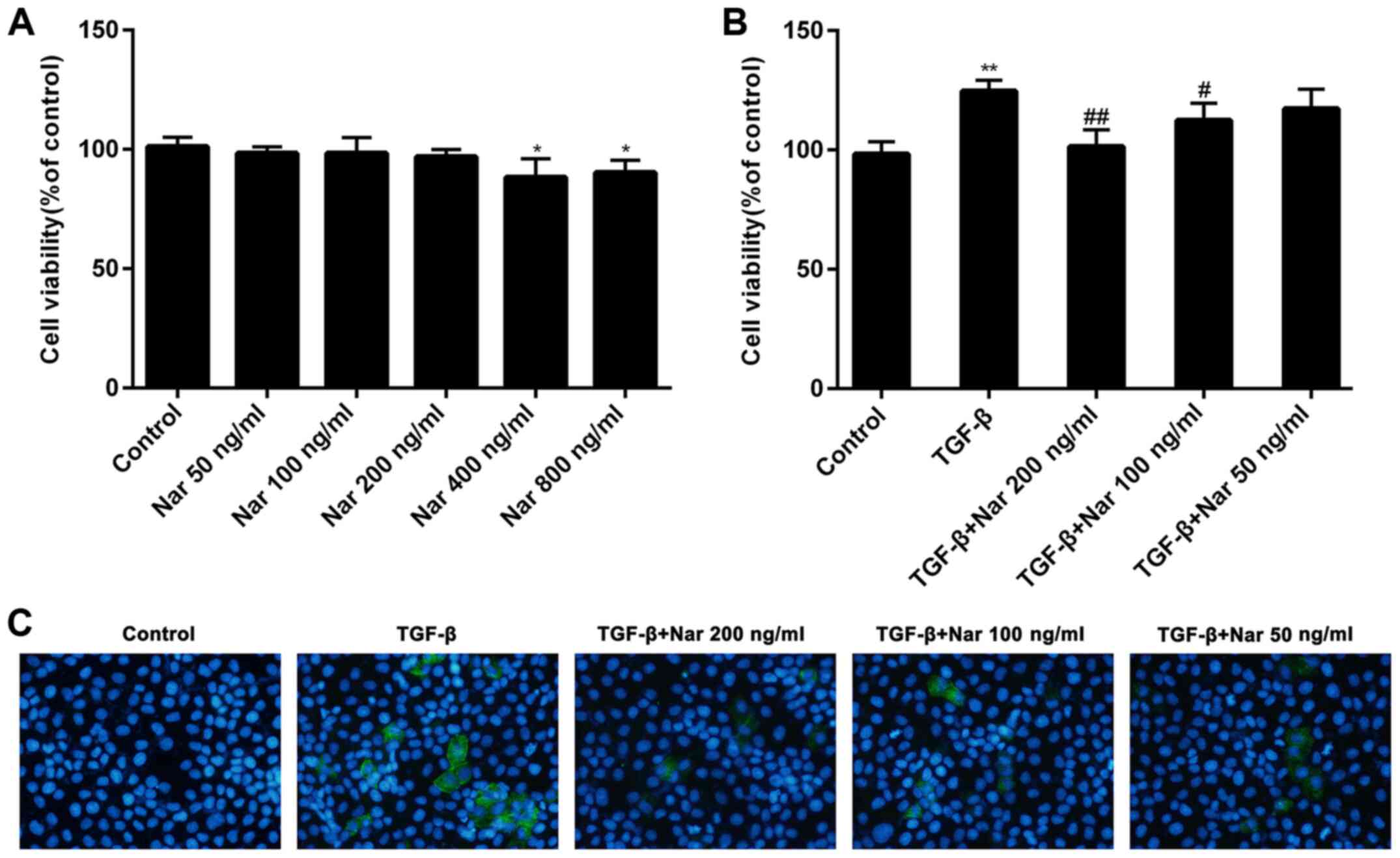
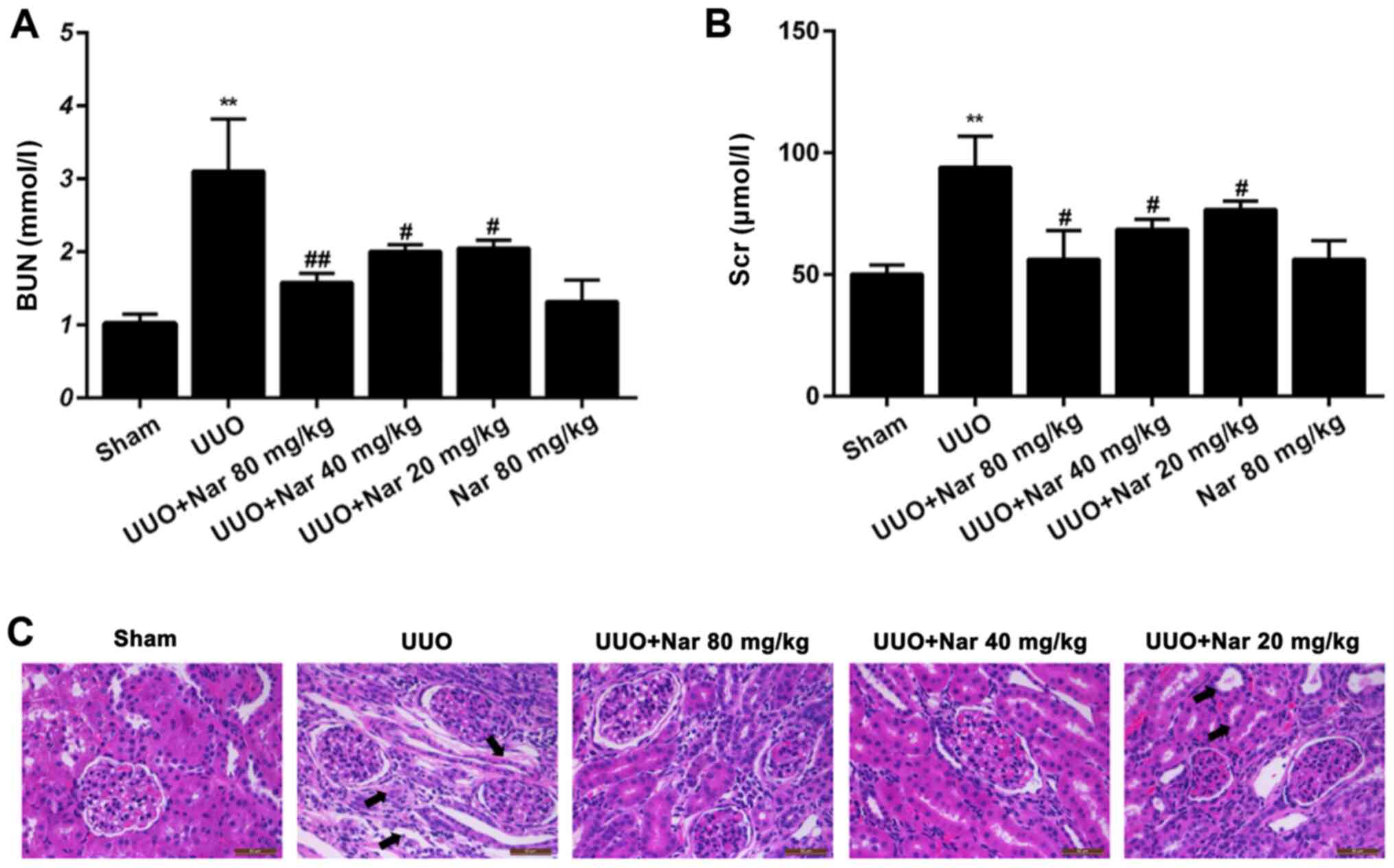
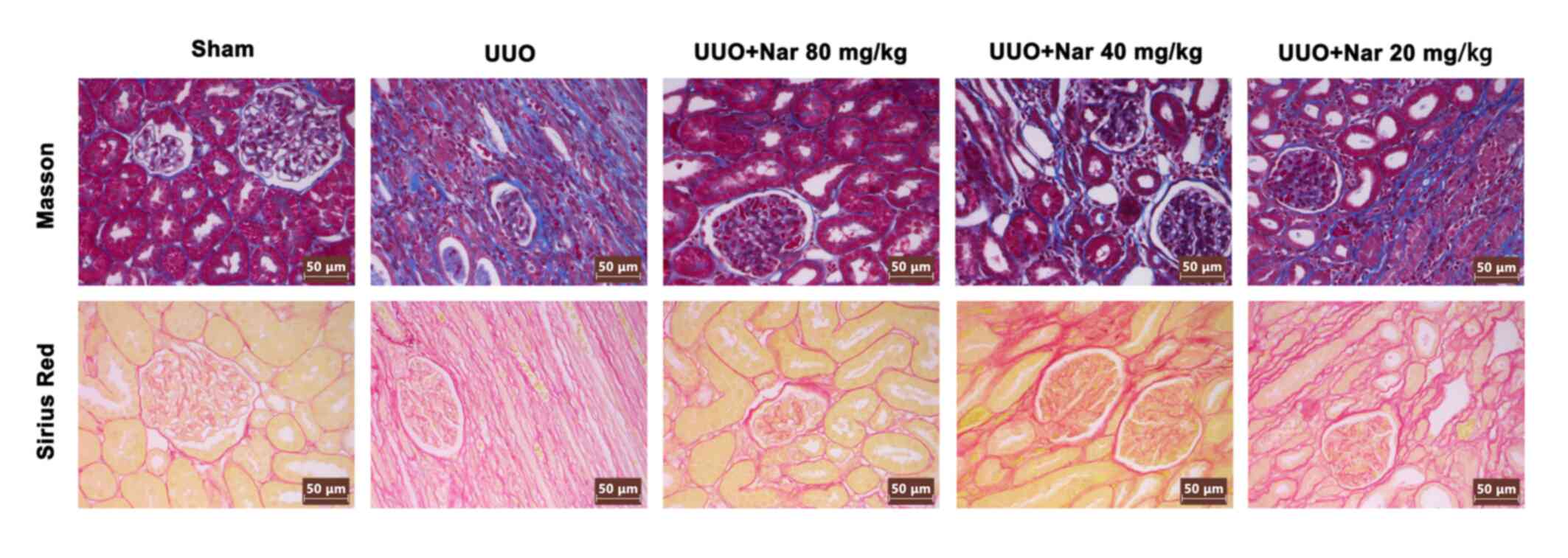
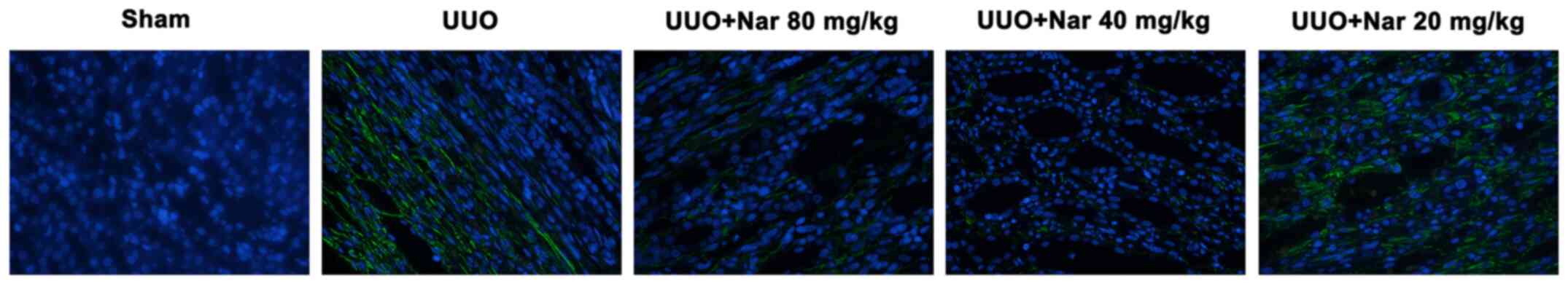
|
1
|
Lawson J, Elliott J, Wheeler-Jones C, Syme
H and Jepson R: Renal fibrosis in feline chronic kidney disease:
Known mediators and mechanisms of injury. Vet J. 203:18–26.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kanasaki K, Taduri G and Koya D: Diabetic
nephropathy: The role of inflammation in fibroblast activation and
kidney fibrosis. Front Endocrinol (Lausanne). 4(7)2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hill NR, Fatoba ST, Oke JL, Hirst JA,
O'Callaghan CA, Lasserson DS and Hobbs FD: Global prevalence of
chronic kidney disease-A systematic review and meta-analysis. PLoS
One. 11(e0158765)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gewin LS: Renal fibrosis: Primacy of the
proximal tubule. Matrix Biol. 68-69:248–262. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen PS, Li YP and Ni HF: Morphology and
evaluation of renal fibrosis. Adv Exp Med Biol. 1165:17–36.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wilson PC, Kashgarian M and Moeckel G:
Interstitial inflammation and interstitial fibrosis and tubular
atrophy predict renal survival in lupus nephritis. Clin kidney J.
11:207–218. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhao D and Luan Z: Oleanolic acid
attenuates renal fibrosis through TGF-β/Smad pathway in a rat model
of unilateral ureteral obstruction. Evid Based Complement Alternat
Med. 2020(2085303)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Deng T, Wei Z, Gael A, Deng X, Liu Y, Lai
J, Hang L, Yan Q, Fu Q and Li Z: Higenamine improves cardiac and
renal fibrosis in rats with cardiorenal syndrome via ASK1 signaling
pathway. J Cardiovasc Pharmacol. 75:535–544. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li W, Cheng F, Songyang YY, Yang SY, Wei J
and Ruan Y: CTRP1 attenuates UUO-induced renal fibrosis via
AMPK/NOX4 pathway in mice. Curr Med Sci. 40:48–54. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu Y, Feng Q, Miao J, Wu Q, Zhou S, Shen
W, Feng Y, Hou FF, Liu Y and Zhou L: C-X-C motif chemokine receptor
4 aggravates renal fibrosis through activating
JAK/STAT/GSK3β/β-catenin pathway. J Cell Mol Med. 24:3837–3855.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
TGF-β: The master regulator of fibrosis. Nat Rev Nephrol.
12:325–338. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tieri P, Termanini A, Bellavista E,
Salvioli S, Capri M and Franceschi C: Charting the NF-κB pathway
interactome map. PloS One. 7(e32678)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Meng XM, Huang XR, Xiao J, Chung AC, Qin
W, Chen HY and Lan HY: Disruption of Smad4 impairs TGF-beta/Smad3
and Smad7 transcriptional regulation during renal inflammation and
fibrosis in vivo and in vitro. Kidney Int. 81:266–279.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-β/Smad signaling in
tissue fibrosis. Chem Biol Interact. 292:76–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Campbell MT, Hile KL, Zhang H, Asanuma H,
Vanderbrink BA, Rink RR and Meldrum KK: Toll-like receptor 4: A
novel signaling pathway during renal fibrogenesis. J Surg Res.
168:e61–69. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pulskens WP, Rampanelli E, Teske GJ,
Butter LM, Claessen N, Luirink IK, van der Poll T, Florquin S and
Leemans JC: TLR4 promotes fibrosis but attenuates tubular damage in
progressive renal injury. J Am Soc Nephrol. 21:1299–1308.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jang HR and Rabb H: Immune cells in
experimental acute kidney injury. Nat Rev Nephrol. 11:88–101.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yao L, Li J, Li L, Li X, Zhang R, Zhang Y
and Mao X: Coreopsis tinctoria Nutt ameliorates high
glucose-induced renal fibrosis and inflammation via the
TGF-β1/SMADS/AMPK/NF-κB pathways. BMC Complement Altern Med.
19(14)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zeng X, Su W, Zheng Y, He Y, He Y, Rao H,
Peng W and Yao H: Pharmacokinetics, tissue distribution,
metabolism, and excretion of naringin in aged rats. Front
Pharmacol. 10(34)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen R, Qi QL, Wang MT and Li QY:
Therapeutic potential of naringin: an overview. Pharm Biol.
54:3203–3210. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Adil M, Kandhare AD, Ghosh P, Venkata S,
Raygude KS and Bodhankar SL: Ameliorative effect of naringin in
acetaminophen-induced hepatic and renal toxicity in laboratory
rats: Role of FXR and KIM-1. Ren Fail. 38:1007–1020.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
El-Desoky AH, Abdel-Rahman RF, Ahmed OK,
El-Beltagi HS and Hattori M: Anti-inflammatory and antioxidant
activities of naringin isolated from Carissa carandas L.: In
vitro and in vivo evidence. Phytomedicine. 42:126–134.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Caglayan C, Temel Y, Kandemir FM, Yildirim
S and Kucukler S: Naringin protects against
cyclophosphamide-induced hepatotoxicity and nephrotoxicity through
modulation of oxidative stress, inflammation, apoptosis, autophagy,
and DNA damage. Environ Sci Pollut Res Int. 25:20968–20984.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ahmed S, Khan H, Aschner M, Hasan MM and
Hassan STS: Therapeutic potential of naringin in neurological
disorders. Food Chem Toxicol. 132(110646)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dong D, Xu L, Yin L, Qi Y and Peng J:
Naringin prevents carbon tetrachloride-induced acute liver injury
in mice. J Functional Foods. 12:179–191. 2015.
|
|
27
|
Adil M, Kandhare AD, Visnagri A and
Bodhankar SL: Naringin ameliorates sodium arsenite-induced renal
and hepatic toxicity in rats: decisive role of KIM-1, Caspase-3,
TGF-β, and TNF-α. Ren Fail. 37:1396–1407. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Adil M, Kandhare AD, Ghosh P and Bodhankar
SL: Sodium arsenite-induced myocardial bruise in rats: Ameliorative
effect of naringin via TGF-β/Smad and Nrf/HO pathways. Chem Biol
Int. 253:66–77. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen F, Zhang N, Ma X, Huang T, Shao Y, Wu
C and Wang Q: Naringin alleviates diabetic kidney disease through
inhibiting oxidative stress and inflammatory reaction. PLoS One.
10(e0143868)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang H, Gao M, Li J, Sun J, Wu R, Han D,
Tan J, Wang J, Wang B, Zhang L and Dong Y: MMP-9-positive
neutrophils are essential for establishing profibrotic
microenvironment in the obstructed kidney of UUO mice. Acta Physiol
(Oxf). 227(e13317)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang ZH, He JQ, Zhao YY, Chen HC and Tan
NH: Asiatic acid prevents renal fibrosis in UUO rats via promoting
the production of 15d-PGJ2, an endogenous ligand of PPAR-γ. Acta
Pharmacol Sin. 41:373–382. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Health NIo: Guide for the care and use of
laboratory animals. 1985.
|
|
33
|
Hosseinian S, Rad AK, Bideskan AE,
Soukhtanloo M, Sadeghnia H, Shafei MN, Motejadded F, Mohebbati R,
Shahraki S and Beheshti F: Thymoquinone ameliorates renal damage in
unilateral ureteral obstruction in rats. Pharmacol Rep. 69:648–657.
2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu H, Li W, He Q, Xue J, Wang J, Xiong C,
Pu X and Nie Z: Mass spectrometry imaging of kidney tissue sections
of rat subjected to unilateral ureteral obstruction. Sci Rep.
7(41954)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lan HY: Diverse roles of TGF-β/Smads in
renal fibrosis and inflammation. Int J Biol Sci. 7:1056–1067.
2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Boor P and Floege J: Chronic kidney
disease growth factors in renal fibrosis. Clin Exp Pharmacol
Physiol. 38:441–450. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Nangaku M: Chronic hypoxia and
tubulointerstitial injury: A final common pathway to end-stage
renal failure. J Am Soc Nephrol. 17:17–25. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Salehi B, Fokou PVT, Sharifi-Rad M, Zucca
P, Pezzani R, Martins N and Sharifi-Rad J: The therapeutic
potential of naringenin: A review of clinical trials.
Pharmaceuticals (Basel) 12: 2019.
|
|
40
|
Zhang M, Guo Y, Fu H, Hu S, Pan J, Wang Y,
Cheng J, Song J, Yu Q, Zhang S, et al: Chop deficiency prevents
UUO-induced renal fibrosis by attenuating fibrotic signals
originated from Hmgb1/TLR4/NFκB/IL-1β signaling. Cell Death Dis.
6(e1847)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gong W, Mao S, Yu J, Song J, Jia Z, Huang
S and Zhang A: NLRP3 deletion protects against renal fibrosis and
attenuates mitochondrial abnormality in mouse with 5/6 nephrectomy.
Am J Physiol Renal Physiol. 310:F1081–1088. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen W, Yan Y, Song C, Ding Y and Du T:
Microvesicles derived from human Wharton's Jelly mesenchymal stem
cells ameliorate ischemia-reperfusion-induced renal fibrosis by
releasing from G2/M cell cycle arrest. Biochem J. 474:4207–4218.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nogueira A, Pires MJ and Oliveira PA:
Pathophysiological mechanisms of renal fibrosis: A review of animal
models and therapeutic strategies. In Vivo. 31:1–22.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xiao Z, Li CW, Shan J, Luo L, Feng L, Lu
J, Li SF, Long D and Li YP: Interventions to improve chronic
cyclosporine A nephrotoxicity through inhibiting renal cell
apoptosis: A systematic review. Chin Med J (Engl). 126:3767–3774.
2013.PubMed/NCBI
|
|
45
|
Humphreys BD, Xu F, Sabbisetti V, Grgic I,
Movahedi Naini S, Wang N, Chen G, Xiao S, Patel D, Henderson JM, et
al: Chronic epithelial kidney injury molecule-1 expression causes
murine kidney fibrosis. J Clin Invest. 123:4023–4035.
2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lan HY, Mu W, Tomita N, Huang XR, Li JH,
Zhu HJ, Morishita R and Johnson RJ: Inhibition of renal fibrosis by
gene transfer of inducible Smad7 using ultrasound-microbubble
system in rat UUO model. J Am Soc Nephrol. 14:1535–1548.
2003.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ishii A, Sakai Y and Nakamura A: Molecular
pathological evaluation of clusterin in a rat model of unilateral
ureteral obstruction as a possible biomarker of nephrotoxicity.
Toxicol Pathol. 35:376–382. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Xin BM, Wang XX, Jin W, Yan HM, Cui B,
Zhang XW, Hua F, Yang HZ and Hu ZW: Activation of Toll-like
receptor 9 attenuates unilateral ureteral obstruction-induced renal
fibrosis. Acta pharmacologica Sinica. 31:1583–1592. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wongmekiat O, Leelarungrayub D and
Thamprasert K: Alpha-lipoic acid attenuates renal injury in rats
with obstructive nephropathy. BioMed Res Int.
2013(138719)2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lucarelli G, Mancini V, Galleggiante V,
Rutigliano M, Vavallo A, Battaglia M and Ditonno P: Emerging
urinary markers of renal injury in obstructive nephropathy. BioMed
Res Int. 2014(303298)2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Shinde AV, Humeres C and Frangogiannis NG:
The role of α-smooth muscle actin in fibroblast-mediated matrix
contraction and remodeling. Biochim Biophys Acta Mol Basis Dis.
1863:298–309. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang Q, Peng Z, Xiao S, Geng S, Yuan J and
Li Z: RNAi-mediated inhibition of COL1A1 and COL3A1 in human skin
fibroblasts. Exp Dermatol. 16:611–617. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Artlett CM, Sassi-Gaha S, Rieger JL,
Boesteanu AC, Feghali-Bostwick CA and Katsikis PD: The inflammasome
activating caspase 1 mediates fibrosis and myofibroblast
differentiation in systemic sclerosis. Arthritis Rheum.
63:3563–3574. 2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zeglinski MR, Hnatowich M, Jassal DS and
Dixon IM: SnoN as a novel negative regulator of TGF-β/Smad
signaling: A target for tailoring organ fibrosis. Am J Physiol
Heart Circ Physiol. 308:H75–H82. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Gu YY, Liu XS, Huang XR, Yu XQ and Lan HY:
Diverse role of TGF-β in kidney disease. Front Cell Dev Biol.
8(123)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang X, Huang H, Zhang G, Li D, Wang H
and Jiang W: Raltegravir attenuates experimental pulmonary fibrosis
in vitro and in vivo. Front Pharmacol. 10(903)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
ten Dijke P and Hill CS: New insights into
TGF-β-Smad signalling. Trends Biochem Sci. 29:265–273.
2004.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Miyazawa K, Shinozaki M, Hara T, Furuya T
and Miyazono K: Two major Smad pathways in TGF-beta superfamily
signalling. Genes Cells. 7:1191–1204. 2002.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Biernacka A, Dobaczewski M and
Frangogiannis NG: TGF-β signaling in fibrosis. Growth Factors.
29:196–202. 2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yan X, Liao H, Cheng M, Shi X, Lin X, Feng
XH and Chen YG: Smad7 protein interacts with receptor-regulated
smads (R-Smads) to inhibit transforming growth factor-β
(TGF-β)/smad signaling. J Biol Chem. 291:382–392. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Masola V, Carraro A, Granata S, Signorini
L, Bellin G, Violi P, Lupo A, Tedeschi U, Onisto M, Gambaro G and
Zaza G: In vitro effects of interleukin (IL)-1 beta inhibition on
the epithelial-to-mesenchymal transition (EMT) of renal tubular and
hepatic stellate cells. J Transl Med. 17(12)2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Liu JH, He L, Zou ZM, Ding ZC, Zhang X,
Wang H, Zhou P, Xie L, Xing S and Yi CZ: A novel inhibitor of
homodimerization targeting MyD88 ameliorates renal interstitial
fibrosis by counteracting TGF-β1-induced EMT in vivo and in vitro.
Kidney Blood Press Res. 43:1677–1687. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Edeling M, Ragi G, Huang S, Pavenstadt H
and Susztak K: Developmental signalling pathways in renal fibrosis:
The roles of Notch, Wnt and Hedgehog. Nature Rev Nephrol.
12:426–439. 2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wang D, Warner GM, Yin P, Knudsen BE,
Cheng J, Butters KA, Lien KR, Gray CE, Garovic VD, Lerman LO, et
al: Inhibition of p38 MAPK attenuates renal atrophy and fibrosis in
a murine renal artery stenosis model. Am J Physiol Renal Physiol.
304:F938–F947. 2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ito Y, Aten J, Bende RJ, Oemar BS,
Rabelink TJ, Weening JJ and Goldschmeding R: Expression of
connective tissue growth factor in human renal fibrosis. Kidney
Int. 53:853–861. 1998.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Caraci F, Gili E, Calafiore M, Failla M,
Rosa CL, Crimi N, Sortino MA, Nicoletti F, Copani A and Vancheri C:
TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK
activation in the transition of human lung fibroblasts into
myofibroblasts. Pharmacol Res. 57:274–282. 2008.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Qi W, Twigg S, Chen X, Polhill TS,
Poronnik P, Gilbert RE and Pollock CA: Integrated actions of
transforming growth factor-beta1 and connective tissue growth
factor in renal fibrosis. Am J Physiol Renal Physiol.
288:F800–F809. 2005.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Naito T, Masaki T, Nikolic-Paterson DJ,
Tanji C, Yorioka N and Kohno N: Angiotensin II induces
thrombospondin-1 production in human mesangial cells via p38 MAPK
and JNK: A mechanism for activation of latent TGF-beta1. Am J
Physiol Renal Physiol. 286:F278–F287. 2004.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Gao Y, Jiang W, Dong C, Li C, Fu X, Min L,
Tian J, Jin H and Shen J: Anti-inflammatory effects of sophocarpine
in LPS-induced RAW 264.7 cells via NF-κB and MAPKs signaling
pathways. Toxicol In Vitro. 26:1–6. 2012.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Ihn H: Pathogenesis of fibrosis: Role of
TGF-beta and CTGF. Curr Opin Rheumatol. 14:681–685. 2002.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Luo K: Signaling cross talk between
TGF-beta/Smad and other signaling pathways. Cold Spring Harb
Perspect Biol. 9(a022137)2017.PubMed/NCBI View Article : Google Scholar
|