Introduction
An estimated 4,292,000 new solid tumor cases and
~2814,000 solid tumor-associated deaths were reported in China in
2015(1). In general, surgery is the
most efficient therapy for early-stage tumors, but most of the
patients experience relapse after radical surgery (2). Furthermore, targeted therapy and
immunotherapy are suitable for specific populations and certain
patients may develop drug resistance after treatment. Multi-line
chemotherapy and local radiotherapy are recommended as salvage
treatments for these recurrent and refractory tumors and no
alternative treatment is available for these patients, but they may
respond to salvage treatment (3).
Tumor cells require nutrients to support
proliferation, growth and metastasis, which causes abundant new
blood vessels to develop (4).
Aberrant angiogenesis is considered to be the key feature of
tumorigenesis, and therefore, tumor growth may be suppressed by
blocking this process (5). The
vascular endothelial growth factor (VEGF) pathway is crucial in
angiogenesis, with VEGF receptor-2 (VEGFR-2) being the leading
signaling receptor involved in this pathway (6,7).
Apatinib, a small-molecule inhibitor of VEGFR-2, is an orally
bioavailable agent that is currently being studied in multiple
tumor types. It may significantly inhibit angiogenesis of tumors
and was proven to be well-tolerated, safe and effective in the
clinic (8). Apatinib has shown
favorable results in gastric cancer, breast cancer, lung cancer and
esophageal cancer in Phase I/II/III trials, with secondary
hypertension, hand-foot syndrome, fatigue and positive urine
protein being the most frequent treatment-associated adverse events
(9-17).
S-1(Tegafur Gimeracil Oteracil Potassium Capsule) is a combination
of novel oral fluoropyrimidine-based agents, exhibiting potent
antitumor activity and low gastrointestinal toxicity (18). Monotherapy with S-1 has provided a
high degree of relief for patients with various types of advanced
tumor (19). In fact, >90% of
patients with malignant tumors succumb due to multidrug resistance
(MDR) (20,21). Apatinib is able to reverse MDR by
inhibiting ATP binding cassette transporters (22). Thus, apatinib combined with
traditional chemotherapy drugs may achieve effective results and
protect against the emergence of MDR. Therefore, the safety and
efficacy of apatinib combined with S-1 in patients with advanced
cancer were explored in the present study.
Materials and methods
Patient eligibility
From April 2016 to March 2019, patients with
advanced cancer who failed two or more lines of chemotherapy and
were then treated with apatinib combined with S-1 at Tianjin
Medical University Cancer Hospital (Tianjin, China) were
retrospectively evaluated. The key inclusion criteria were as
follows: i) Patients with malignant tumors, age ≥18 years; ii)
patients who had received second-line treatment (radiotherapy or
chemotherapy), and were refractory or had relapsed iii) at least
one measurable lesion; iv) Karnofsky performance status (KPS) ≥80
at enrollment; v) failed to tolerate another chemotherapy; vi) took
the combination of apatinib and S-1 for at least one cycle. All
patients provided written informed consent and the present study
was approved by the Ethics Committee of Tianjin Medical University
Cancer Hospital (Tianjin, China). The trial is registered at
ClinicalTrials.gov (no. NCT04128800).
Treatment methods
All patients received oral apatinib 250 mg once plus
S-1 60 mg/m2 twice daily on days 1-14, repeated every 3
weeks. The examination of hematological, liver and kidney function,
urinalysis, tumor markers and electrocardiogram were routinely
performed during treatment with apatinib plus S-1 and all patients
had a reassessment after every treatment cycle. The color
ultrasound of the upper abdomen and neck lymph nodes, bone scan,
enhanced CT of the chest and MRI of the brain were performed every
two cycles. In the meantime, adverse reactions of patients were
recorded in detail, including myelosuppression, nausea and
vomiting, hand-foot syndrome (HFS), fatigue, proteinuria and
hypertension, prior to and after each taking the drug cycle. During
treatment, if a patient experienced grade 3 or 4 neutropenia or
thrombocytopenia, recombinant human granulocyte colony-stimulating
factor or interleukin-11 was administered by subcutaneous
injection. Treatment was continued until disease progression or the
occurrence of intolerable adverse effects. Of note, patients with a
bleeding tendency were closely monitored.
Outcome assessments
Complete response (CR), partial response (PR),
progressive disease (PD) and stable disease (SD) were defined
according to the Response Evaluation Criteria in Solid Tumors 1.1
(RECIST guideline version 1.1). The objective response rate (ORR)
was defined as ORR=CR+PR rates. The disease control rate (DCR) was
defined as DCR=CR+PR+SD rates. Toxic reactions were assessed in
accordance with the National Cancer Institute-Common Toxicity
Criteria for Adverse Events version 4.0 (NCI-CTCAE guideline
version 4.0).
Progression-free survival (PFS) time was defined as
the time from the first day of each treatment cycle to the first
found evidence of PD on imaging. Overall survival (OS) time was
defined as the time from the first day of each treatment cycle to
the time-point of death or the last follow-up of patients.
Statistical analysis
SPSS version 24.0 (IBM Corp.) was used for
statistical analysis. Only patients who received at least one cycle
of treatment were included in the analysis. The association between
the patients' characteristics and curative effects was analyzed
using the χ2 test. The association between clinical
characteristics and severe adverse effects was also analyzed using
the χ2 test. Survival analysis was performed using the
Kaplan-Meier method by log-rank test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
A total of 33 patients were enrolled in the present
study. Their baseline characteristics are summarized in Table I. Among them, 22 patients were male
and 11 were female with a median age of 60 years (range, 40-77
years). There were 13 (41.9%) patients with NSCLC and also 13
(41.9%) patients with SCLC. The NSCLC patients included 7 patients
(21.2%) with adenocarcinomas and 6 (18.2%) with squamous-cell
carcinomas; furthermore, there were 3 patients (9.1%) with cervical
cancer and 4 patients (12.1%) with esophageal cancer. Prior to
receiving their treatment as part of the present study, 32 patients
(97.0%) had received palliative chemotherapy, 26 (78.8%) had
received radiotherapy, 12 (36.4%) had received concurrent
chemoradiotherapy and only 2 patients (6.1%) had undergone surgery.
A total of 114 cycles of treatment were recorded, with a median of
3 cycles per patient (range, 1-6 cycles). The characteristics of
the patients in the concurrent chemo-radiotherapy group are
presented in Table SI.
 | Table ICharacteristics of patients
(n=33). |
Table I
Characteristics of patients
(n=33).
|
Characteristics | Value |
|---|
| Age (years) | 60 (40-77) |
| Sex | |
|
Male | 22 (66.7) |
|
Female | 11 (33.3) |
| KPS score | |
|
80 | 25 (75.8) |
|
90 | 8 (24.2) |
|
100 | 0 |
| Cancer type | |
|
Lung
squamous cell carcinomas | 6 (18.2) |
|
Lung
adenocarcinoma | 7 (21.2) |
|
Small cell
lung cancer | 13 (39.4) |
|
Esophageal
cancer | 4 (12.1) |
|
Cervical
cancer | 3 (9.1) |
| Previous treatment
received | |
|
Surgery | 2 (6.1) |
|
Radiotherapy | 26 (78.8) |
|
Chemotherapy | 32 (97.0) |
|
Concurrent
chemoradiotherapy | 12 (36.4) |
| Treatment during
medication | |
|
Primary
tumor radiotherapy | 0 |
|
Metastasis
radiotherapy | 4 (12.1) |
| Cycles (apatinib +
S-1) | |
|
1 | 5 (15.2) |
|
2 | 5 (15.2) |
|
3 | 1 (33.3) |
|
4 | 2 (6.1) |
|
5 | 2 (6.1) |
|
6 | 8 (24.2) |
|
Average | 3 |
Adverse effects
As presented in Table
II, the most common treatment-associated toxic effects and
adverse events were anemia (23.7%), hypertension (22.8%),
leukopenia (19.3%), increase of bilirubin (15.0%) and proteinuria
(13.0%). These side effects were of grade 1-2 and were tolerable
and controllable. However, the incidence of other side effects,
including thrombocytopenia, nausea, HFS, hemorrhagic tendency and
diarrhea, were low. As presented in Table III, all of the 33 patients had
experienced toxicities and side effects and 15 of them had severe
adverse effects (grade 3-4) with an incidence rate of 45.5%. The
top three severe adverse effects (grade 3-4) were hypertension
(15.2%), thrombocytopenia (12.1%) and proteinuria (9.1%). As
presented in Table IV, there was
no significant association with age, gender, KPS score, treatment
cycles and cancer type. A total of 5 patients discontinued
treatment after cycle 1, which was due to disease progression in 3
cases and due to unknown reasons in 2 cases. Furthermore, 5
patients discontinued treatment after two cycles, which was due to
personal will in 3 cases, due to cerebral embolism in 1 case and
due to radiation pneumonia in 1 case. The treatment-associated
toxicities and side effects in all these patients were mild and
manageable.
 | Table IIToxicity and adverse effects (n=114
cycles). |
Table II
Toxicity and adverse effects (n=114
cycles).
| Grade | 1 | 2 | 3 | 4 |
|---|
| Anemia | 23 (20.2) | 4 (3.5) | 1 (0.9) | 1 (0.9) |
| Leukopenia | 8 (7.0) | 14 (12.3) | 2 (1.8) | - |
|
Thrombocytopenia | 8 (7.0) | 2 (1.8) | 2 (1.8) | 1 (0.9) |
| Nausea | 3 (2.6) | - | - | - |
| Fatigue | 6 (5.3) | - | - | - |
| Hypertension | 20 (17.5) | 6 (5.3) | 5 (4.4) | - |
| Proteinuria | 10 (8.8) | 4 (3.5) | 3 (2.6) | - |
| Hand-foot
syndrome | 8 (7.0) | 3 (2.6) | 2 (1.8) | - |
| Hemorrhagic
tendency | 2 (1.8) | 1 (0.9) | - | 2 (1.8) |
| Bilirubin
increased | 15 (13.2) | 2 (1.8) | - | - |
| Diarrhea | 7 (6.1) | - | 2 (1.8) | - |
 | Table IIIAnalysis of safety in the cohort
(n=33). |
Table III
Analysis of safety in the cohort
(n=33).
| Adverse events | Any grade | Grade 3 or 4 |
|---|
| Anemia | 16 (48.5) | 2 (6.1) |
| Leukopenia | 11 (33.3) | 2 (6.1) |
|
Thrombocytopenia | 12 (36.4) | 4 (12.1) |
| Nausea | 4 (12.1) | - |
| Fatigue | 4 (12.1) | - |
| Hypertension | 17 (51.5) | 5 (15.2) |
| Proteinuria | 8 (24.2) | 3 (9.1) |
| Hand-foot
syndrome | 10 (30.3) | 1 (3.0) |
| Hemorrhagic
tendency | 6 (18.2) | 2 (6.1) |
| Bilirubin
increased | 12 (36.4) | - |
| Diarrhea | 4 (12.1) | 1 (3.0) |
 | Table IVAssociation between clinical
characteristics and adverse effects in patients with advanced solid
tumor treated with apatinib plus S-1. |
Table IV
Association between clinical
characteristics and adverse effects in patients with advanced solid
tumor treated with apatinib plus S-1.
| Clinical
characteristic | N | Grade 1-2 adverse
effects | Grade 3-4 adverse
effects | χ2 | P-value |
|---|
| Age (years) | | | | 2.695 | 0.101 |
|
<60 | 16 | 12 (36.4) | 4 (12.1) | | |
|
≥60 | 17 | 8 (24.2) | 9 (27.3) | | |
| Sex | | | | 0.639 | 0.672 |
|
Male | 22 | 13 (43.3) | 9 (30.0) | | |
|
Female | 8 | 6 (20.0) | 2 (6.7) | | |
| KPS scores | | | | 0.016 | 1.000 |
|
<80 | 25 | 15 (45.5) | 10 (30.3) | | |
|
≥80 | 8 | 5 (15.2) | 3 (9.1) | | |
| Treatment
cycles | | | | 0.002 | 1.000 |
|
<3 | 10 | 6 (18.2) | 4 (12.1) | | |
|
≥3 | 23 | 14 (42.4) | 9 (27.3) | | |
| Cancer type | | | | 0.045 | 1.000 |
|
Respiratory
system | 26 | 16 (48.5) | 10 (30.3) | | |
|
Non-respiratory
system | 7 | 4 (12.1) | 3 (9.1) | | |
A total of 2 deaths (6.1%) were recorded during the
treatment. These 2 patients with lung squamous-cell carcinomas died
of massive hemorrhage instead of disease progression after four and
two cycles of treatment, respectively. Both of these were
treatment-associated deaths.
Therapeutic outcomes and survival
analyses
Until March 01, 2019, 22 of the patients of the
present study had died and 11 survived. The disease had progressed
in 9 cases and 24 had no disease progression. No CR was achieved
and 10 patients achieved PR, resulting in an ORR of 30.30%.
Furthermore, 14 patients achieved SD and 9 patients had PD,
resulting in a DCR of 72.73%. The 13 patients with NSCLC had an ORR
and DCR of 30.77 and 84.62%, respectively. Furthermore, the 13
patients with SCLC had an ORR of 30.77% and a DCR of 53.85%. The
ORR of the patients with cervical cancer was25% and the DCR was
75%. Patients with esophageal cancerhad an ORR of 33.33% and a DCR
of 100%. However, for patients with advanced NSCLC and SCLC, there
was nosignificant difference in DCR or ORR among the age
groups,gender groups, KPS score groups and treatment cycle
groups(Tables V and VI). The treatment efficacy for the 3
patients with cervical cancer and 4 patients with esophageal cancer
is presented in Table VII.
 | Table VAssociation between clinical
characteristics and curative effect in patients with small-cell
lung cancer treated with apatinib plus S-1. |
Table V
Association between clinical
characteristics and curative effect in patients with small-cell
lung cancer treated with apatinib plus S-1.
| Item | Total | PR | SD | PD | DCR (%) | χ2 | P-value | ORR (%) | χ2 | P-value |
|---|
| Age (years) | | | | | | 0.066 | 1.000 | | 0.034 | 1.000 |
|
<60 | 7 | 2 | 2 | 3 | 57.1 | | | 28.57 | | |
|
≥60 | 6 | 2 | 1 | 3 | 50 | | | 33.33 | | |
| Sex | | | | | | 0.627 | 0.592 | | 3.611 | 0.105 |
|
Male | 8 | 4 | 1 | 3 | 62.5 | | | 50 | | |
|
Female | 5 | 0 | 2 | 3 | 40 | | | 0 | | |
| KPS scores | | | | | | 6.964 | 0.021 | | 0.325 | 1.000 |
|
<80 | 8 | 2 | 0 | 6 | 25 | | | 25 | | |
|
≥80 | 5 | 2 | 3 | 0 | 100 | | | 40 | | |
| Treatment
cycles | | | | | | 1.040 | 0.559 | | 1.003 | 0.530 |
|
<3 | 9 | 2 | 2 | 5 | 44.4 | | | 22.22 | | |
|
≥3 | 4 | 2 | 1 | 1 | 75 | | | 50 | | |
 | Table VIAssociation between clinical
characteristics and curative effect in patients with non-small cell
lung cancer treated with apatinib plus S-1. |
Table VI
Association between clinical
characteristics and curative effect in patients with non-small cell
lung cancer treated with apatinib plus S-1.
| Item | Total | PR | SD | PD | DCR (%) | χ2 | P-value | ORR (%) | χ2 | P-value |
|---|
| Age (years) | | | | | | 2.758 | 0.192 | | 0.034 | 1.000 |
|
<60 | 6 | 2 | 2 | 2 | 66.67 | | | 33.33 | | |
|
≥60 | 7 | 2 | 5 | 0 | 100 | | | 28.57 | | |
| Sex | | | | | | 0.965 | 0.423 | | 1.733 | 0.497 |
|
Male | 10 | 4 | 5 | 1 | 90 | | | 40 | | |
|
Female | 3 | 0 | 2 | 1 | 66.67 | | | 0 | | |
| KPS scores | | | | | | 0.197 | 1.000 | | 0.481 | 1.000 |
|
<80 | 12 | 4 | 6 | 2 | 83.33 | | | 33.33 | | |
|
≥80 | 1 | 0 | 1 | 0 | 100 | | | 0 | | |
| Treatment
cycles | | | | | | 2.758 | 0.192 | | 1.935 | 0.266 |
|
<3 | 6 | 3 | 1 | 2 | 66.67 | | | 50 | | |
|
≥3 | 7 | 1 | 6 | 0 | 100 | | | 14.29 | | |
| Cancer type | | | | | | 0.014 | 1.000 | | 1.040 | 0.559 |
|
Lung
squamous-cell carcinomas | 6 | 1 | 4 | 1 | 83.33 | | | 16.67 | | |
|
Lung
adenocarcinoma | 7 | 3 | 3 | 1 | 85.71 | | | 42.86 | | |
 | Table VIICurative effect in patients with
advanced esophageal cancer and cervical cancer. |
Table VII
Curative effect in patients with
advanced esophageal cancer and cervical cancer.
| Cancer type | Total | PR | SD | PD | DCR (%) | ORR (%) |
|---|
| Esophageal
cancer | 4 | 1 | 2 | 1 | 75 | 25 |
| Cervical
cancer | 3 | 1 | 2 | 0 | 100 | 33 |
For all patients receiving oral apatinib combined
with S-1 therapy, the follow-up data suggested that the median PFS
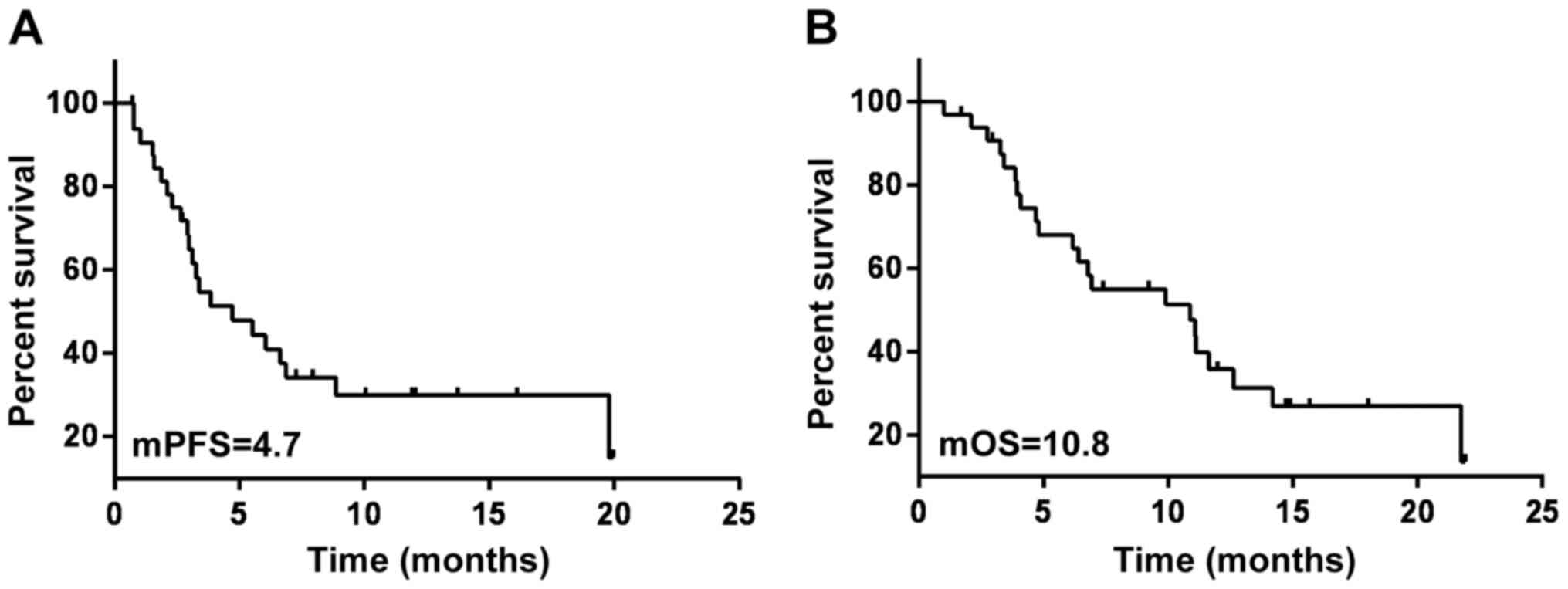
(mPFS) was 4.7 months and the median OS (mOS) was 10.8 months
(Fig. 1). The mPFS of the 13
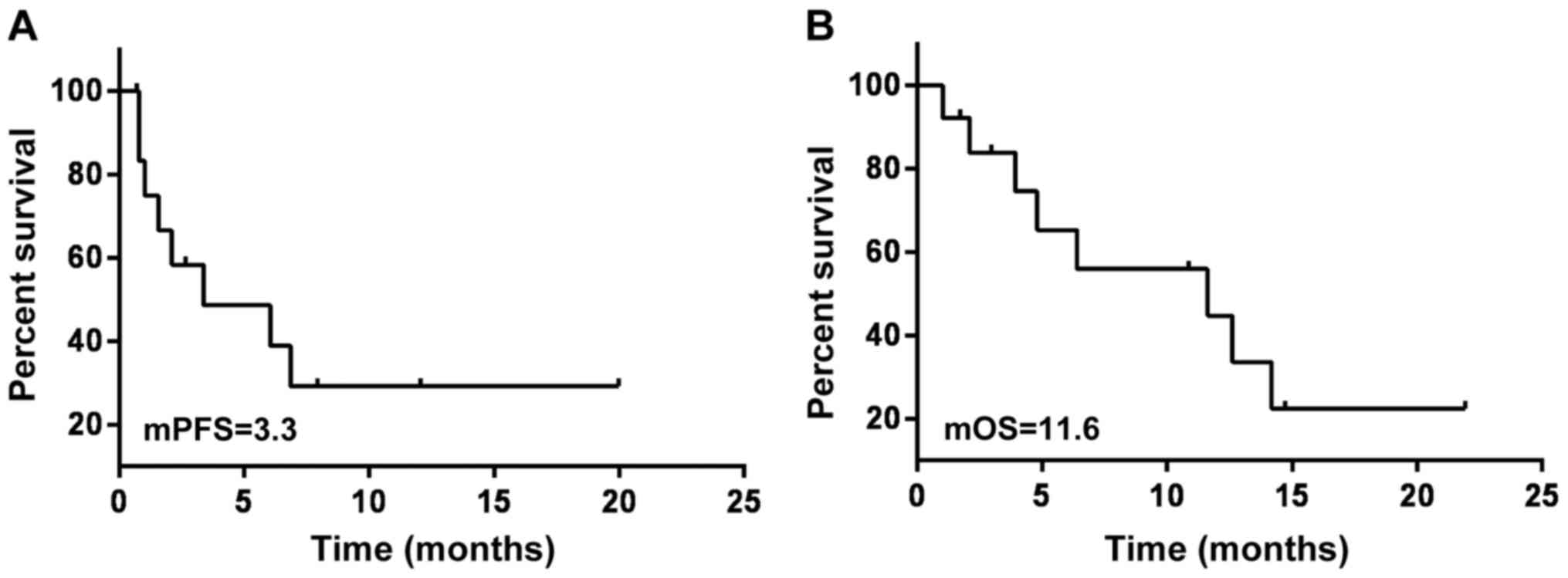
patients with SCLC was 3.3 months and the mOS was 11.6 months
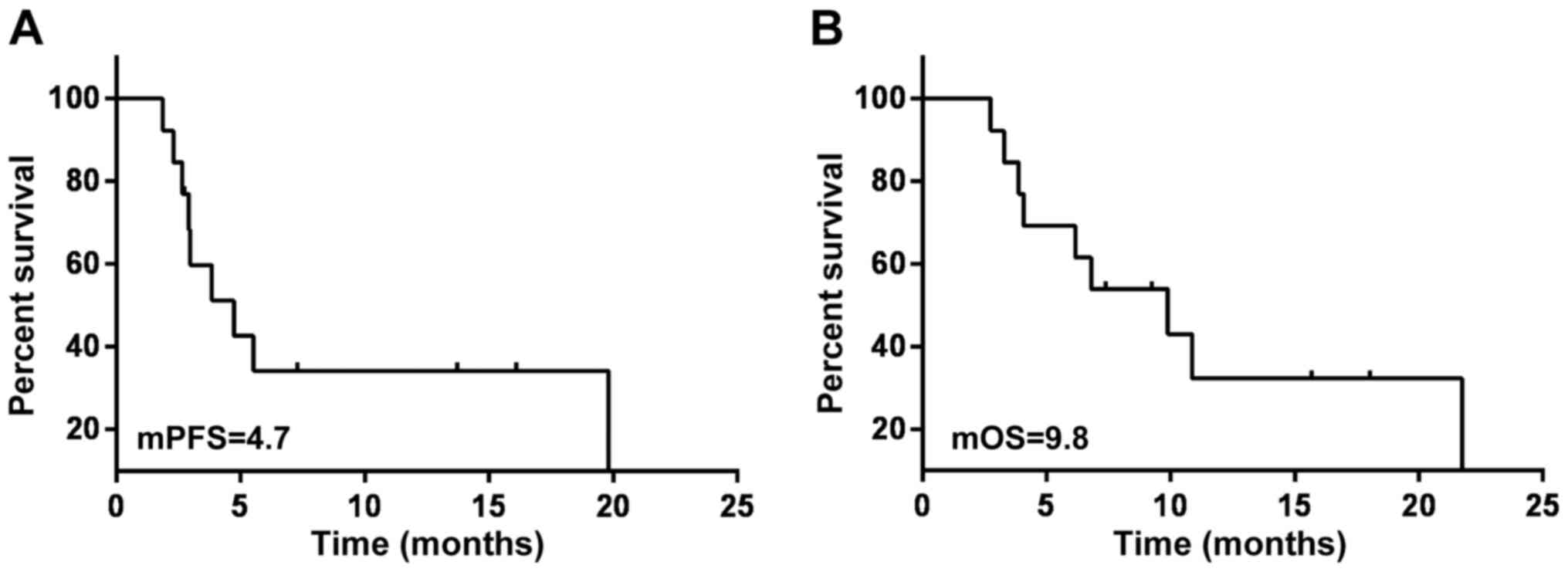
(Fig. 2). The mPFS of the 13
patients with NSCLC was 4.7 months and the mOS was 9.8 months
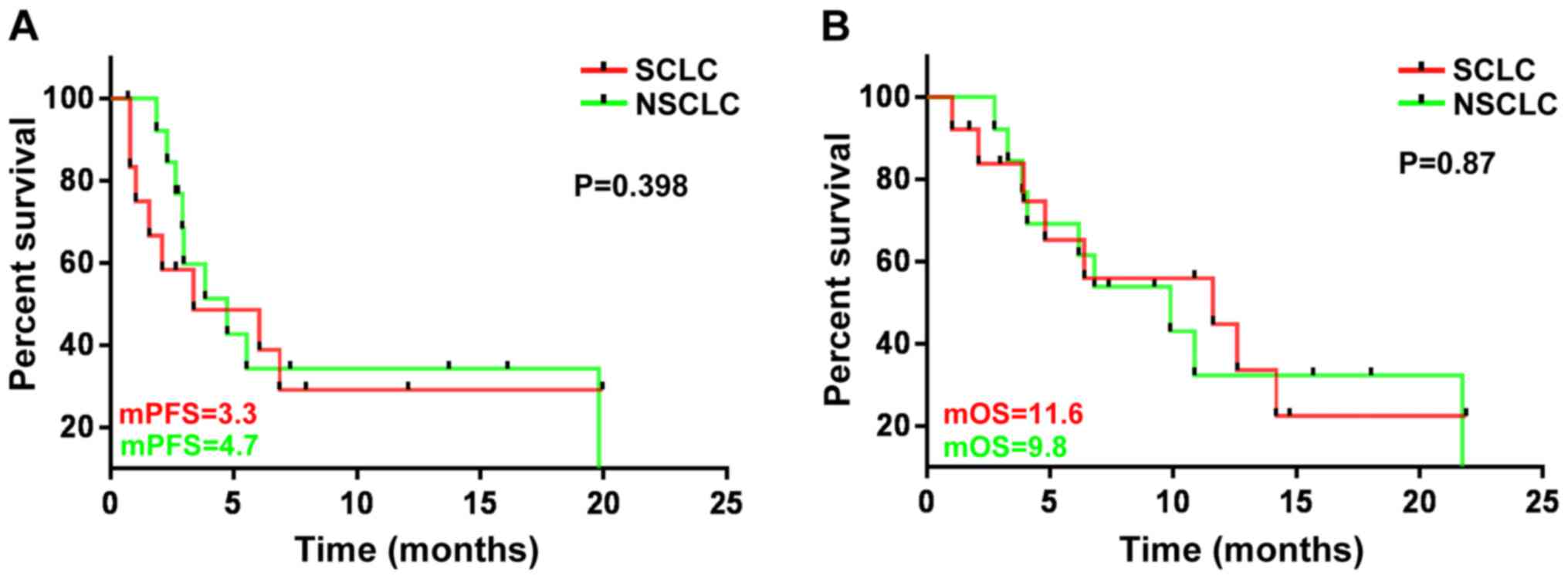
(Fig. 3). There was no significant
difference in the mPFS and mOS between the SCLC and NSCLC patients
(P=0.398 and 0.87, respectively) (Fig.
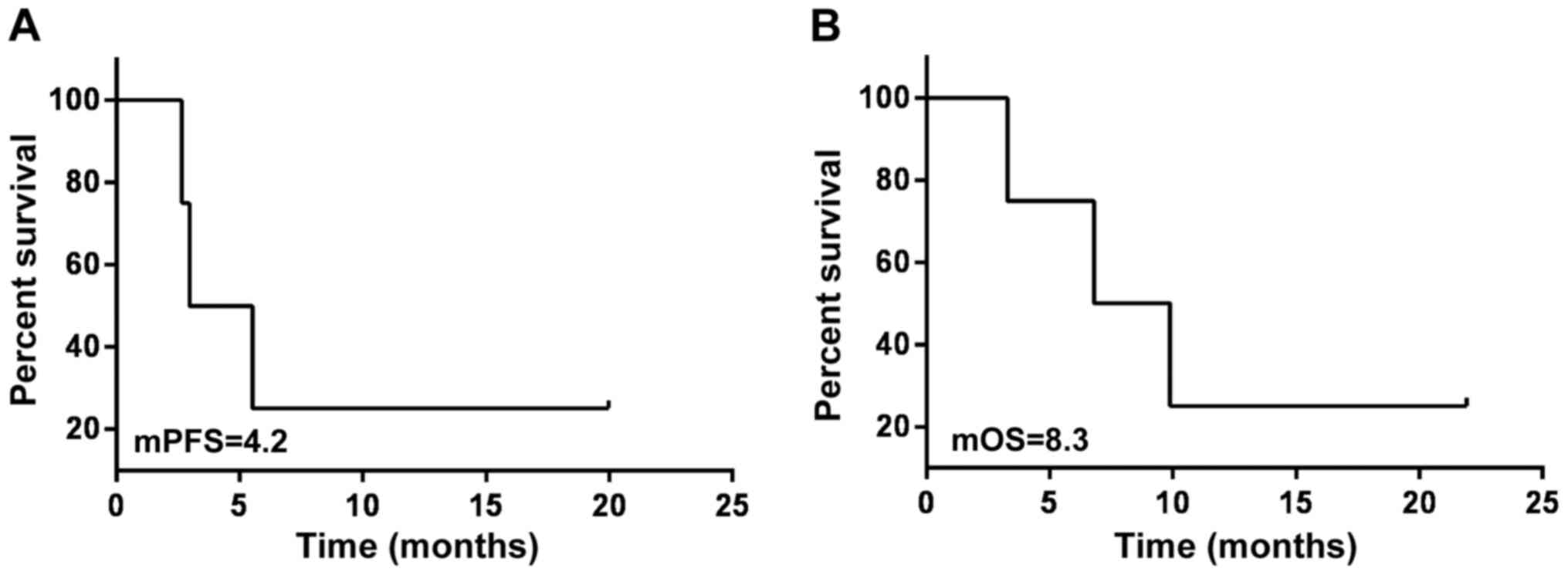
4). For the patients treated with radiotherapy during
medication, the follow-up data suggested that the mPFS and mOS was
4.2 and 8.3 months, respectively (Fig.
5). Furthermore, the mPFS and mOS of the 4 patients with
esophageal cancer was 9.2 and 12.9 months, respectively. The mPFS
and mOS of the 3 patients with cervical cancer was 3.2 and 6.9
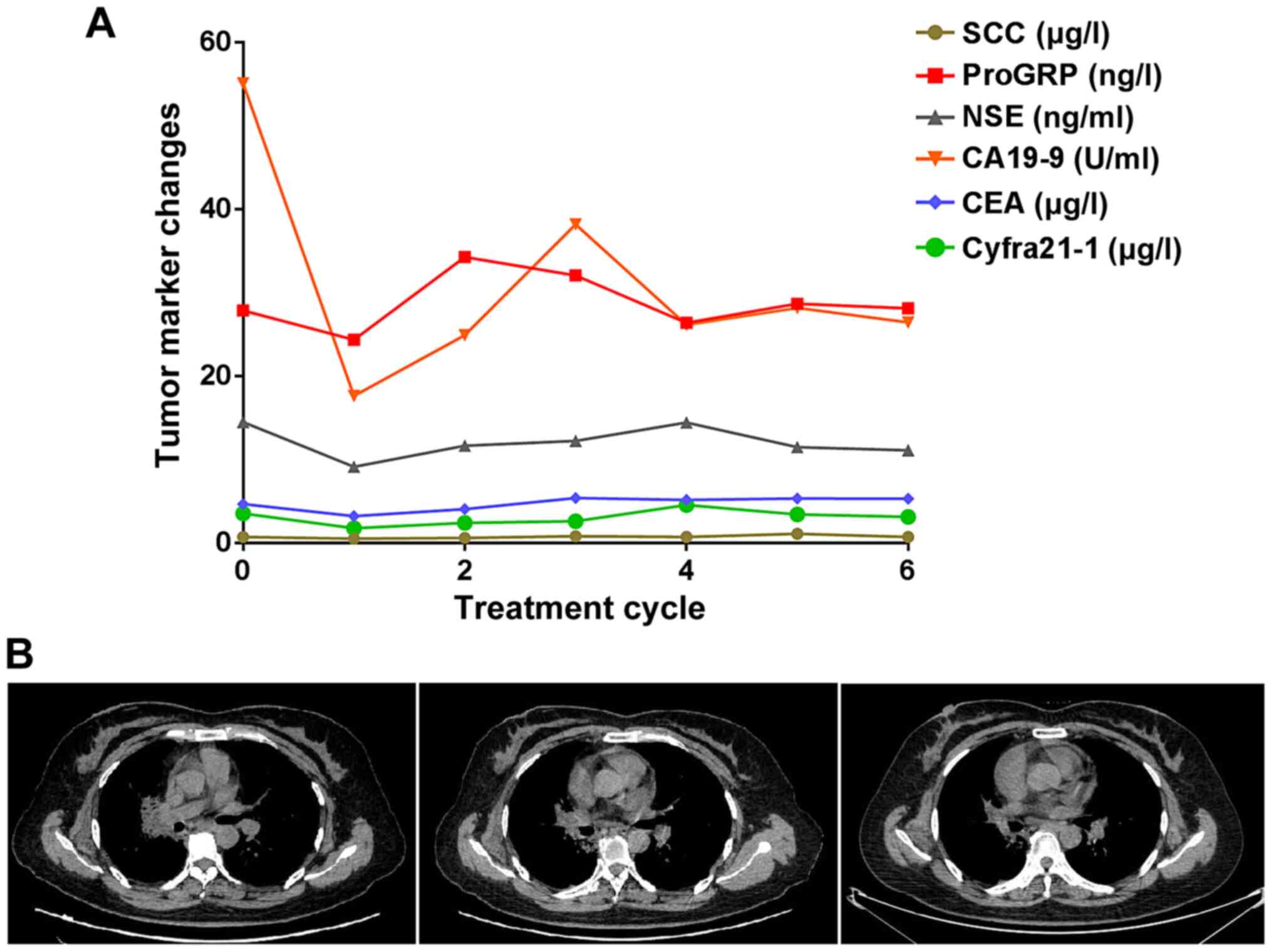
months, respectively (data not shown). In Fig. 6, a representative case of a patient
with lung adenocarcinoma in whom a good effect was achieved is
presented. After taking the medicine for 2, 4 and 6 cycles, the CT
reexamination showed that the lesions were diminished than prior to
taking the medicine and the tumor markers were also reduced.
Discussion
The present study was the first to evaluate the
curative effect and safety of apatinib and S-1 in the treatment of
refractory and recurrent solid tumors, to the best of our
knowledge. The total DCR was 72.7% and the incidence rate of
adverse effects (grade 3-4) was 45.5%. The major side effects were
hypertension, thrombocytopenia and proteinuria. In brief, apatinib
combined with S-1 appeared to be effective and relatively safe in
the treatment of solid tumor, but caution should be taken for
patients with squamous-cell lung carcinoma.
Targeted therapy, immunotherapy, radiotherapy and
chemotherapy are standard treatments for most types of carcinoma
(23). A previous study indicated
that the ORR and DCR was 42.2 and 51.5% in patients with NSCLC who
received apatinib treatment (250 mg/day) (24). In comparison, the present study
suggested that the ORR and DCR of the 13 patients with NSCLC were
30.77 and 84.62%; for the 13 patients with SCLC, the ORR and DCR
rates were 30.77 and 53.85%, respectively. In the present study,
the efficacy of apatinib combined with S-1 was confirmed and a
significant antitumor effect was observed in patients with advanced
lung cancer. However, the mPFS and mOS of the patients of the
present study was only 3.3 and 11.6 months, respectively, which was
considered similar to another study (25). As an example, in a single-center
retrospective study, 23 cases of extensive-stage SCLC had received
apatinib as maintenance therapy and the mPFS and mOS was 4.1 and
12.5 months, respectively (25). A
major reason may be that the patients with SCLC in the present
study who received multi-course treatment had a poor KPS score.
VEGF is highly expressed in numerous types of solid
tumor (26), which has an important
role in promoting endothelial cell survival and maintaining
vascular integrity. Bleeding is a common adverse reaction of VEGFR
inhibitor, which may be directly related to the inhibitory effect
of the VEGF signaling pathway (27). Apatinib, targeting the VEGF
signaling pathway, has been indicated to be a promising treatment
for numerous types of cancer in preclinical and clinical trials
(28). The majority of these
patients (n=24; 72.7%) exhibited tumor shrinkage or stabilization,
which is better than the effect of other VEGFR-TKIs. The potential
reasons may be as follows: i) When combined with chemotherapy,
inhibition of VEGF may impair the ability of endothelial cells to
repair or regenerate during tumor shrinkage, resulting in an
increased risk of bleeding (29).
As an example, the SD rate reported in a phase-I study of sorafenib
for advanced refractory solid tumors was only 26% (30). In a pooled analysis of 137 patients
with advanced refractory solid tumors in 4 phase-I trials of
sorafenib, only 2 patients (1.4%) achieved PR and 38 (28%) achieved
SD, while the majority of patients (70.8%) exhibited PD on
radiological imaging (31). ii) The
combination treatment S-1 was proven effective in the treatment of
certain types of solid cancer, including gastric (32), breast (33), colorectal (34) and pancreatic (35) cancer, and NSCLC (36). S-1 is a combination of the
fluoropyrimidine-based anticancer agent tegafur (FT) as the
effector drug with two modulators, 5-chloro-2,4-dihydroxypyridine
(CDHP) and potassium oxonate (Oxo), at a molar ratio of
1:0.4:1(37). The degradation of
FT-derived 5-fluorouracil (5-FU) is inhibited by CDHP, resulting in
enhancement of the antitumor effect (38,39).
Oxo reduces the gastrointestinal toxicity of 5-FU. After its oral
administration, Oxo is distributed selectively to the small and
large intestines, thereby reducing the incidence of diarrhea
(40). A phase-II trial determined
that the adverse event profile of the combination S-1 was
neutropenia (10.3%), leukopenia (13.8%), anemia (3.4%) and
thrombocytopenia (3.4%) (41).
In the present study, anemia, hypertension,
leukopenia, increase of bilirubin, proteinuria and HFS were the
most common treatment-associated toxicities. Among them, the
incidence rate of grade 3-4 anemia, leukopenia and thrombocytopenia
was only 6.1, 6.1 and 12.1%, respectively. Most of the remaining
adverse events were not severe and controllable (grade 1/2). These
side effects may mainly be due to apatinib. While 69% of patients
in the present study experienced myelosuppression, the cause of
bone marrow suppression is the presence of VEGF receptors on bone
marrow progenitor cells (42).
Although 66.7% of the patients experienced hypertension, it was
easy to lower blood pressure with medication. The key mechanism of
hypertension is thought to be that VEGF inhibition diminishes
nitric oxide synthesis and promotes vasoconstriction, thereby
increasing peripheral resistance (43). Although 36.4% of patients
experienced an increase of bilirubin, this was transient and
disappeared gradually after the treatment was terminated.
Furthermore, the incidence of HFS and proteinuria was low and of
grade 1 or 2 in most cases. Of note, a patient with cervical cancer
developed grade-3 HFS after taking two cycles of medication. A
study has indicated that the mechanism is still unclear and may not
be produced by blockade of the epidermal growth factor receptor,
but rather by effects on intracellular downstream pathways
(44). The use of lotions or
moisturizers may help ease symptoms. In the present study, two
patients died of massive hemorrhage, but neither of them had PD
according to CT scan. This may be associated with the location of
the lesion, which was close to the aorta in those patients.
Therefore, apatinib should be used in patients with squamous-cell
lung carcinoma with caution. Similar side effects have been
reported for bevacizumab (avastin) and sunitinib malates (sutent,
sorafenib and nexavar) (27,45-47).
In the case of cough or high pressure in the thoracic region,
massive hemoptysis due to the broken integrity of the vessel wall
may be present. Thereby, the safety of apatinib in patients with
advanced squamous-cell carcinoma may be worthy of recognition. The
underlying mechanisms of treatment-associated pulmonary hemorrhage
and its possible clinical risk factors should be further explored.
It was indicated that squamous-cell histology, tumor erosion,
necrosis or cavitation, tumor location close to major blood vessels
or trachea may be considered as potential risk factors for
apatinib-associated hemorrhage.
Of note, the present study had certain limitations.
First, as it was an exploratory study, only a small number of
patients were recruited without the availability of any better
treatments, which may lead to unreliable statistical results.
Furthermore, PFS and OS analysis were performed among different
groups, but it may not be possible to generalize the conclusions
from the subgroup analyses within the small heterogeneous cohort.
It is necessary to perform further studies in the future.
In conclusion, the present study indicated that the
combination of apatinib and S-1 had manageable toxicities and
promising efficacy in patients with recurrent and refractory solid
tumors, but the regimen should be used with caution in patients
with squamous-cell lung carcinoma.
Supplementary Material
Characteristics of patients subjected
to radiotherapy during medication.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the
National Natural Science Foundation of China (grant no. 81372518 to
PW) and the Tianjin Science and Technology Commission Project
(grant no. 16JCYBJC25300 to NL).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SC, JS, HL, JL and DJ collected the clinical cases;
SC performed statistical analysis; NL designed the study and
obtained funding; SC drafted the manuscript; and LZ, YGS, YCS
provided the cases and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures in studies involving human
participants were performed in accordance with the ethical
standards of the institutional research committee and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards. This study was approved by the Tianjin Medical
University Cancer Institute and Hospital Human Research Ethics
Committee (Tianjin, China). Comprehensive informed consent was
obtained from all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
van Hagen P, Hulshof MC, van Lanschot JJ,
Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ,
Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al: Preoperative
chemoradiotherapy for esophageal or junctional cancer. N Engl J
Med. 366:2074–2084. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Song Z, Yu X, Lou G, Shi X and Zhang Y:
Salvage treatment with apatinib for advanced non-small-cell lung
cancer. Onco Targets Ther. 10:1821–1825. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Roudsari LC and West JL: Studying the
influence of angiogenesis in in vitro cancer model systems. Adv
Drug Deliv Rev. 97:250–259. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676.
2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ebos JM, Bocci G, Man S, Thorpe PE,
Hicklin DJ, Zhou D, Jia X and Kerbel RS: A naturally occurring
soluble form of vascular endothelial growth factor receptor 2
detected in mouse and human plasma11ontario graduate scholarship in
science and technology (J.M.L. Ebos); sunnybrook trust for medical
research (G. Bocci); and NIH grant CA41223, Canadian institutes for
health research, and national cancer institute of Canada (R.S.
Kerbel). Mol Cancer Res. 2:315–326. 2004.
|
|
8
|
Geng R, Song L, Li J and Zhao L: The
safety of apatinib for the treatment of gastric cancer. Expert Opin
Drug Saf. 17:1145–1150. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brower V: Apatinib in treatment of
refractory gastric cancer. Lancet Oncol. 17(e137)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ding L, Li QJ, You KY, Jiang ZM and Yao
HR: The use of apatinib in treating nonsmall-cell lung cancer: Case
report and review of literature. Medicine (Baltimore).
95(e3598)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li F, Liao Z, Zhao J, Zhao G, Li X, Du X,
Yang Y and Yang J: Efficacy and safety of Apatinib in stage IV
sarcomas: Experience of a major sarcoma center in China.
Oncotarget. 8:64471–64480. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Scott A, Messersmith W and Jimeno A:
Apatinib: A promising oral antiangiogenic agent in the treatment of
multiple solid tumors. Drugs Today (Barc). 51:223–229.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y,
Liu W, Tong J, Liu Y, Xu R, et al: Randomized, double-blind,
placebo-controlled phase III trial of apatinib in patients with
chemotherapy-refractory advanced or metastatic adenocarcinoma of
the stomach or gastroesophageal junction. J Clin Oncol.
34:1448–1454. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hu X, Zhang J, Xu B, Jiang Z, Ragaz J,
Tong Z, Zhang Q, Wang X, Feng J, Pang D, et al: Multicenter phase
II study of apatinib, a novel VEGFR inhibitor in heavily pretreated
patients with metastatic triple-negative breast cancer. Int J
Cancer. 135:1961–1969. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li J and Wang L: Efficacy and safety of
apatinib treatment for advanced esophageal squamous cell carcinoma.
Onco Targets Ther. 10:3965–3969. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li F, Zhu T, Cao B, Wang J and Liang L:
Apatinib enhances antitumour activity of EGFR-TKIs in non-small
cell lung cancer with EGFR-TKI resistance. Eur J Cancer.
84:184–192. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hu X, Cao J, Hu W, Wu C, Pan Y, Cai L,
Tong Z, Wang S, Li J, Wang Z, et al: Multicenter phase II study of
apatinib in non-triple-negative metastatic breast cancer. BMC
Cancer. 14(820)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shirasaka T, Nakano K, Takechi T, Satake
H, Uchida J, Fujioka A, Saito H, Okabe H, Oyama K, Takeda S, et al:
Antitumor activity of 1 M tegafur-0.4 M
5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against
human colon carcinoma orthotopically implanted into nude rats.
Cancer Res. 56:2602–2606. 1996.PubMed/NCBI
|
|
19
|
Yumine K and Kawahara M: Phase II study of
S-1, a novel oral fluorouracil, in advanced non-small-cell lung
cancer. Gan To Kagaku Ryoho. 33 (Suppl 1):189–192. 2006.PubMed/NCBI(In Japanese).
|
|
20
|
Zhang H, Xiong J, Guo L, Patel N and Guang
X: Integrated traditional Chinese and western medicine modulator
for overcoming the multidrug resistance with carbon nanotubes. RSC
Adv. 5:71287–71296. 2015.
|
|
21
|
Wu P, Li S and Zhang H: Design real-time
reversal of tumor multidrug resistance cleverly with shortened
carbon nanotubes. Drug Des Devel Ther. 8:2431–2438. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP,
Wang F, Tao LY, Zhang CZ, Dai CL, Tiwari AK, et al: Apatinib
(YN968D1) reverses multidrug resistance by inhibiting the efflux
function of multiple ATP-binding cassette transporters. Cancer Res.
70:7981–7991. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gotwals P, Cameron S, Cipolletta D,
Cremasco V, Crystal A, Hewes B, Mueller B, Quaratino S,
Sabatos-Peyton C, Petruzzelli L, et al: Prospects for combining
targeted and conventional cancer therapy with immunotherapy. Nat
Rev Cancer. 17:286–301. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang SY, Liu Z, Ou W, Li N, Wu HQ, Mao XY
and Yuan C: Apatinib monotherapy for advanced non-small cell lung
cancer after the failure of chemotherapy or other targeted therapy.
J Clin Oncol. 35 (15 Suppl)(e20626)2017.
|
|
25
|
Yan X, Wang Q, Wang H, Li P, Zhang G,
Zhang M, Zheng X, Yang J, Zhang X and Ma Z: Apatinib as maintenance
therapy in extensive-stage small-cell lung cancer: Results from a
single-center retrospective study. J Cancer Res Clin Oncol.
145:235–240. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Roskoski R Jr: Vascular endothelial growth
factor (VEGF) signaling in tumor progression. Crit Rev Oncol
Hematol. 62:179–213. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kamba T and McDonald D: Mechanisms of
adverse effects of anti-VEGF therapy for cancer. Br J Cancer.
96:1788–1795. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Scott LJ: Apatinib: A review in advanced
gastric cancer and other advanced cancer. Drugs. 78:747–758.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kilickap S, Abali Hs and Celik I:
Bevacizumab, bleeding, thrombosis, and warfarin. J Clin Oncol.
21:3542–3543. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Clark JW, Eder JP, Ryan D, Lathia C and
Lenz HJ: Safety and pharmacokinetics of the dual action Raf kinase
and vascular endothelial growth factor receptor inhibitor, BAY
43-9006, in patients with advanced, refractory solid tumors. Clin
Cancer Res. 11:5472–5480. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Strumberg D, Clark JW, Awada A, Moore MJ,
Richly H, Hendlisz A, Hirte HW, Eder JP, Lenz HJ, Schwartz B, et
al: Safety, pharmacokinetics, and preliminary antitumor activity of
sorafenib: A review of four phase I trials in patients with
advanced refractory solid tumors. Oncologist. 12:426–437.
2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Saeki T, Takashima S, Sano M, Horikoshi N,
Miura S, Shimizu S, Morimoto K, Kimura M, Aoyama H, Ota J, et al: A
phase II study of S-1 in patients with metastatic breast cancer-a
Japanese trial by the S-1 cooperative study group, breast cancer
working group. Breast Cancer. 11:194–202. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Goto A, Yamada Y, Yasui H, Kato K,
Hamaguchi T, Muro K, Shimada Y and Shirao K: Phase II study of
combination therapy with S-1 and irinotecan in patients with
advanced colorectal cancer. Ann Oncol. 17:968–973. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Okusaka T, Funakoshi A, Furuse J, Boku N,
Yamao K, Ohkawa S and Saito H: A late phase II study of S-1 for
metastatic pancreatic cancer. Cancer Chemother Pharmacol.
61:615–621. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Takakuwa O, Oguri T, Maeno K, Ozasa H,
Iwashima Y, Miyazaki M, Kunii H, Takano Y, Mori T, Sato S and Ueda
R: Efficacy of S-1 monotherapy for non-small cell lung cancer after
the failure of two or more prior chemotherapy regimens. Oncol Lett.
1:147–150. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shirasaka T, Shimamato Y, Ohshimo H,
Yamaguchi M, Kato T, Yonekura K and Fukushima M: Development of a
novel form of an oral 5-fluorouracil derivative (S-1) directed to
the potentiation of the tumor selective cytotoxicity of
5-fluorouracil by two biochemical modulators. Anticancer Drugs.
7:548–557. 1996.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tatsumi K, Fukushima M, Shirasaka T and
Fujii S: Inhibitory effects of pyrimidine, barbituric acid and
pyridine derivatives on 5-fluorouracil degradation in rat liver
extracts. Jpn J Cancer Res. 78:748–755. 1987.PubMed/NCBI
|
|
39
|
Oguri T, Achiwa H, Bessho Y, Muramatsu H,
Maeda H, Niimi T, Sato S and Ueda R: The role of thymidylate
synthase and dihydropyrimidine dehydrogenase in resistance to
5-fluorouracil in human lung cancer cells. Lung Cancer. 49:345–351.
2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shirasaka T, Shimamoto Y and Fukushima M:
Inhibition by oxonic acid of gastrointestinal toxicity of
5-fluorouracil without loss of its antitumor activity in rats.
Cancer Res. 53:4004–4009. 1993.PubMed/NCBI
|
|
41
|
Kaira K, Sunaga N, Yanagitani N, Imai H,
Utsugi M, Iwasaki Y, Shimizu K, Iijima H, Tsurumaki H, Tomizawa Y,
et al: Phase 2 study of S-1 plus carboplatin in patients with
advanced non-small cell lung cancer. Lung Cancer. 68:253–257.
2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hattori K, Heissig B, Wu Y, Dias S, Tejada
R, Ferris B, Hicklin DJ, Zhu Z, Bohlen P, Witte L, et al: Placental
growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+)
stem cells from bone-marrow microenvironment. Nat Med. 8:841–849.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
43
|
Hood JD, Meininger CJ, Ziche M and Granger
HJ: VEGF upregulates ecNOS message, protein, and NO production in
human endothelial cells. Am J Physiol. 274:H1054–H1058.
1998.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Alexandrescu D, Vaillant J and Dasanu C:
Effect of treatment with a colloidal oatmeal lotion on the acneform
eruption induced by epidermal growth factor receptor and multiple
tyrosine-kinase inhibitors. Clin Exp Dermatol. 32:71–74.
2007.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Johnson DH, Fehrenbacher L, Novotny WF,
Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF III,
Gaudreault J, Damico LA, et al: Randomized phase II trial comparing
bevacizumab plus carboplatin and paclitaxel with carboplatin and
paclitaxel alone in previously untreated locally advanced or
metastatic non-small-cell lung cancer. J Clin Oncol. 22:2184–2191.
2004.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Socinski MA, Novello S, Sanchez JM,
Brahmer JA, Govindan R, Belani CP, Atkins JN, Gillenwater HH,
Palleres C and Chao RC: Efficacy and safety of sunitinib in
previously treated, advanced non-small cell lung cancer (NSCLC):
Preliminary results of a multicenter phase II trial. J Clin Oncol.
24 (18 Suppl)(S7001)2006.
|