Introduction
Osteoarthritis (OA) is one of the most common
degenerative joint diseases, affecting millions of individuals
worldwide. OA is characterized by chondrocyte apoptosis and
hypertrophy (1,2), formation of osteophytes and
inflammation of the synovial membrane (3). An increasing number of studies have
reported that chronic inflammation with mild synovitis, elevated
proinflammatory cytokine levels and infiltration of inflammatory
cells (4) are linked to the
progression of OA (5). During OA,
cartilage integrity is lost and hypertrophic chondrocytes exhibit
increased synthetic activity in an attempt to repair the damage.
However, as a result of the increased synthetic activity,
proinflammatory mediator products [including tumor necrosis factor
(TNF)-α to interleukin (IL)-6 and IL-1β)] deregulate chondrocyte
function and act on the adjacent synovium to stimulate
proliferative and inflammatory responses (6). Proliferating synoviocytes also release
proinflammatory products into the synovial fluid (SF), leading to a
proinflammatory microenvironment (6). The altered SF microenvironment affects
inflammatory cell infiltration, polarization and apoptosis. In
addition, various proinflammatory factors such as IL-1, IL-1β, IL-6
and TNF-α secreted by the increased number of inflammatory cells,
including M1 macrophages, promote the proinflammatory
microenvironment of SF further to exacerbate OA. Previous studies
have reported that mononuclear cells, including macrophages
(7), B (8) and T cells (9), which aggregate during OA synovial
tissues and fluid (4,6-8),
contribute to chondrocyte degradation, local inflammation and
activation of innate immune cells. A number of studies have
demonstrated that the elevated number of macrophages present in the
inflamed synovium and SF serve a major role during OA progression
(10-12).
Therefore, identifying the potential mechanism underlying the
effects of the SF microenvironment on macrophage infiltration,
apoptosis and polarization is important for identifying the
mechanism underlying OA.
Macrophages serve important roles in innate
immunity, displaying a high degree of plasticity and polarize into
two main subtypes: Classically activated M1 macrophages and
alternatively activated M2 macrophages (13,14).
Previous studies have reported that M1 macrophages can be activated
by interferon-γ and toll-like receptors, which display a
proinflammatory phenotype by producing proinflammatory cytokines,
including IL-1, IL-6, TNF-α and IL-12 (15,16).
Macrophages can also be polarized into an anti-inflammatory
phenotype, known as M2 macrophages, which promote T helper cell 2
responses and contribute to tissue repair and healing (17,18).
During OA, macrophages can be identified by measuring the
expression of their respective cell markers using flow cytometry
(FCM), including CD14, CD163 and CD68(19). A previous study reported that local
inflammation and activation of innate immune cells, primarily M1
macrophages, are major factors that contribute to the acceleration
of OA progression (20). However,
few studies have explored the impact of the proinflammatory
microenvironment, including synovial tissues and SF, on macrophage
infiltration, apoptosis and polarization during OA progression.
MicroRNAs (miRNAs/miRs) are small, single-stranded
RNAs that are associated with a number of different diseases,
including OA, diabetes and cancer (21-23).
miRNAs control the differentiation and function of myeloid and
lymphoid cells, among other cell types (24). A recent study on miRNAs in the
immune system demonstrated that miR-146a and miR-155-5p are
associated with OA (25).
Additionally, it has been reported that increasing the expression
of miR-155-5p leads to M1 polarization in the RAW264.7 macrophage
cell line (26,27). It has also been reported that
miR-155-5p promoted M1 macrophage polarization by targeting
suppressor of cytokine signaling 1 (SOCS1), which is involved in
STAT3 and AKT signaling (28-30).
In the present study, the impact of knee SF from patients with knee
OA (KOA) on macrophage polarization and apoptosis was investigated.
miR-155-5p was identified as a potential target that affected
macrophage apoptosis and polarization in KOA SF.
Materials and methods
Collection of clinical samples
A total of 53 SF samples were collected by needle
aspiration from the knee joints of patients with KOA (28 males and
25 females; age range, 50-79 years; average age, 66.7 years;
admitted from January 2017 to December 2018; last follow up
occurred 1 year after admission) receiving treatment at the Yantai
Yuhuangding Hospital (Yantai, China). The present study was
approved by the Ethics Committee of Yantai Yuhuangding Hospital and
written informed consent was obtained from all participants. Blood
samples (20 ml) from healthy subjects (20 males, 22 females; age
range, 50-79 years; average age, 68.1 years; admitted from January
2017 to December 2018; last follow up occurred 1 year after
admission) and patients with KOA were collected into sodium heparin
Vacutainer® tubes (cat. no. 366480; Becton-Dickinson and
Company). The severity of radiographic X-ray KOA was graded using
the Kellgren/Lawrence scoring system (24), which is scored from 0 to 4. The
X-ray presentations of the patients were as follows: i) Stage 2
KOA, 9 patients; ii) stage 3 KOA, 28 patients; and iii) stage 3
KOA, 15 patients. KOA SF was carefully cross-examined and processed
within 2 h of fluid collection. KOA SF supernatant was collected by
centrifugation at 16,000 x g at 4˚C and stored at -80˚C until
further analysis.
Cell culture and stimulation
293T cells (American Type Culture Collection) were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) and penicillin and
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37˚C in a
humidified incubator containing 5% CO2. Peripheral blood
mononuclear cells (PBMCs) were obtained from whole blood samples by
density gradient centrifugation (400 x g at room temperature for 30
min) using Ficoll® 400 (Sigma-Aldrich; Merck KGaA),
according to the manufacturer's protocol. After washing twice with
PBS, PBMCs were seeded (3x106 cells/well) into 12-well
plates with RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.)
containing 10% FBS and 10 ng/ml macrophage colony-stimulating
factor (R&D Systems, Inc.). After incubation for 5 days at
37˚C, PBMC-derived macrophages were obtained, seeded
(1x106 cells/well) into 12-well plates and co-incubated
with 500 µl RPMI-1640-0.4% BSA (Sigma-Aldrich; Merck KGaA) and 500
µl SF for 12, 24 or 48 h at 37˚C. Control cells were treated with 1
ml control medium (CM; RPMI-1640-0.4% BSA). CD14+
monocytes/macrophages (MON/Mc) were isolated from the KOA SF and
PBMCs of patients with KOA or normal subjects using human CD14
MicroBeads (cat no. 130-050-201; MACS; Miltenyi Biotec, Inc.),
according to the manufacturer's protocols. PBMC-derived macrophage
apoptosis was induced by incubation with 1% DMSO (Sigma-Aldrich;
Merck KGaA) for 12 h at 37˚C.
Cell transfection
PBMC-derived macrophages were seeded into 12-well
plates at a density of 1.5x105 cells per well and
transfected with 100 nmol/l miR-155 mimic (5'-UUAAUG
CUAAUCGUGAUAGGGGU-3'), 100 nmol/l miR-155 inhibitor
(5'-ACCCCUAUCACGAUUAGCAUUAA-3') or 100 nmol/l miR-155
mimic/inhibitor negative control (NC; 5'-UCACAA
CCUCCUAGAAAGAGUAGA-3'; Shanghai GenePharma Co., Ltd.) using
Lipofectamine® 2000 reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Following
transfection for 24 or 48 h at 37˚C, PBMC-derived macrophages were
co-incubated with 500 µl RPMI-0.4% BSA and 500 µl SF for a further
24 h at 37˚C. Control cells were treated with 1 ml CM.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from macrophages and 293T
cells used TRIzol® reagent (Thermo Fisher Scientific,
Inc.). Total RNA was reverse transcribed into cDNA using the
PrimeScript™ RT reagent kit (Takara Bio, Inc.), according to the
manufacturer's protocol. Subsequently, cDNA was subjected to qPCR
using SYBR® Premix Ex Taq™ (Takara Bio, Inc.). The
thermocycling conditions were as follows: 5 min at 95˚C, followed
by 40 cycles at 95˚C for 15 sec and 60˚C for 30 sec. The primers
used for qPCR are presented in Table
I. mRNA and miRNA expression levels were quantified using the
2-ΔΔCq method (31) and
normalized to the internal reference genes GAPDH and U6,
respectively.
 | Table IPrimers used for quantitative
PCR. |
Table I
Primers used for quantitative
PCR.
| Gene | Primer sequence
(5'→3') |
|---|
| IL-1β | F: |
ATGATGGCTTATTACAGTGGCAA |
| | R: |
GTCGGAGATTCGTAGCTGGA |
| IL-6 | F: |
ACTCACCTCTTCAGAACGAATTG |
| | R: |
CCATCTTTGGAAGGTTCAGGTTG |
| NOS2 | F: |
TTCAGTATCACAACCTCA |
| | R: |
TGGACCTGCAAGTTAAAAT |
| IL-10 | F: |
GACTTTAAGGGTTACCTGGGTTG |
| | R: |
TCACATGCGCCTTGATGTCTG |
| ARG1 | F: |
GTGGAAACTTGCATGGAC |
| | R: |
AATCCTGGCACATCGGGAAT |
| SOCS1 | F: |
CACGCACTTCCGCACATTC |
| | R: |
TAAGGGCGAAAAAGCAGTT |
| CASP3 | F: |
CATGGAAGCGAATCAATGGACT |
| | R: |
CTGTACCAGACCGAGATGTCA |
| Ym1 | F: | TCACAAACAAAAGG |
| | R: |
GAATATGTAACACATTCAA |
| miR-155 | F: |
ATTGCCAATTTCTCTACCAC |
| | R: |
AGTAACAGGCATCATACACT |
| GAPDH | F: |
CAAGGTCATCCATGACAACTTTG |
| | R: |
GTCCACCACCCTGTTGCTGTAG |
| U6 | F: |
CTCGCTTCGGCAGCACA |
| | R: |
AACGCTTCACGAATTTGCGT |
FCM staining, analysis and apoptosis
detection
PBMC-derived macrophage single-cell suspensions
(1x106 cells/100 µl) were incubated at 4˚C for 30 min
the with LIVE/DEAD™ Fixable Aqua Dead Cell Stain kit (Thermo Fisher
Scientific, Inc.), which are a class of viability dyes suitable for
identifying dead cells in samples that are sent for fixation.
FcR-blocking reagent (10 µl; 10 min at 4˚C) and fluorescently
labeled antibodies targeted against CD86 (1:100; cat. no.
12-0869-42; eBioscience; Fluorophores, R-phycoerythrin; Thermo
Fisher Scientific, Inc.), inducible nitric oxide synthase (1:100;
iNOS; cat. no. 53-5920-82; eBioscience; Fluorophores, Alexa Fluor
488; Thermo Fisher Scientific, Inc.) and CD206 (1:100; cat. no.
12-2069-41; eBioscience; Fluorophores: PE-Cy7; Thermo Fisher
Scientific, Inc.) were then added. The cell pellet was resuspended
in 200 µl PBS and reacted with Alexa Fluor® 488-labeled
goat anti-mouse IgG antibody (1:200; Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature for 30 min in darkness.
Apoptotic cells were detected using the Annexin V/PI Cell Apoptosis
kit (Sungene Biotech Co., Ltd.), according to the manufacturer's
protocol. FCM analyses were performed using a flow cytometer (BD
Accuri C6 cytometer; BD Biosciences) and FlowJo software (version
7.6.1; FlowJo LLC).
Western blotting
Total protein was isolated from macrophages and
cells using protein extraction kit (Bio-Rad Laboratories, Inc.),
after which protein concentration was determined by a BCA assay
(Beyotime Institute of Biotechnology). Subsequently, samples were
separated by 8-12% SDS-PAGE and transferred onto nitrocellulose
membranes using a semidry transfer apparatus. PVDF membranes were
washed with TBST (0.05% Tween-20) three times before being blocked
with 5% skimmed milk at room temperature for 2 h. The membranes
were then incubated at 4˚C overnight with primary antibodies
targeted against: Phosphorylated (p)-STAT1 (1:1,000; cat. no.
ab30645; Abcam), STAT1 (1:1,000; cat. no. ab2415; Abcam), p-STAT6
(1:1,000; cat. no. ab54461; Abcam), STAT6 (1:1,000; cat. no.
ab44718; Abcam), SOCS1 (1:1,000; cat. no. 3950; Cell Signaling
Technology, Inc.), caspase-3 (CASP3) (1:1,000; cat. no. 9662; Cell
Signaling Technology, Inc.) and GAPDH (1:1,000; cat. no.
60004-1-Ig; ProteinTech, Inc.). The membranes were washed with TBST
three times, incubated with horseradish peroxidase-conjugated goat
anti-rabbit (1:1,000; cat. no. ab6721; Abcam) and goat anti-mouse
(cat. no. ab97040; 1:5,000; Abcam) secondary antibodies for 2 h at
room temperature. ECL chemiluminescence solution and bicinchoninic
acid protein quantitative detection kits were purchased from
Beyotime Institute of Biotechnology. Finally, ImageJ (version 1.38;
National Institutes of Health) was used to analyze the gray
value.
Luciferase reporter assay
The interaction sites between miR-155-5p and CASP3
were predicted using miRanda (http://www.microrna.org), miRwalk (http://mirwalk.umm.uniheidelberg.de) and
miRTarbase (http://mirtarbase.mbc.nctu.edu.tw) databases. The
online resource microRNA.org (http://www.microrna.org) predicted the targeted
binding site between miR-155-5p and the 3'-untranslated region
(3'-UTR) SOCS1. The wild-type (WT) or mutant (MUT) 3'-UTR of SOCS1
or CASP3 (constructed by Guangzhou RiboBio Co., Ltd.) were cloned
into the pMIR vector (Guangzhou RiboBio Co., Ltd.). 293T cells were
seeded (4x105 cells/well) into plates with 0.5 ml
complete growth medium. At 70-90% confluency, 293T cells were
co-transfected with the miR-155 mimic or miR-155 inhibitor,
negative control (miR-NC) and 50 ng pMIR-SOCS1-3'-UTR-WT or
pMIR-SOCS1-3'-UTR-MUT using Lipofectamine 3000® reagent
(Thermo Fisher Scientific, Inc.). For each well, 1 µg/µl DNA was
diluted in 50 µl Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.),
whilst in a separate tube, 2.5 µl Lipofectamine® 3000
was diluted in 50 µl Opti-MEM. Each tube was gently mixed and
incubated for 20 min at room temperature. Subsequently, the two
solutions were added to each well and gently mixed. Cells were
incubated for 48 h before assessing transfection efficiency. The
same operating procedures were used for the miR-155-5p/CASP3
luciferase reporter assay using pMIR-CASP3-3'-UTR-WT and
pMIR-CASP3-3'-UTR-MUT vectors. At 48 h post-transfection, cells
were harvested and luciferase activities were measured using the
Dual-Luciferase® Reporter System (Promega Corporation),
according to the manufacturer's protocol. Firefly luciferase
activity was normalized to that of Renilla luciferase.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 7; GraphPad Software Inc.). Data are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Comparisons among groups were analyzed using one-way ANOVA followed
by Tukey's post hoc test.
Results
KOA SF promotes PBMC-derived M1
macrophage polarization and inhibits macrophage apoptosis
The effects of SF from patients with KOA on
PBMC-derived macrophages were assessed using RT-qPCR and FCM. KOA
SF significantly promoted the expression of M1-type markers by the
macrophages (Fig. 1A), whilst
partially reducing the expression of M2-type markers (Fig. 1B) compared with macrophages
incubated in CM. The FCM results indicated that SF significantly
promoted the expression of CD86 and iNOS, whilst reducing the
expression of CD206 in PBMC-derived macrophages compared with those
incubated with CM (Fig. 1C and
D). During OA, cartilage loses its
integrity, where the number of apoptotic chondrocytes and immune
cells increases (6). The mechanism
underlying this increase in the presence of KOA SF was therefore
investigated by assessing its effects on macrophage apoptosis. In
the presence and absence of 1% DMSO, KOF SF inhibited PBMC-derived
macrophage apoptosis compared with those incubated with CM
(Fig. 1E and F). The results suggested that the increase
in M1 macrophages in KOA SF may be caused by the proinflammatory SF
during the progression of OA.
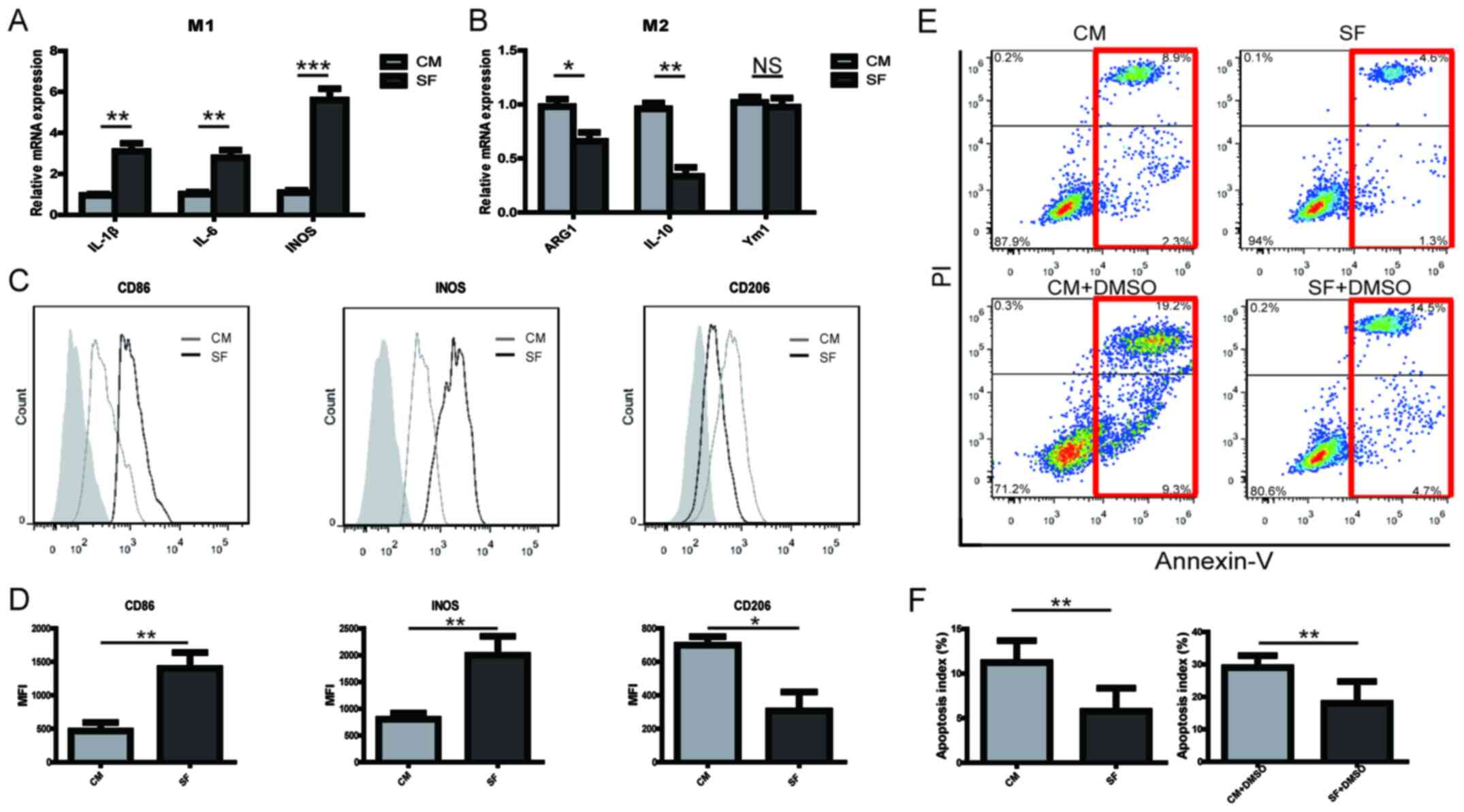 | Figure 1KOA SF promotes PBMC-derived
macrophage M1 polarization and inhibits macrophage apoptosis. The
relative expression of (A) M1 and (B) M2 markers in PBMC-derived
macrophages cultured with either KOA SF or CM for 12 h. CD86, iNOS
and CD206 expression levels in PBMC-derived macrophages cultured
with KOA SF or CM for 24 h were (C) determined by flow cytometry
and (D) quantified. The grey shadow represents the isotype control.
Following treatment with or without 1% DMSO for 12 h in RPMI-1640
containing 10% FBS, PBMC-derived macrophages were cultured with KOA
SF or CM for 24 h. Apoptotic cells were (E) detected by flow
cytometry (red box indicates apoptotic cells) and (F) quantified.
*P<0.05, **P<0.01 and
***P<0.001. KOA, knee osteoarthritis; SF, synovial
fluid; PBMC, peripheral blood mononuclear cells; CM, control
medium; iNOS, inducible nitric oxide synthase; IL, interleukin;
ARG1, arginase 1; Ym1, chitinase-like 3; NS, not significant; PI,
propidium iodide. |
Expression of miR-155-5p is
upregulated in KOA SF-derived macrophages and KOA SF-cultured
PBMC-derived macrophages
A recent study reported that miR-155-5p was
associated with OA (21). The
expression level of miR-155-5p was found to be upregulated in
CD14+ MON/Mc derived from the SF of patients with KOA
compared with that in CD14+ MON/Mc isolated from the
PBMCs of patients with KOA or healthy individuals (Fig. 2A). In addition, the expression of
SOCS1, a target of miR-155-5p, was revealed to be downregulated in
CD14+ MON/Mc derived from the SF of patients with KOA
compared with CD14+ MON/Mc isolated from the PBMCs of
patients with KOA or healthy individuals (Fig. 2B). PBMC-derived macrophages cultured
in KOA SF expressed significantly higher levels of miR-155-5p
compared with macrophages cultured in CM (Fig. 2C). These results suggested that
miR-155-5p may serve an important role in polarizing macrophages
during OA progression.
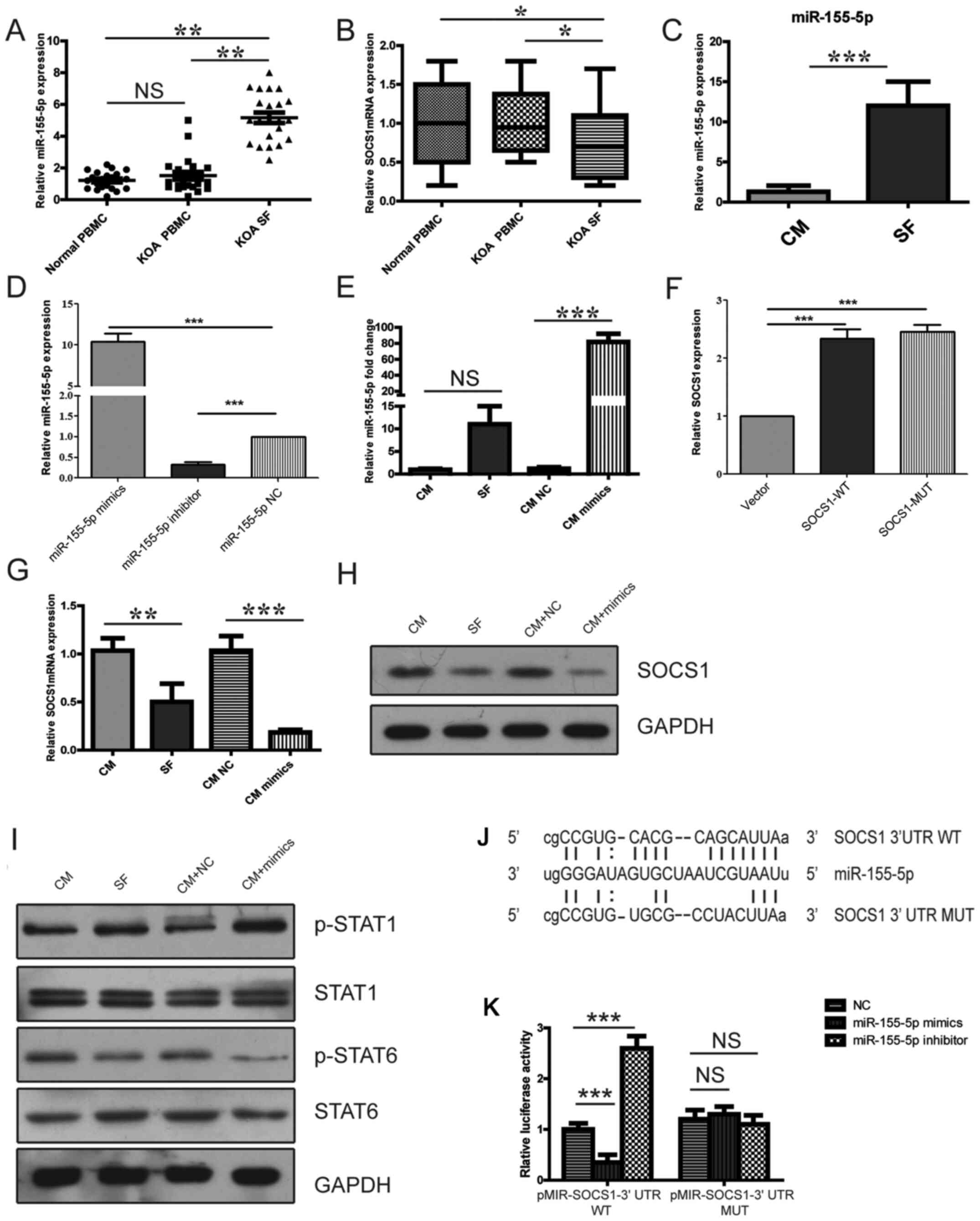 | Figure 2M1 polarization is associated with
the miR-155-5p/SOCS1 signaling pathway. The relative expression of
(A) miR-155-5p and (B) SOCS1 in PBMC-derived macrophages isolated
from the blood samples of normal individuals, patients with KOA and
PBMC-derived macrophages isolated from the KOA SF. (C) The
expression of miR-155-5p in PBMC-derived macrophages cultured with
KOA SF or CM for 12 h was detected by reverse
transcription-quantitative PCR. The relative expression of
miR-155-5p (D) in cells transfected with either NC, miR-155-5p
mimic or the miR-155-5p inhibitor, (E) in transfected cells
cultured with CM or KOA and in cells transfected with either NC or
miR-155-5p cultured in CM. (F) The relative expression of SOCS1 in
PBMC-derived macrophages transfected with control vector, SOCS-WT
or SOCS-MUT. The relative expression of SOCS1 (G) mRNA and (H)
protein in PBMC-derived macrophages treated and/or transfected with
CM, KOA SF, CM + NC or CM + miR-155-5p mimic. (I) Levels of STAT1
and STAT6 phosphorylation in PBMC-derived macrophages treated
and/or transfected with CM, KOA SF, CM + NC or CM + miR-155-5p
mimic. (J) The potential interaction between miR-155-5p and SOCS1
3’-UTR-WT or SOCS1 3’-UTR-MUT. (K) The interaction between miR-155
and SOCS1 was confirmed using a dual-luciferase reporter assay.
*P<0.05, **P<0.01 and
***P<0.001. miR, microRNA; SOCS1, suppressor of
cytokine signaling 1; KOA, knee osteoarthritis; SF, synovial fluid;
PBMC, peripheral blood mononuclear cells; CM, control medium; NC,
negative control; p, phosphorylated; 3’-UTR, 3’-untranslated
region; WT, wild-type; MUT, mutant; NS, not significant. |
KOA SF-induced promotion of
PBMC-derived M1 macrophage polarization is associated with the
miR-155-5p/SOCS1 signaling pathway
As a potential target of miR-155-5p, SOCS1-mediated
inhibition of p-STAT1 activity and promotion of p-STAT6 activity to
induce macrophage M2 polarization whilst inhibiting M1 macrophage
polarization has been extensively studied in RAW264.7 cells
(16). Therefore, it was
hypothesized that miR-155-p serves a role in KOA-SF-induced
PBMC-derived M1 macrophage polarization via SOCS1. Transfection
with the miR-155-5p mimic significantly increased miR-155-5p
expression, whilst transfection with the miR-155-5p inhibitor
significantly reduced miR-155-5p expression in PBMC-derived
macrophages (Fig. 2D). Following
transfection with NC or miR-155-5p mimic for 24 h, PBMC-derived
macrophages were cultured with CM or KOA SF. The expression of
miR-155-5p in PBMC-derived macrophages transfected with miR-155-5p
mimic was upregulated compared with the CM NC group, while the
upregulation of miR-155-5p expression in PBMC-derived macrophages
cultured with KOA SF was not significantly different compared with
PBMC-derived macrophages cultured with CM (Fig. 2E). Additionally, RT-qPCR results
indicated that SOCS1 expression was significantly increased in the
SOCS1-WT and SOCS1-MUT groups compared with that in the control
vector group (Fig. 2F). The
expression of SOCS1 mRNA and protein in PBMC-derived macrophages
cultured with KOA SF or transfected with the miR-155-5p mimic was
found to be downregulated compared with that in the corresponding
control groups (Fig. 2G and
H). In addition, the activation of
STAT1 was revealed to be increased whereas the activation of STAT6
was found to be reduced in PBMC-derived macrophages cultured with
KOA SF or transfected with miR-155-5p mimic compared with those in
the corresponding control groups (Fig.
2I). The online resource microRNA.org
(http://www.microrna.org) predicted the targeted
binding site between miR-155-5p and the 3'-UTR SOCS1. Subsequently,
dual-luciferase reporters encoding the WT or MUT 3'-UTR of SOCS1
were constructed (Fig. 2J).
Co-transfection with the miR-155-3p mimic significantly inhibited
the dual-luciferase activity of the SOCS1 WT 3'-UTR SOCS1 plasmid
but not that of the SOCS1 MUT 3'-UTR plasmid. By contrast, the
miR-155-5p inhibitor significantly enhanced the dual-luciferase
activity of the SOCS1 WT 3'-UTR plasmid but not that of the SOCS1
MUT 3'-UTR (Fig. 2K). These results
suggested a regulatory relationship between miR-155 and SOCS1
mRNA.
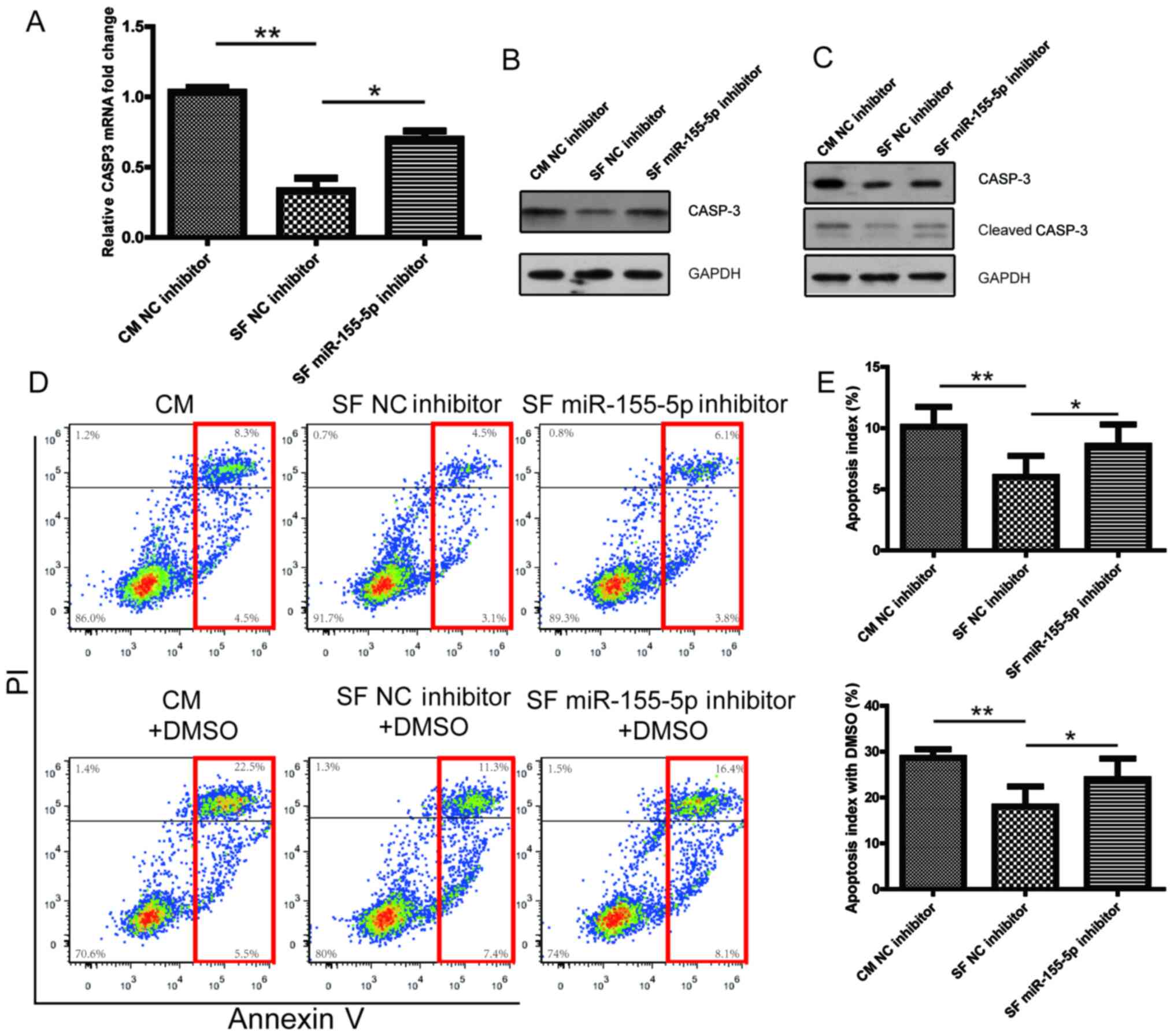
Downregulation of miR-155-5p promotes
SOCS1 expression and inhibits M1 polarization of KOA SF-stimulated
PBMC-derived macrophages
Following transfection with the NC inhibitor or
miR-155-5p inhibitor for 24 h, PBMC-derived macrophages were
cultured with CM or KOA SF. The downregulation of miR-155-5p
reversed the reduced SOCS1 mRNA and protein expression by KOA SF
(Fig. 3A-C). Furthermore,
PBMC-derived macrophages cultured in KOA SF exhibited increased
p-STAT1 and decreased p-STAT6 levels compared with those in the CM
group. However, in PBMC-derived macrophages transfected with the
miR-155-5p inhibitor and cultured in KOA SF, the KOA SF-induced
effects on STAT1 and STAT6 phosphorylation were reversed (Fig. 3C). In PBMC-derived macrophages
transfected with the miR-155-5p inhibitor, the KOA SF-induced
increased expression of IL-1β, IL-6, iNOS and CD86 was reversed,
however the reduced expression of IL-10 and CD206 as a result of
KOA-SF treatment was not reversed (Fig.
3D, E, F and G).
These observations indicated further that KOA SF promoted M1
macrophage polarization by increasing miR-155-5p, which targeted
SOCS1 to upregulate p-STAT1 activity whilst inhibiting p-STAT6
activity.
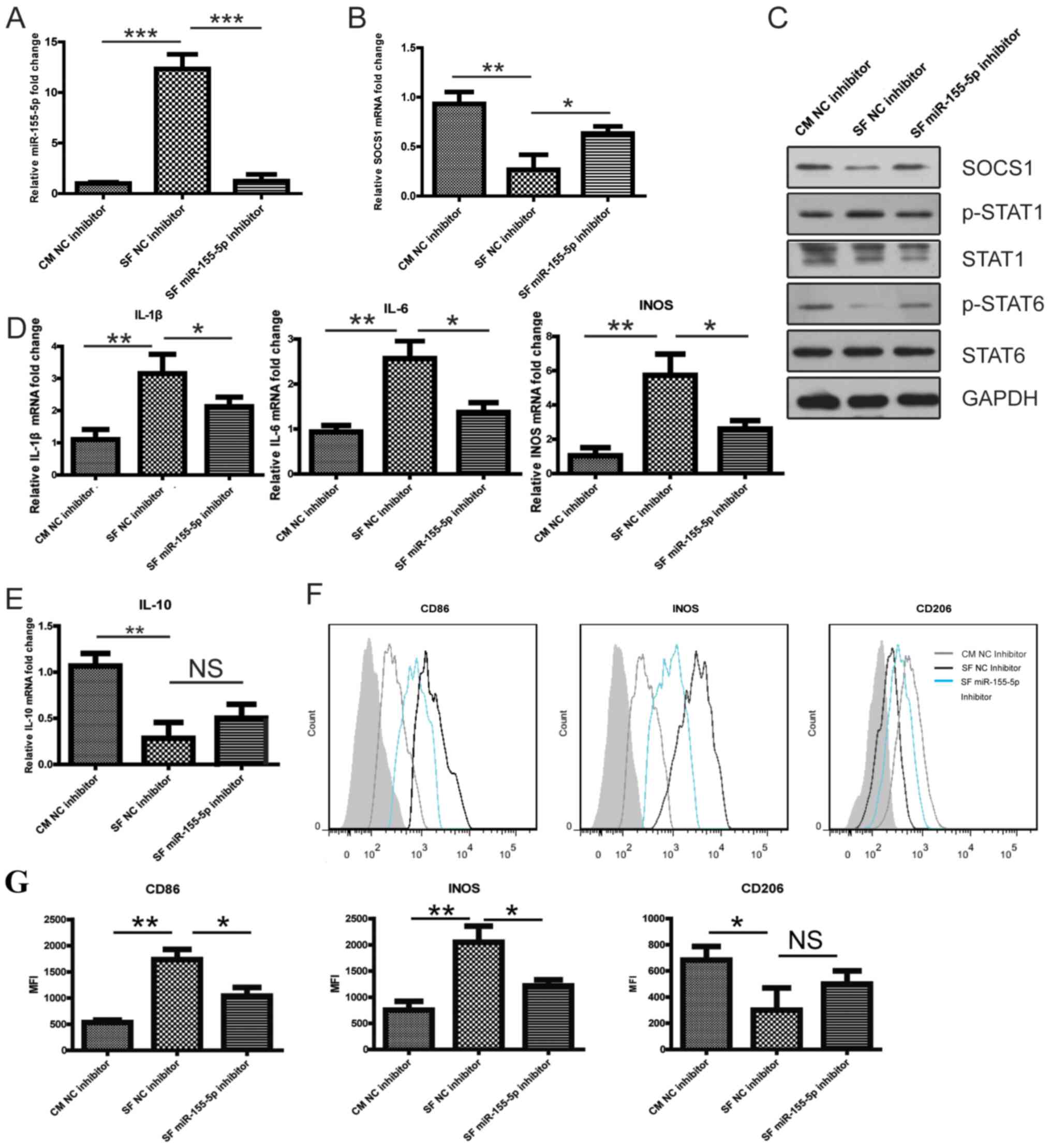 | Figure 3miR-155-5p downregulation promotes
SOCS1 expression. (A-G) Following transfection with the NC
inhibitor or miR-155-5p inhibitor for 24 h, PBMC-derived
macrophages were cultured with CM or KOA SF. The relative
expression of (A) miR-155-5p and (B) SOCS1 mRNA in PBMC-derived
macrophages. (C) SOCS1 protein expression and the phosphorylation
levels of STAT1 and STAT6 in PBMC-derived macrophages. The relative
expression of (D) IL-1β, IL-6, iNOS and (E) IL-10 mRNA in
PBMC-derived macrophages. The expression levels of CD86, iNOS and
CD206 expression in PBMC-derived macrophages was (F) determined by
flow cytometry and (G) quantified. The grey shadow represents the
isotype control. *P<0.05, **P<0.01 and
***P<0.001, as indicated. miR, microRNA; SOCS1,
suppressor of cytokine signaling 1; NC, negative control; PBMC,
peripheral blood mononuclear cells; CM, control medium; KOA, knee
osteoarthritis; SF, synovial fluid; p, phosphorylated; IL,
interleukin; iNOS, inducible nitric oxide synthase; NS, not
significant. |
KOA SF-induced inhibition of
PBMC-derived macrophage apoptosis occurs via the miR-155-5p/CASP3
signaling pathway
miR-155-5p has been previously associated with
apoptosis in several cancer cell lines and carcinomas (23); therefore, it was hypothesized that
miR-155-5p may serve an important role in KOA SF-induced
suppression of PBMC-derived macrophage apoptosis. The online
microRNA, miRanda (http://www.microrna.org), miRwalk (http://mirwalk.umm.uniheidelberg.de) and
miRTarbase (http://mirtarbase.mbc.nctu.edu.tw) databases predicted
that CASP3 was a target gene of miR-155-5p, whilst the microRNA.org database identified the target binding
site for miR-155 on the 3'-UTR of CASP3 mRNA (Fig. 4E). The expression of CASP3 mRNA was
found to be significantly reduced in PBMC-derived macrophages
cultured with KOA SF or transfected with miR-155-5p mimic compared
with that in the corresponding control groups (Fig. 4A). The expression of CASP3 and
cleaved CASP3 were also revealed to be decreased in PBMC-derived
macrophages cultured with KOA SF or transfected with miR-155-5p
mimic compared with those in the corresponding control groups
(Fig. 4B and C). Additionally, the RT-qPCR results
indicated that CASP3 expression was significantly increased in
CASP3-WT and CASP3-MUT groups compared with that in the control
vector group (Fig. 4D). To further
investigate the interaction between miR-155-5p and CASP3, WT and
MUT CASP3 3'-UTRs were cloned into the pMIR-reporter plasmid
(Fig. 4E). The dual-luciferase
reporter assay indicated that the luciferase activity of
pMIR-CASP3-3'-UTR-WT, but not pMIR-CASP3-3'-UTR-MUT, was
significantly decreased by co-transfection with the miR-155-5p
mimic and significantly enhanced by transfection with the
miR-155-5p inhibitor compared that in the miR-NC group (Fig. 4F). These findings indicated that KOA
SF increased miR-155-5p-induced inhibition of macrophage apoptosis
by targeting CASP3.
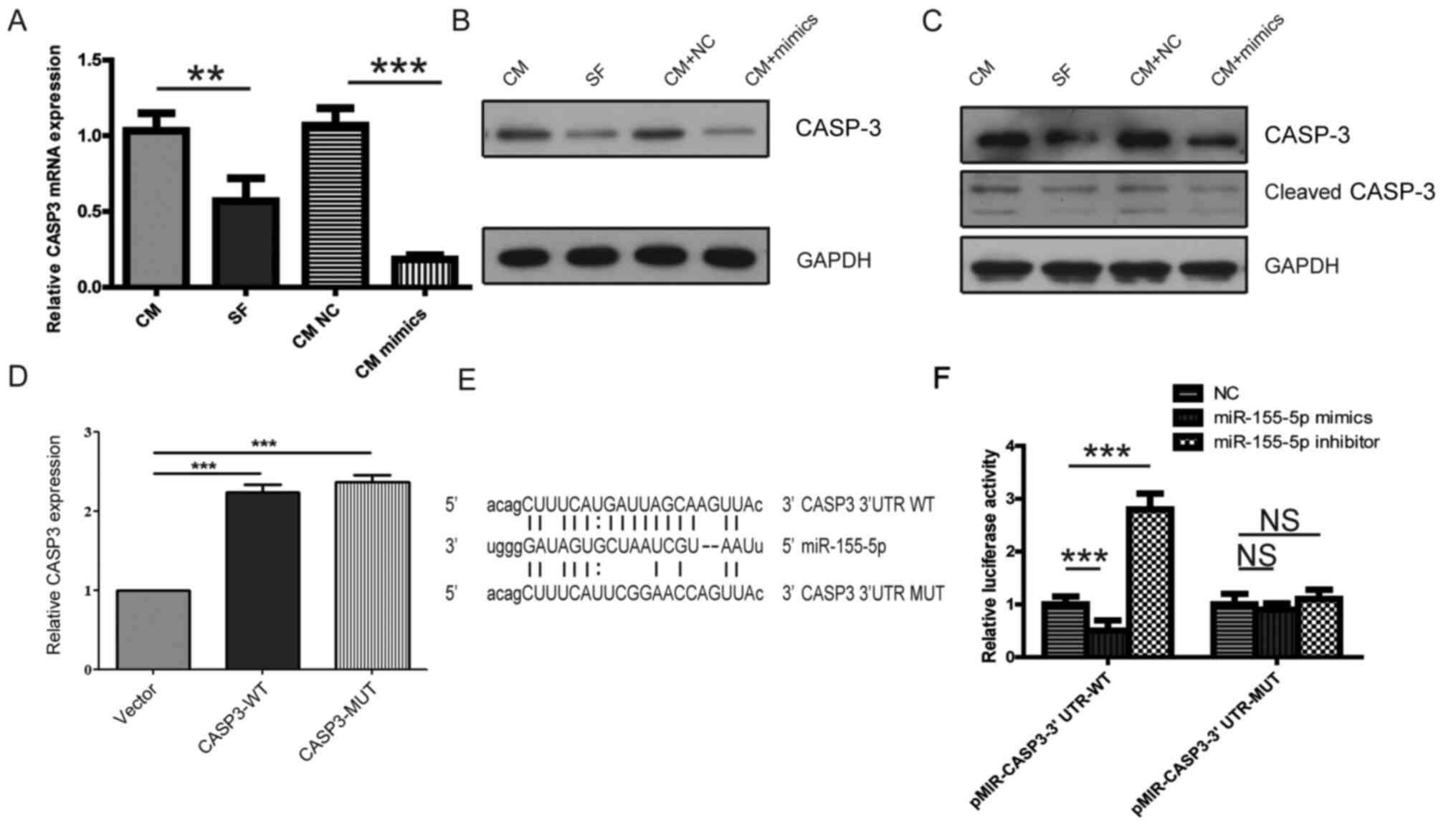 | Figure 4PBMC-derived macrophages are
regulated by the miR-155-5p/CASP3 signaling pathway. (A-C)
Following transfection with NC or miR-155-5p mimic for 24 h,
PBMC-derived macrophages were cultured with CM or KOA SF. The
relative expression of CASP3 (A) mRNA and (B) protein. (C) The
relative expression of CASP3 and cleaved CASP3 in PBMC-derived
macrophages treated with 1% DMSO. (D) The relative expression of
CASP3 following transfection with the control vector, CASP-WT or
CASP-MUT. (E) The binding sites of miR-155-p on CASP3 3’-UTR-WT or
CASP3 3’-UTR-MUT. (F) The potential interaction between miR-155 and
CASP3 was verified using a dual-luciferase reporter assay.,
**P<0.01 and ***P<0.001. PBMC,
peripheral blood mononuclear cells; miR, microRNA; CASP3,
caspase-3; NC, negative control; CM, control medium; KOA, knee
osteoarthritis; SF, synovial fluid; 3’-UTR, 3’-untranslated region;
WT, wild-type; MUT, mutant; NS, not significant. |
miR-155-5p downregulation promotes
CASP3 expression and enhances macrophage apoptosis
Following transfection with the NC inhibitor or
miR-155-5p inhibitor for 24 h, PBMC-derived macrophages were
cultured with CM or KOA SF. miR-155-5p downregulation was found to
reverse the KOA SF-induced downregulation of CASP3 mRNA and protein
expression (Fig. 5A and B). Furthermore, in 1% DMSO-treated
PBMC-derived macrophages, KOA SF reduced the expression of CASP3
and cleaved CASP3 (Fig. 5C).
However, in PBMC-derived macrophages transfected with the
miR-155-5p inhibitor, the reduced expression of CASP3 and cleaved
CASP3 by KOA SF was abolished (Fig.
5C). In addition, both in the presence and absence of 1% DMSO
treatment, KOA SF treatment suppressed PBMC-derived macrophage
apoptosis, which was reversed by transfection with the miR-155-5p
inhibitor (Fig. 5D and E). These results indicated that CASP3 is a
novel target of miR-155-5p and that KOA SF inhibited macrophage
apoptosis via the miR-155-5p/CASP3 signaling pathway.
Discussion
OA is a debilitating condition that affects millions
of individuals worldwide, where the only effective treatment
strategies available are knee and hip replacement surgeries
(2). Therefore, identifying the
mechanism underlying OA is of substantial importance. OA has a
complex pathogenesis where its etiology is remains to be completely
elucidated (1). A previous study
has revealed that macrophages may serve as a major factor in the
acceleration of OA progression (7).
In particular, miR-155-5p has been previously found to associate
with OA, where increased miRNA-155-5p expression leads to the M1
polarization of the RAW 264.7 macrophage cell line (27). It has also been reported that
miR-155 is involved in TNF-α-mediated inhibition of osteogenesis
differentiation, which directly targets a suppressor of cytokine
signaling 1 (SOCS1) to inhibit bone morphogenetic protein 2-induced
osteoblast differentiation (32).
In the present study, PBMC-derived macrophages cultured in KOA SF
displayed upregulated expression of M1 markers, reduced apoptosis
and increased miR-155-5p expression compared with those cultured in
CM. The present study investigated the relationship between
macrophage phenotype, apoptosis and KOA. The results indicated that
the proinflammatory microenvironment of KOA SF may favor M1
macrophage polarization by upregulating miR-155-5p whilst
inhibiting M1 macrophage apoptosis. Therefore, the results of the
present study may provide an explanation for the increased ratio of
total to M1-like macrophages in the granulocyte cell population
during KOA progression. In addition, the results of the present
study suggested that KOA SF altered PBMC-derived macrophages M1
polarization via the miR-155-5p/SOCS1 signaling pathway. CASP3 was
subsequently identified as a novel target of miR-155-5p, where the
results indicated that KOA SF inhibited macrophage apoptosis
through the miR-155-5p/CASP3 signaling pathway. Based on the
results of the aforementioned study (33), the present study suggested that KOA
SF promoted PBMC-derived M1 macrophage polarization via the
miR-155-5p/SOCS1 signaling pathway and inhibited macrophage
apoptosis via the same signaling pathway.
It should be noted that the present study has a
number of limitations. Since the infiltration of MON/Mcs in
patients with OA was not investigated, the possibility that MON/Mc
infiltration may affect the ratio of macrophages in the granulocyte
cell population cannot not be ruled out. In addition, miR-155-5p
was one of the most important elements that affected macrophage
polarization phenotype and apoptosis in KOA SF vs. CM, but other
factors can be involved. Further studies are required to explore
the mechanism underlying the effect of the KOA SF microenvironment
on macrophage polarization, phenotype and apoptosis. Based on the
results of the present study, it was hypothesized that targeting
the inflammatory KOA SF and macrophage polarization may delay or
prevent OA progression. Further investigation into the potential of
miR-155-5p as a therapeutic target for KOA is required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Joint Special Foundation of Shandong Province (grant no.
20160841).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GDW designed the study, collected the data and wrote
the manuscript. GSL and LC performed the experiments and
interpreted the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Yantai Yuhuangding Hospital (Yantai, China).
Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Losina E, Weinstein AM, Reichmann WM,
Burbine SA, Solomon DH, Daigle ME, Rome BN, Chen SP, Hunter DJ,
Suter LG, et al: Lifetime risk and age at diagnosis of symptomatic
knee osteoarthritis in the US. Arthritis Care Res (Hoboken).
65:703–711. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Boilard E, Nigrovic PA, Larabee K, Watts
GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O'Donnell E,
Farndale RW, Ware J, et al: Platelets amplify inflammation in
arthritis via collagen-dependent microparticle production. Science.
327:580–583. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kriegova E, Manukyan G, Mikulkova Z,
Gabcova G, Kudelka M, Gajdos P and Gallo J: Gender-related
differences observed among immune cells in synovial fluid in knee
osteoarthritis. Osteoarthritis Cartilage. 26:1247–1256.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pers YM, Quentin J, Feirreira R, Espinoza
F, Abdellaoui N, Erkilic N, Cren M, Dufourcq-Lopez E, Pullig O,
Nöth U, et al: Injection of adipose-derived stromal cells in the
knee of patients with severe osteoarthritis has a systemic effect
and promotes an anti-inflammatory phenotype of circulating immune
cells. Theranostics. 8:5519–5528. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hunter DJ and Bierma-Zeinstra S:
Osteoarthritis. Lancet. 393:1745–1759. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kraus VB, McDaniel G, Huebner JL, Stabler
TV, Pieper CF, Shipes SW, Petry NA, Low PS, Shen J, McNearney TA,
et al: Direct in vivo evidence of activated macrophages in human
osteoarthritis. Osteoarthritis Cartilage. 24:1613–1621.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun H, Zhang Y, Song W, Yin L, Wang G, Yu
D, Zhang Q, Yan X and Li S: IgM+CD27+ B cells
possessed regulatory function and represented the main source of B
cell-derived IL-10 in the synovial fluid of osteoarthritis
patients. Hum Immunol. 80:263–269. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Arkestål K, Mints M, Enocson A, Linton L,
Marits P, Glise H, Andersson J and Winqvist O: CCR2 upregulated on
peripheral T cells in osteoarthritis but not in bone marrow. Scand
J Immunol. 88(e12722)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Deligne C, Casulli S, Pigenet A, Bougault
C, Campillo-Gimenez L, Nourissat G, Berenbaum F, Elbim C and Houard
X: Differential expression of interleukin-17 and interleukin-22 in
inflamed and non-inflamed synovium from osteoarthritis patients.
Osteoarthritis Cartilage. 23:1843–1852. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
de Munter W, Geven EJ, Blom AB, Walgreen
B, Helsen MM, Joosten LA, Roth J, Vogl T, van de Loo FA, Koenders
MI, et al: Synovial macrophages promote TGF-β signaling and protect
against influx of S100A8/S100A9-producing cells after
intra-articular injections of oxidized low-density lipoproteins.
Osteoarthritis Cartilage. 25:118–127. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bondeson J, Wainwright SD, Lauder S, Amos
N and Hughes CE: The role of synovial macrophages and
macrophage-produced cytokines in driving aggrecanases, matrix
metalloproteinases, and other destructive and inflammatory
responses in osteoarthritis. Arthritis Res Ther.
8(R187)2006.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Barros MH, Hauck F, Dreyer JH, Kempkes B
and Niedobitek G: Macrophage polarisation: An immunohistochemical
approach for identifying M1 and M2 macrophages. PLoS One.
8(e80908)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Varol C, Mildner A and Jung S:
Macrophages: Development and tissue specialization. Annu Rev
Immunol. 33:643–675. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bi D, Zhou R, Cai N, Lai Q, Han Q, Peng Y,
Jiang Z, Tang Z, Lu J, Bao W, et al: Alginate enhances Toll-like
receptor 4-mediated phagocytosis by murine RAW264.7 macrophages.
Int J Biol Macromol. 105:1446–1454. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sanjuan MA, Dillon CP, Tait SW, Moshiach
S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff
S, et al: Toll-like receptor signalling in macrophages links the
autophagy pathway to phagocytosis. Nature. 450:1253–1257.
2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hallowell RW, Collins SL, Craig JM, Zhang
Y, Oh M, Illei PB, Chan-Li Y, Vigeland CL, Mitzner W, Scott AL, et
al: mTORC2 signalling regulates M2 macrophage differentiation in
response to helminth infection and adaptive thermogenesis. Nat
Commun. 8(14208)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qing L, Fu J, Wu P, Zhou Z, Yu F and Tang
J: Metformin induces the M2 macrophage polarization to accelerate
the wound healing via regulating AMPK/mTOR/NLRP3 inflammasome
singling pathway. Am J Transl Res. 11:655–668. 2019.PubMed/NCBI
|
|
19
|
Daghestani HN, Pieper CF and Kraus VB:
Soluble macrophage biomarkers indicate inflammatory phenotypes in
patients with knee osteoarthritis. Arthritis Rheumatol. 67:956–965.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Benito MJ, Veale DJ, FitzGerald O, van den
Berg WB and Bresnihan B: Synovial tissue inflammation in early and
late osteoarthritis. Ann Rheum Dis. 64:1263–1267. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dragomir MP, Knutsen E and Calin GA:
SnapShot: Unconventional miRNA functions. Cell. 174:1038–1038.e1.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
D'Adamo S, Alvarez-Garcia O, Muramatsu Y,
Flamigni F and Lotz MK: MicroRNA-155 suppresses autophagy in
chondrocytes by modulating expression of autophagy proteins.
Osteoarthritis Cartilage. 24:1082–1091. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu J, Li Y, Pan Y, Liu Y, Xing H, Xie X,
Wan D and Jiang Z: Deficient regulatory innate lymphoid cells and
differential expression of miRNAs in acute myeloid leukemia
quantified by next generation sequence. Cancer Manag Res.
11:10969–10982. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Soyocak A, Kurt H, Ozgen M, Turgut Cosan
D, Colak E and Gunes HV: miRNA-146a, miRNA-155 and JNK expression
levels in peripheral blood mononuclear cells according to grade of
knee osteoarthritis. Gene. 627:207–211. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang Y, Zhang M, Zhong M, Suo Q and Lv K:
Expression profiles of miRNAs in polarized macrophages. Int J Mol
Med. 31:797–802. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ma C, Wang Y, Shen A and Cai W:
Resveratrol upregulates SOCS1 production by
lipopolysaccharide-stimulated RAW264.7 macrophages by inhibiting
miR-155. Int J Mol Med. 39:231–237. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xu F, Kang Y, Zhang H, Piao Z, Yin H, Diao
R, Xia J and Shi L: Akt1-mediated regulation of macrophage
polarization in a murine model of Staphylococcus aureus pulmonary
infection. J Infect Dis. 208:528–538. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang Y, Yang L, Liang X and Zhu G:
MicroRNA-155 promotes atherosclerosis inflammation via targeting
SOCS1. Cell Physiol Biochem. 36:1371–1381. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cai X, Yin Y, Li N, Zhu D, Zhang J, Zhang
CY and Zen K: Re-polarization of tumor-associated macrophages to
pro-inflammatory M1 macrophages by microRNA-155. J Mol Cell Biol.
4:341–343. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yu XM, Meng HY, Yuan XL, Wang Y, Guo QY,
Peng J, Wang AY and Lu SB: MicroRNAs' involvement in osteoarthritis
and the prospects for treatments. Evid Based Complement Alternat
Med. 2015(236179)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lu L, McCurdy S, Huang S, Zhu X, Peplowska
K, Tiirikainen M, Boisvert WA and Garmire LX: Time series
miRNA-mRNA integrated analysis reveals critical miRNAs and targets
in macrophage polarization. Sci Rep. 6(37446)2016.PubMed/NCBI View Article : Google Scholar
|