et al
(10) revealed that TLR4 mRNA
expression is upregulated in an ischemic lower limb mouse model
following downregulation of miR-329-3p. The results obtained in the
present study demonstrated that TLR4 is a target gene of
miR-329-3p. In addition, downregulation of miR-329-3p increased
TLR4 expression and its associated downstream genes, increased the
nuclear translocation of NF-κB and increased the apoptotic rate of
endothelial cells under high glucose conditions. In addition,
upregulation of miR-329-3p protected endothelial cells from the
apoptosis induced by high glucose by inhibiting the TLR4 signaling
pathway. Overexpression of TLR4 abolished the protective effect of
miR-329-3p, which indicated that high glucose stimulation may lead
to inhibition of miR-329-3p expression in endothelial cells,
activate the TLR4 signaling pathway, promote nuclear translocation
of NF-κB, reduce the viability of endothelial cells and enhance
apoptosis.
In conclusion, the present study demonstrated that
plasma miR-329-3p expression is decreased in patients with T2DM. In
addition, miR-329-3p may affect the development of T2DM by
regulating the TLR4/TRAF6/NF-κB signaling pathway and high
glucose-induced endothelial cell injury. The results of the present
study provide experimental evidence for the role of miR-329-3p in
high glucose-associated endothelial dysfunction, which may help to
understand the role of miRNA in endothelial injury induced by
hyperglycemia.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
The final version of the manuscript has been read
and approved by all authors and each author considers the
manuscript to represent honest work. GS and LL collaborated to
design the study and analyzed the data. GS, LL and YY were
responsible for performing the experiments. All authors
collaborated to interpret the results and develop the
manuscript.
Ethical approval and consent to
participate
All procedures performed in the current study were
approved by the Ethics Committee of The First People's Hospital of
Jinan (Shandong, China). Written informed consent was obtained from
all patients or their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hurlow JJ, Humphreys GJ, Bowling FL and
McBain AJ: Diabetic foot infection: A critical complication. Int
Wound J. 15:814–821. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Knapp M, Tu X and Wu R: Vascular
endothelial dysfunction, a major mediator in diabetic
cardiomyopathy. Acta Pharmacol Sin. 40:1–8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Eelen G, de Zeeuw P, Simons M and
Carmeliet P: Endothelial cell metabolism in normal and diseased
vasculature. Circ Res. 116:1231–1244. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nakagami H, Kaneda Y, Ogihara T and
Morishita R: Endothelial dysfunction in hyperglycemia as a trigger
of atherosclerosis. Curr Diabet Rev. 1:59–63. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mirra P, Raciti GA, Nigro C, Fiory F,
D'Esposito V, Formisano P, Beguinot F and Miele C: Circulating
miRNAs as intercellular messengers, potential biomarkers and
therapeutic targets for Type 2 diabetes. Epigenomics. 7:653–667.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hou LJ, Han JJ and Liu Y: Up-regulation of
microRNA-503 by high glucose reduces the migration and
proliferation but promotes the apoptosis of human umbilical vein
endothelial cells by inhibiting the expression of insulin-like
growth factor-1 receptor. Eur Rev Med Pharmacol Sci. 22:3515–3523.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hui Y and Yin Y: MicroRNA-145 attenuates
high glucose-induced oxidative stress and inflammation in retinal
endothelial cells through regulating TLR4/NF-kappaB signaling. Life
Sci. 207:212–218. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Seitz H, Royo H, Bortolin ML, Lin SP,
Ferguson-Smith AC and Cavaille J: A large imprinted microRNA gene
cluster at the mouse Dlk1-Gtl2 domain. Genome Res. 14:1741–1748.
2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Benetatos L, Hatzimichael E, Londin E,
Vartholomatos G, Loher P, Rigoutsos I and Briasoulis E: The
microRNAs within the DLK1-DIO3 genomic region: Involvement in
disease pathogenesis. Cell Mol Life Sci. 70:795–814.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Welten SM, Bastiaansen AJ, de Jong RC, de
Vries MR, Peters EA, Boonstra MC, Sheikh SP, La Monica N,
Kandimalla ER, Quax PH and Nossent AY: Inhibition of 14q32
MicroRNAs miR-329, miR-487b, miR-494, and miR-495 increases
neovascularization and blood flow recovery after ischemia. Circ
Res. 115:696–708. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang P, Luo Y, Duan H, Xing S, Zhang J, Lu
D, Feng J, Yang D, Song L and Yan X: MicroRNA 329 suppresses
angiogenesis by targeting CD146. Mol Cell Biol. 33:3689–3699.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vaure C and Liu YL: ‘A comparative review
of toll-like receptor 4 expression and functionality in different
animal species’. Front Immunol. 5(316)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hu L, Yang H, Ai M and Jiang S: Inhibition
of TLR4 alleviates the inflammation and apoptosis of retinal
ganglion cells in high glucose. Graefes Arch Clin Exp Ophthalmol.
255:2199–2210. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ye EA and Steinle JJ: miR-146a attenuates
inflammatory pathways mediated by TLR4/NF-κB and TNFα to protect
primary human retinal Microvascular endothelial cells grown in high
glucose. Mediators Inflamm. 2016(3958453)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang TH, Huang CM, Gao X, Wang JW, Hao LL
and Ji Q: Gastrodin inhibits high glucose-induced human retinal
endothelial cell apoptosis by regulating the SIRT1/TLR4/NF-κBp65
signaling pathway. Mol Med Rep. 17:7774–7780. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta c(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang X, Lu X, Zhang T, Wen C, Shi M, Tang
X, Chen H, Peng C, Li H, Fang Y, et al: mir-329 restricts tumor
growth by targeting grb2 in pancreatic cancer. Oncotarget.
7:21441–21453. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jiang W, Liu J, Xu T and Yu X: MiR-329
suppresses osteosarcoma development by downregulating Rab10. FEBS
Lett. 590:2973–2981. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Z, Yu X, Wang Y, Shen J, Wu WK, Liang J
and Feng F: By downregulating TIAM1 expression, microRNA-329
suppresses gastric cancer invasion and growth. Oncotarget.
6:17559–17569. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xiao B, Tan L, He B, Liu Z and Xu R:
MiRNA-329 targeting E2F1 inhibits cell proliferation in glioma
cells. J Transl Med. 11(172)2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gordon S: Pattern recognition receptors:
Doubling up for the innate immune response. Cell. 111:927–930.
2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Devaraj S, Dasu MR, Rockwood J, Winter W,
Griffen SC and Jialal I: Increased toll-like receptor (TLR) 2 and
TLR4 expression in monocytes from patients with type 1 diabetes:
Further evidence of a proinflammatory state. J Clin Endocrinol
Metab. 93:578–583. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dasu MR, Devaraj S, Park S and Jialal I:
Increased toll-like receptor (TLR) activation and TLR ligands in
recently diagnosed type 2 diabetic subjects. Diabetes Care.
33:861–868. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Garibotto G, Carta A, Picciotto D, Viazzi
F and Verzola D: Toll-like receptor-4 signaling mediates
inflammation and tissue injury in diabetic nephropathy. J Nephrol.
30:719–727. 2017.PubMed/NCBI View Article : Google Scholar
|