Introduction
Since the discovery of endothelial progenitor cells
(EPCs) by Asahara et al in 1997(1), it has been reported that EPCs are
involved in vascular repair and postnatal angiogenesis (2). Following stimulation with various
factors such as velvet antler, hydrogen sulfide and danhong, EPCs
migrate to sites of injury to promote angiogenesis and repair
vascular endothelial cell damage (3-5).
The mechanism underlying EPC-mediated repair of injured endothelium
is not only associated with differentiation and
transdifferentiation into EPCs, but also with the biological
functions of EPCs, including proliferation, migration and tube
formation (6,7). EPCs can also prevent neointima
formation and participate in re-endothelialization in damaged
vascular tissue (8). Moreover,
previous studies have revealed that EPCs can produce new blood
vessels during tumor growth, development and metastasis (9-11).
Cerebrovascular disease (12),
cardiovascular disease (13),
chronic kidney disease (14) and
cancer (15) development are
associated with EPC dysfunction. Therefore, investigating the
biological features of EPCs and the underlying mechanisms will be
useful for identifying the therapeutic potential of EPCs for the
repair of damaged vascular endothelium, and also for the synthesis
of effective anti-angiogenic drugs to prevent EPC-mediated
vasculogenesis during tumor growth and progression.
Stromal cell derived factor-1 (SDF-1), a member of
the CXC chemokine family, is constitutively expressed on stromal
cells in various tissues (16) and
can regulate multiple physiological processes such as
organogenesis, regeneration and tumorigenesis (17). SDF-1 is a small chemotactic
signaling protein that promotes downstream effects primarily via
the C-X-C Motif Chemokine Receptor (CXCR)4, a specific G
protein-coupled receptor (18).
Previous studies have reported that SDF-1 is a strong
chemoattractant for CD34+ cells, including hematopoietic
stem cells and EPCs, which highly express CXCR4 (19,20).
In addition, SDF-1 overexpression in the peripheral circulation
induces mobilization of hematopoietic progenitors and stem cells,
including EPCs (21). The increased
expression of SDF-1 in ischemic muscles acts as a chemoattractant
to support the homing of CXCR4+ EPCs (22). A previous study also revealed that
locally administered SDF-1 promoted EPCs accumulation at the site
of ischemia, which was associated with ischemic neovascularization
(23). Furthermore, it has been
reported that the SDF-1/CXCR4 axis in EPCs serves an important role
during bone fracture healing (24).
A review has shown that SDF-1 binding to CXCR4
initiates several signaling pathways, which can result in a variety
of responses that are important during the process of angiogenesis,
such as chemotaxis, cell proliferation, migration and the secretion
of angiopoietic factors (25). The
PI3K/Akt and mitogen-activated protein kinase (MAPK)/ERK signal
transduction pathways, which are mediated by SDF-1, contribute to
cell migration, proliferation, tube formation, apoptosis and
chemotaxis (26,27). For example, SDF-1-induced Akt and
ERK activation results in lung cancer cell invasion and metastasis
(28), sacral chondrosarcoma and
glioblastoma cell cycle progression and epithelial-mesenchymal
transition (29,30), as well as ovarian cancer cell
proliferation (31), and pre-B
(26), F5M2 osteosarcoma (32) and epitheloid carcinoma (33) cell migration. It has been reported
that SDF-1 serves a critical role in the regulation of EPC cellular
functions, including cell proliferation and migration (23,34).
Zheng et al revealed that the PI3K/Akt signaling pathway,
but not the MAPK/ERK signaling pathway, was required for
SDF-1-mediated regulation of EPC migration (35). However, to the best of our
knowledge, the effects of SDF-1 on EPC proliferation and tube
formation, as well as the underlying mechanisms, have not been
fully elucidated. Therefore, the present study aimed to investigate
the effects of SDF-1 on EPC biological properties and to identify
the possible underlying mechanisms.
Materials and methods
Ethics and animals
A total of 42 male Sprague-Dawley rats (weight,
150-180 g; age, 5-6 weeks) were provided by The Experimental Animal
Center of General Hospital of Central Theater Command. All rats
were housed in a temperature-controlled room (22˚C) with 50%
humidity and maintained on a 12-h light/12-h dark cycle with food
and water available ad libitum. The animal use and all
experimental protocols were conducted in accordance with the
National Institutes of Health Guide for Care and Use of Laboratory
Animals (36) and were approved by
the Animal Research Committee of The General Hospital of Central
Theater Command (Wuhan, China).
EPC isolation and culture
Sprague Dawley rats were anesthetized with ether (50
mg/kg) and rapidly decapitated after the rats were fully
anesthetized, which was determined with no response to a paw pinch
and disappearance of righting and eyelid reflex. Then, the tibias
and femurs were dissected and cleaned. The bone marrow was slowly
flushed out from tibias and femurs using a syringe filled with PBS
and the mononuclear cells were isolated using the density gradient
centrifugation method (Lymphoprep 1.077; Axis-Shield Diagnostics
Ltd.) at 1,600 x g for 15 min at 4˚C. After three washes with PBS,
mononuclear cells were resuspended in fresh EGM-2 media (Lonza
Group, Ltd.) supplemented with 5% FBS (Hyclone; Cytiva), seeded on
fibronectin-coated cell culture plates (2x105
cells/cm2) and incubated at 37˚C with 5% CO2.
Following incubation for 24 h at 37˚C, the medium was changed,
non-adherent cells were removed and fresh EGM-2 media was replaced
every 3 days before cells were used for subsequent experiments.
Alterations in cell morphology were observed using an IX81 inverted
phase-contrast microscope (Olympus Corporation; magnification,
x200).
EPC characterization
The cellular morphologies at days 7 and 14 were used
to identify the EPCs. To assess the endothelial phenotype of EPCs,
Dil-labelled acetylated low-density lipoprotein (Dil-ac-LDL;
Invitrogen; Thermo Fisher Scientific, Inc.) uptake was evaluated.
Following culture for 7 days, cells were harvested and washed with
PBS and incubated with Dil-ac-LDL (10 µg/ml) for 4 h at 37˚C with
5% CO2. Following fixation with 4% phosphate-buffered
paraformaldehyde for 10 min at room temperature, cells were stained
with FITC-labeled Ulex europaeus agglutinin-1 (UEA-1;
Sigma-Aldrich; Merck KGaA) for 1 h at 37˚C with 5% CO2.
Subsequently, cells were washed with PBS and stained for 15 min
with Hoechst at room temperature. Triple-positive cells
(Dil-ac-LDL, FITC-UEA-1 and Hoechst) were identified as EPCs.
Stained cells were observed using a fluorescence microscope
(Olympus Corporation; magnification, x200).
Surface antigen expression was assessed by flow
cytometry using a FC500 flow cytometer (Beckman Coulter, Inc.).
Following culture for 7 days, cells were harvested and washed in
PBS, and then resuspended in flow cytometry wash buffer (2% FBS;
Hyclone; Cytiva) and 0.1% sodium azide in PBS). The cells were
blocked in 10% (v/v) normal goat serum (Abcam) for 15 min at 4˚C.
Subsequently, cells (1x106) were suspended in PBS, and
then incubated with monoclonal antibodies: FITC-conjugated
anti-CD133 antibody (cat. no. orb467002; Biorbyt Ltd.; 1:300),
FITC-conjugated anti-CD34 antibody (cat. no. sc-7324; Santa Cruz
Biotechnology, Inc.; 1:300) and FITC-conjugated anti-vascular
endothelial growth factor receptor 2 antibody (VEGFR-2; cat. no.
ab184903; Abcam; 1:300) for 30 min at 4˚C. The stained samples were
analyzed using the flow cytometer.
Cell proliferation assay
To investigate whether SDF-1 treatment regulated EPC
proliferation, cells (1x105 cells/well) were treated
with different concentrations of SDF-1 (10, 100 and 500 ng/ml;
Abcam) in 6-well plates for 24 h at 37˚C with 5% CO2.
Then, EPCs were harvested and seeded (1x104 cells/well)
into 96-well culture plates and incubated with SDF-1 (10, 100 and
500 ng/ml) again for another 48 h.
To investigate the mechanisms underlying
SDF-1-mediated EPC viability, EPCs (1x105 cells/well)
were pretreated with CXCR4 antagonist AMD3100 (60 µM;
Sigma-Aldrich; Merck KGaA), PI3K inhibitor LY294002 (20 µM; Selleck
Chemicals) or MEK inhibitor PD98059 (20 µM; Sigma-Aldrich; Merck
KGaA) for 2 h at 37˚C, and then stimulated with SDF-1 (100 ng/ml)
for 24 h in 6-well plates at 37˚C with 5% CO2. EPCs were
harvested and seeded (1x104 cells/well) into 96-well
culture plates and incubated with SDF-1 (100 ng/ml) again for 48
h.
Cell proliferation was measured using the
colorimetric MTS assay (Cell Titer 96 Aqueous; Promega
Corporation), according to the manufacturer's protocol. MTS reagent
(20 µl) was added to each well and incubated for 1 h at 37˚C. The
optical density (OD) value of each well was measured at a
wavelength of 490 nm using a 96-well plate reader (Bio-Rad
Laboratories, Inc.).
Cell migration assay
Cell migration assays were performed using the 8-µm
pore 24-well Cell Migration assay kit (BD Biosciences). Cells were
pretreated with AMD3100 (60 µM), LY294002 (20 µM), PD98059 (20 µM)
or control EGM-2 medium for 2 h at 37˚C with 5% CO2.
Subsequently, cells were incubated in plates with SDF-1 (100 ng/ml)
or control EGM-2 medium for 24 h at 37˚C (n=6 wells/group). Cells
(1x105 cells/well) were serum-starved for 24 h at 37˚C
with 5% CO2 and seeded into the upper chamber with
serum-free culture medium. EGM-2 medium supplemented with 5% FBS
containing SDF-1 (100 ng/ml) or control EGM-2 medium was added into
the lower chambers. Following incubation at 37˚C for 24 h with 5%
CO2, cells on the upper surface were removed using
cotton-tipped swabs. Cells on the lower surface of the membrane
were fixed using 95% dehydrated alcohol for 30 min at room
temperature and stained with crystal violet for 15 min at room
temperature. After washing three times with PBS, stained cells were
observed in five random fields of view using an IX81 inverted
phase-contrast microscope (Olympus Corporation; magnification,
x100).
Tube formation assay
The tube formation assay was performed using
Matrigel® (BD Biosciences), according to the
manufacturer's instructions. Matrigel® was thawed
overnight at 4˚C. The 96-well plate and 100 µl pipette tips were
also maintained at 4˚C overnight. Subsequently,
Matrigel® (30 µl/well) was added to the 96-well plate
and incubated at 37˚C for 1 h. To investigate the effect of SDF-1
on tube formation of EPCs and the underlying mechanism, AMD3100 (60
µM), LY294002 (20 µM) or PD98059 (20 µM) was added to culture
medium for 2 h at 37˚C with 5% CO2. Then, cells were
incubated with SDF-1 (100 ng/ml) for 24 h at 37˚C 5%
CO2. EPCs (1x104 cells/well) were added to
the surface of the Matrigel® in the 96-well plate and
incubated at 37˚C for 4 h. Tube formation was observed using an
IX81 inverted phase-contrast microscope (Olympus Corporation;
magnification, x100). Images were acquired at the same
magnification. The total branching length of the vascular network
was determined using ImageJ software version 1.51 (National
Institutes of Health).
Western blotting
EPCs (1x105 cells/well) were cultured in
6-well plates and at 80% confluence pretreated with AMD3100 (60
µM), LY294002 (20 µM) or PD98059 (20 µM) for 2 h at 37˚C with 5%
CO2. Subsequently, EPCs were stimulated with 100 ng/ml
SDF-1 for 1 h at 37˚C with 5% CO2. The concentrations of
inhibitor used in the present study were based on previous studies
(17,25,26).
Total protein was extracted using RIPA lysis buffer (Santa Cruz
Biotechnology, Inc.) with phosphatase inhibitor and
phenylmethanesulfonyl fluoride. Total protein was quantified using
the Bradford method (37). The
whole cell extracts (30 µg) were separated via 10% SDS-PAGE and
transferred onto PVDF membranes (Bio-Rad Laboratories, Inc.), which
were blocked with 5% non-fat milk for 1 h at 37˚C. Subsequently,
the membranes were incubated at 4˚C overnight with the following
primary antibodies: Anti-Akt (cat. no. 2920S; Cell Signaling
Technology, Inc.; 1:2,000), anti-phosphorylated (p)-Akt (cat. no.
4051S; Cell Signaling Technology, Inc.; 1;2,000), anti-ERK (cat.
no. 4696S; Cell Signaling Technology, Inc.; 1:1,000), anti-p-ERK
(cat. no. 9106S; Cell Signaling Technology, Inc.; 1:1,000) and
anti-β-actin (cat. no. 3700S; Cell Signaling Technology, Inc.;
1:1,000). Following primary incubation, the membranes were washed
with TBST [TBS with 0.1% (v/v) Tween-20] and incubated with
corresponding horseradish peroxidase-conjugated goat anti-rat
immunoglobulin G secondary antibody (cat. no. 7077S; Santa Cruz
Biotechnology, Inc.; 1:3,000) for 1 h at 37˚C. The membranes were
washed with TBST and protein bands were visualized using
chemiluminescent solution (EMD Millipore). Protein expression
levels were quantified using Quantity One software (version 4.4;
Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation
of ≥3 individual experiments. In cell experiments, six replicate
wells were performed for each group. Statistical analyses were
performed using SPSS software (version 18.0; SPSS, Inc.).
Differences among groups were analyzed using one-way ANOVA followed
by Bonferroni's post hoc test. P<0.05 was considered to indicate
a statistically significant difference.
Results
EPC characterization
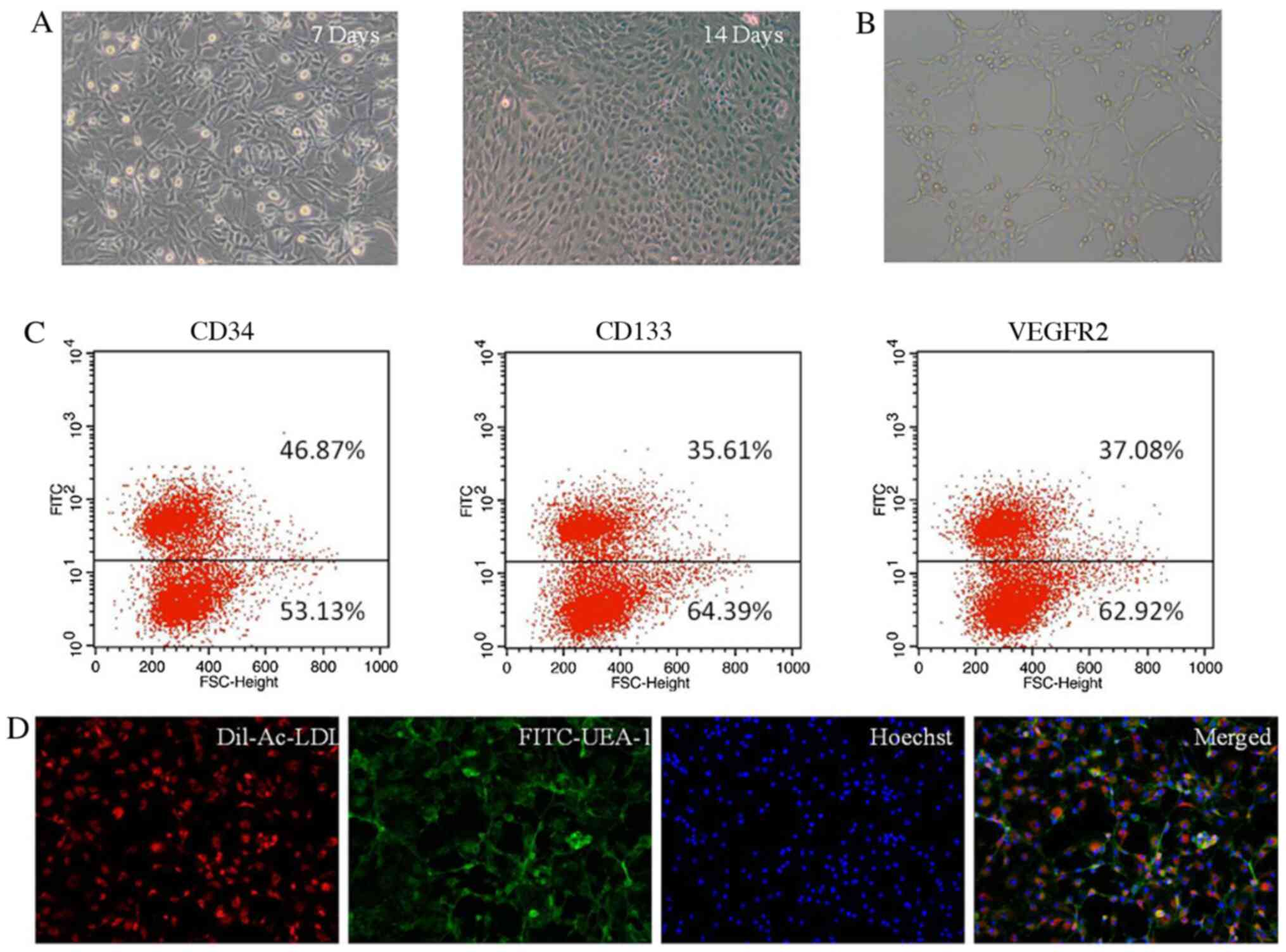
Cells displayed spindle-like morphology at day 7 and
cobblestone-like morphology at day 14 (Fig. 1A). Following cell culture for 7 days
in EGM-2 media, the tube formation ability of the attached cells
was determined using the Matrigel® network formation
assay (Fig. 1B). Following culture
for 7 days in EGM-2 media, cells were harvested and assessed by
flow cytometry. FACS analysis indicated that the adherent cells
expressed progenitor or stem cell markers CD133 and CD34, and the
endothelial cell marker VEGFR2 (Fig.
1C). EPCs were further characterized by performing a Dil-ac-LDL
uptake and FITC-UEA-1 binding assay after 7 days of culture in
EGM-2 media (Fig. 1D), and it was
identified that the cultured cells were EPCs.
Effect of SDF-1 on EPC proliferation
and the underlying mechanism
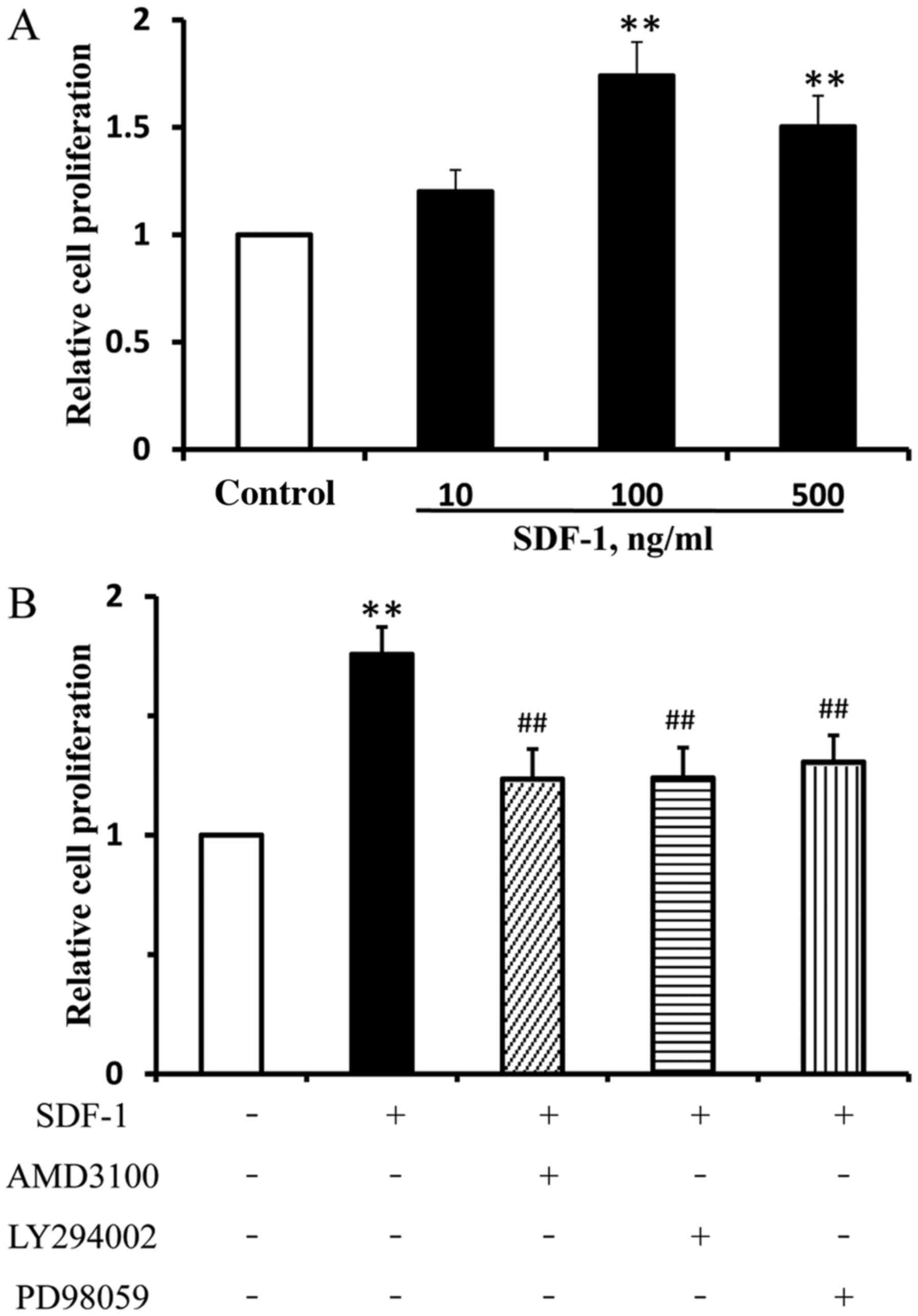
The effects of SDF-1 on EPC proliferation were
investigated. The maximum increase in EPC proliferation was
observed in the 100 ng/ml SDF-1 treatment group, and no further
increase in EPC proliferation was observed in the 500 ng/ml SDF-1
treatment group. SDF-1 (100 and 500 ng/ml) significantly enhanced
EPC proliferation compared with the control, and 100 ng/ml SDF-1
was selected for subsequent experiments (Fig. 2A). Moreover, there was no
statistical difference in the OD values between the 10 ng/ml SDF-1
group and the control group.
To investigate the underlying signaling pathway
associated with SDF-1-induced EPC proliferation, the MTS assay was
performed. SDF-1 group could significantly increase the
proliferation of EPCs compared with the control group.
SDF-1-induced proliferation was significantly inhibited by
pretreatment with AMD3100, LY294002 or PD98059, compared with the
SDF-1-treated group (Fig. 2B).
Collectively, the results suggested that the Akt and ERK signal
transduction pathways were associated with SDF-1-induced EPC
proliferation.
LY294002 reverses SDF-1-induced EPC
migration and tube formation
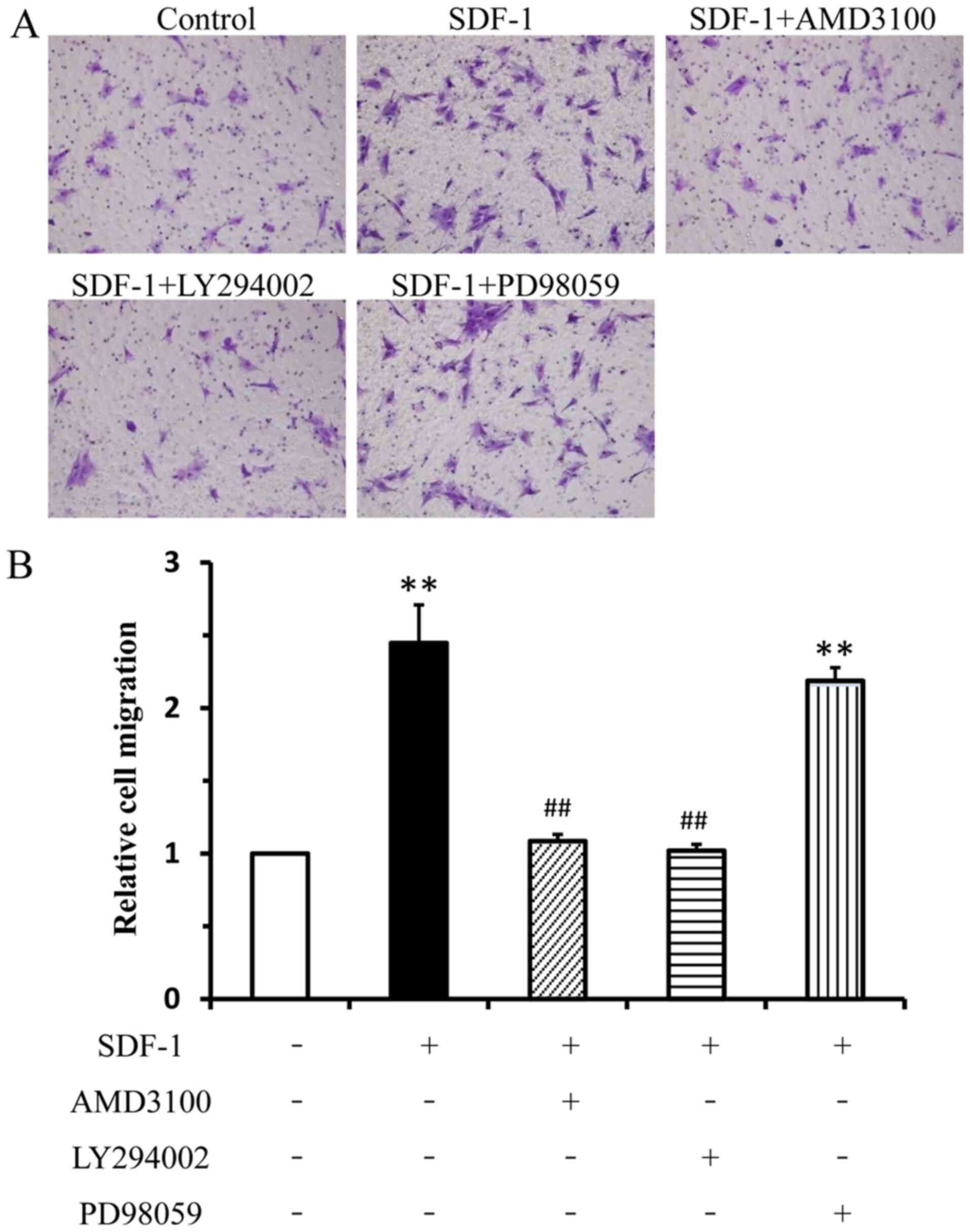
To investigate the effect of SDF-1 on EPC migration,
the migratory ability of EPCs was evaluated using the Transwell
assay. The number of migratory cells in the SDF-1-treatment group
was significantly increased compared with the negative control
group. Furthermore, AMD3100 and LY294002 significantly reduced
SDF-1-induced EPC migration compared with the SDF-1 group. There
was no significant difference in the migration of EPCs between the
SDF-1 group and PD98059 group (Fig.
3). Therefore, the results demonstrated that the Akt signaling
pathway served an important role during SDF-1-induced EPC
migration.
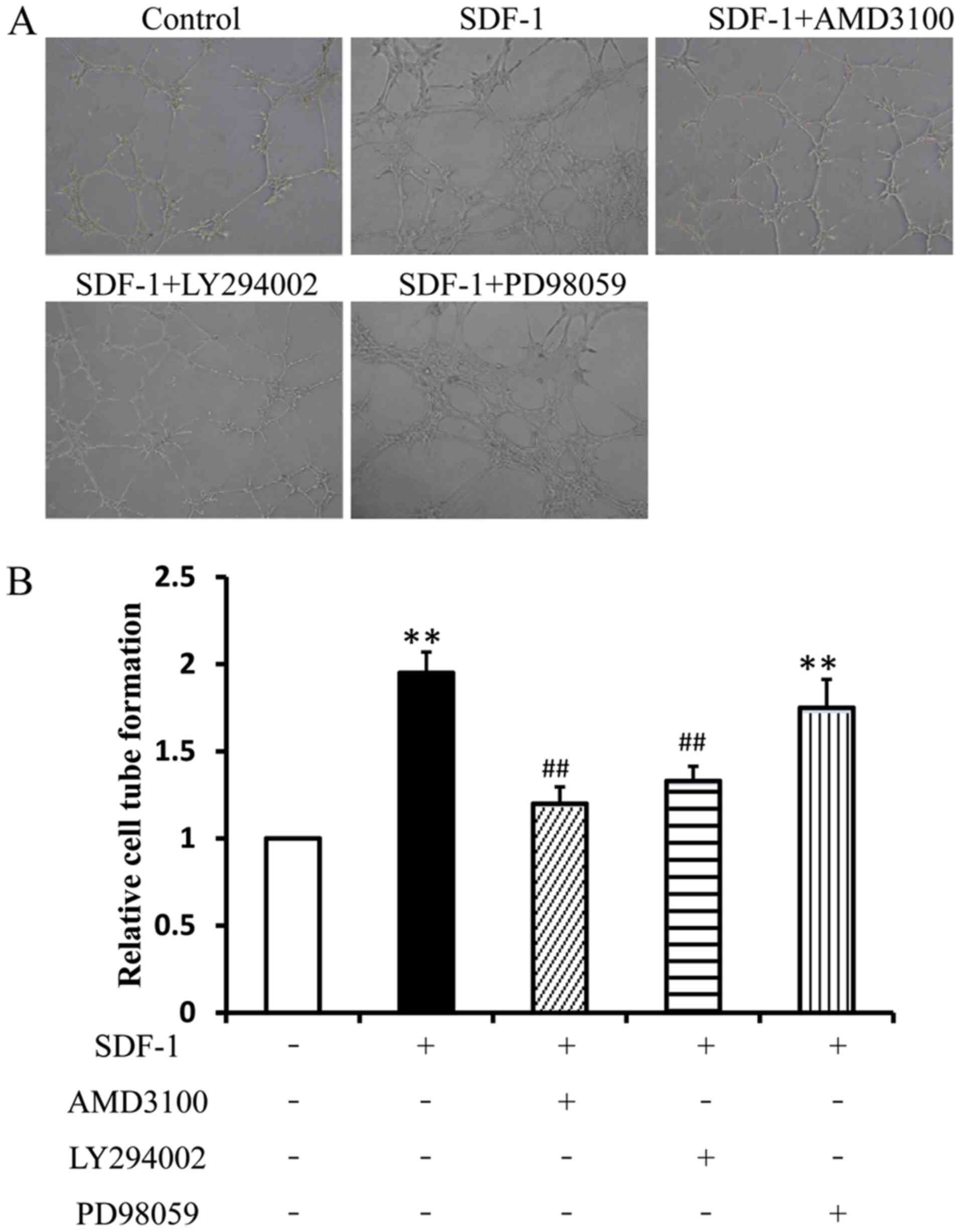
The tube-like formation assay was performed to
examine the effect of SDF-1 on tube-like structure formation.
Compared with the control group, the number of tube-like structures
in the SDF-1 group was significantly increased (Fig. 4). Pretreatment with AMD3100 and
LY294002 significantly attenuated SDF-1-induced EPC tube-like tube
formation. No significant difference in tube formation of EPCs was
found between the SDF-1 group and PD98059 group. Thus, the results
suggested that the Akt signaling pathway, but not ERK signaling,
was associated with SDF-1-induced EPC tube-like formation.
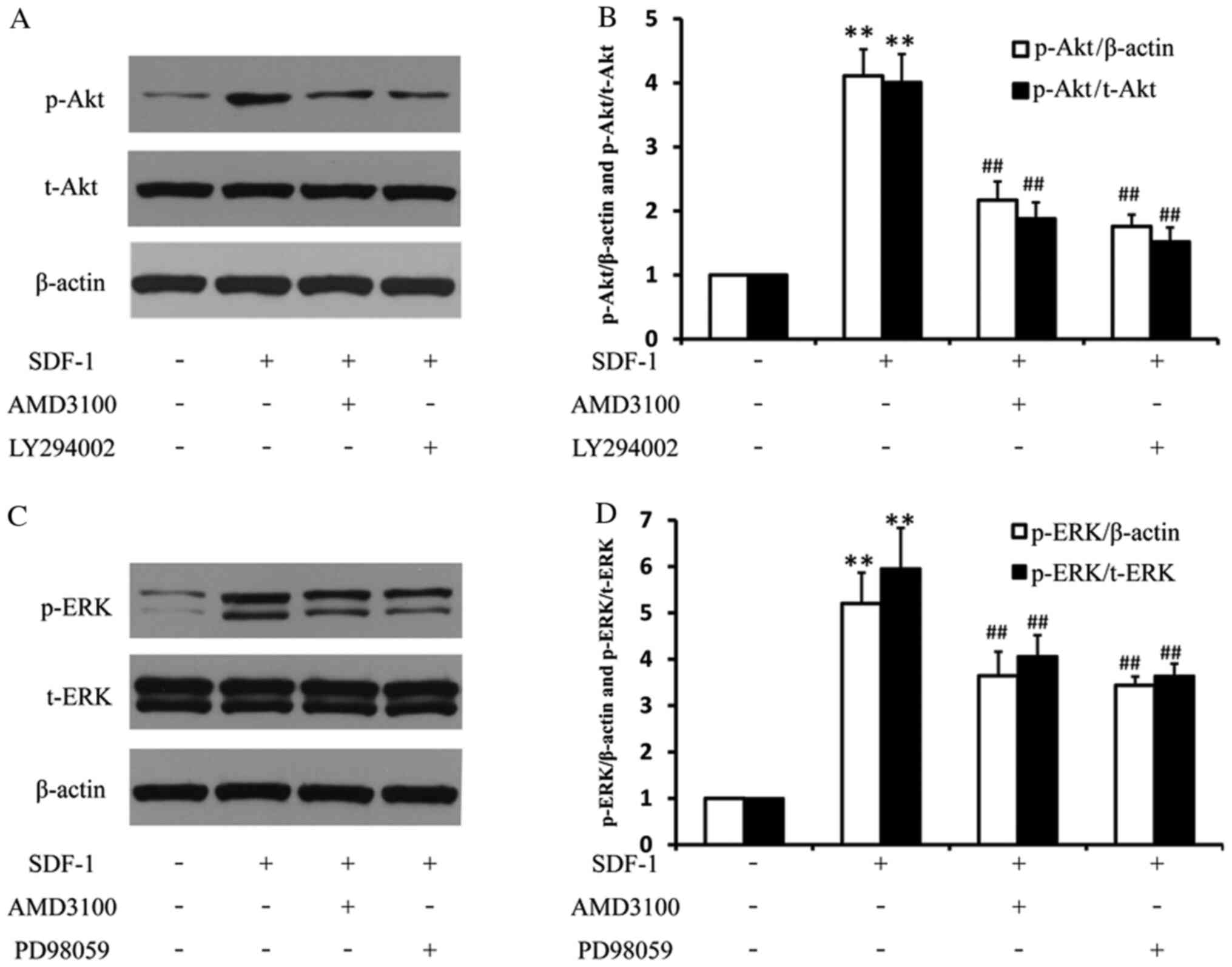
Various inhibitors attenuate
SDF-1-stimulated Akt and ERK phosphorylation
To investigate whether the PI3K/Akt signaling
pathway was involved in EPC biological functions, the
phosphorylation/activation levels of Akt were examined by western
blotting following stimulation with SDF-1. SDF-1 treatment
significantly upregulated the protein expression of p-Akt, which
was reversed by AMD3100 or LY294002 (Fig. 5A and B), indicating activation of the Akt
signaling pathway. Based on the finding that SDF-1-induced EPC
proliferation, migration and tube formation were attenuated by
LY294002, it was speculated that the Akt signaling pathway was
associated with SDF-1-mediated EPC proliferation, migration and
tube formation.
SDF-1 also upregulated the protein expression of
p-ERK in EPCs, and pretreatment with AMD3100 or PD98059
significantly reduced SDF-1-induced increases in ERK
phosphorylation (Fig. 5C and
D), thus suggesting that the ERK
signaling pathway was required for SDF-1-induced EPC
proliferation.
Discussion
In the present study, the potential effects of SDF-1
on EPCs and the underlying mechanisms were investigated. The
results suggested that SDF-1 significantly stimulated EPC
functional characteristics, such as cell proliferation, migration
and tube formation. SDF-1-induced EPC proliferation was inhibited
by the CXCR4 antagonist (AMD3100), the PI3K inhibitor (LY294002)
and the MEK inhibitor (PD98059). However, SDF-1-induced migration
and tube formation were only attenuated by AMD3100 and LY294002. To
further investigate the underlying molecular mechanisms, western
blotting was performed. The results indicated that SDF-1 treatment
stimulated Akt and ERK phosphorylation; however, pretreatment with
AMD3100, LY294002 and PD98059 blocked SDF-1-induced
phosphorylation. Moreover, SDF-1-induced EPC proliferation was
accompanied by Akt and ERK phosphorylation. Thus, the Akt signaling
pathway via CXCR4 may serve critical roles during SDF-1-induced EPC
proliferation, migration and tube formation. In addition, ERK
activation was associated with EPC proliferation, but it was not
required for SDF-1-induced EPC migration and tube formation.
Since Asahara et al (1) first described human circulating
CD34+ cells as EPCs, other studies have reported that
EPCs migrate to sites of injured blood vessels, differentiate into
mature endothelial cells and participate in re-endothelialization
(38-40).
It has also been revealed that EPCs have important roles during
neovascularization (41), vascular
repair (42) and various diseases,
such as cerebrovascular disease (12), cardiovascular disease (13), chronic kidney disease (14) and cancer (15).
Numerous cytokines are released in injured or
ischemic tissue, of which SDF-1 and VEGF are the most important
(43). VEGF is the most specific
and potent angiogenic factor (44).
A previous study showed that SDF-1 can mobilize and recruit EPCs to
participate in angiogenesis, and VEGF is an important factor
involved in this process (35). A
recent study has also reported that VEGF and SDF-1 have significant
synergistic effects on the angiogenic properties of EPCs (45). Collectively, it was speculated that
VEGF and SDF-1 exert significant synergistic effects on the
angiogenesis of EPCs.
SDF-1 is primarily secreted by stromal fibroblasts
and vascular endothelial cells, and is constitutively expressed in
multiple tissues, including the liver, lung, brain, kidney, heart,
colon, lymph nodes, skin and bone marrow (18). SDF-1 binds to the transmembrane G
protein-coupled receptors CXCR4 and CXCR7(46). CXCR4 was previously considered to be
the only SDF-1 receptor until the identification of CXCR7 in T
lymphocytes (47,48). Compared with CXCR4, the affinity of
CXCR7 to SDF-1 is higher (47).
Furthermore, SDF-1 binding to CXCR4 leads to the activation of G
protein signaling kinases, including PI3K and MAPK signaling
pathways, as well as the NF-κB signaling pathway (18). However, binding of SDF-1 to CXCR7
signals via the β-arrestin pathway, which is not via a G
protein-mediated signaling pathway (49). CXCR7 can form heterodimers with
CXCR4 to form a structural trigger of the downstream signaling
pathway (50). Although the exact
function of CXCR7 is not completely understood, previous studies
have reported that CXCR7 is closely related to cell survival
(51), proliferation (52) and adhesion (53), as well as the formation of the SDF-1
concentration gradient (54).
SDF-1 binds to the G-coupled protein receptor CXCR4
to initiate downstream signaling molecules and induce diverse
biological processes, including cell proliferation, migration,
survival and senescence (55).
Previous studies have revealed that CXCR4 expression is associated
with EPC homing, subsequent endothelial regeneration and the
angiogenic response (56,57). The present results suggested that
SDF-1 treatment increased EPC migration, which was consistent with
the results of previous studies (23,35).
In addition, SDF-1-induced proliferation and angiogenesis were
significantly blocked by the CXCR4 antagonist AMD3100. In line with
previous studies (35,56,57),
the present study identified a potential role for CXCR4 for the
integration of EPCs into the vascular bed, and further indicated
that SDF-1 modulated EPC proliferation, migration and angiogenesis.
However, the underlying downstream pathways of SDF-1 that are
associated with EPC viability, migration and angiogenesis require
further investigation.
Previous studies have revealed that the SDF-1/CXCR4
axis is associated with cell proliferation and migration via
activation of several signal transduction pathways, including the
PI3K/Akt signaling pathway (29,33).
Akt serves an important role during cell proliferation,
differentiation and survival, and regulates a number of genes that
are downstream targets of PI3K (58). The PI3K/Akt signaling pathway is
also required for the biological features of EPCs that are induced
by diverse pathophysiological and interventional interventions
(59). A recent study revealed that
SDF-1 increased EPC proliferation, colony formation, migration and
angiogenesis (60); however, the
molecular mechanisms underlying the biological activities of SDF-1
on EPCs are not fully understood. Consistent with a previous study
(60), the present results also
demonstrated that SDF-1 promoted the functional activities of EPCs.
Furthermore, inhibition of Akt blocked SDF-1-induced EPC biological
functions, such as proliferation, migratory and angiogenesis. To
further investigate the involvement of the Akt signal transduction
pathway in the process, the effect of SDF-1 on the protein
expression levels of Akt and p-Akt in EPCs were assessed by western
blotting. The results demonstrated that SDF-1-treated EPCs had
increased Akt phosphorylation, and pretreatment with the PI3K
inhibitor LY294002 significantly decreased SDF-1-induced p-Akt
expression. It was also found that the Akt signaling pathway was
required for SDF-1-induced EPC biological functions. Moreover,
CXCR4 inhibition by ADM3100 significantly attenuated SDF-1-induced
EPC biological functions and p-Akt activity. Therefore, the
CXCR4-mediated PI3K/Akt signaling pathway may serve a key role
during SDF-1-induced EPC viability, migratory and angiogenesis.
The MAPK/ERK signaling pathway is a chain of
proteins in the cell that can transduce extracellular information
into intracellular responses, and are associated with the
regulation of a variety of growth and differentiation signaling
pathways via several phosphorylation cascades (58). Previous studies have reported that
low-dose radiation and basic fibroblast growth factor can promote
EPC proliferation and migration via activation of the ERK signaling
pathway (61,62). In addition, the ERK signaling
pathway is associated with the regulation of EPC angiogenesis
(63). Thus, the aforementioned
studies revealed that the ERK signaling pathway may serve an
important role during EPC proliferation, migration and
angiogenesis. SDF-1/CXCR4 signal transduction stimulates ERK
activation during lung cancer (28), sacral chondrosarcoma (29), glioblastoma (30) and in ovarian cancer (31) cell lines. To the best of our
knowledge, the association between SDF-1-induced EPC functions and
the ERK signaling pathway has not been previously reported.
Therefore, the present study investigated whether the ERK signaling
pathway was regulated in EPCs in response to SDF-1. The results
indicated that SDF-1-induced proliferation was accompanied by ERK
phosphorylation. Moreover, the MEK inhibitor PD98059 decreased
SDF-1-induced p-ERK protein expression and EPC proliferation, but
had no effect on SDF-1-induced migration and tube formation.
Therefore, it was speculated that the ERK signaling pathway was
associated with SDF-1-induced EPC proliferation, but not EPC
migration and tube formation.
The present study had a number of limitations that
require consideration. For example, the SDF-1/CXCR4 axis activates
several signaling pathways (18),
including the PI3K/Akt, MEK/ERK, NF-κB, Ras-activated, Janus
kinase/STAT and G protein-coupled receptor kinase-β-arrestin
signaling pathways. However, the present study primarily focused on
the PI3K/Akt and MAPK/ERK signaling pathways. Thus, whether SDF-1
promotes EPC proliferation, migration and tube formation via other
signaling pathways should be examined in future studies.
Furthermore, the other SDF-1 receptor, CXCR7, requires further
investigation. The roles of the SDF-1/CXCR4 and SDF-1/CXCR7
signaling pathways, as well as their crosstalk in EPC biological
functions should also be investigated in future studies.
In conclusion, to the best of our knowledge, the
present study was the first to demonstrate that SDF-1 stimulated
EPC proliferation via activation of the CXCR4-dependent Akt and ERK
signaling pathways. Moreover, the Akt signaling pathway also
contributed to SDF-1-induced EPC migration and tube formation. The
results of the present study may further the understanding of the
molecular mechanisms underlying SDF-1-mediated EPC functions, as
well as provide an insight into potential therapeutic targets for
cerebrovascular disease, cardiovascular disease, chronic kidney
disease and cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the China
Postdoctoral Science Foundation (grant no. 2017M623431).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YC, LM and ZR conceived and designed the study. BD
and ZZ interpreted and analyzed the data. YC drafted the
manuscript. LM and ZR critically revised the manuscript. YC, GW and
JY performed the cell culture and experimental procedures. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Experimental protocols involving the use of animals
were approved by the Animal Research Committee of the General
Hospital of Central Theater Command (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–966. 1997.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang M, Rehman J and Malik AB:
Endothelial progenitor cells and vascular repair. Curr Opin
Hematol. 21(224)2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li Y, Wang Z, Mao M, Zhao M, Xiao X, Sun
W, Guo J, Liu C, Yang D, Qiao J, et al: Velvet antler mobilizes
endothelial progenitor cells to promote angiogenesis and repair
vascular endothelial injury in rats following myocardial
infarction. Front Physiol. 9(1940)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hu Q, Ke X, Zhang T, Chen Y, Huang Q, Deng
B, Xie S, Wang J and Nie R: Hydrogen sulfide improves vascular
repair by promoting endothelial nitric oxide synthase-dependent
mobilization of endothelial progenitor cells. J Hypertens.
37:972–984. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hu Z, Wang H, Fan G, Zhang H, Wang X, Mao
J, Zhao Y, An Y, Huang Y, Li C, et al: Danhong injection mobilizes
endothelial progenitor cells to repair vascular endothelium injury
via upregulating the expression of Akt, eNOS and MMP-9.
Phytomedicine. 61(152850)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wei H, Mao Q, Liu L, Xu Y, Chen J, Jiang
R, Yin L, Fan Y, Chopp M, Dong J and Zhang J: Changes and function
of circulating endothelial progenitor cells in patients with
cerebral aneurysm. J Neurosci Res. 89:1822–1828. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xu Y, Tian Y, Wei HJ, Chen J, Dong JF,
Zacharek A and Zhang JN: Erythropoietin increases circulating
endothelial progenitor cells and reduces the formation and
progression of cerebral aneurysm in rats. Neuroscience.
181:292–299. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Urbich C and Dimmeler S: Endothelial
progenitor cells: Functional characterization. Trends Cardiovasc
Med. 14:318–322. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Moccia F, Zuccolo E, Poletto V, Cinelli M,
Bonetti E, Guerra G and Rosti V: Endothelial progenitor cells
support tumour growth and metastatisation: Implications for the
resistance to anti-angiogenic therapy. Tumor Biol. 36:6603–6614.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Flamini V, Jiang WG, Lane J and Cui YX:
Significance and therapeutic implications of endothelial progenitor
cells in angiogenic-mediated tumour metastasis. Crit Rev Oncol
Hematol. 100:177–189. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao X, Liu HQ, Li J and Liu XL:
Endothelial progenitor cells promote tumor growth and progression
by enhancing new vessel formation. Oncol Lett. 12:793–799.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jung KH and Roh JK: Circulating
endothelial progenitor cells in cerebrovascular disease. J Clin
Neurol. 4:139–147. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sheng ZQ, Li YF, Zheng KL, Lu HH, Xie J,
Wu H and Xu B: The relationship between number and function of EPCs
and concentration of VEGF165 and SDF-1 in coronary artery spasm.
Eur Rev Med Pharmacol Sci. 22:2767–2777. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Coppolino G, Cernaro V, Placida G,
Leonardi G, Basile G and Bolignano D: Endothelial progenitor cells
at the interface of chronic kidney disease: From biology to
therapeutic advancement. Curr Med Chem. 25:4545–4551.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ammendola M, Leporini C, Luposella M,
Sacco R, Sammarco G, Russo E, Patruno R, De Sarro G and Ranieri G:
Targeting endothelial progenitor cells in cancer as a novel
biomarker and anti-angiogenic therapy. Curr Stem Cell Res Ther.
10:181–187. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nagasawa T, Kikutani H and Kishimoto T:
Molecular cloning and structure of a pre-B-cell growth-stimulating
factor. Proc Natl Acad Sci USA. 91:2305–2309. 1994.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ratajczak M, Zuba-Surma E, Kucia M, Reca
R, Wojakowski W and Ratajczak J: The pleiotropic effects of the
SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis.
Leukemia. 20:1915–1924. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Möhle R, Bautz F, Rafii S, Moore MA,
Brugger W and Kanz L: The chemokine receptor CXCR-4 is expressed on
CD34+ hematopoietic progenitors and leukemic cells and mediates
transendothelial migration induced by stromal cell-derived
factor-1. Blood. 91:4523–4530. 1998.PubMed/NCBI
|
|
20
|
Walter DH, Haendeler J, Reinhold J,
Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R,
Arenzana-Seisdesdos F, et al: Impaired CXCR4 signaling contributes
to the reduced neovascularization capacity of endothelial
progenitor cells from patients with coronary artery disease. Circ
Res. 97:1142–1151. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hattori K, Heissig B, Tashiro K, Honjo T,
Tateno M, Shieh JH, Hackett NR, Quitoriano MS, Crystal RG, Rafii S
and Moore MA: Plasma elevation of stromal cell-derived factor-1
induces mobilization of mature and immature hematopoietic
progenitor and stem cells. Blood. 97:3354–3360. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hoenig MR, Bianchi C and Sellke FW:
Hypoxia inducible factor-1α, endothelial progenitor cells,
monocytes, cardiovascular risk, wound healing, cobalt and
hydralazine: A unifying hypothesis. Curr Drug Targets. 9:422–435.
2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yamaguchi JI, Kusano KF, Masuo O, Kawamoto
A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner
JM and Asahara T: Stromal cell-derived factor-1 effects on ex vivo
expanded endothelial progenitor cell recruitment for ischemic
neovascularization. Circulation. 107:1322–1328. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kawakami Y, Ii M, Matsumoto T, Kuroda R,
Kuroda T, Kwon SM, Kawamoto A, Akimaru H, Mifune Y, Shoji T, et al:
SDF-1/CXCR4 axis in Tie2-lineage cells including endothelial
progenitor cells contributes to bone fracture healing. J Bone Miner
Res. 30:95–105. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Ganju RK, Brubaker SA, Meyer J, Dutt P,
Yang Y, Qin S, Newman W and Groopman JE: The α-chemokine, stromal
cell-derived factor-1alpha, binds to the transmembrane
G-protein-coupled CXCR-4 receptor and activates multiple signal
transduction pathways. J Biol Chem. 273:23169–23175.
1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Libura J, Drukala J, Majka M, Tomescu O,
Navenot JM, Kucia M, Marquez L, Peiper SC, Barr FG,
Janowska-Wieczorek A and Ratajczak MZ: CXCR4-SDF-1 signaling is
active in rhabdomyosarcoma cells and regulates locomotion,
chemotaxis, and adhesion. Blood. 100:2597–2606. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zeng Y, Wang X, Yin B, Xia G, Shen Z, Gu W
and Wu M: Role of the stromal cell derived factor-1/CXC chemokine
receptor 4 axis in the invasion and metastasis of lung cancer and
mechanism. J Thorac Dis. 9:4947–4959. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang P, Wang G, Huo H, Li Q, Zhao Y and
Liu Y: SDF-1/CXCR4 signaling up-regulates survivin to regulate
human sacral chondrosarcoma cell cycle and epithelial-mesenchymal
transition via ERK and PI3K/AKT pathway. Med Oncol.
32(377)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liao A, Shi R, Jiang Y, Tian S, Li P, Song
F, Qu Y, Li J, Yun H and Yang X: SDF-1/CXCR4 axis regulates cell
cycle progression and epithelial-mesenchymal Transition via
up-regulation of survivin in glioblastoma. Mol Neurobiol.
53:210–215. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Porcile C, Bajetto A, Barbieri F, Barbero
S, Bonavia R, Biglieri M, Pirani P, Florio T and Schettini G:
Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) stimulates
ovarian cancer cell growth through the EGF receptor
transactivation. Exp Cell Res. 308:241–253. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lu Y, Hu B, Guan GF, Chen J, Wang CQ, Ma
Q, Wen YH, Qiu XC, Zhang XP and Zhou Y: SDF-1/CXCR4 promotes F5M2
osteosarcoma cell migration by activating the Wnt/β-catenin
signaling pathway. Med Oncol. 32(194)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Peng SB, Peek V, Zhai Y, Paul DC, Lou Q,
Xia X, Eessalu T, Kohn W and Tang S: Akt activation, but not
extracellular signal-regulated kinase activation, is required for
SDF-1α/CXCR4-mediated migration of epitheloid carcinoma cells. Mol
Cancer Res. 3:227–236. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Segal MS, Shah R, Afzal A, Perrault CM,
Chang K, Schuler A, Beem E, Shaw LC, Li Calzi S, Harrison JK, et
al: Nitric oxide cytoskeletal-induced alterations reverse the
endothelial progenitor cell migratory defect associated with
diabetes. Diabetes. 55:102–109. 2006.PubMed/NCBI
|
|
35
|
Zheng H, Fu G, Dai T and Huang H:
Migration of endothelial progenitor cells mediated by stromal
cell-derived factor-1α/CXCR4 via PI3K/Akt/eNOS signal transduction
pathway. J Cardiovasc Pharmacol. 50:274–280. 2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: In: Guide for the Care and Use of Laboratory Animals
National Academies Press (US) Copyright©. 2011, National
Academy of Sciences, Washington (DC), 2011.
|
|
37
|
Kruger NJ: The Bradford method for protein
quantitation. Methods Mol Biol. 32:9–15. 1994.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Balaji S, King A, Crombleholme TM and
Keswani SG: The role of endothelial progenitor cells in postnatal
vasculogenesis: Implications for therapeutic neovascularization and
wound healing. Adv Wound Care (New Rochelle). 2:283–295.
2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lu C, Zhang J, Zhang D, Uzan G and Li M:
EPCs in vascular repair: How can we clear the hurdles between bench
and bedside? Front Biosci (Landmark Ed). 19(34)2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li L, Liu H, Xu C, Deng M, Song M, Yu X,
Xu S and Zhao X: VEGF promotes endothelial progenitor cell
differentiation and vascular repair through connexin 43. Stem Cell
Res Ther. 8(237)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li DW, Liu ZQ, Wei J, Liu Y and Hu LS:
Contribution of endothelial progenitor cells to neovascularization.
Int J Mol Med. 30:1000–1006. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dimmeler S and Zeiher AM: Vascular repair
by circulating endothelial progenitor cells: The missing link in
atherosclerosis? J Mol Med (Berl). 82:671–677. 2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu SQ, Tefft BJ, Zhang D, Roberts D,
Schuster DJ and Wu A: Cardioprotective mechanisms activated in
response to myocardial ischemia. Mol Cell Biomech. 8:319–338.
2011.PubMed/NCBI
|
|
44
|
Ahluwalia A, Jones MK, Matysiakbudnik T
and Tarnawski AS: VEGF and colon cancer growth beyond angiogenesis:
Does VEGF directly mediate colon cancer growth via a non-angiogenic
mechanism? Curr Pharm Des. 20:1041–1044. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Odent Grigorescu G, Rosca AM, Preda MB,
Tutuianu R, Simionescu M and Burlacu A: Synergic effects of VEGF-A
and SDF-1 on the angiogenic properties of endothelial progenitor
cells. J Tissue Eng Regen Med. 11:3241–3252. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lipfert J, Odemis V, Wagner D, Boltze J
and Engele J: CXCR4 and CXCR7 form a functional receptor unit for
SDF-1/CXCL12 in primary rodent microglia. Neuropathol Appl
Neurobiol. 39:667–680. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Balabanian K, Lagane B, Infantino S, Chow
KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M and
Bachelerie F: The chemokine SDF-1/CXCL12 binds to and signals
through the orphan receptor RDC1 in T lymphocytes. J Biol Chem.
280:35760–35766. 2005.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Burns JM, Summers BC, Wang Y, Melikian A,
Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ,
et al: A novel chemokine receptor for SDF-1 and I-TAC involved in
cell survival, cell adhesion, and tumor development. J Exp Med.
203:2201–2213. 2006.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Rajagopal S, Kim J, Ahn S, Craig S, Lam
CM, Gerard NP, Gerard C and Lefkowitz RJ: β-arrestin-but not G
protein-mediated signaling by the ‘decoy’ receptor CXCR7. Proc Natl
Acad Sci USA. 107:628–632. 2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Puchert M and Engele J: The peculiarities
of the SDF-1/CXCL12 system: In some cells, CXCR4 and CXCR7 sing
solos, in others, they sing duets. Cell Tissue Res. 355:239–253.
2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Badillo AT, Chung S, Zhang L, Zoltick P
and Liechty KW: Lentiviral gene transfer of SDF-1α to wounds
improves diabetic wound healing. J Surg Res. 143:35–42.
2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yoshida D, Nomura R and Teramoto A:
Signalling pathway mediated by CXCR7, an alternative chemokine
receptor for stromal-cell derived factor-1α, in AtT20 mouse
adrenocorticotrophic hormone-secreting pituitary adenoma cells. J
Neuroendocrinol. 21:481–488. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hartmann TN, Grabovsky V, Pasvolsky R,
Shulman Z, Buss EC, Spiegel A, Nagler A, Lapidot T, Thelen M and
Alon R: A crosstalk between intracellular CXCR7 and CXCR4 involved
in rapid CXCL12-triggered integrin activation but not in
chemokine-triggered motility of human T lymphocytes and CD34+
cells. J Leukoc Biol. 84:1130–1140. 2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Boldajipour B, Mahabaleshwar H, Kardash E,
Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q and Raz E:
Control of chemokine-guided cell migration by ligand sequestration.
Cell. 132:463–473. 2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pozzobon T, Goldoni G, Viola A and Molon
B: CXCR4 signaling in health and disease. Immunol Lett. 177:6–15.
2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Chen L, Wu F, Xia WH, Zhang YY, Xu SY,
Cheng F, Liu X, Zhang XY, Wang SM and Tao J: CXCR4 gene transfer
contributes to in vivo reendothelialization capacity of endothelial
progenitor cells. Cardiovasc Res. 88:462–470. 2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wu Q, Shao H, Darwin Eton D, Li J, Li J,
Yang B, Webster KA and Yu H: Extracellular calcium increases CXCR4
expression on bone marrow-derived cells and enhances
pro-angiogenesis therapy. J Cell Mol Med. 13:3764–3773.
2009.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Rai SN, Dilnashin H, Birla H, Singh SS,
Zahra W, Rathore AS, Singh BK and Singh SP: The role of PI3K/Akt
and ERK in neurodegenerative disorders. Neurotox Res. 35:775–795.
2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Everaert BR, Van Craenenbroeck EM, Hoymans
VY, Haine SE, Van Nassauw L, Conraads VM, Timmermans JP and Vrints
CJ: Current perspective of pathophysiological and interventional
effects on endothelial progenitor cell biology: Focus on
PI3K/AKT/eNOS pathway. Int J Cardiol. 144:350–366. 2010.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Keshavarz S, Nassiri SM, Siavashi V and
Alimi NS: Regulation of plasticity and biological features of
endothelial progenitor cells by MSC-derived SDF-1. Biochim Biophys
Acta Mol Cell Res. 1866:296–304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Guo S, Yu L, Cheng Y, Li C, Zhang J, An J,
Wang H, Yan B, Zhan T, Cao Y, et al: PDGFRβ triggered by bFGF
promotes the proliferation and migration of endothelial progenitor
cells via p-ERK signalling. Cell Biol Int. 36:945–950.
2012.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Wang P, Zhang H, Li Z, Liu X, Jin Y, Lei
M, Jiao Z, Bi Y and Guo W: Low-dose radiation promotes the
proliferation and migration of AGE-treated endothelial progenitor
cells derived from bone marrow via activating SDF-1/CXCR4/ERK
signaling pathway. Radiat Res. 191:518–526. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Rosell A, Arai K, Lok J, He T, Guo S,
Navarro M, Montaner J, Katusic ZS and Lo EH: Interleukin-1β
augments angiogenic responses of murine endothelial progenitor
cells in vitro. J Cereb Blood Flow Metab. 29:933–943.
2009.PubMed/NCBI View Article : Google Scholar
|