1. Introduction
Rheumatic heart disease (RHD) poses a major threat
to the health of primarily patients under the age of 40 years old
(1). As many as 250,000 people
succumb to RHD annually (2).
Although valve replacement surgery is currently the most effective
treatment, this surgery is expensive and requires high technical
skills and specialized equipment. Therefore, RHD represents a major
health concern in less developed regions. In order to effectively
treat RHD, it is important to fully elucidate its pathogenic
process; however, the exact pathogenesis of RHD remains elusive
(3). Signalling pathways are key
factors in the occurrence and development of several diseases, and
research on signalling pathways is an important approach to
elucidating the pathogenesis of disease. A number of drugs exert
their pharmacological effects by acting on signalling pathways
(4-6).
To date, there are no effective drugs for the prevention or
treatment of RHD. Therefore, it is crucial to study the signalling
pathways involved in RHD pathogenesis, as drug intervention in
RHD-related pathways for prevention or treatment would markedly
benefit RHD patients in underdeveloped areas. Previous studies have
investigated the signalling pathways associated with the
pathogenesis of RHD. Therefore, the aim of the present review was
to discuss the research progress on 6 major signalling pathways
implicated in the pathogenesis of RHD, to summarize the available
results in order to further elucidate the pathogenesis of RHD, and
to serve as a reference for researchers worldwide investigating the
pathogenesis of RHD.
2. Selection and search criteria
English literature databases were searched using the
following terms: ‘RHD’ (all fields) and ‘pathway or signal’ (all
fields). The following criteria were considered for the review of
studies: i) The research content was signalling pathways associated
with the pathogenesis of RHD; and ii) the research made significant
progress, such as the discovery of a certain RHD-related signalling
pathway, or examined the mechanism of the RHD-related signalling
pathway. The number of studies on the signalling pathways
associated with the pathogenesis of RHD worldwide is limited. A
total of 6 important signalling pathways that are implicated in the
pathogenesis of RHD and on which significant research progress was
made were identified.
RhoA/Rho-associated protein kinase
(RhoA/ROCK) signalling pathway and RHD
In 2008, Jiang et al (7) investigated the association between
extracellular matrix (ECM) and RHD. First, it was observed that the
concentration of tenascin-C (TN-C), which is the key component of
the ECM in patients with RHD, was significantly higher compared
with that in controls. The transcription of TN-C is closely
associated with the RhoA/ROCK signalling pathway. Therefore, it was
ultimately discovered that interferon (IFN)-γ and tumour necrosis
factor (TNF)-α were involved in the pathogenesis of RHD via the
RhoA/ROCK signalling pathway, which regulated the expression of
TN-C and the ECM remodelling process (8). That study identified two important
factors associated with RHD: The RhoA/ROCK signalling pathway and
the ECM. The RhoA/ROCK signalling pathway regulates an important
component of the ECM, as mentioned earlier, but it is also involved
in TNF-α-mediated endothelial apoptosis (9) and the development of the sinoatrial
node (10). Previous studies
demonstrated that ECM remodelling in the heart was implicated in
cardiac fibrosis (11), and the ECM
is also a therapeutic target for myocardial repair (12). Whether the RhoA/ROCK signalling
pathway and ECM cooperate with endothelial apoptosis, cardiac
fibrosis and myocardial repair in the pathogenesis of RHD is
unclear, and it may be investigated to further elucidate the
pathogenesis of RHD in the future. A previous study (7) also highlighted two intervention
points: Neutralization of IFN-γ and TNF-α with antibodies
attenuated TN-C transcription in patients with RHD, as IFN-γ and
TNF-α induce TN-C transcription via the RhoA/ROCK signalling
pathway. A number of studies demonstrated that IFN-γ and TNF-α were
closely associated with the pathogenesis of RHD (13-16).
Therefore, IFN-γ and TNF-α may be potential targets for therapeutic
intervention in RHD. However, a previous study (7) only revealed that neutralization of
IFN-γ and TNF-α suppressed the transcription of TN-C, but it did
not investigate the effects of the lower transcription of TN-C on
the occurrence and development of RHD. IFN-γ and TNF-α as potential
targets for intervention require further investigation in future
studies.
Mitogen-activated protein kinase
(MAPK) signalling pathway and RHD
In 2013, Li et al (17) divided patients with RHD into atrial
fibrillation (AF) and sinus rhythm (SR) groups according to their
heart rhythm. When comparing the expression levels of basic
fibroblast growth factor (bFGF)-related factors and MAPK signalling
pathway-related factors between these two groups, they were found
to be higher in the AF group. It was concluded that bFGF may be
involved in the process of atrial fibrosis, and the MAPK signalling
pathway may be the molecular basis of this process. The focus of
that study was atrial fibrosis in patients with RHD, and the
association among bFGF, the MAPK signalling pathway and RHD was
initially examined.
A 2014 study also examined the role of the MAPK
pathway in the process of atrial fibrosis in patients with RHD.
Zhang et al (18) also
compared the SR and AF groups of RHD patients. The sample size was
larger, and the focus was on transforming growth factor (TGF)-β1
and tumour necrosis factor receptor-associated factor (TRAF)6 in
addition to MAPK. It was demonstrated that the MAPK/TGF-β1/TRAF6
signalling pathway was involved in atrial fibrosis in patients with
chronic AF, indicating that TRAF6 may be a new intervention target
for the treatment of atrial fibrosis. The study also examined the
role of the MAPK pathway in atrial fibrosis due to RHD.
In 2020, Zhao et al (19) also investigated the association
between the MAPK pathway and RHD. The pathological process
investigated in that study was RHD-induced valve fibrosis. Blood
samples and valve specimens from patients with RHD were compared to
age- and sex-matched RHD-negative patients. The research focused on
CD4+ T cells, the MAPK signalling pathway and TGF-β1.
The findings confirmed that the MAPK signalling pathway and TGF-β1
participated in the process of RHD-induced valve fibrosis, and
concluded that CD4+ T cells also played an important
role in the fibrotic process.
Therefore, the MAPK signalling pathway plays an
important role in the process of RHD-induced fibrosis. MAPK is a
cellular serine/threonine protein kinase that plays a key role in
biological reactions involved in cell proliferation,
differentiation, transformation and apoptosis (20). All three aforementioned studies
examined the close association between the MAPK signalling pathway
and the process of fibrosis of cardiac tissue caused by RHD. The
three most important factors in the fibrotic process of RHD
involved with MAPK were bFGF, TGF-β1 and CD4+ T cells.
bFGF promoted the growth of fibroblasts and was closely associated
with the pathological process of atrial fibrosis caused by RHD.
These studies also demonstrated that the concentrations of bFGF and
MAPK were increased in the AF group, but no specific mechanism was
further examined, and no suitable intervention target was
identified. TGF-β1 is known to play an important role in the
pathogenesis of RHD, which is discussed below. CD4+ T
cells are important immune cells in the human body. It is widely
hypothesised that immunity plays a key role in the pathogenesis of
RHD. However, the types of immune cells that are involved in this
process are not known, although a role for CD4+ T cells
has been suggested. The studies (17-19)
aforementioned only reported that the numbers of CD4+ T
cells were significantly increased in the peripheral blood and
valve tissues of patients with RHD, but did not further examine
their mechanism of action. The mechanism of action of
CD4+ T cells in RHD was later examined, which is
discussed below.
Protein kinase B(AKT)/S6 kinase (S6K)
signalling pathway and RHD
In 2013, Zhang et al (21) found that focal adhesion kinase (FAK)
regulated the AKT/S6K pathway and participated in the process of
RHD-induced atrial fibrosis. Specifically, FAK mediated the process
of atrial fibrosis in patients with RHD via the AKT/S6K pathway.
Research on RHD patients and animal models uncovered that the
expression of AKT/S6K signalling pathway-related factors was
increased in patients with AF. Animal experiments revealed
suppressed α-smooth muscle actin (α-SMA) expression in
TGF-β1-induced fibroblasts after inhibition of the expression of
FAK and AKT. This study (21)
focused on RHD-induced fibrotic lesions. Inhibition of the
expression of AKT/S6K signalling pathway-related factors inhibited
TGF-β1-induced cardiac fibroblast activation, which was confirmed
in other studies (22). Both
studies suggested that the AKT/S6K signalling pathway was closely
associated with the fibrosis of heart tissue, and inhibition of its
expression inhibited cardiac fibroblast activation. Therefore, the
AKT/S6K signalling pathway may be an effective intervention target
for the prevention or treatment of fibrotic lesions caused by RHD.
The aforementioned study (21)
again emphasizes the important role of TGF-β1 in fibrotic lesions
due to RHD.
TGF-β1/Smad signalling pathway and
RHD
Zhang et al (23) also found that the TGF-β1/Smad
signalling pathway increased the expression of α-actinin-2 and
participated in the process of RHD-induced atrial fibrosis. Patients
with RHD-induced AF were used as the experimental group and
patients with congenital heart disease and SR who underwent heart
surgery were used as controls. The atrial specimens of the two
groups were compared. The levels of TGF-β1/Smad signalling
pathway-related factors and α-actinin-2 in the experimental group
were significantly higher compared with those in the control group.
The TGF-β1/Smad signalling pathway is closely associated with scar
tissue formation, and disruption of the TGF-β1/Smad pathway is an
important pathogenic mechanism of tissue fibrosis (24). The results of a previous study
(23) suggest that disorder of this
pathway caused atrial fibrosis. However, the exact cause of
TGF-β1/Smad pathway dysfunction is not known, and the upstream
stimuli for this pathway must be investigated in future studies.
This study also highlights the important role of TGF-β1 in
RHD-induced fibrosis. TGF-β1 was the focus of a previous study
(19) on the association between
the MAPK signalling pathway and RHD and the association between the
AKT/S6K signalling pathway and RHD. In the aforementioned study by
Zhang et al (23), it was
once again shown that TGF-β1 may play an important role in the
pathogenesis of RHD. The process of fibrotic valve injury during
the pathogenesis of RHD is also regulated by the general fibrosis
status of the body, and it is not an independent process. The view
of this study (23) is that the
increased expression of Smad2 and Smad3, which causes atrial
fibrosis, is due to the regulation of TGF-β1. Smad2 and Smad3 are
also key factors in endothelial-to-mesenchymal transition (EMT), as
the phosphorylation of Smad2 and Smad3 regulates the transcription
factors lymphoid enhancer factor-1, Snail1, TWIST and zinc finger
E-box binding homeobox 1/2 to induce EMT (25,26).
However, although EMT was not the focus of this study (23), it suggests that the occurrence and
development of RHD may involve EMT.
Wnt signalling pathway and RHD
Guo et al (27) found that the Wnt signalling pathway
increased the expression of Snail1 and participated in the process
of RHD-induced atrial fibrosis. The method they used was similar to
the three studies on MAPK mentioned above, i.e., comparison of the
SR and AF groups of patients with RHD. The Wnt signalling pathway
involves multiple signal molecule-mediated transduction pathways,
and the activation of this pathway is observed in several
cardiovascular pathologies (28).
This study (27) extended the
prevalence of the Wnt signalling pathway in cardiovascular disease
to RHD and highlighted Snail1. The Wnt signalling pathway and
Snail1 are implicated in the EMT process. The Wnt signalling
pathway is closely associated with EMT. For example, the activation
of the Wnt signalling pathway after myocardial infarction triggers
EMT to promote the cardiac repair process (29). Snail1 is one of the transcription
factors that induces EMT (25,26).
These data suggest that EMT may play a role in the pathogenesis of
RHD. Therefore, whether the results of this research (increased
expression of Snail1 participated in the process of RHD-induced
atrial fibrosis) include the participation of EMT requires future
in-depth investigation.
Signal transducer and activator of
transcription 3 (STAT3) signalling pathway and RHD
Wu et al (3)
demonstrated that the sphingosine 1-phosphate receptor 1
(S1PR1)/STAT3 signalling pathway was activated during RHD-induced
valve damage. The authors established an RHD rat model to detect
differences in the expression of S1PR1, STAT3, phosphorylated
(p)-STAT3 and T helper (Th) 17 cell-related cytokines in the
cardiac valves of RHD and control rats, and found that the
expression of S1PR1 in the cardiac valves of RHD rats was lower
compared with that in controls. The expression levels of p-STAT3
and the Th17 cell-related cytokines interleukin (IL)-17 and IL-21
were higher compared with those in the control group. The
inflammation and fibrosis of the valves in the RHD group were also
more prominent compared with the control group.
Chen et al (30) found that inhibiting the expression
of miR-155-5p inhibited the expression of p-STAT3 in an RHD rat
model and reduced the expression of the Th17 cell-related cytokines
IL-6 and IL17 and valve fibrotic damage and inflammation caused by
RHD.
The focus of both studies was on STAT3-related
signalling pathways and valvular disease due to RHD. Valvular
disease is the hallmark of RHD, which may also cause other
secondary heart diseases. These two studies directly investigated
valvular disease, which is relatively more meaningful in the
pathogenesis of RHD. The research content of these two studies
gradually deepened the understanding of STAT3 signalling pathway.
The first study observed that the STAT3 signalling pathway was
activated in the RHD rat model, and the second study examined
methods for interfering with the STAT3 signalling pathway.
STAT3 is a cellular signal transcription factor that
is involved in numerous cell activities, and has been extensively
investigated (31). Both studies
emphasized that the main role of STAT3 in RHD-induced valve damage
was to induce CD4+ T-cell differentiation into Th17
cells, which was described in previous studies (32). The important role of CD4+
T cells in the pathogenesis of RHD is therefore confirmed.
Both studies investigated Th17 immune cells and
their specific mechanism of action, which included the increased
phosphorylation of STAT3 to promote the differentiation of
CD4+ T cells into Th17 cells, which release the
inflammatory factors that participate in valve damage induced by
RHD. Both studies involved animal experiments, but an increase in
the Th17 cell-related cytokine IL-17A was also reported in RHD
patients (33). Therefore, Th17
cells play an important role in RHD-induced valve inflammation.
In summary, the two studies (3,30)
focused on the important role of the STAT3 signalling pathway and
Th17 cells in the process of valve inflammation and fibrosis caused
by RHD. The mechanism of valve injury in RHD is closely associated
with inflammatory factors, and STAT3-related signalling pathways
play an important role in regulating related inflammatory factors.
miR-155-5p may be an effective intervention target. Further
research into the STAT3 pathway may uncover that this pathway may
represent a promising target for the prevention or treatment of RHD
with.
3. Discussion and conclusions
The conclusions of the studies on these signalling
pathways associated with the pathogenesis of RHD may be summarized
as follows:
Multiple signalling pathways regulate the
pathogenesis of RHD. A schematic diagram of the role of the
signalling pathways described in this review in the pathogenesis of
RHD is presented in Fig. 1.
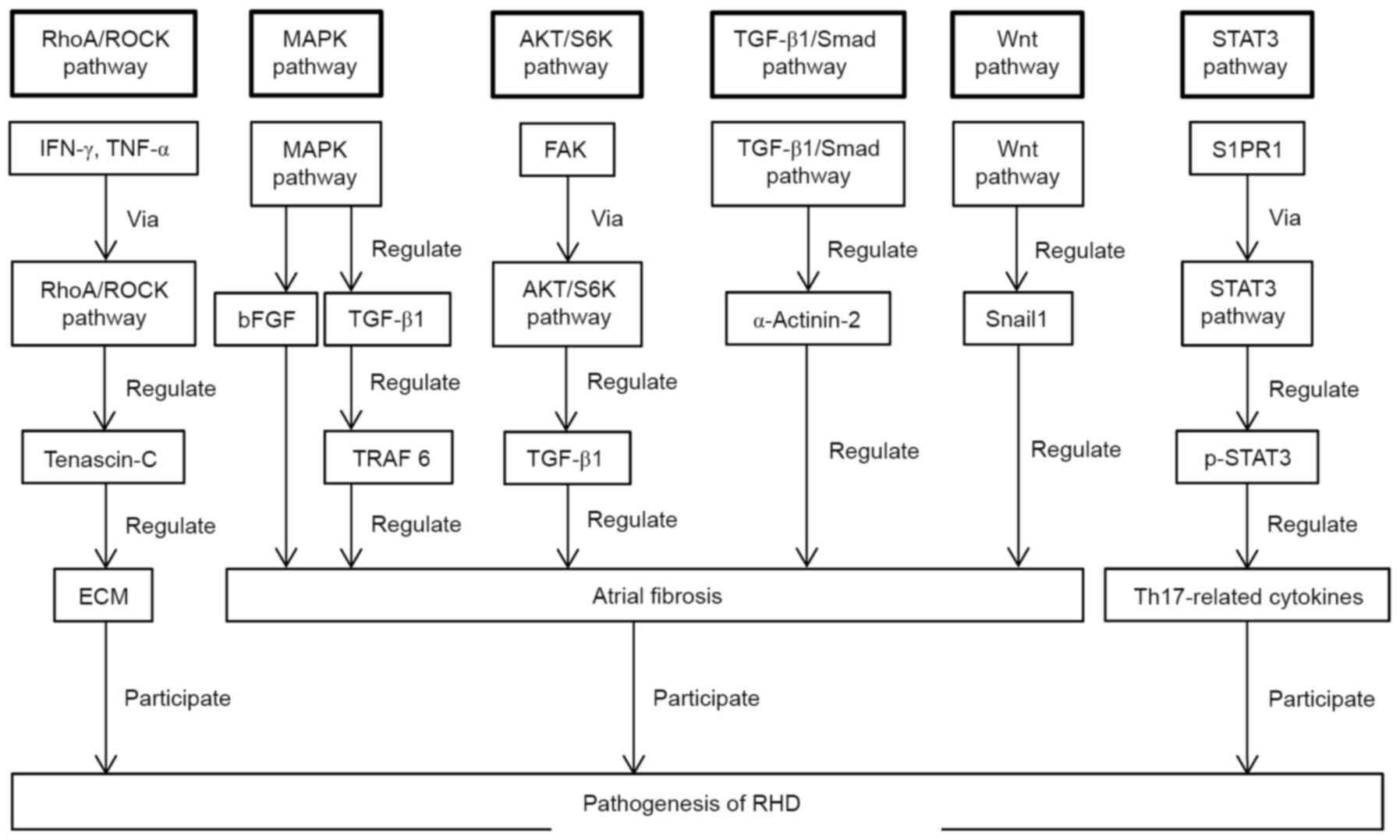 | Figure 1Schematic presentation of the
discussed signalling pathways that are implicated in the
pathogenesis of RHD. RHD, rheumatic heart disease; RhoA/ROCK,
RhoA/Rho-dependent kinase; IFN-γ, interferon-γ; TNF-α, tumour
necrosis factor-α; ECM, extracellular matrix; MAPK,
mitogen-activated protein kinase; bFGF, basic fibroblast growth
factor; TGF-β1, transforming growth factor-β1; TRAF 6, tumour
necrosis factor receptor-associated factor 6; AKT/S6K, protein
kinase B/S6 kinase; FAK, focal adhesion kinase; S1PR1, sphingosine
1-phosphate receptor 1; STAT3, signal transducer and activator of
transcription 3; p-STAT3, phosphorylated STAT3; Th17, T helper
17. |
These signalling pathways serve their respective
physiological or pathological roles in the occurrence and
development of RHD. Some of these signalling pathways play the same
role in the occurrence and development of RHD as in other diseases.
However, further research is needed to determine whether the
functions of these signalling pathways in other diseases are also
involved in the occurrence and development of RHD. In the future,
the present review may help determine the impact of new RHD
prevention and treatment methods on other diseases that use the
same signalling pathways.
The pathogenesis of RHD may not be a simple immune
response but may involve other conditions, including dysfunction of
signalling pathways, such as the TGF-β1/Smad signalling pathway.
Targeted intervention in the expression process of some of the
signalling pathways may reduce the heart damage caused by RHD. The
pathogenesis of RHD involves several pathological processes,
primarily inflammatory reactions and fibrosis. Multiple signalling
pathways regulate each process, such as atrial fibrosis.
CD4+ T cells are the main immune cells in the
pathogenesis of RHD. Important factors in the occurrence and
development of RHD include bFGF, TGF-β1 and CD4+ T
cells.
EMT is most likely implicated in the occurrence and
development of RHD and its role requires further investigation.
Some progress has been made in the field of RHD-related signalling
pathway research. Unfortunately, only research on the RhoA/ROCK,
AKT/S6K and STAT3 signalling pathways identified intervention
targets, and the effect of such intervention in humans requires
further investigation.
There are currently three intervention targets that
may be used for the prevention or treatment of RHD: Intervention in
IFN-γ and TNF-α-mediated ECM remodelling; intervention in the
AKT/S6K pathway to suppress α-SMA expression in TGF-β1-induced
fibroblasts; and interference with the phosphorylation of STAT3 to
inhibit Th17 cell-related cytokine release and reduce RHD-induced
valve damage.
Each RHD-related signalling pathway study described
in the present review has limitations. No specific mechanism for
the regulation of each signalling pathway on RHD was investigated
in depth. If the specific mechanisms of the regulation of each
signalling pathway in RHD are further investigated on the basis of
existing research and further effective drug intervention targets
are identified in humans, the prevention and treatment of RHD may
improve.
There is some cross-talk between these signalling
pathways. The MAPK, AKT/S6K, TGF-β1/Smad and Wnt signalling
pathways are all involved in the pathogenesis of RHD via regulation
of atrial fibrosis; both the MAPK and AKT/S6K signalling pathways
regulate TGF-β1; both the MAPK and STAT3 signalling pathways
regulate CD4+ T cells; the TGF-β1/Smad and Wnt
signalling pathways have the potential to regulate EMT in the
pathogenesis of RHD.
There was a limitation to the present study.
Meta-analysis has statistical power and a better ability to
extrapolate to the greater population, and it should be performed
in our future studies further elucidate the pathogenesis of
RHD.
The number of studies on the pathogenesis of RHD
worldwide is limited. Although the researchers mentioned in this
article did not further examine the mechanism of action of these
signalling pathways based on existing research, their efforts have
produced valuable results, which will prompt future research that
may ultimately fully elucidate the pathogenesis of RHD and identify
prevention and treatment methods accessible to patients in
underdeveloped areas.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81960082), the
Guangxi Key Laboratory Base of Precision Medicine in
Cardio-cerebrovascular Disease Control and Prevention (grant no.
17-259-85), the Guangxi Clinical Research Center for
Cardio-cerebrovascular Diseases (grant no. AD17129014) and the
Guangxi Medical High-level Backbone Talents ‘139’ Program (grant
no. G201901006).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
ZZ and SX conceived and designed the study. SX
collected and analyzed the data. SX wrote the manuscript. All the
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leal MTBC, Passos LSA, Guarçoni FV, de
Souza Aguiar JM, da Silva RBR, de Paula TMN, Santos RFD, Nassif
MCL, Gomes NFA, Tan TC and Nunes MCP: Rheumatic heart disease in
the modern era: Recent developments and current challenges. Rev Soc
Bras Med Trop. 52(e20180041)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mirabel M, Narayanan K, Jouven X and
Marijon E: Cardiology patient page. Prevention of acute rheumatic
fever and rheumatic heart disease. Circulation. 130:e35–e37.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wu XD, Zeng ZY, Gong DP, Wen JL and Huang
F: Potential involvement of S1PR1/STAT3 signaling pathway in
cardiac valve damage due to rheumatic heart disease. Biotech
Histochem. 94:398–403. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang Y, He G, Tang H, Shi Y, Kang X, Lyu
J, Zhu M, Zhou M, Yang M, Mu M, et al: Aspirin inhibits
inflammation and scar formation in the injury tendon healing
through regulating JNK/STAT-3 signalling pathway. Cell Prolif.
52(e12650)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu CH, Hua N, Fu X, Pan YL, Li B and Li
XD: Metformin regulates atrial SK2 and SK3 expression through
inhibiting the PKC/ERK signaling pathway in type 2 diabetic rats.
BMC Cardiovasc Disord. 18(236)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sung JY and Choi HC: Nifedipine inhibits
vascular smooth muscle cell proliferation and reactive oxygen
species production through AMP-activated protein kinase signaling
pathway. Vascul Pharmacol. 56:1–8. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jiang L, Wei XF, Yi DH, Xu P, Liu H, Chang
Q, Yang SM, Li ZF, Gao HB and Hao GJ: Synergistic effects of cyclic
strain and Th1-like cytokines on tenascin-C production by rheumatic
aortic valve interstitial cells. Clin Exp Immunol. 155:216–223.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zeisberg M, Hanai J, Sugimoto H, Mammoto
T, Charytan D, Strutz F and Kalluri R: BMP-7 counteracts
TGF-beta1-induced epithelial-to-mesenchymal transition and reverses
chronic renal injury. Nat Med. 9:964–968. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Yang L, Tang L, Dai F, Meng G, Yin R, Xu X
and Yao W: Raf-1/CK2 and RhoA/ROCK signaling promote TNF-α-mediated
endothelial apoptosis via regulating vimentin cytoskeleton.
Toxicology. 389:74–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vicente-Steijn R, Kelder TP, Tertoolen LG,
Wisse LJ, Pijnappels DA, Poelmann RE, Schalij MJ, deRuiter MC,
Gittenberger-de Groot AC and Jongbloed MRM: RHOA-ROCK signalling is
necessary for lateralization and differentiation of the developing
sinoatrial node. Cardiovasc Res. 113:1186–1197. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li L, Zhao Q and Kong W: Extracellular
matrix remodeling and cardiac fibrosis. Matrix Biol. 68-69:490–506.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dziki JL and Badylak SF: Extracellular
matrix for myocardial repair. Adv Exp Med Biol. 1098:151–171.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Diamantino Soares AC, Araújo Passos LS,
Sable C, Beaton A, Ribeiro VT, Gollob KJ, Dutra WO and Nunes MCP:
Circulating cytokines predict severity of rheumatic heart disease.
Int J Cardiol. 289:107–109. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rehman S, Akhtar N, Saba N, Munir S, Ahmed
W, Mohyuddin A and Khanum A: A study on the association of
TNF-α(-308), IL-6(-174), IL-10(-1082) and IL-1Ra(VNTR) gene
polymorphisms with rheumatic heart disease in Pakistani patients.
Cytokine. 61:527–531. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Deng H, Xue YM, Zhan XZ, Liao HT, Guo HM
and Wu SL: Role of tumor necrosis factor-alpha in the pathogenesis
of atrial fibrillation. Chin Med J (Engl). 124:1976–1982.
2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Guilherme L, Cury P, Demarchi LM, Coelho
V, Abel L, Lopez AP, Oshiro SE, Aliotti S, Cunha-Neto E,
Pomerantzeff PMA, et al: Rheumatic heart disease: Proinflammatory
cytokines play a role in the progression and maintenance of
valvular lesions. Am J Pathol. 165:1583–1591. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li M, Yi X, Ma L and Zhou Y: Hepatocyte
growth factor and basic fibroblast growth factor regulate atrial
fibrosis in patients with atrial fibrillation and rheumatic heart
disease via the mitogen-activated protein kinase signaling pathway.
Exp Ther Med. 6:1121–1126. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang D, Liu X, Chen X, Gu J, Li F, Zhang
W and Zheng Y: Role of the MAPKs/TGF-β1/TRAF6 signaling pathway in
atrial fibrosis of patients with chronic atrial fibrillation and
rheumatic mitral valve disease. Cardiology. 129:216–223.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhao Z, He D, Ling F, Chu T, Huang D, Wu H
and Ge J: CD4+ T cells and TGFβ1/MAPK signal pathway
involved in the valvular hyperblastosis and fibrosis in patients
with rheumatic heart disease. Exp Mol Pathol.
114(104402)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee S, Rauch J and Kolch W: Targeting MAPK
signaling in cancer: Mechanisms of drug resistance and sensitivity.
Int J Mol Sci. 21(1102)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang P, Wang W, Wang X, Wang X, Song Y,
Zhang J and Zhao H: Focal adhesion kinase mediates atrial fibrosis
via the AKT/S6K signaling pathway in chronic atrial fibrillation
patients with rheumatic mitral valve disease. Int J Cardiol.
168:3200–3207. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Narikawa M, Umemura M, Tanaka R, Fujita T,
Yokoyama U, Ishigami T, Kimura K, Tamura K and Ishikawa Y: Acute
hyperthermia inhibits TGF-β1-induced cardiac fibroblast activation
via suppression of Akt signaling. Sci Rep. 8(6277)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang L, Zhang N, Tang X, Liu F, Luo S and
Xiao H: Increased α-actinin-2 expression in the atrial myocardium
of patients with atrial fibrillation related to rheumatic heart
disease. Cardiology. 135:151–159. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-β/Smad signaling in
tissue fibrosis. Chem Biol Interact. 292:76–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vincent T, Neve EPA, Johnson JR, Kukalev
A, Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold
PL, et al: A SNAIL1-SMAD3/4 transcriptional repressor complex
promotes TGF-beta mediated epithelial-mesenchymal transition. Nat
Cell Biol. 11:943–950. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Thuault S, Tan EJ, Peinado H, Cano A,
Heldin CH and Moustakas A: HMGA2 and Smads co-regulate SNAIL1
expression during induction of epithelial-to-mesenchymal
transition. J Biol Chem. 283:33437–33446. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Guo F, Yi X, Li M, Fu J and Li S: Snail1
is positively correlated with atrial fibrosis in patients with
atrial fibrillation and rheumatic heart disease. Exp Ther Med.
14:4231–4237. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Foulquier S, Daskalopoulos EP, Lluri G,
Hermans KCM, Deb A and Blankesteijn WM: WNT signaling in cardiac
and vascular disease. Pharmacol Rev. 70:68–141. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aisagbonhi O, Rai M, Ryzhov S, Atria N,
Feoktistov I and Hatzopoulos AK: Experimental myocardial infarction
triggers canonical Wnt signaling and endothelial-to-mesenchymal
transition. Dis Model Mech. 4:469–483. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen A, Wen J, Lu C, Lin B, Xian S, Huang
F, Wu Y and Zeng Z: Inhibition of miR-155-5p attenuates the
valvular damage induced by rheumatic heart disease. Int J Mol Med.
45:429–440. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hu YS, Han X and Liu XH: STAT3: A
potential drug target for tumor and inflammation. Curr Top Med
Chem. 19:1305–1317. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu X, Hu H, Fan H, Zuo D, Shou Z, Liao Y,
Nan Z and Tang Q: The role of STAT3 and AhR in the differentiation
of CD4+ T cells into Th17 and Treg cells. Medicine
(Baltimore). 96(e6615)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bas HD, Baser K, Yavuz E, Bolayir HA,
Yaman B, Unlu S, Cengel A, Bagriacik EU and Yalcin R: A shift in
the balance of regulatory T and T helper 17 cells in rheumatic
heart disease. J Investig Med. 62:78–83. 2014.PubMed/NCBI View Article : Google Scholar
|















