Introduction
Laparoscopic surgery is a widely recommended
procedure for excision of gynecological lesions, such as a cyst or
cancer of the ovary or the uterine, owing to lower postoperative
pain, a better aesthetic result and earlier discharge and recovery
compared with laparotomy (1).
Laparoscopic surgery is usually performed under general anesthesia,
and a target-controlled intravenous infusion of
propofol-remifentanil (PR) is the most common anesthetic regimen
administered during laparoscopic gynecological surgery (2,3).
Previous studies have suggested that this combination allows rapid
onset and recovery from anesthesia, and reduces the incidence of
complications, such as postoperative nausea and vomiting (PONV),
pain, agitation or other various adverse sequelae, thereby
improving the quality of recovery (4-6).
However, cardiovascular depression and hemodynamic instability,
which are potentially fatal, have been reported to develop during
the induction of anesthesia (7,8).
Therefore, additional anesthetic agents that do not result in
cardiovascular depression and hemodynamic instability may be
required to be combined with PR.
In addition to intravenous anesthesia, inhaled
anesthetics are also another commonly used approach in clinical
practice (9). It has been reported
that inhaled anesthetics regulate the hemodynamic response of the
patients and resulted in muscle relaxation (10). However, inhaled anesthetics have
also been indicated to exhibit certain disadvantages, such as
prolonged recovery time after surgery and a higher incidence of
postoperative agitation, which lessen patient satisfaction
(11). Therefore, researchers have
been making efforts to identify novel inhaled anesthetics.
Desflurane is a novel fluorine halogenated methyl ether and is
categorized as an inhaled anesthetic. The blood gas solubility of
desflurane is only 0.49, which is lower compared with that of other
inhaled anesthetics (such as isoflurane, 1.27; sevoflurane, 0.62;
halothane, 2.46) (12), and
therefore, allows for a fast alveolar equilibration of desflurane
and exhibits rapid onset/recovery characteristics (13-15).
Accordingly, we hypothesized that supplementary inhalation of
desflurane may not only prevent the adverse effects of
cardiovascular depression and hemodynamic instability, but also may
not influence the anesthetizing effects of the PR regimen, which
has not yet been investigated in laparoscopic gynecological
surgery, to the best of our knowledge (16).
The purpose of the present study was to determine
whether the combination of inhaled desflurane is superior to PR
total intravenous anesthesia alone in patients undergoing
laparoscopic gynecological surgery, especially with regard to the
effects on the hemodynamic stability.
Materials and methods
Patients
The present study was approved by the Ethics
Committee of the Second Hospital of Jilin University (Changchun,
China; approval no. 2018-010) and registered in the Chinese
Clinical Trial Register (trial registration no. ChiCTR1800015017;
http://www.chictr.org.cn/index.aspx). The objective
and methods of the present study were explained to all patients,
and written informed consent was obtained. All protocols were
performed in accordance with the principles of the Declaration of
Helsinki.
A total of 60 adult female patients (median age, 41
years) who were scheduled to undergo laparoscopic gynecological
surgery at the Second Hospital of Jilin University (Changchun,
China) were enrolled between January 2018 and June 2018. All
patients had to meet the following inclusion criteria: i) aged
between 18 and 60 years; ii) classified as American Society of
Anesthesiologists (ASA) physical status I and II (17); iii) exhibit no heart, lung or brain
diseases; iv) exhibit no history of diabetes and hypertension; v)
present normal liver and kidney function, electrolytes, blood
routine and coagulation test results preoperatively; and vi)
present no abnormality in the electrocardiogram and chest X-ray.
Patients who exhibited a history of: i) alcohol and/or drug abuse;
ii) cardiovascular diseases with cardiovascular agents used and New
York Heart Association classification as III or IV (18); iii) bradyarrhythmia (sinus
bradycardia, left bundle branch block or third-degree
atrioventricular block); iv) abnormal liver and kidney function;
and v) allergy to any of the study drugs, were excluded from the
present study.
Patients were randomly allocated via a
computer-based random distribution to receive either PR or combined
PR and desflurane (PRD) for the maintenance of anesthesia.
Anesthetic protocol
Upon arrival to the surgical room, all patients
routinely received two-lead electrocardiography, peripheral
oximetry, capnography, non-invasive blood pressure and bispectral
index (BIS) monitoring. Following pre-oxygenation for 3 min,
midazolam at 0.05 mg/kg, fentanyl at 4 µg/kg, etomidate at 0.3
mg/kg and cisatracurium at 0.15 mg/kg were administered to the
patients for the induction of anesthesia. Anesthesia was maintained
by an intravenous infusion of propofol and remifentanil, which were
designed to achieve a target effect-site concentration of 2 mg/ml
and 4 ng/ml, respectively, via a target-controlled infusion system
(Orchestra® Base Primea; Fresenius Vial S.A.S.).
Following endotracheal intubation, the patients in the PRD group
received inhalation of desflurane at an oxygen flow rate of 2 l min
and an expired end-tidal concentration of 3%.
During the surgery, the concentrations of propofol
and remifentanil were titrated to maintain the mean arterial blood
pressure (MAP) within 20% of the baseline values. When MAP was
continuously >10% of the baseline values for 1 min and BIS was
>60, the concentration of propofol and remifentanil was
increased by 0.5 µg/kg and 0.5 ng/ml, respectively; if BIS was
40-60, only the concentration of remifentanil was increased by 0.5
ng/ml. When MAP was continuously <10% of the baseline values for
1 min and BIS was <40, the concentration of propofol and
remifentanil was decreased by 0.5 µg/kg and 0.5 ng/ml,
respectively; if BIS was 40-60, only the concentration of
remifentanil was decreased by 0.5 ng/ml. When MAP was <20% of
the baseline values, ephedrine at 10 mg was administered. If the
patient's heart rate (HR) was decreased to <45 beats per minute
(BPM), atropine (0.5 mg) was administered.
Postoperatively, the oxygen flow rate of desflurane
was adjusted to 6 l/min to promote the removal of desflurane,
followed by the removal of the laparoscopic instruments, the suture
and the termination of propofol infusion. Atropine (0.01 mg/kg) and
neostigmine (0.02 mg/kg) were administered to counteract the
cisatracurium-induced neuromuscular block, while flumazenil (0.5
mg) was administered for antagonism of the residual sedative
effects of midazolam. No patients received naloxone for awakening.
Patients were extubated when the following conditions were met: i)
stable autonomic respiratory rhythm; ii) tidal volume >6 ml/kg;
iii) peripheral capillary oxygen saturation >95% for 5 min; iv)
patient end-tidal carbon dioxide <45 mmHg; and v) recovery of
protective reflex and ability to open their eyes on verbal
commands, followed by transfer from the operating room to the
staffed post-anesthesia care unit. When modified Aldrete Recovery
Score was ≥9(19), the patients
were discharged to the ward.
Measurement
Hemodynamics, including MAP and HR, were measured
upon arrival to the surgical room (T0), immediately at intubation
(T1), immediately at operation initiation (T2), 5 min
post-pneumoperitoneum (T3), at removal of pneumoperitoneum needle
(T4), immediately at post-operation (T5), immediately at extubation
(T6), following extubation for 5 min (T7) and 10 min (T8).
Records were made on intraoperative intake,
estimated blood loss, intraoperative urine output, consumption of
remifentanil and propofol and the time of operation, anesthesia,
eye-opening on verbal commands, extubation, orientation recovery
and achievement of a modified Aldrete recovery score (19) ≥9.
Observer's Assessment of Alertness and Sedation
(OAA/S) score was assessed preoperatively and postoperatively to
predict the sedation status, which was rated on a 5-point scale as
follows: 5, alert; 4, lethargic; 3, awakened by voice; 2, awakened
by shaking; and 1, deep sleep (20). The Sedation-agitation scale (SAS)
was evaluated at T6 and T8 to predict the agitation status, which
was rated on a 7-point scale, with a ≥5 score diagnosed as
emergence agitation (21).
Postoperative pain was assessed using the visual analogue scale
[VAS; range, 0-10 (0 represents no pain and 10 represents the worst
imaginable pain)] (22) at T8 and 1
h after the operation. VAS >4 indicated the occurrence of
postoperative pain. Intravenous fentanyl (0.1 mg) was the
first-line rescue analgesic, and pethidine (50 mg) was used as the
second-line rescue analgesic on demand. All scores were assessed by
the same anesthesiologists in a blinded fashion to the grouping of
the trial.
Postoperative nausea was defined as a subjectively
unpleasant sensation associated with an awareness of the urge to
vomit, whereas an episode of vomiting was defined as vomiting
(forceful expulsion of gastric contents from the mouth) and
retching (spasmodic, labored and rhythmic contractions of the
respiratory muscles without expulsion of gastric contents). When
the patients either vomited or retched, 0.3 mg ramosetron was
injected intravenously as a rescue treatment, if treatment was
requested. In addition, other postoperative complications,
including respiratory depression, shivering and bradycardia, were
also recorded.
Statistical analysis
The sample size was calculated using GraphPad InStat
version 3.0 software (GraphPad Software, Inc.). The calculation
revealed that 27 subjects per group were required to achieve a
power of 90% with a type I error of 0.05. To allow for a dropout
rate of up to 10%, 30 subjects were designed to be enrolled in each
group. Categorical data are presented as number (%) and are
compared between groups using χ2 test or Fisher's exact
test. Non-Gaussian continuous data are presented as the median
(minimum-maximum) and are compared between groups using the
Mann-Whitney U test. Normally distributed continuous data are
presented as the mean ± SD and are compared between groups using
two-sample independent Student's t-test. A mixed-design repeated
measures ANOVA followed by Bonferroni's multiple comparisons test
was used to compare MAP and HR within (different time points) and
between PR and PRD groups. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using SPSS v23.0 software (IBM Corp.).
Results
Study population
Between January 2018 and June2018, 60 patients were
enrolled in the present study, and no dropout occurred. These 60
patients were subsequently randomly allocated to receive PR or
combined PRD for the maintenance of anesthesia, with 30 patients in
each group (Fig. 1). No significant
differences were observed between the two groups with regard to the
patients' demographics, including age, body weight, ASA
classification and the cause of the laparoscopic gynecological
surgery, indicating that both groups were comparable (Table I).
 | Table IDemographic data of the population of
the present study. |
Table I
Demographic data of the population of
the present study.
| Variables | PR group (n=30) | PRD group (n=30) | P-value |
|---|
| Mean age ± SD,
years | 41.46±10.97 | 38.87±9.63 | 0.333 |
| Mean weight ± SD,
kg | 62.23±7.34 | 59.80±5.00 | 0.139 |
| Median height
(minimum-maximum), cm | 160 (151-170) | 160 (155-169) | 0.834 |
| ASA physical status,
n (%) | | | 0.756 |
|
I | 6 (20.0) | 7 (23.3) | |
|
II | 24 (80.0) | 23 (76.7) | |
| Type of surgery, n
(%) | | | 0.873 |
|
Ovarian
cystectomy | 6 (20.0) | 7 (23.3) | |
|
Hysterectomy | 9 (30.0) | 10 (33.3) | |
|
Myomectomy | 15 (50.0) | 13 (43.3) | |
Impact on perioperative
characteristics
Surgery and anesthesia were uneventful in all
patients. The perioperative characteristics were also recorded and
compared. The results indicated no significant differences were
observed in the intra-operative intake, estimated blood loss,
intra-operative urine output, operation time and anesthesia time
between the two groups, but the addition of desflurane
significantly decreased the consumption of propofol and
remifentanil (Table II),
indicating that the incidence of PR-induced complications, such as
hemodynamic instability, may be reduced. This hypothesis was
verified by the measurement of MAP and HR, which indicated that
both MAP (Fig. 2A) and HR (Fig. 2B) were significantly higher at T3,
but significantly lower at T4 and T5 in the PR group compared with
the PRD group. In addition, within the PRD group, the MAP and HR
were generally stable, with significant alterations only between a
few time points (Table III). By
contrast, significant differences in MAP and HR were observed at
multiple time points within the PR group (Table III).
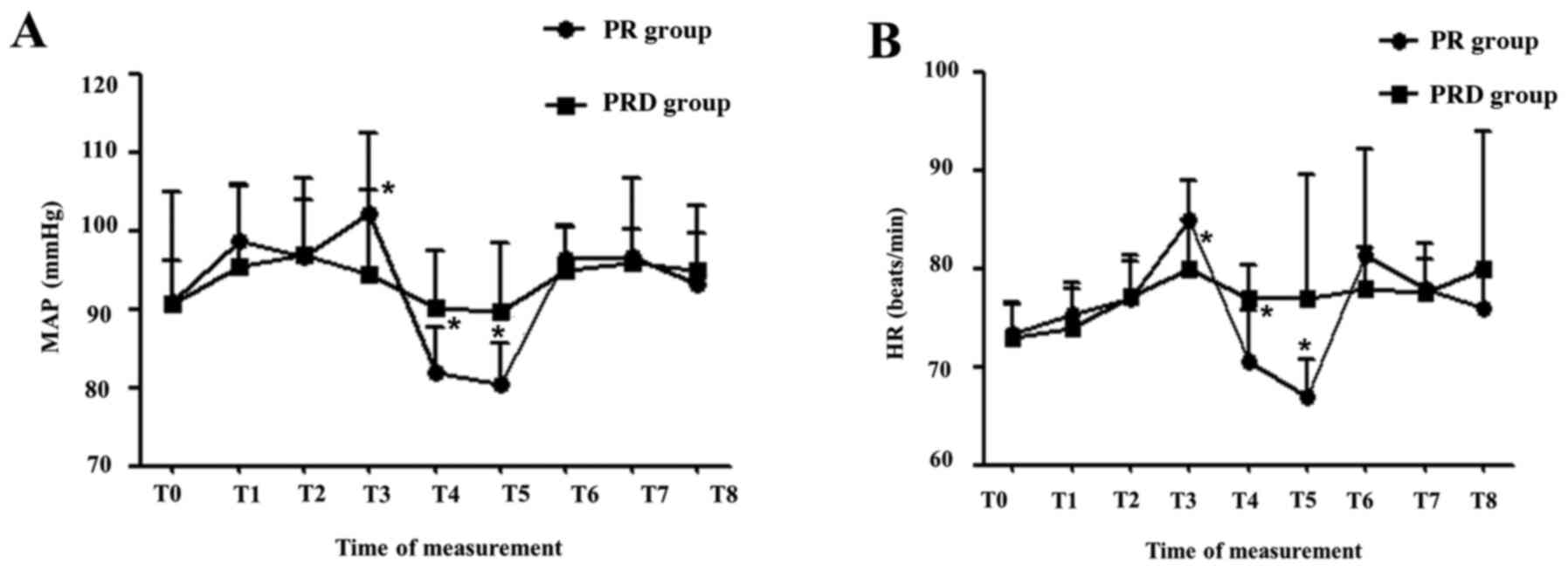 | Figure 2Hemodynamic responses to various
stimuli during the perioperative period. (A) MAP. (B) HR. T0, upon
arrival to the surgical room; T1, immediately at intubation; T2,
immediately at operation initiation; T3, 5 min
post-pneumoperitoneum; T4, removal of pneumoperitoneum needle; T5,
immediately post-operation; T6, immediately at extubation; T7, 5
min following extubation; T8, 10 min following extubation.
*P<0.05 vs. PRD group. PR, propofol-remifentanil;
PRD, PR and low-dose desflurane; MAP, mean arterial blood pressure,
HR, heart rate. |
 | Table IIPatients' perioperative
characteristics. |
Table II
Patients' perioperative
characteristics.
| Variables | PR group (n=30) | PRD group (n=30) | P-value |
|---|
| Intra-operative
intake, ml | 500 (300-800) | 500 (300-800) | 0.515 |
| Estimated blood loss,
ml | 100 (50-300) | 55 (50-300) | 0.271 |
| Intra-operative urine
output, ml | 100 (50-280) | 90 (50-350) | 0.847 |
| Operation time,
min | 70 (50-150) | 65 (40-169) | 0.853 |
| Anesthesia time,
min | 99 (65-165) | 85 (75-185) | 0.911 |
| Consumption of
anesthetics | | | |
|
Cisatracurium,
mg | 14 (12-35) | 12.5 (12-25) | 0.242 |
|
Remifentanil,
mg | 1.56 (0.96-4.36) | 0.90
(0.20-2.88) | <0.001 |
|
Propofol,
mg | 358.33±100.18 | 212.33±62.85 | <0.001 |
 | Table IIIHemodynamic alterations at different
points for each group. |
Table III
Hemodynamic alterations at different
points for each group.
| Hemodynamic
parameter | Time point | PR group
(n=30) | PRD group
(n=30) |
|---|
| MAP | T0 | 91.00±5.09 | 90.73±14.32 |
| | T1 |
98.63±6.99a | 95.47±10.49 |
| | T2 | 96.60±7.34 | 96.93±9.71 |
| | T3 |
102.10±10.24a,c | 94.33±10.92 |
| | T4 |
82.03±5.58a-d |
90.17±7.25c |
| | T5 |
80.50±5.05a-d |
89.77±8.59c |
| | T6 |
96.50±3.98a,e,f | 95.00±5.63 |
| | T7 |
96.60±10.05e,f |
95.97±4.20e,f |
| | T8 |
93.23±9.82d-f,g | 95.00±4.71 |
| HR | T0 | 73.30±3.03 | 73.00±3.55 |
| | T1 |
75.30±3.17a | 74.00±3.91 |
| | T2 |
77.00±4.39a |
77.23±3.46a,b |
| | T3 |
85.03±3.96a-c |
80.00±4.91a,b |
| | T4 |
70.63±5.12b-d |
77.00±3.35a |
| | T5 |
67.00±3.80a-d | 76.87±12.67 |
| | T6 |
81.33±10.75a,e,f |
78.00±4.18a |
| | T7 |
78.00±4.56a,d-f |
77.57±3.41a |
| | T8 |
76.00±3.85d-f | 79.87±14.12 |
Impact on postoperative outcomes
Furthermore, postoperative recovery parameters and
adverse events were also recorded and are presented in Tables IV and V. The results indicated that the two
groups were comparable in eye-opening time, extubation time,
orientation recovery time, time to achieve Aldrete score ≥9, OAA/S
score and SAS score (Table IV). No
significant difference was observed in the number of patients who
experienced complications, including bradycardia, hypotension,
agitation, PONV, nausea, vomiting and pain, between the two groups.
The number of patients requiring rescue antiemetic and postsurgical
analgesia also did not differ between the two groups (Table V).
 | Table IVPost-anesthesia recovery
parameters. |
Table IV
Post-anesthesia recovery
parameters.
| Variables | PR group
(n=30) | PRD group
(n=30) | P-value |
|---|
| Eye-opening time,
min | 7.00±1.97 | 7.40±1.61 | 0.392 |
| Extubation time,
min | 10.00±2.44 | 10.70±2.32 | 0.259 |
| Orientation
recovery time, min | 12.43±2.18 | 13.47±2.32 | 0.080 |
| Time to achieve
Aldrete score ≥9, min | 15.97±2.55 | 16.53±2.54 | 0.393 |
| OAA/S, n (5/4) | | | 0.401 |
|
Preoperatively | 30/0 | 30/0 | |
|
1 h
post-operation | 30/0 | 29/1 | |
| SAS, n
(0-4/5-7) | | | 0.492 |
|
Extubation | 30/0 | 28/2 | |
|
10 min after
extubation | 30/0 | 30/0 | |
 | Table VIncidence of postoperative adverse
reactions. |
Table V
Incidence of postoperative adverse
reactions.
| Variables | PR group
(n=30) | PRD group
(n=30) | P-value |
|---|
| Hypotension, n
(%) | 2 (6.7) | 0 (0) | 0.492 |
| Bradycardia, n
(%) | 5 (16.7) | 2 (6.7) | 0.424 |
| Agitation, n
(%) | 0 (0.0) | 2 (6.7) | 0.492 |
| PONV, n (%) | 1 (3.3) | 1 (3.3) | 1.000 |
| Nausea, n
(none/mild/moderate/severe) | 29/1/0/0 | 29/1/0/0 | 1.000 |
| Vomiting, n
(%) | 0 (0.0) | 0 (0.0) | 1.000 |
| Rescue antiemetic,
n (%) | 0 (0.0) | 0 (0.0) | |
| VAS, n
(0-3/4-6/7-10) | | | |
|
10 min after
extubation | 30/0/0 | 30/0/0 | 1.000 |
|
1 h
post-operation | 27/3/0 | 22/8/0 | 0.098 |
| Pain, n (%) | 3 (10.0) | 8 (26.7) | 0.095 |
| Postsurgical
analgesia, n (%) | | | |
|
None | 27 (90.0) | 22 (73.3) | 0.098 |
|
Fentanyl | 3 (10.0) | 8 (26.7) | |
|
Fentanyl +
pethidine | 0 (0.0) | 0 (0.0) | |
Discussion
Intravenously infused propofol and inhaled
desflurane are two commonly used anesthetics that can be combined
with the ultra-short-acting μ-opioid receptor agonist remifentanil
for the induction and maintenance of anesthesia during surgery.
However, which combination is optimal remains unclear, and may be
attributed to certain disadvantages of each anesthetic (23-27).
For example, Cho et al (23)
reported that tissue oxygen saturation was higher in the desflurane
group compared with that in the propofol group at 30 and 60 min of
ventilation. The recovery slope during the vascular occlusion test,
reflecting microvascular reperfusion adequacy, was also higher in
the desflurane compared with that in the propofol group during
surgery (23). Mahli et al
(24) reported that the general
mean values of MAP and HR for the PR group were higher compared
with that of the desflurane-remifentanil group (89.3 mmHg and 72.4
BPM vs. 77.1 mmHg and 69.5 BPM, respectively). These findings
indicated that desflurane-remifentanil anesthesia may be associated
with an improved microcirculation and hemodynamic stability
compared with PR anesthesia (24).
Yoo et al (6) demonstrated
that the incidence of nausea in the post-anesthetic care unit (22.6
vs. 6.5%; P=0.001) and at 1-6 h postoperatively (54.8 vs. 16.1%;
P=0.001) was significantly higher in the desflurane-remifentanil
compared with that in the PR group. Zaballos et al (25) reported that the
desflurane-remifentanil group received an increased amount of
fentanyl as rescue analgesia compared with the PR group (200±65 vs.
113±38 µg). Gritti et al (26) and Gozdemir et al (27) demonstrated that the recovery times
for spontaneous ventilation, extubation, time to awakening, eye
opening and ability to provide name and date of birth were shorter
in the PR group compared with those in the desflurane group. These
results suggested that PR may be more effective for recovery and
associated with a decreased number of complications. Moreover, the
concentration of each anesthetic was higher when only propofol or
desflurane was used, resulting in non-negligible adverse outcomes
(such as unstable hemodynamic responses and PONV) in the clinic
(28). Therefore, we hypothesized
that a combination of three drugs (desflurane, propofol and
remifentanil) in lower doses may prevent their respective
shortcomings and achieve improved anesthetizing effects. Although a
previous study has recommended the supplementation of intravenous
anesthesia with desflurane, it has not compared the effects of PR
and PRD, but only compared the PRD with the desflurane group
(16). Therefore, this is the first
time, to the best of our knowledge, that a study compared the
anesthetizing effects of a combination of lower-dose desflurane
with PR. The results of the present study indicated that the PRD
group not only exhibited a similar recovery potential and
complications (such as low PONV) to the PR group, but also
maintained stable hemodynamics. Although three drugs were used, the
combined cost may be similar for the patients, as the dose of PR
was significantly decreased in the PRD compared with the PR group,
and the price of propofol and desflurane has been reported to be
similar (29,30).
Several limitations to the present study exist.
Firstly, although the power analysis indicated that the number of
patients who were required in the present study was sufficient, the
population size was relatively small and this was a single-center
study. This may be an underlying reason explaining statistically
non-significant differences in postoperative adverse reactions
between the PRD and PR groups and the lower PONV observed (1/30
patients, 3.3%) compared with previous reports (20-50%) (4,31,32).
Secondly, the enrolled patients were relatively young and whether
the conclusion is similar in an older population requires further
validation. Thirdly, a desflurane-remifentanil control group should
have been included. Fourthly, only one kind of surgery, such as
ovarian cystectomy, should be included in future trials, in order
to more easily control the operation and anesthesia time and reduce
its influence on the postoperative pain. For example, the fact that
the incidence of pain was relatively higher in the PRD compared
with that in the PR group may be attributed to the increased number
of patients (3/8) who underwent myomectomy among the patients with
pain. Fifthly, a cost analysis with the use of the three agents was
not a part of the present study. Finally, more outcomes, including
the incidence of intraoperative awareness and body movement, and
mechanism parameters, such as alterations in the stress response
(catecholamines, noradrenaline, adrenaline, adrenocorticotropic
hormone and cortisol) or inflammation factors (interleukin-6, tumor
necrosis factor-α, C-reactive protein and nitric oxide) (33,34)
should be recorded to comprehensively assess the anesthetizing
effects of lower-dose desflurane combined with PR.
The present study suggested that combining low-dose
desflurane with PR may represent an efficient anesthesia regimen to
prevent the hemodynamic instability of total intravenous anesthesia
for patients undergoing laparoscopic gynecological surgery.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
PZ and XS participated in the conception and design
of the study. PZ and YC collected the data and performed the
statistical analysis. LS was involved in the interpretation of the
data. PZ drafted the manuscript. XS revised the manuscript for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Second Hospital of Jilin University (Changchun,
China; approval no. 2018-010) and written informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Koo YJ, Kim JE, Kim YH, Hahn HS, Lee IH,
Kim TJ, Lee KH, Shim JU and Lim KT: Comparison of laparoscopy and
laparotomy for the management of early-stage ovarian cancer:
Surgical and oncological outcomes. J Gynecol Oncol. 25:111–117.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang D and Nie A: Assessment of different
anesthesia depth under total intravenous anesthesia on
postoperative cognitive function in laparoscopic patients. J Res
Med Sci. 21(73)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Carli D, Meletti JFA, Neto NEU, Martinez
G, Kim ALC and de Camargo RPS: General anesthesia technique and
perception of quality of postoperative recovery in women undergoing
cholecystectomy: A randomized, double-blinded clinical trial. PLoS
One. 15(e0228805)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lee WK, Kim MS, Kang SW, Kim S and Lee JR:
Type of anaesthesia and patient quality of recovery: A randomized
trial comparing propofol-remifentanil total i.v. anaesthesia with
desflurane anaesthesia. Br J Anaesth. 114:663–668. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ryu JH, Kim JH, Park KS and Do SH:
Remifentanil-propofol versus fentanyl-propofol for monitored
anesthesia care during hysteroscopy. J Clin Anesth. 20:328–332.
2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yoo YC, Bai SJ, Lee KY, Shin S, Choi EK
and Lee JW: Total intravenous anesthesia with propofol reduces
postoperative nausea and vomiting in patients undergoing
robot-assisted laparoscopic radical prostatectomy: A prospective
randomized trial. Yonsei Med J. 53:1197–1202. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Son I, Oh CS, Choi JW and Kim SH: The
effect of sufentanil administration on remifentanil-based
anaesthesia during laparoscopic gynaecological surgery: A
double-blind randomized controlled trial. ScientificWorldJournal.
2014(701329)2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Beier SL, Mattoso CR, Aguiar AJ, Vianna PT
and Massone F: Hemodynamic effects of target-controlled infusion of
propofol alone or in combination with a constant-rate infusion of
remifentanil in dogs. Can J Vet Res. 79:309–315. 2015.PubMed/NCBI
|
|
9
|
Herling SF, Dreijer B, Wrist Lam G,
Thomsen T and Møller AM: Total intravenous anaesthesia versus
inhalational anaesthesia for adults undergoing transabdominal
robotic assisted laparoscopic surgery. Cochrane Database Syst Rev.
4(CD011387)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bharti N, Chari P and Kumar P: Effect of
sevoflurane versus propofol-based anesthesia on the hemodynamic
response and recovery characteristics in patients undergoing
microlaryngeal surgery. Saudi J Anaesth. 6:380–384. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ionescu D, Mărgărit S, Vlad L, Iancu C,
Alexe A, Deac D, Răduţ A, Tudorică G, Necula A and Pop T: TIVA-TCI
(Total IntraVenous Anesthesia-Target Controlled Infusion) versus
isoflurane anesthesia for laparoscopic cholecystectomy.
Postoperative nausea and vomiting, and patient satisfaction.
Chirurgia (Bucur). 104:167–172. 2009.PubMed/NCBI(In Romanian).
|
|
12
|
Yasuda N, Eger EI II, Weiskopf RB,
Tanifuji Y and Kobayashi K: Solubility of desflurane (I-653),
sevoflurane, isoflurane, and halothane in human blood. Masui.
40:1059–1062. 1991.PubMed/NCBI(In Japanese).
|
|
13
|
Eger EI II: New inhaled anesthetics.
Anesthesiology. 80:906–922. 1994.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jindal R, Kumra VP, Narani KK and Sood J:
Comparison of maintenance and emergence characteristics after
desflurane or sevoflurane in outpatient anaesthesia. Indian J
Anaesth. 55:36–42. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kotwani MB and Malde AD: Comparison of
maintenance, emergence and recovery characteristics of sevoflurane
and desflurane in pediatric ambulatory surgery. J Anaesthesiol Clin
Pharmacol. 33:503–508. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Boat A, Mahmoud M, Michelfelder EC, Lin E,
Ngamprasertwong P, Schnell B, Kurth CD, Crombleholme TM and
Sadhasivam S: Supplementing desflurane with intravenous anesthesia
reduces fetal cardiac dysfunction during open fetal surgery.
Paediatr Anaesth. 20:748–756. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Daabiss M: American Society of
Anaesthesiologists physical status classification. Indian J
Anaesth. 55:111–115. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bredy C, Ministeri M, Kempny A,
Alonso-Gonzalez R, Swan L, Uebing A, Diller GP, Gatzoulis MA and
Dimopoulos K: New York Heart Association (NYHA) classification in
adults with congenital heart disease: Relation to objective
measures of exercise and outcome. Eur Heart J Qual Care Clin
Outcomes. 4:51–58. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ead H: From Aldrete to PADSS: Reviewing
discharge criteria after ambulatory surgery. J Perianesth Nurs.
21:259–267. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chernik DA, Gillings D, Laine H, Hendler
J, Silver JM, Davidson AB, Schwam EM and Siegel JL: Validity and
reliability of the Observer's Assessment of Alertness/Sedation
Scale: Study with intravenous midazolam. J Clin Psychopharmacol.
10:244–251. 1990.PubMed/NCBI
|
|
21
|
Namigar T, Serap K, Esra AT, Özgül O, Can
ÖA, Aysel A and Achmet A: The correlation among the Ramsey sedation
scale, Richmond agitation sedation scale and Riker sedation
agitation scale during midazolam-remifantanil sedation. Braz J
Anesthesiol. 67:347–354. 2016.
|
|
22
|
Langley GB and Sheppeard H: The visual
analogue scale: Its use in pain measurement. Rheumatol Int.
5:145–148. 1985.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cho YJ, Bae J, Kim TK, Hong DM, Seo JH,
Bahk JH and Jeon Y: Microcirculation measured by vascular occlusion
test during desflurane-remifentanil anesthesia is superior to that
in propofol-remifentanil anesthesia in patients undergoing thoracic
surgery: Subgroup analysis of a prospective randomized study. J
Clin Monit Comput. 31:989–997. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mahli A, Coskun D, Karaca GI, Akcali DT,
Karabiyik L and Karadenizli Y: Target-controlled infusion of
remifentanil with propofol or desflurane under bispectral index
guidance: Quality of anesthesia and recovery profile. J Res Med
Sci. 16:611–620. 2011.PubMed/NCBI
|
|
25
|
Zaballos M, Reyes A, Etulain J, Monteserín
C, Rodríguez M and Velasco E: Desflurane versus propofol in
post-operative quality of recovery of patients undergoing day
laparoscopic cholecystectomy. Prospective, comparative,
non-inferiority study. Rev Esp Anestesiol Reanim. 65:96–102.
2018.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
26
|
Gritti P, Carrara B, Khotcholava M,
Bortolotti G, Giardini D, Lanterna LA, Benigni A and Sonzogni V:
The use of desflurane or propofol in combination with remifentanil
in myasthenic patients undergoing a video-assisted
thoracoscopic-extended thymectomy. Acta Anaesthesiol Scand.
53:380–389. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gozdemir M, Sert H, Yilmaz N, Kanbak O,
Usta B and Demircioglu RI: Remifentanil-propofol in vertebral disk
operations: Hemodynamics and recovery versus desflurane-n(2)o
inhalation anesthesia. Adv Ther. 24:622–631. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen PN, Lu IC, Chen HM, Cheng KI, Tseng
KY and Lee KT: Desflurane reinforces the efficacy of propofol
target-controlled infusion in patients undergoing laparoscopic
cholecystectomy. Kaohsiung J Med Sci. 32:32–37. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lin CY, Wong CS and Wu CT: Cost analysis
of three anesthetic regimens under auditory evoked potential
monitoring in gynecologic laparoscopic surgery. Acta Anaesthesiol
Taiwan. 46:53–54. 2008.PubMed/NCBI
|
|
30
|
Demeere JL, Merckx Ch and Demeere N: Cost
minimisation and cost effectiveness in anaesthesia for total hip
replacement surgery, in Belgium? A study comparing three general
anaesthesia techniques. Acta Anaesthesiol Belg. 57:145–151.
2006.PubMed/NCBI
|
|
31
|
Seki H, Furumoto K, Sato M, Kagoya A,
Hashimoto H, Sekiguchi Y and Nakatsuka I: Effects of epidural
anesthesia on postoperative nausea and vomiting in laparoscopic
gynecological surgery: A randomized controlled trial. J Anesth.
32:608–615. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Uchinami Y, Takikawa S, Takashima F, Maeda
Y, Nasu S, Ito A and Saito T: Incidence of postoperative nausea and
vomiting is not increased by combination of low concentration
sevoflurane and propofol compared with propofol alone in patients
undergoing laparoscopic gynecological surgery. JA Clin Rep.
5(70)2019.PubMed/NCBIDOI: 10.1186/s40981-019-0292-4.
|
|
33
|
Marana E, Russo A, Colicci S, Polidori L,
Bevilacqua F, Viviani D and Di Stasio E: Desflurane versus
sevoflurane: a comparison on stress response. Minerva
anestesiologica. 79:7–14. 2013.PubMed/NCBI
|
|
34
|
Roh GU, Song Y, Park J, Ki YM and Han DW:
Effects of propofol on the inflammatory response during
robot-assisted laparoscopic radical prostatectomy: A prospective
randomized controlled study. Sci Rep. 9(5242)2019.PubMed/NCBI View Article : Google Scholar
|