Introduction
Pulmonary embolism (PE) is a fatal disease of the
cardiovascular system with nonspecific clinical symptoms and
significant potential morbidity and mortality (1). In the US, PE is considered to be the
third leading cause of cardiovascular death after myocardial
infarction and stroke (2,3). In China, the annual incidence of PE
sharply increased from 0.0% in 1997 to 0.1% in 2003, and then
remained at 0.1% through to 2008; the case fatality rate decreased
from 25.1% in 1997 to 8.6% in 2008 in a multicenter registration
study of 16,972,182 hospital admissions, among which a total 18,206
patients were confirmed to have PE (4). Patients with no definitive diagnosis
had a mortality rate of 30% if untreated, 11% of patients died
within the first hour of admission to hospitals (5) but the mortality rate may be <10% if
patients are diagnosed and treated in time (6,7). CT
pulmonary angiography (CTPA) is currently considered as the
first-line modality and the reference standard for PE diagnosis due
to its high diagnostic accuracy (8,9).
However, avoiding X-ray radiation is of great concern for younger
patients and pregnant females. Furthermore, certain patients have
allergic reactions to CT iodinated contrast material. The incidence
of contrast material-related nephropathy after CTPA may reach 4%
overall and 12% in patients with paired (pre- and post-CTPA)
creatinine measurements (10).
As an ionizing radiation-free method, MRI offers an
alternative to CTPA for pulmonary vasculature evaluation (11,12)
that does not require iodinated contrast material, and the
combination of a comprehensive MR scanning protocol is able to
achieve a performance close to that of CTPA (13,14),
including various MR sequences such as true fast imaging with
steady-state precession (true FISP), T2-weighted single-shot
half-Fourier turbo spin echo, MR pulmonary perfusion imaging and
contrast-enhanced MR pulmonary angiography (MRPA) by using a
three-dimensional (3D) fast low-angle shot spoiled gradient echo
(FLASH) and contrast-enhanced volumetric interpolated body
examination (VIBE). However, the longer examination time and the
requirements for patients to hold their breath multiple times
during MR pulmonary imaging have limited the widespread application
of MRI for the primary diagnosis of PE in clinical practice. For
certain critically ill patients who cannot tolerate a long scanning
time and numerous breath-hold sequences (15), it is necessary and important to scan
the most accurate MR sequences as fast as possible in order to
obtain the highest accuracy for PE diagnosis with a relatively
short acquisition time.
The accuracy of MRI for PE detection has been
studied based on different MR protocols in different centers and
the evaluations were generally performed on per-patient,
per-embolus or per-lobe bases (12,13,15,16),
but the diagnostic performance of MR sequences for displaying
different levels of pulmonary arteries affected by emboli has
rarely been reported. Therefore, the present study aimed to
investigate the diagnostic accuracy of three MR sequences that were
clinically used at our hospital for PE diagnosis on per-patient and
per-vessel bases, including true FISP, contrast-enhanced MRPA and
VIBE compared with CTPA, in order to focus on a more relatively
time-effective MR sequence, particularly for emergency
patients.
Materials and methods
Study population
The present prospective study was approved by The
Medical Ethics Committee of Huazhong University of Science and
Technology and written informed consent was obtained from all
patients. One of the patients was 17 years old and written informed
consent was obtained from this patient himself and his legal
guardians. A total of 21 patients [15 males, 6 females; mean age,
51.38±5.70 years (range, 17 to 66 years)] with confirmed deep
venous thrombosis with suspected acute PE underwent
320-multidetector CTPA between January 2017 and December 2017, and
they all agreed to undergo MRI examinations within 24 h after CTPA.
Of all the patients, 3 had received recent surgical treatment, 2
had a history of malignancy, 2 had a recent history of trauma, 3
had moderate to severe arterial stenosis of the lower limbs, 2 had
a history of varicose veins of the lower extremities and the
remaining patients had no definite reason for leg swelling and
pain. The most commonly present chest symptoms were chest pain,
cough and sudden shortness of breath. The exclusion criteria were
as follows: i) Pregnancy; ii) inability to undergo MRI (e.g. due to
severe dyspnea, continuous cough or shock); iii) contraindications
for MRI examination, including adverse reactions to MR contrast
material; and iv) acute or chronic severe renal impairment
(estimated glomerular filtration rate <30 ml/min/1.73
m2).
CTPA protocol
CTPA scans were acquired on a 320-multidetector CT
scanner (Aquilion One; Toshiba Medical Systems). A single bolus of
40 ml nonionic contrast agent (300 mgI/ml; Lomeron; Patheon Italia
S.P.A.) was injected by a power injector (Stellant, CT injection
system MedRad; Bayer Healthcare) through an intravenous antecubital
catheter at 4.5 ml/sec, followed by a 40 ml saline bolus at 4.5
ml/sec. The threshold level for triggering was set at 80 HU and the
region of interest (ROI) was placed in the main trunk of the
pulmonary artery. Image acquisition was started 3 sec after
exceeding the threshold of the measured ROI. The scanning
parameters were as follows: Field of view, 35 cm; tube voltage, 100
kV; tube current, 100 mA with an automatic tube current modulation
technique; gantry rotation time, 0.5 sec; collimation, 160x0.5 mm;
and slice thickness, 1 mm with an 0.8-mm interval. Patients were
instructed to hold their breath 2 times for 8-10 sec each time.
MRI
MRI was performed with a 1.5 T MR scanner (Magnetom
Aera; Siemens Medical Systems). Patients were positioned in a
supine position with the head oriented toward the magnet. One
abdominal surface flex coil with 18 channels covered the chest
area. Gadopentetate dimeglumine (Magnevist; Bayer Schering Pharma
AG) was injected into the right antecubital vein via a 22-gauge
needle using a power injector (Spectris; MedRad). The MRPA sequence
was initiated by using the bolus tracking technique, with 0.1
mmol/kg contrast material injected at 2.5 ml/sec and followed by a
15-ml saline flush at 2.5 ml/sec.
MR scanning was performed in a fixed order with the
following sequences: i) True FISP in coronal and axial orientations
without contrast material and no need for breath-hold; ii)
contrast-enhanced MRPA scanned by subtraction of 3D-FLASH sequences
from prior to and after administration of the contrast agent in
coronal orientation, with 3 times for breath-hold, including one
time for pre-contrast and the other two times for post-contrast for
18 sec each time; and iii) T1-weighted fat-suppressed VIBE in
coronal and axial orientations with 3 times for breath-hold, 18 sec
each time. The detailed parameters are listed in Table I.
 | Table IDetailed parameters of true FISP,
contrast-enhanced MRPA and VIBE sequences. |
Table I
Detailed parameters of true FISP,
contrast-enhanced MRPA and VIBE sequences.
| Parameters | true FISP | MRPA | VIBE |
|---|
| Field of view
(mm) | 400 | 400 | 400 |
| Repetition time
(msec) | 3.78 | 2.82 | 3.55 |
| Echo time (msec) | 1.89 | 0.94 | 1.35 |
| Flip angle
(degrees) | 70 | 25 | 25 |
| Slice thickness
(mm) | 4.0 | 1.4 | 1.5 |
| Base
resolution | 256 | 384 | 448 |
| Phase resolution
(%) | 100 | 70 | 75 |
| Bandwidth
(Hz/Px) | 1028 | 450 | 470 |
Data analysis
PE diagnosis on MR scans was made according to the
presence of an intraluminal filling defect in the pulmonary
arteries or the complete absence of vessel enhancement (16,17).
The results of three sequences were initially confirmed by using
CTPA as the reference standard.
CTPA images were retrospectively reviewed by two
radiologists [X.K. and H.M. with 13 and 15 years of experience,
respectively, in both chest CT and MRI] who were blinded to the MR
images. If there was a disagreement between judgements of the two
radiologists, a consensus was obtained for the final results after
discussion. The location and presence of emboli on CTPA were
recorded in each pulmonary artery by anatomic distribution. To
determine the effect of recall bias on accuracy in the current
study, >3 months later, the two radiologists analyzed the same
MR images for the evidence of pulmonary emboli, which was
classified into central, lobar and segmental levels by pulmonary
arterial distribution. The central level contained the main
pulmonary trunk, the left pulmonary artery and the right pulmonary
artery. The lobar level was divided into the right upper, middle
and lower lobes; left upper lobe; lingula and left lower lobe. The
segmental level contained 18 segmental pulmonary arteries.
Therefore, emboli were assessed for 27 vessel segments per patient
for a total of 567 vessel segments in 21 patients.
If examination of more than three lobar or half of
the segmental pulmonary arteries failed to achieve diagnostic
quality, the entire sequence was defined as nondiagnostic. If
images of all three of the sequences were nondiagnostic, the whole
MR scan for the patient was defined as a failed MR examination. A
single vessel was considered nondiagnostic if the vessels were not
recognizable for identification or exclusion of PE due to vascular
ambiguity (18). If there were no
significant respiratory motion artifacts and there was clear
visualization of the segmental pulmonary arteries, the images were
defined as satisfactory (19). The
number and anatomical location of emboli were recorded for further
calculation. The evaluation was performed and represented a
consensus interpretation. The CT and MR results previously obtained
were compared >2 months later to examine if emboli detected on
MRI were observed as corresponding lesions on CTPA.
Statistical analysis
Data of three sequences for presence of PE was
classified into 1 (PE positive) and 0 (PE negative). The
sensitivity, specificity, positive predictive value (PPV) and
negative predictive value (NPV) of each sequence for PE detection
were calculated. Receiver operating characteristic (ROC) curve
analysis was used to calculate the area under the curve (AUC) using
95% confidence intervals (CIs). The method by Delong et al
(20) was used to compare the AUCs
between different sequences by MedCalc software (version 18.11.3;
MedCalc Software, Ltd). P<0.05 was considered to indicate a
statistically significant difference.
Results
General
None of the patients failed the whole MR scans.
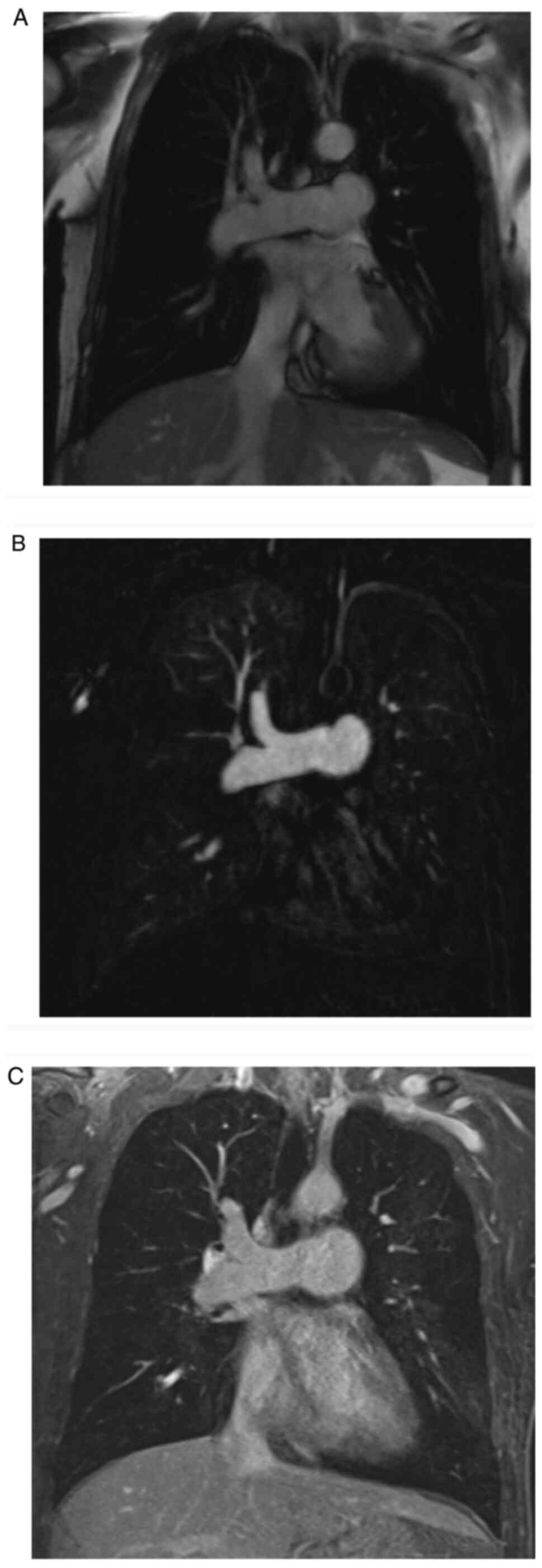
Representative images of true FISP, MRPA and VIBE with negative PE
are presented in Fig. 1. Only one
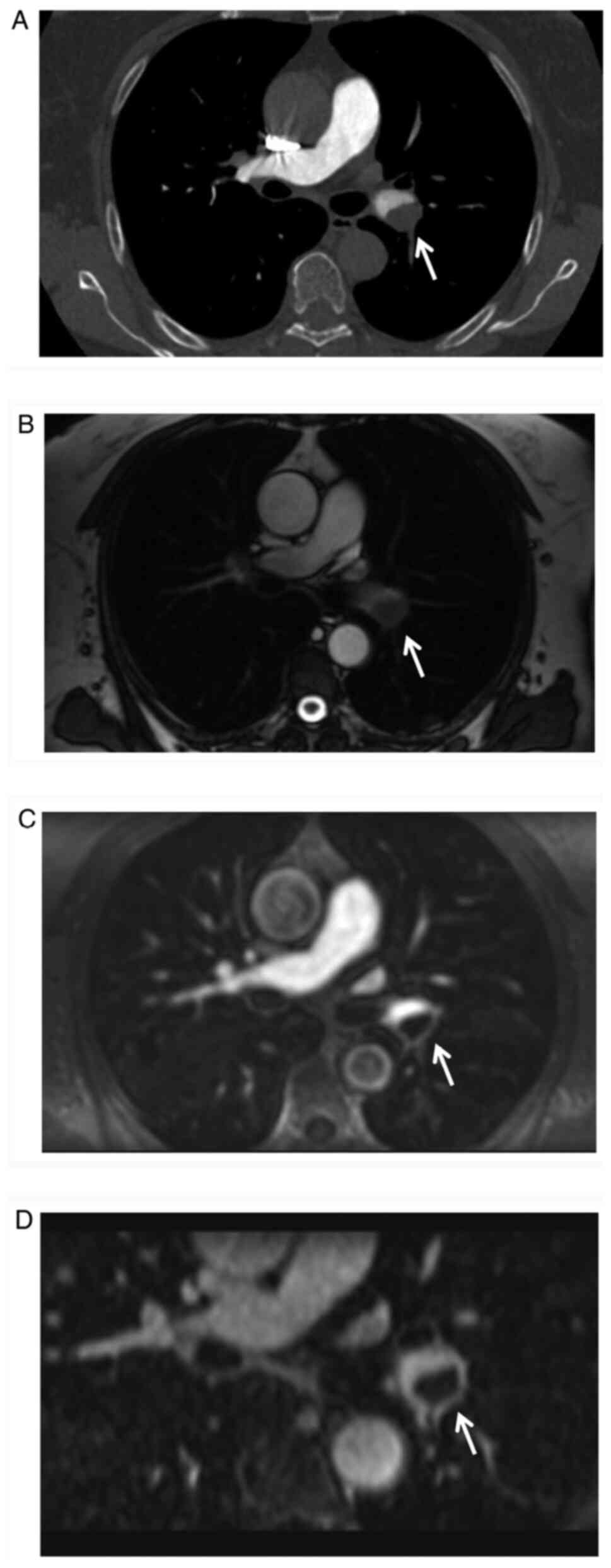
patient exhibited a mild allergic reaction after CTPA scanning
(Fig. 2), while all other patients
completed MR scanning successfully without any allergic reactions.
CTPA revealed 45 pulmonary emboli in total, while MRI detected 36
in total, including 23 with true FISP, 25 with MRPA and 36 with
VIBE. Of the 9 emboli not identified by MRI, 7 were in the
segmental pulmonary arterial branches and the other 2 were in the
truncation between lobar and segmental levels.
Analysis on a per-patient basis
CTPA revealed PE in 18 patients, while MRI detected
PE in 17 patients. The one patient missed by MRI had only one small
isolated embolus in a segmental pulmonary arterial branch. True
FISP indicated one false-positive finding due to misinterpretation
of the pulmonary vein as a pulmonary artery. MRPA of one patient
was excluded from the final results due to severe respiratory
motion artifacts caused by coughing during the acquisition. The
differences for PE detection were not significant (true FISP vs.
MRPA: P=0.2466; true FISP vs. VIBE: P=0.1414; MRPA vs. VIBE:
P=0.1441; Table II). The ROC
curves are displayed in supplementary material of Fig. S1A.
 | Table IIDiagnostic performance and
statistical results of MR sequences for pulmonary emboli detection
on a per-patient basis (A) and per-vessel basis (B). |
Table II
Diagnostic performance and
statistical results of MR sequences for pulmonary emboli detection
on a per-patient basis (A) and per-vessel basis (B).
| A, Per-patient
basis |
|---|
| | Number of
patients | MR diagnostic
performance | ROC curve analysis
results |
|---|
| MR sequence | TP | FP | FN | TN | Sens. (%) | Spec. (%) | PPV (%) | NPV (%) | AUC | 95% CI |
|---|
| True FISP | 13 | 1 | 3 | 4 | 81.3 | 80.0 | 92.9 | 57.1 | 0.716 | 0.473-0.892 |
| MRPA | 14 | 0 | 3 | 3 | 82.4 | 100.0 | 100.0 | 50.0 | 0.912 | 0.698-0.991 |
| VIBE | 17 | 0 | 1 | 3 | 94.4 | 100.0 | 100.0 | 75.0 | 0.971 | 0.782-1.000 |
| B, Per-vessel
basis |
| | Number of
patients | MR diagnostic
performance | ROC curve analysis
results |
| MR sequence | TP | FP | FN | TN | Sens. (%) | Spec. (%) | PPV (%) | NPV (%) | AUC | 95% CI |
| True FISP | 58 | 1 | 100 | 408 | 36.7 | 99.8 | 98.3 | 80.3 | 0.692 | 0.647-0.734 |
| MRPA | 63 | 3 | 49 | 365 | 56.3 | 99.2 | 95.5 | 88.2 | 0.785 | 0.744-0.822 |
| VIBE | 94 | 3 | 44 | 389 | 68.1 | 99.2 | 96.9 | 89.8 | 0.873 | 0.839-0.902 |
Analysis on a per-vessel basis
A total of 158 pulmonary vessels (27.9%, 158/567)
were affected by emboli on CTPA and 97 of them (17.1%, 97/567) were
identified on MRI. Altogether, 29 vessels (5.1%, 29/567) were
excluded from comparison between CTPA and MRI, as those vessels
were excluded simultaneously in two MR sequences. A total of 58
vessels (10.2%, 58/567) with emboli involvement were detected by
true FISP, 62 by MRPA (10.9%, 62/567) and 94 by VIBE (16.6%,
94/567). By contrast, 87 vessels from MRPA (15.3%, 87/567) and 37
from VIBE (6.5%, 37/567) were excluded from the evaluation due to
severe respiratory motion artifacts, pleural effusion and pulmonary
atelectasis.
The differences in the AUCs among the three
sequences based on pulmonary arterial vessels were significant
(true FISP vs. MRPA: P=0.0016; true FISP vs. VIBE: P<0.0001;
MRPA vs. VIBE: P=0.0004; Table
II). The ROC curves are displayed in Fig. S1B. On per-patient and per-vessel
bases, true FISP had an accuracy of 81.0 and 82.2% respectively,
MRPA had an accuracy of 85.0 and 89.2% respectively, and VIBE had
an accuracy of 95.2 and 91.1% respectively; the sensitivity for
true FISP was 81.3 and 36.7% respectively, for MRPA was 82.4 and
56.3% respectively, and for VIBE was 94.4 and 68.1% respectively;
the specificity for true FISP was 80.0 and 99.8% respectively, for
MRPA was 100 and 99.2% respectively, and for VIBE was 100 and 99.2%
respectively. The respective PPV was 92.9 and 98.3% for true FISP,
100 and 95.5% for MRPA, 100 and 96.9% for VIBE. The NPV was 57.1
and 80.3% respectively for true FISP, 50.0 and 88.2% respectively
for MRPA, and 75.0 and 89.8% respectively for VIBE.
All the central pulmonary arteries were classified
as assessable for PE detection. CTPA revealed 10 central pulmonary
arteries with emboli; 9 were detected by true FISP, 8 by MRPA and
10 by VIBE. The differences in the AUCs among the three sequences
based on the pulmonary arterial segments were not significant (true
FISP vs. MRPA: P=0.3173; true FISP vs. VIBE: P=0.3173; MRPA vs.
VIBE: P=0.1336; Table III). The
ROC curves are displayed in supplementary material of Fig. S2A.
 | Table IIIDiagnostic accuracy of MR sequences
for detecting pulmonary embolism at the central, lobar and
segmental pulmonary levels. |
Table III
Diagnostic accuracy of MR sequences
for detecting pulmonary embolism at the central, lobar and
segmental pulmonary levels.
| A, Central
pulmonary arterial level |
|---|
| | Number of
vessels | MR diagnostic
performance | ROC curve analysis
results |
|---|
| MR sequences | TP | FP | FN | TN | Sens. (%) | Spec. (%) | PPV (%) | NPV (%) | AUC | 95% CI |
|---|
| True FISP | 9 | 0 | 1 | 53 | 90.0 | 100.0 | 100.0 | 98.1 | 0.950 | 0.864-0.989 |
| MRPA | 8 | 0 | 2 | 53 | 80.0 | 100.0 | 100.0 | 96.4 | 0.900 | 0.798-0.961 |
| VIBE | 10 | 0 | 0 | 53 | 100.0 | 100.0 | 100.0 | 100.0 | 1.000a | 0.943-1.000 |
| B, Lobar level |
| MR sequences | TP | FP | FN | TN | Sens. (%) | Spec. (%) | PPV (%) | NPV (%) | AUC | 95% CI |
| True FISP | 29 | 0 | 12 | 85 | 70.7 | 100.0 | 100.0 | 87.6 | 0.862 | 0.778-0.923 |
| MRPA | 24 | 1 | 10 | 69 | 70.6 | 98.6 | 96.0 | 87.3 | 0.914 | 0.840-0.961 |
| VIBE | 31 | 1 | 5 | 81 | 86.1 | 98.8 | 96.9 | 94.2 | 0.941 | 0.875-0.978 |
| C, Segmental
level |
| MR sequences | TP | FP | FN | TN | Sens. (%) | Spec. (%) | PPV (%) | NPV (%) | AUC | 95% CI |
| True FISP | 20 | 1 | 88 | 269 | 18.5 | 99.6 | 95.2 | 75.4 | 0.580 | 0.521-0.637 |
| MRPA | 30 | 2 | 42 | 239 | 41.7 | 99.2 | 93.8 | 85.1 | 0.712 | 0.657-0.763 |
| VIBE | 53 | 2 | 39 | 255 | 73.6 | 99.2 | 96.4 | 86.7 | 0.824 | 0.776-0.866 |
For the analysis at the pulmonary lobar level, 22
pulmonary arterial vessels from MRPA and 8 from VIBE were excluded
from the assessment due to severe respiratory motion artifacts.
CTPA revealed 40 lobar pulmonary arteries with emboli, while true
FISP detected 29; MRPA 24 and VIBE detected 31. The differences in
the AUCs among the three sequences based on the pulmonary lobes
were not significant (true FISP vs. MRPA: P=0.3173; true FISP vs.
VIBE: P=0.1179; MRPA vs. VIBE: P=0.4375; Fig. 2; Table
III). The ROC curves are displayed in Fig. S2B.
Regarding the evaluation of the segmental pulmonary
vessels, 65 from MRPA and 29 from VIBE were excluded from
evaluation due to severe respiratory motion artifacts, pleural
effusion and pulmonary atelectasis. CTPA revealed 108 segmental
pulmonary arteries affected by emboli, while true FISP detected 20,
MRPA 30 and VIBE detected 53. The differences in the AUCs among the
three sequences were significant (true FISP vs. MRPA: P=0.0008;
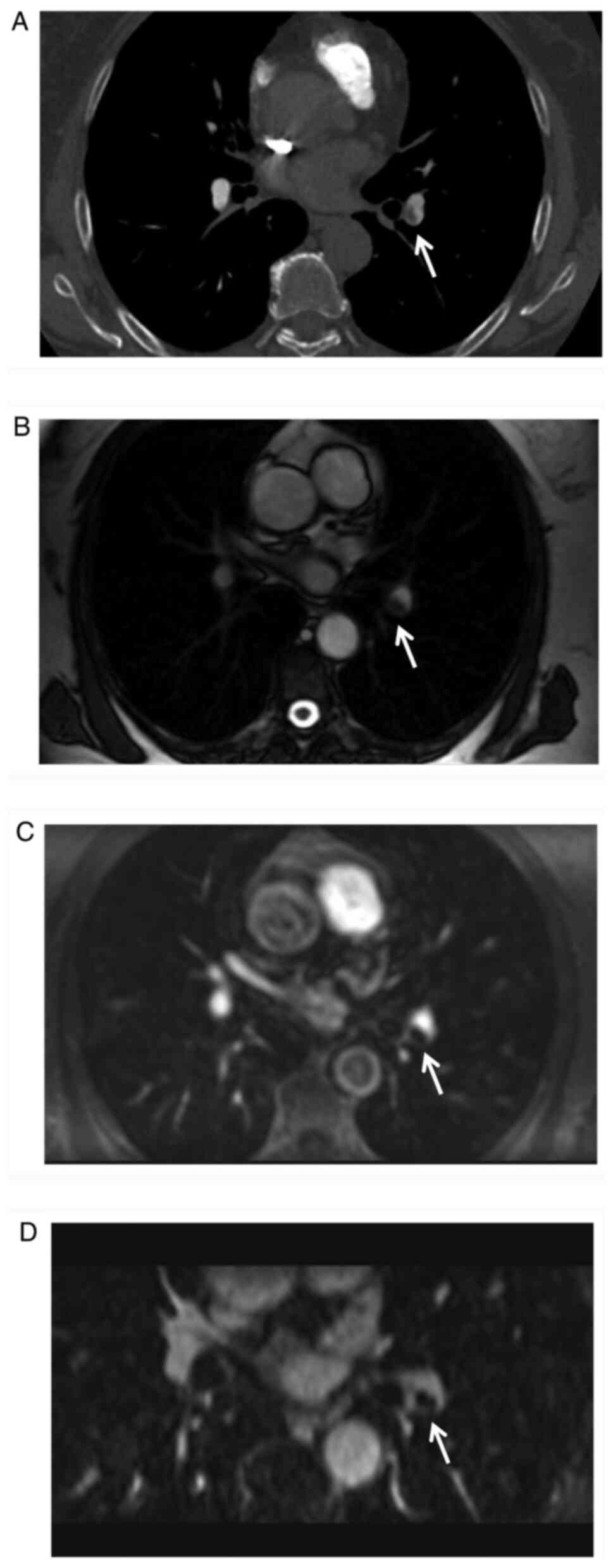
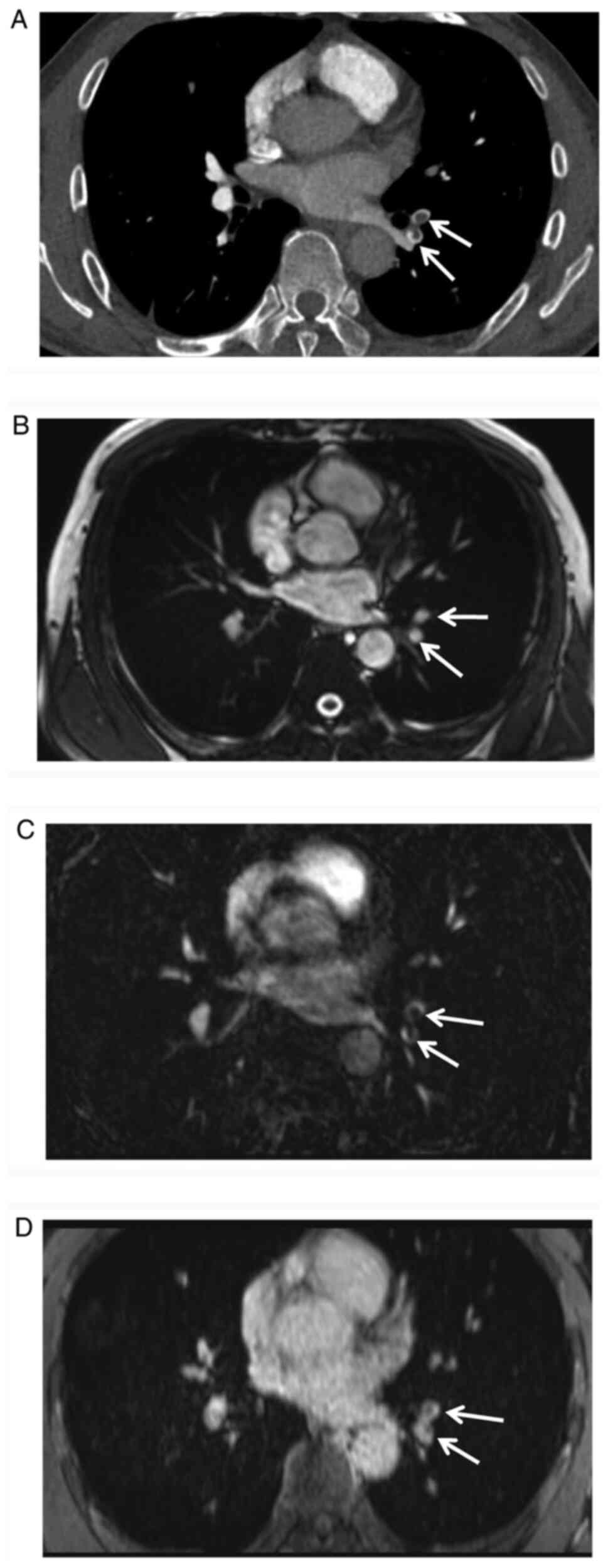
true FISP vs. VIBE: P<0.0001; MRPA vs. VIBE: P=0.0014; Figs. 3 and 4; Table
III). The ROC curves were displayed in supplementary material
of Fig. S2C. From central-lobar
level to the segmental level, sensitivity of true FISP changed from
70.7-90.0 to 18.5%, specificity ranged from 99.6-100%; sensitivity
of MRPA changed from 70.6-80.0 to 41.7%, specificity ranged from
98.6-100%; sensitivity of VIBE changed from 86.1-100 to 73.6%,
specificity ranged from 98.8-100%.
Discussion
The present study aimed to investigate the
diagnostic value of three MR sequences (true FISP, MRPA and VIBE)
for PE detection compared with CTPA. The results revealed enhanced
VIBE to be the most accurate sequence, with 95.2% accuracy on a
per-patient basis and 91.1% accuracy on a per-vessel basis.
The three sequences evaluated in the present study
were most commonly used in everyday clinical practice at our
hospital and patients were only required to hold their breath 6
times during the whole protocol. The total time for patients on the
scanner bed was controlled within 10 min, which achieved a
relatively fast MR protocol for patients suspected of having PE.
Indeed, radiologists were not able to diagnose PE on MRI by
evaluating only one sequence in clinical practice, but it is
necessary to determine the diagnostic ability of different
sequences for PE, particularly for emergency patients and
critically ill patients. For those patients, particularly when they
have contraindications for CTPA, contrast-enhanced VIBE should be
preferentially considered.
True FISP in MRI has been proven to be useful for
depicting lung parenchyma and the pulmonary vasculature without the
requirement for breath-holding or contrast material (18). Since it is a true fast imaging
technique with steady-state precession sequences that can generate
predominantly T2-weighted contrast images, true FISP is able to
depict the vessel wall and embolus clearly as the thrombi displays
hypointensity. Furthermore, this sequence is not sensitive to
motion degradation, allowing patients who cannot successfully hold
their breath to be scanned. It has been reported to achieve 62-93%
sensitivity and 95-100% specificity (13,15,16,21).
Similar to those results, the present study determined that true
FISP had a specificity of 80.0 and 99.8% on a per-patient basis and
per-vessel basis, respectively, and the sensitivity was 81.3% in
the per-patient analysis but only 36.7% in the per-vessel analysis.
For different levels of the pulmonary arteries, the specificity
ranged from 99.6 to 100% irrespective of the location of pulmonary
vessels, while the sensitivity decreased from the central-lobar
level (70.7-90.0%) to the segmental level (18.5%) and its ability
to depict the peripheral pulmonary vasculature was diminished more
than that for the central pulmonary arteries (22), which is in accordance with previous
studies (13,23); however, the sensitivity at the
segmental level (18.5%) was lower than that determined in previous
studies (41.7 and 62.0%). This discrepancy may be due to the
different analyses, as a per-vessel analysis was employed to detect
the vessels that were involved with emboli at different levels;
this discrepancy may also be attributed to different thrombus
distributions in different studies. The present results initially
validated true FISP to be feasible for PE detection in the central
and lobar pulmonary arteries, but inadequate for the segmental
emboli with only 18.5% sensitivity.
It has been reported that the incidence of severe
acute adverse reactions associated with gadolinium-based contrast
material is lower than that of iodinated contrast agents (24,25),
and a large number of clinical studies related to MR for PE
diagnosis have been performed using contrast-enhanced MR (12,13,16,23).
As contrast-enhanced MRPA is a fast and flow-independent method for
assessing pulmonary vasculature, it is able to avoid saturation
effects and offer a substantially higher resolution than
non-contrast techniques. On per-patient and per-vessel bases, MRPA
in the present study had an accuracy of 85.0 and 89.2%,
respectively, a sensitivity of 82.4 and 56.3%, respectively, and a
specificity of 100 and 99.2%, respectively. For different levels of
the pulmonary arteries, the sensitivity of MRPA decreased from the
central-lobar level (70.6-80.0%) to the segmental level (41.7%),
but the specificity ranged from 98.6 to 100%, irrespective of the
different levels of the pulmonary vessels. These results were
consistent with the largest report that focused on the accuracy of
contrast-enhanced MRPA for acute PE diagnosis (12), in which technically adequate MRPA
had a 78% sensitivity and 99% specificity; the sensitivity for PE
at the main or lobar level was 79% and the specificity was 98-100%
irrespective of the order of the pulmonary vessels. The sensitivity
of MRPA on a per-lobe basis in the present study was similar to
that determined by Zhang et al (26), in which lobar, segmental and
subsegmental emboli were assigned to the distribution of the lobar
pulmonary artery-supplying territory. By contrast, in the present
study, the diagnostic performance was evaluated based on different
pulmonary arterial levels; thus, sensitivity on a per-vessel basis
(56.3%) and on a per-segmental basis (41.7%) was lower than those
determined by the above study.
By analyzing emboli that were not depicted by MRPA
at the central and lobar levels, it was determined that those
emboli were located at the truncation of central and lobar
pulmonary vessels and adhered to the vessel wall instead of
localizing at the center of the vessel lumen. The subtraction of
MRPA provides visualization of the vessel lumen with the help of
contrast material, but it has a limited ability to display the
vessel wall, which makes it difficult to visualize an eccentric
thrombus, particularly when the emboli are adherent to the vessel
wall (16).
Enhanced VIBE has been widely used for delineating
vasculatures such as the pulmonary arteries (27) and the venous system of the lower
legs (23,27-29),
as this sequence enables the vessel wall to be visualized and the
intraluminal emboli to be outlined with the help of contrast
material. With a high resolution and low slice thickness, on a
per-patient basis and per-vessel basis in the present study, this
sequence had an accuracy of 95.2 and 91.1%, respectively, a
sensitivity of 94.4 and 68.1%, respectively, and a specificity of
100 and 99.2% respectively; the highest AUC in the ROC curve
evaluation was obtained at the central, lobar and segmental levels,
and a significant difference was detected, particularly at the
segmental level, which proved VIBE to be the most accurate sequence
for PE detection among the three sequences. This result is
consistent with that of Kalb et al (16), who evaluated the presence of PE
based on eight pulmonary arteries and/or vascular territories, and
segmental and subsegmental emboli were assigned to the vascular
distribution of the lobar pulmonary arteries. However, in contrast
with their investigation, the present study included patients with
identified deep venous thrombosis who were suspected to have acute
PE, and an evaluation was performed based on the central, lobar,
segmental pulmonary arteries to investigate the abilities of three
sequences to depict the vessels affected by emboli. Due to the
recirculation phases of contrast enhancement in VIBE sequence, it
demands less experience for MR operators as it is able to eliminate
the timing failures in capturing the peak enhancement of the
pulmonary arteries, which may be a limitation of contrast-enhanced
MRPA.
CTPA has been applied in routine work, even for
severe cases and dyspneic patients in emergency settings, and only
requires short, infrequent breath-holds (30); however, for patients who are
allergic to iodinated contrast material and particularly young
patients who are aware of the ionizing radiation involved within
CTPA, MRI serves as an alternative method for pulmonary imaging, as
it requires neither ionizing radiation nor the use of iodinated
contrast material. However, it is still challenging in severely ill
patients because of the longer scan times and multiple breath-holds
required in different MR protocols. A reduced duration and
frequency of breath-holds is associated with better patient
cooperation. Of note, shorter breath-holds resulted in decreased
motion artifacts; therefore, it is crucial to keep the breath-hold
time as short as possible. In the present study, even though
patients were only required to hold their breath 6 times for 18 sec
each time, the fact that certain pulmonary vessels with severe
motion artifacts failed to receive a clear diagnosis was still a
major drawback for MRI. Therefore, it is necessary to determine the
sequence with the most accurate results. When patients have
difficulty in completely cooperating for the whole MR scanning
protocol, contrast-enhanced VIBE is adequate only with 2-3 times of
breath-hold for axial and coronal PE imaging (31). However, among the excluded vessels
in the per-vessel analysis, including 15.3% (87/567) of the
pulmonary arteries from MRPA, 6.5% (37/567) of those from VIBE and
an overall 5.1% (29/567) of the pulmonary arteries from the
combined MR protocol, 38.6% (61/158) of the vessels affected by
emboli were still missed by MRI; these results also supported the
lower diagnostic performance of MRI as compared with that of CTPA
for PE diagnosis.
The present study had several limitations: First,
the number of enrolled patients was relatively small. Furthermore,
evaluation of subsegmental pulmonary arteries was not performed,
because the sensitivity of MRI for subsegmental emboli remains
insufficient (12,16,21,30).
In addition, in a previous study (9), which focused on the comparison of
diagnostic accuracy for acute PE between CTPA and pulmonary
angiography, subsegmental PE were detected in 15 patients in
pulmonary angiography, whilst CTPA failed to demonstrate the
subsegmental vessel involvement in 8 of the 15 patients tested
(53%). Certain other advanced MR techniques, such as time-resolved
contrast-enhanced MR angiography, radial VIBE, dynamic
contrast-enhanced MR perfusion and quiescent-interval single-shot
techniques (32) should also be
considered in future studies. As another limitation, the higher
cost of MRI in comparison with that of CTPA has limited its
wide-spread application in practice. Finally, bias may exist due to
the fixed order and different slice thicknesses of the three
sequences in the present study.
In conclusion, enhanced VIBE surpassed the other two
sequences in revealing PE, with 95.2% accuracy for the per-patient
analysis and 91.1% accuracy for the per-vessel analysis,
particularly for segmental pulmonary PE, which is essential for
emergency patients who have contraindications to iodinated contrast
and those who have great concerns about ionizing radiation.
Supplementary Material
ROC curves. Analysis (A) based on
per-patient basis and (B) based on all pulmonary arterial vessel
segments. Since CT angiography was used as the gold standard for
presence/absence of pulmonary emboli, raw data of the three
sequences were displayed as either 1, which represents PE positive
and 0, which representing PE negative. Therefore, the ROC curves
contained straight lines rather than the standard curves with more
steps. AUC, area under the curve; CI, confidence interval; FISP,
fast imaging with steady-state precession; VIBE,
volume-interpolated body examination; MR, magnetic resonance.
ROC curves. Analysis based on (A)
central, (B) lobar and (C) segmental pulmonary levels (C). Since CT
angiography was used as the gold standard for presence/absence of
pulmonary emboli, raw data of the three sequences were displayed as
either 1, which represents PE positive and 0, which representing PE
negative. Therefore, the ROC curves contained straight lines rather
than the standard curves with more steps. AUC, area under the
curve; CI, confidence interval; FISP, fast imaging with
steady-state precession; VIBE, volume-interpolated body
examination; MR, magnetic resonance.
Acknowledgements
Not applicable.
Funding
No funding was recieved.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QF: Conceptualization, writing, project
administration, review and editing; QC: Methodology, review and
editing; XK and HM: Visualization, formal analysis; ZL:
Methodology, formal analysis. All authors contributed to the study
conception and design. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This prospective study was approved by the Medical
Ethics Committee of Tongji Medical College, Huazhong University of
Science and Technology (Wuhan, China). Patients were informed of
the procedures and the purpose of this study, and written consent
was obtained.
Patient consent for publication
All the patients provided written informed consent
for the publication of the associated data and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Uresandi F, Monreal M, García-Bragado F,
Domenech P, Lecumberri R, Escribano P, Zamorano JL, Jimenez S,
Ruiz-Artacho P, Lozano F, et al: National consensus on the
diagnosis, risk stratification and treatment of patients with
pulmonary embolism. Spanish society of pneumology and thoracic
surgery (SEPAR). Society Española Internal Medicine (SEMI). Spanish
Society of Thrombosis and Haemostasis (SETH). Spanish Society of
Cardiology (ESC). Spanish Society of Medicine Accident and
Emergency (SEMES). Spanish Society of Angiology and Surgery
Vascular (SEACV). Arch Bronconeumol. 49:534–547. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dronkers CE, Klok FA and Huisman MV:
Current and future perspectives in imaging of venous
thromboembolism. J Thromb Haemost. 14:1696–1710. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Goldhaber SZ and Bounameaux H: Pulmonary
embolism and deep vein thrombosis. Lancet. 379:1835–1846.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang Y, Liang L, Zhai Z, He H, Xie W, Peng
X and Wang C: Investigators for National Cooperative Project for
Prevention and Treatment of PTE-DVT. Pulmonary embolism incidence
and fatality trends in Chinese hospitals from 1997 to 2008: A
multicenter registration study. PLoS One. 6(e26861)2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dalen JE: Pulmonary embolism: What have we
learned since Virchow? Natural history, pathophysiology, and
diagnosis. Chest. 122:1440–1456. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kuriakose J and Patel S: Acute pulmonary
embolism. Radiol Clin North Am. 48:31–50. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nikolaou K, Thieme S, Sommer W, Johnson T
and Reiser MF: Diagnosing pulmonary embolism: New computed
tomography applications. J Thorac Imaging. 25:151–160.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stein PD, Fowler SE, Goodman LR,
Gottschalk A, Hales CA, Hull RD, Leeper KJ, Popovich JJ, Quinn DA,
Sos TA, et al: Multidetector computed tomography for acute
pulmonary embolism. N Engl J Med. 354:2317–2327. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Winer-Muram HT, Rydberg J, Johnson MS,
Tarver RD, Williams MD, Shah H, Namyslowski J, Conces D, Jennings
SG, Ying J, et al: Suspected acute pulmonary embolism: Evaluation
with multi-detector row CT versus digital subtraction pulmonary
arteriography. Radiology. 233:806–815. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mitchell AM and Kline JA: Contrast
nephropathy following computed tomography angiography of the chest
for pulmonary embolism in the emergency department. J Thromb
Haemost. 5:50–54. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mudge CS, Healey TT, Atalay MK and
Pezzullo JA: Feasibility of detecting pulmonary embolism using
noncontrast MRI. ISRN Radiol. 2013(729271)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stein PD, Chenevert TL, Fowler SE, Goodman
LR, Gottschalk A, Hales CA, Hull RD, Jablonski KA, Leeper KV Jr,
Naidich DP, et al: Gadolinium-enhanced magnetic resonance
angiography for pulmonary embolism: A multicenter prospective study
(PIOPED III). Ann Intern Med. 152:434–443. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hosch W, Schlieter M, Ley S, Heye T,
Kauczor HU and Libicher M: Detection of acute pulmonary embolism:
Feasibility of diagnostic accuracy of MRI using a stepwise
protocol. Emerg Radiol. 21:151–158. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Revel MP, Sanchez O, Lefort C, Meyer G,
Couchon S, Hernigou A, Niarra R, Chatellier G and Frija G:
Diagnostic accuracy of unenhanced, Contrast-enhanced perfusion and
angiographic MRI sequences for pulmonary embolism diagnosis:
Results of independent sequence readings. Eur Radiol. 23:2374–2382.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pasin L, Zanon M, Moreira J, Moreira AL,
Watte G, Marchiori E and Hochhegger B: Magnetic resonance imaging
of pulmonary embolism: Diagnostic accuracy of unenhanced MR and
influence in mortality rates. Lung. 195:193–199. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kalb B, Sharma P, Tigges S, Ray GL,
Kitajima HD, Costello JR, Chen Z and Martin DR: MR imaging of
pulmonary embolism: Diagnostic accuracy of contrast-enhanced 3D MR
pulmonary angiography, contrast-enhanced low-flip angle 3D GRE, and
nonenhanced Free-induction FISP sequences. Radiology. 263:271–278.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mamlouk MD, VanSonnenberg E, Gosalia R,
Drachman D, Gridley D, Zamora JG, Casola G and Ornstein S:
Pulmonary embolism at CT angiography: Implications for
appropriateness, cost, and radiation exposure in 2003 patients.
Radiology. 256:625–632. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kluge A, Muller C, Hansel J, Gerriets T
and Bachmann G: Real-time MR with TrueFISP for the detection of
acute pulmonary embolism: Initial clinical experience. Eur Radiol.
14:709–718. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Schiebler ML, Nagle SK, Francois CJ,
Repplinger MD, Hamedani AG, Vigen KK, Yarlagadda R, Grist TM and
Reeder SB: Effectiveness of MR angiography for the primary
diagnosis of acute pulmonary embolism: Clinical outcomes at 3
months and 1 year. J Magn Reson Imaging. 38:914–925.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
DeLong ER, DeLong DM and Clarke-Pearson
DL: Comparing the areas under two or more correlated receiver
operating characteristic curves: A nonparametric approach.
Biometrics. 44:837–845. 1988.PubMed/NCBI
|
|
21
|
Kluge A, Mueller C, Strunk J, Lange U and
Bachmann G: Experience in 207 combined MRI examinations for acute
pulmonary embolism and deep vein thrombosis. AJR Am J Roentgenol.
186:1686–1696. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ohno Y, Yoshikawa T, Kishida Y, Seki S and
Karabulut N: Unenhanced and Contrast-enhanced MR angiography and
perfusion imaging for suspected pulmonary thromboembolism. AJR Am J
Roentgenol. 208:517–530. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kaya F, Ufuk F and Karabulut N: Diagnostic
performance of contrast-enhanced and unenhanced combined pulmonary
artery MRI and magnetic resonance venography techniques in the
diagnosis of venous thromboembolism. Br J Radiol.
92(20180695)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Prince MR, Zhang H, Zou Z, Staron RB and
Brill PW: Incidence of immediate gadolinium contrast media
reactions. AJR Am J Roentgenol. 196:W138–W143. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dillman JR, Ellis JH, Cohan RH, Strouse PJ
and Jan SC: Frequency and severity of acute Allergic-like reactions
to Gadolinium-containing i.v. Contrast media in children and
adults. AJR Am J Roentgenol. 189:1533–1538. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang LJ, Luo S, Yeh BM, Zhou CS, Tang CX,
Zhao Y, Li L, Zheng L, Huang W and Lu GM: Diagnostic accuracy of
Three-dimensional Contrast-enhanced MR angiography at 3-T for acute
pulmonary embolism detection: Comparison with multidetector CT
angiography. Int J Cardiol. 168:4775–4783. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hansch A, Betge S, Poehlmann G, Neumann S,
Baltzer P, Pfeil A, Waginger M, Boettcher J, Kaiser WA, Wolf G, et
al: Combined magnetic resonance imaging of deep venous thrombosis
and pulmonary arteries after a single injection of a blood pool
contrast agent. Eur Radiol. 21:318–325. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pfeil A, Betge S, Poehlmann G, Boettcher
J, Drescher R, Malich A, Wolf G, Mentzel HJ and Hansch A: Magnetic
resonance VIBE venography using the blood pool contrast agent
gadofosveset Trisodium-an interrater reliability study. Eur J
Radiol. 81:547–552. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fu Q, Cheng Q, Wu S and Kong X:
Fat-suppressed magnetic resonance volume interpolated examination
for deep venous thrombosis compared with duplex sonography. Exp
Ther Med. 19:2632–2640. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Squizzato A, Pomero F, Allione A, Priotto
R, Riva N, Huisman MV, Klok FA, Stein PD, Guasti L, Fenoglio L, et
al: Diagnostic accuracy of magnetic resonance imaging in patients
with suspected pulmonary embolism: A bivariate Meta-analysis.
Thromb Res. 154:64–72. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fu Q, Liu D, Kong X and Lei Z: Combined MR
imaging for pulmonary embolism and deep venous thrombosis by
Contrast-enhanced MR volume interpolated body examination. Curr Med
Sci. 40:192–198. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Edelman RR, Silvers RI, Thakrar KH, Metzl
MD, Nazari J, Giri S and Koktzoglou I: Nonenhanced MR angiography
of the pulmonary arteries using single-shot radial
quiescent-interval slice-selective (QISS): A technical feasibility
study. J Cardiovasc Magn Reson. 19(48)2017.PubMed/NCBI View Article : Google Scholar
|