Introduction
Osteoarthritis (OA) is a type of disease in which
articular cartilage becomes fibrotic and ulcerated and can be lost
(1). The common clinical
manifestations of OA are joint pain and tenderness, joint
stiffness, joint swelling, bone friction and joint weakness
(2). In general, OA can be
pathologically summarized as cartilage degeneration and synovitis,
and its main features are the degradation of articular cartilage
tissue and the apoptosis of chondrocytes (3). Although the cause of OA is still
unclear, studies have shown that microRNAs (miRNAs/miRs) are
closely related to OA (4-7).
Therefore, discovering and exploring the functions of miRNAs may
provide new strategies for OA treatment in the near future.
In recent years, 990 known miRNAs and 1,621
potential miRNAs have been found in human OA chondrocytes (5). It has been demonstrated that miRNAs
are not only involved in cartilage development, including growth
plate development, osteogenic and chondrogenic differentiation, and
osteoclast formation, but also participate in the development of OA
related to chondrocyte apoptosis and abnormal cartilage metabolism
(6,8). Moreover, miRNAs and cytokines
accelerate the course of disease or delay the course of disease
through mutual synergy and antagonism (9).
miRNAs are a group of endogenous small noncoding
RNAs (22-25 nucleotides) that regulate gene expression at the
post-transcriptional level by directly targeting the
3'-untranslated region (3'-UTR) of a gene. They are important
regulators of different biological processes, including cell
proliferation, differentiation, migration, apoptosis and
tumorigenesis (10). Under normal
conditions, miRNA expression has strict tissue and temporal
specificity, and is highly conserved in evolution, while abnormally
expressed miRNAs may cause disease. miR-335-5P was originally
discovered as a tumour metastasis suppressor targeting the
transcription factors Sry- box transcription factor 4 and tenascin
C and is encoded by the second intron of the mesoderm-specific
transcript gene (11). miR-335-5P
can inhibit the proliferation and migration of primary bone
marrow-derived human mesenchymal stem cells (MSCs) (12). In addition, it has been reported
that the expression of four miRNAs, namely miR-138-5P, miR-146a-5P,
miR-335-5P and miR-9-5P, was significantly upregulated in human
knee joint OA according to gene chip and in situ
hybridization techniques, suggesting that miR-335-5P may play an
important role in the development of OA (13). However, the biological function of
miR-335-5P in OA is not well understood. Thus, the present study
investigated the effect of miR-335-5P on chondrocytes and whether
miR-335-5P may be a potential target for OA treatment.
Materials and methods
Ethics statement
Human articular cartilage was harvested from a
patient following a traumatic amputation. The patient provided
their written informed consent. All protocols were approved by the
Ethics Committee of Fuzhou Second Hospital Affiliated to Xiamen
University (Fuzhou China).
Isolation, culture and transfection of
human primary articular chondrocytes
Under sterile conditions, cartilage slices were
dissected from a sample of a male patient with traumatic amputation
(age, 32 years) on May 10, 2018 with no history of OA. The excess
fibrous connective tissue in the specimen was removed, and the
cartilage tissue was cut to a size of ~1 mm3 and washed
with PBS containing 1% penicillin and gentamicin double antibiotic
solution. Then, 5 µl 0.25% trypsin were added, the tissue was
digested in a 37˚C incubator for 30 min, and the supernatant was
discarded. Next, 6ml 0.2% type II collagenase (Sigma-Aldrich; Merck
KGaA) was added, the tissue was digested in a 37˚C incubator for 16
h, and cells were collected every 4 h. The cell suspension was
filtered through a 75 µm mesh filter and centrifuged at 4˚C, 500 x
g for 5 min, and the supernatant was discarded. The suspension was
washed three times with DMEM Complete Medium (Beijing Dingguo
Changsheng Biotechnology Co., Ltd). containing 10% foetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). Finally, the
cells were seeded into a culture flask at a density of
1x105 cells/ml and cultured at 37˚C in a 5%
CO2 incubator as described previously (5). For the primary cell culture, only
chondrocytes from the normal articular cartilage harvested from the
femoral condyles and tibial plateaus of the tissue donor was used.
The cells were identified by toluidine blue (Sigma-Aldrich; Merck
KGaA) staining and type II collagen (cat. no. ab34712; Abcam)
immunohistochemistry staining (Fig.
S1). The immunohistochemistry staining procedure was as
follows: The slides were rewarmed at 37˚C for 45 min after
incubating with 50 µl of type II collagen antibody (1:500) at 4˚C
overnight. After washing with PBS three times, the slides were
incubated with 40-50 µl goat anti-rabbit IgG antibody (1:500; cat.
no. ab 205718; Abcam) at room temperature for 1 h. Slides were then
observed under a light microscope after incubation with freshly
prepared 1 mg/ml DAB solution (Sigma-Aldrich; Merck KGaA) for 5-10
min in the dark at 37˚C. After rinsing with tap water for 10 min,
the slides were counterstained with hematoxylin (Sigma-Aldrich;
Merck KGaA) at 37˚C for 1 min. After thoroughly rinsing each sample
in water, the cells were immersed in 1% hydrochloric acid alcohol
and then 1% aqueous ammonia, followed by thorough washing in water.
The cells from each sample were then dehydrated in 70% ethanol for
2 min, 80% ethanol for 2 min, 90% ethanol twice for 2 min, 95%
ethanol twice for 2 min and 100% ethanol twice for 2 min. The cells
were then immersed in xylene solution twice for 2 min and mounted
on a glass slide in neutral resin, sealed with a neutral gum seal
and finally examined using a Nikon TE2000 microscope.
A total of 5x104 chondrocytes per well
were seeded into six-well plates, and the cells grown to ~80%
confluence. Transfection was performed according to the
instructions for Lipofectamine® 3000 transfection
reagent (cat. no. L3000015; Invitrogen; Thermo Fisher Scientific,
Inc.). Briefly, mimics or inhibitors (75 nM of each) were mixed
with the transfection agent in Opti-MEM for 20 min, and then added
to serum-free medium. After 6 h of transfection, the solution was
changed. Cells were cultured for another 48 h, subjected to
TRIzol® (Thermo Fisher Scientific, Inc.) RNA isolation
and cell images were captured using a Nikon TE2000 light microscope
(magnification, x100) or cells were cultured for 72 h to be used
for western blot analysis. The hsa-miR-335-5P mimic and inhibitor
were biosynthesized by Shanghai GenePharma Co., Ltd. The
hsa-miR-335-5P mimic sequence was 5'-UACAGUACUGUGAUAACUGAA-3' and
the mimic negative control (NC) sequence was
5'-GTTCTCCGAACGTGTCACGT-3', the hsa-miR-335-5P inhibitor sequence
was 5'-ACAUUUUUCGUUAUUGCUCUUGA-3' and the inhibitor NC sequence was
5'-CAGUACUUUUGUGUAGUACAA-3'. The overexpression vector
pCMV3-C-Myc-HMG-box transcription factor 1 (HBP1) and empty vector
were purchased from Sino Biological, Inc.
Reverse transcription quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. To detect miR-335-5P expression, the
TaqMan MicroRNA RT system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used to generate cDNA, and the expression of
miR-335-5P was measured using the miR-335-5P TaqMan microRNA assay
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Data were
analysed using the comparative 2-ΔΔCq method (6), and values were normalised to U6
expression. For the detection of gene expression, RT was performed
using the PrimeScript II 1st Strand cDNA Synthesis kit (Takara Bio,
Inc.), followed by qPCR according to the instructions provided with
the SYBR Premix Ex Taq kit (Takara Bio, Inc.). The expression
levels of genes were normalized to the expression of GAPDH. The
primers for the genes and miR-335-5P are shown in Table I. For RT-qPCR, the following thermal
and thermocycling conditions were used: 50˚C for 3 min and 95˚C for
3 min for RT; and 40 cycles of 94˚C for 1 min and 56˚C for 1 min
for the q-PCR. For miRNA, the following thermal and thermocycling
conditions were used: 95˚C for 10 min for RT; followed by 40 cycles
of 94˚C for 1 min, 56˚C for 1 min and 72˚C for 1 min for qPCR.
Relative expression of the genes was evaluated with the
2-ΔΔCq method (6).
 | Table IPrimer sequences for aggrecan,
collagen II, MMP13, collagen X and GAPDH. |
Table I
Primer sequences for aggrecan,
collagen II, MMP13, collagen X and GAPDH.
| Gene | Primer sequence
(5'to 3') |
|---|
| Aggrecan | F:
CTTCCGCTGGTCAGATGGAC |
| | R:
CGTTTGTAGGTGGTGGCTGT |
| Collagen II | F:
CCTACAATAATAATATATACCCCACCA |
| | R:
ATGTGTTTTCAGTGATCATGTTTTC |
| MMP13 | F:
GCACTTCCCACAGTGCCTAT |
| | R:
AGTTCTTCCCTTGATGGCCG |
| Collagen X | F:
AAAGGCCCACTACCCAACAC |
| | R:
GTGGACCAGGAGTACCTTGC |
| IL-1α | F:
AGCTATGGCCCACTCCATGAAG |
| | R:
ACATTAGGCGCAATCCAGGTGG |
| IL-6 | F:
AGACAGCCACTCACCTCTTCA |
| | R:
CACCAGGCAAGTCTCCTCATT |
| GAPDH | F:
GGAAGGTGAAGGTCGGAGTCA |
| | R:
CTGGAAGATGGTGATGGGATTTC |
Western blot analysis
A total of 48 h post-transfection, chondrocyte
proteins were extracted using cell lysis buffer (cat. no. 9803S;
Cell Signaling Technologies, Inc). The protein concentration was
determined by the BCA method. A total of 30 µg protein was loaded
per lane. The proteins were subjected to SDS-PAGE on 10% gels and
then transferred to a PVDF membrane. After blocking with 5% skim
milk powder at room temperature for 1 h, anti-aggrecan (ACAN; cat.
no. ab3778; Abcam), anti-collagen II (cat. no. ab34712; Abcam),
anti-collagen X (cat. no. ab58632; Abcam), anti-matrix
metalloproteinase (MMP)13 (cat. no. ab39012; Abcam), anti-Bax (cat.
no. ab32503; Abcam), anti-Bcl2 (cat. no. ab32124; Abcam), anti-
NF-κB (cat. no. ab16502; Abcam), anti-IκB (cat. no. ab32518;
Abcam), anti-IL-1α (cat. no. ab7632; Abcam), anti-IL-6 (cat. no.
ab6672; Abcam) and anti-β-actin (cat. no. MA5-15739; Sigma-Aldrich;
Merck KGaA) primary antibodies (1:1,000) were incubated with the
membrane overnight at 4˚C. After rinsing three times with TBS-Tween
(TBST; 0.1% Tween-20; 10 min/wash), the horseradish
peroxidase-conjugated AffiniPure Goat anti-rabbit IgG (cat. no.
31460) or goat anti-mouse IgG (cat. no. A24512) secondary
antibodies (1:5,000) (Pierce; Thermo Fisher Scientific, Inc.) were
incubated with the membrane for 1 h at 4˚C. The blots were washed
with TBST three times (10 min/wash) the protein of interest was
developed by electrochemiluminescence (Thermo Fisher Scientific,
Inc.). The intensities of the proteins in western blots were
semi-quantified using ImageJ version 1.52 (National Institute of
Health).
Cell Counting kit-8 (CCK-8) assay
Cell viability was assessed using a CCK-8 kit
(Dojindo Molecular Technologies, Inc.), according to the
manufacturer's instructions. Chondrocytes were seeded at a density
of 1x104 cells/well into 96-well culture plates and
cultured in DMEM/F12 containing 10% FBS at 37˚C in a 5%
CO2 incubator, with five duplicate wells in each group.
At 24, 48 and 72 h after transfection, the cells were incubated
with 100 µl WST-8 at 37˚C for 4 h. The absorbance of cells was
measured by a spectrophotometer at 570 nm.
Flow cytometry
An Annexin V-Fluorescein Isothiocyanate (FITC)
Apoptosis Detection kit (Invitrogen; Thermo Fisher Scientific,
Inc.) was used to detect apoptotic activity according to the
manufacturer's instructions. Chondrocytes were seeded into six-well
plates at a density of 1x106 cells/well in DMEM
containing 10% FBS. When the cells reached 60% confluence, they
were transfected. After 48 h, the cells were digested with 0.5%
trypsin and resuspended in 300 µl binding buffer containing 5 µl
Annexin V-FITC and 1 µl propidium iodide solution (100 µg/ml).
After incubation for 20 min in the dark at room temperature, the
stained cells were analysed by BD FACS Calibur with CellQuest Pro
Software 5.1 (BD Biosciences).
Luciferase reporter assay
The miR-335-5P response element in the 3'-UTR of
HBP1 (both wild-type and mutant HBP1) was cloned into the
pMIR-REPORT miRNA luciferase reporter vector (Ambion, Inc.; Thermo
Fisher Scientific, Inc.) containing firefly luciferase confirmed by
sequencing. The region of HBP1 was predicted using TargetScan 7.2
(http://www.targetscan.org/). 293T cells
(The Cell Bank of Type Culture Collection of the Chinese Academy of
Science) were co-transfected with the miR-335-5P mimic and
wild-type or mutant HBP1 3'-UTR luciferase reporters together with
the Renilla plasmid for 48 h using the miR-335-5P mimic NC
as the negative control. Then, firefly and Renilla
luciferase activities were measured according to the manufacturer's
instructions using a Dual-Luciferase Reporter Assay System (Promega
Corporation), and the firefly luciferase activity was normalized to
the value of the Renilla luciferase activity. Each
experiment was repeated in triplicate. The statistical data are
expressed as the mean ± standard error of the mean (SEM).
Statistical analysis
Data were analysed using GraphPad Prism 6 software
(GraphPad Software, Inc.). The statistical data are expressed as
the mean ± SEM. A two-tailed Student's t-test was used to compare
two groups. One-way ANOVA with Bonferroni post hoc test was used
for multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
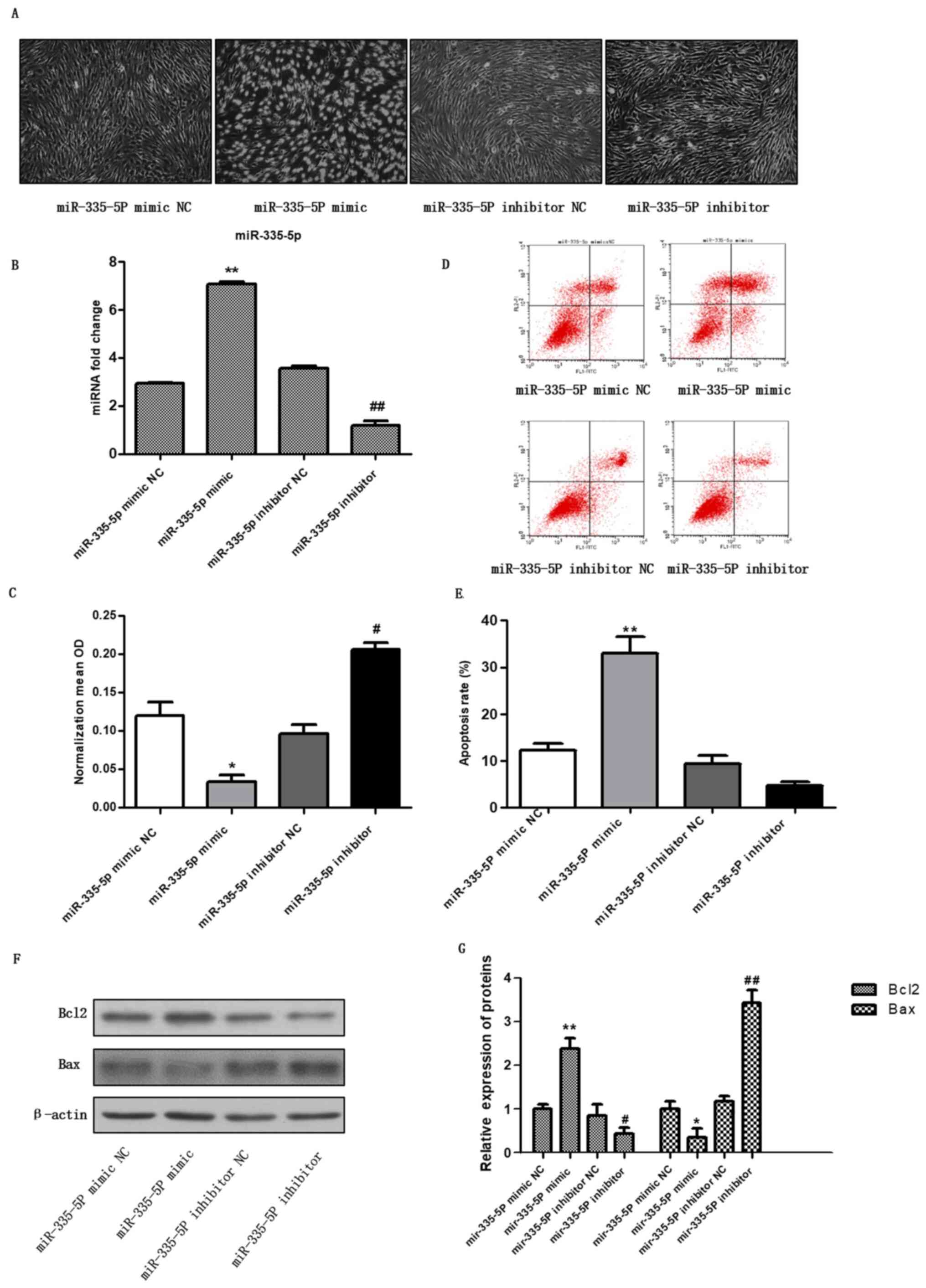
miR-335-5P mimic promotes the
apoptosis of chondrocytes
To investigate the effect of miR-335-5P on
chondrocyte apoptosis, the miR-335-5P mimic and the miR-335-5P
inhibitor were transfected into chondrocytes. After 48 h, the
expression levels of miR-335-5P were detected by RT-qPCR (Fig. 1B). The results showed that the level
of miR-335-5P was significantly higher in the mimic group than in
the mimic NC group, and the level in the inhibitor group was
significantly lower than that in the inhibitor NC group (Fig. 1B), indicating that the miR-335-5P
mimic and inhibitor were successfully transfected into
chondrocytes. Images of cell morphology were captured (Fig. 1A), cell activity was detected by
CCK-8 assay (Fig. 1C, P<0.05,
vs. control group), apoptosis was detected using flow cytometry
(Fig. 1D and E), and Bax and Bcl2 expression were
detected by western blotting (Fig.
1F and G, P<0.01, vs.
control group). For miR-335-5p mimic, miR-335-5p mimic NC was used
as the control, for miR-335-5p inhibitor, miR-335-5p inhibitor NC
was used as the control. The results showed that compared with the
mimic control group, miR-335-5P mimic transfection inhibited the
viability of chondrocytes and promoted chondrocyte apoptosis.
miR-335-5P regulates
cartilage-specific genes in human chondrocytes in vitro
To determine the regulation of cartilage-specific
genes by miR-335-5P, cells were harvested 48 h after transfection,
and the expression of cartilage-specific genes was detected by
western blotting and RT-qPCR. As shown in Fig. 2A and B, the miR-335-5P mimic significantly
downregulated the protein expression of ACAN (P<0.05) and
collagen II (P<0.05), and significantly upregulated the
expression levels of MMP13 (P<0.01) and collagen X (P<0.001)
compared with the mimic control. Conversely, the miR-335-5P
inhibitor significantly upregulated the expression levels of ACAN
(P<0.05) and collagen II (P<0.05) and downregulated the
protein expression of MMP13 (P<0.01) and collagen X (P<0.01)
compared with the inhibitor control. In addition, compared with the
mimic NC group, the overexpression of miR-335-5P significantly
downregulated the mRNA expression levels of collagen II
(P<0.05), and upregulated the mRNA expression levels of MMP13
(P<0.001) and collagen X (P<0.01). Conversely, inhibition of
miR-335-5P significantly upregulated the mRNA expression levels of
collagen II (P<0.05) and downregulated the mRNA expression
levels of MMP13 (Fig. 2C,
P<0.05) compared with the inhibitor NC. These results suggested
that miR-335-5P may inhibit the expression of the anabolic genes
ACAN and collagen II, and promote the expression of the OA-related
genes MMP13 and collagen X. Because inflammation usually
accompanies OA, the NF-κB signalling pathway and inflammatory
factors were measured in cells. The results showed that the NF-κB
signalling pathway was activated, and the levels of IL-1α
(P<0.01) and IL-6 (P<0.01) in cells transfected with
miR-335-5p mimics were significantly increased compared with the NC
group. Conversely, the NF-κB signalling pathway was suppressed and
the expression of IL-1α (P<0.05) and IL-6 (P<0.05) in cells
transfected with miR-335-5p inhibitors was significantly decreased
compared with the inhibitor control (Fig. S2).
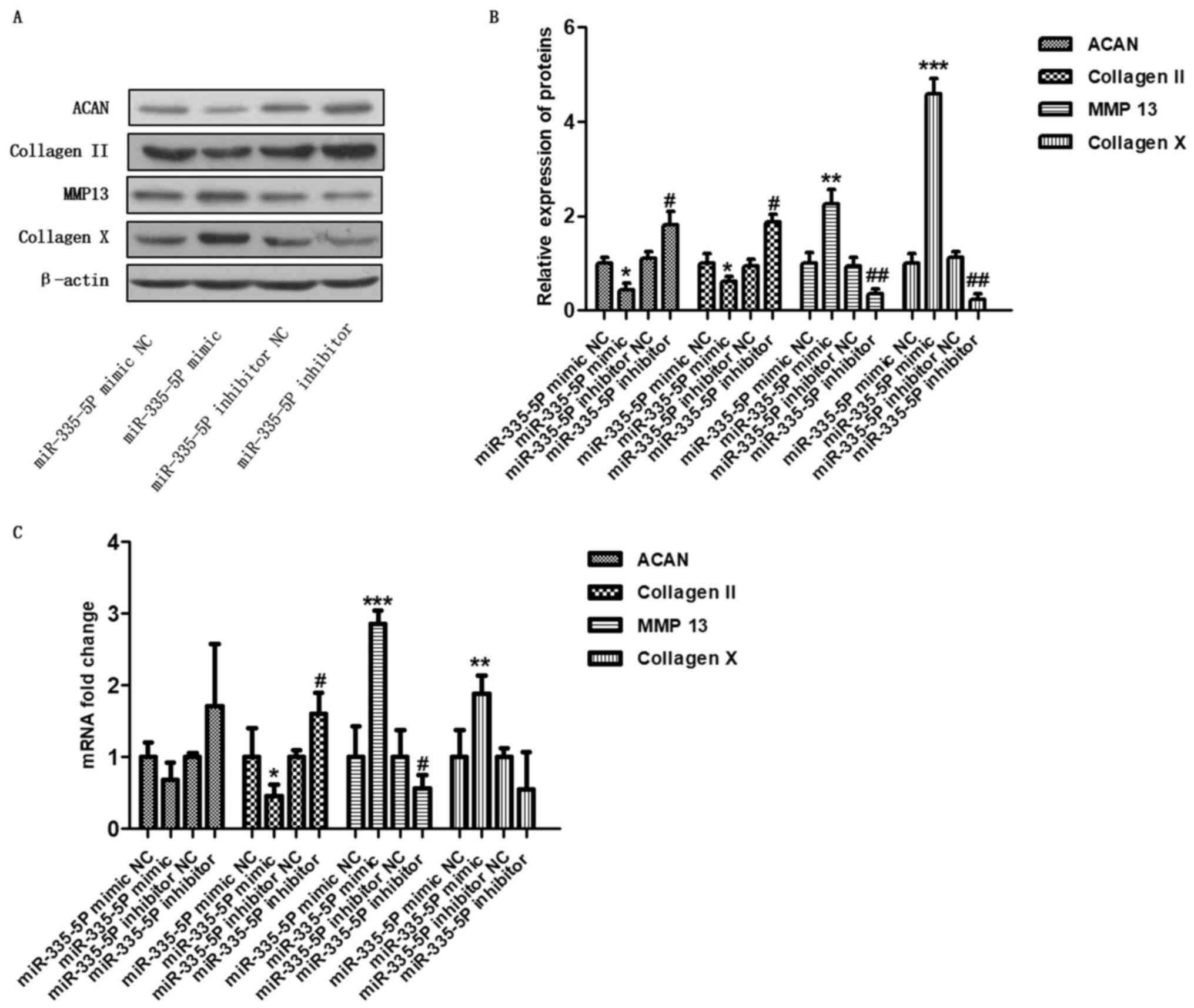 | Figure 2miR-335-5P regulates
cartilage-specific genes in human chondrocytes in vitro.
Chondrocytes were transfected with the miR-335-5P mimic NC,
miR-335-5P mimic, miR-335-5P inhibitor NC or miR-335-5P inhibitor.
After 48 h, the levels of ACAN, collagen II, MMP13, and collagen X
were analysed by (A and B) western blotting and (C) reverse
transcription-quantitative PCR. *P<0.05,
**P<0.01, ***P<0.001 compared with the
miR-335-5P mimic NC group; #P<0.05,
##P<0.01 compared with the miR-335-5P inhibitor NC
group. ACAN, aggrecan; MMP13, matrix metalloproteinase 13; miR,
microRNA; NC, negative control. |
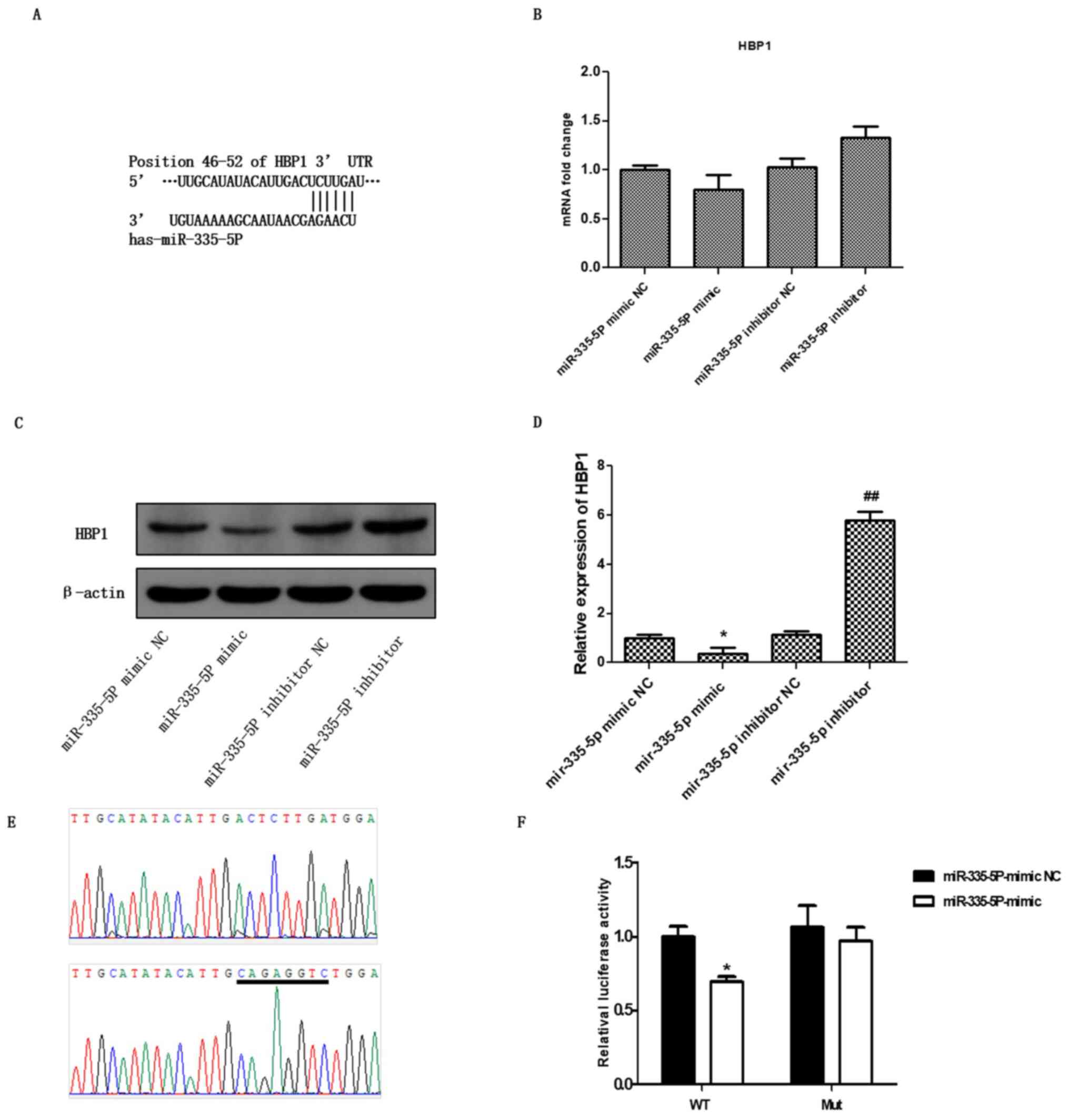
Prediction of HBP1 as a target of
miR-335-5P
To further investigate the molecular mechanism of
miR-335-5P in OA, the miR-335-5P mimic and miR-335-5P inhibitor
were transfected into chondrocytes. After 48 h, the expression of
HBP1 was detected by RT-qPCR and western blot analysis. As
expected, the overexpression of miR-335-5P non-significantly
restricted the mRNA expression of HBP1, whereas the downregulation
of miR-335-5P non-significantly enhanced the mRNA expression of
HBP1 (Fig. 3B; P>0.05;). In
addition, the western blotting results showed that the miR-335-5P
mimic significantly downregulated the protein expression of HBP1
(P<0.05) compared with the mimic NC. By contrast, the miR-335-5P
inhibitor significantly upregulated the levels of HBP1 (Fig. 3C, P<0.01) compared with the
inhibitor NC.
Subsequent bioinformatics analysis was used to
assess the targeted regulatory relationship between miR-335-5P and
HBP1. It was found that the 3'-UTR of HBP1 has a potential binding
site for miR-335-5P (Fig. 3A). This
result suggested that HBP1 may be a potential target gene of
miR-335-5P. To verify whether miR-335-5P directly binds to the
3'-UTR (46-52 bp) of HBP1, the 3'-UTR (+1 to +1,000 bp) of HBP1 and
the fragment containing the binding site mutation were inserted
into the pMIR-REPORT miRNA luciferase reporter vector. The
pMIR-Report-HBP1 wild-type and pMIR-Report-HBP1 mutant were
constructed and confirmed by sequencing (Fig. 3D). Then, the luciferase reporter
assay was used to confirm whether miR-335-5P regulates HBP1
transcriptional activity. The miR-335-5P mimic NC and the
miR-335-5P mimic were transfected into pMIR-Report-HBP1 wild-type
and pMIR-Report-HBP1-mutant 293T cells. After 48 h, the firefly and
Renilla luciferase activities were assessed. The results
showed that the luciferase activity of the miR-335-5P mimic group
was significantly lower than that of the mimic control group in the
transfected pMIR-Report-HBP1 wild-type cells but not in the
pMIR-Report-HBP1-mutant cells (Fig.
3E, P<0.05). These results indicated that miR-335-5P may
specifically bind to the 3'-UTR of HBP1 and that HBP1 could be a
novel specific target gene of miR-335-5P.
Regulation of chondrocyte apoptosis
and cartilage-specific genes by miR-335-5P via targeting HBP1
To further confirm whether miR-335-5P regulated HBP1
expression, promoted chondrocyte apoptosis and regulated the
expression of cartilage-specific genes, miR-335-5 and
pcDNA3.1-Myc-HBP1 plasmids were cotransfected into chondrocytes.
After 48 h, RT-qPCR was performed to detect the miR-335-5P
expression level (P<0.05; Fig.
4A), and western blotting was performed to detect HBP1
overexpression (Fig. 4A). The
expression levels of ACAN (P<0.05, vs. control group) and
collagen II (P<0.05) were found to be significantly upregulated,
and the expression levels of MMP13 (P<0.001) and collagen X
(P<0.01) were significantly downregulated after cotransfection
with the miR-335-5P and pcDNA3.1-Myc-HBP1 plasmids compared with
cotransfection with the miR-335-5P and vector (Fig. 4B and C). For pcDNA3.1-Myc-HBP1, empty vector was
used as the control, for miR-335-5P mimic + Myc-HBP1, miR-335-5P
mimic + empty vector was used as the control. Furthermore, cell
viability was detected by CCK-8 assays (Fig. 4D, P<0.05, vs. control group), and
apoptosis was detected using flow cytometry (Fig. 4G and H, P<0.05, vs. control group). Bax and
Bcl2 expression was detected by western blotting to test
chondrocyte apoptosis (Fig. 4E and
F). These results suggested that
HBP1 may have a role in the downstream effects of miR-335-5P on
chondrocyte apoptosis and cartilage-specific gene expression to
some degree.
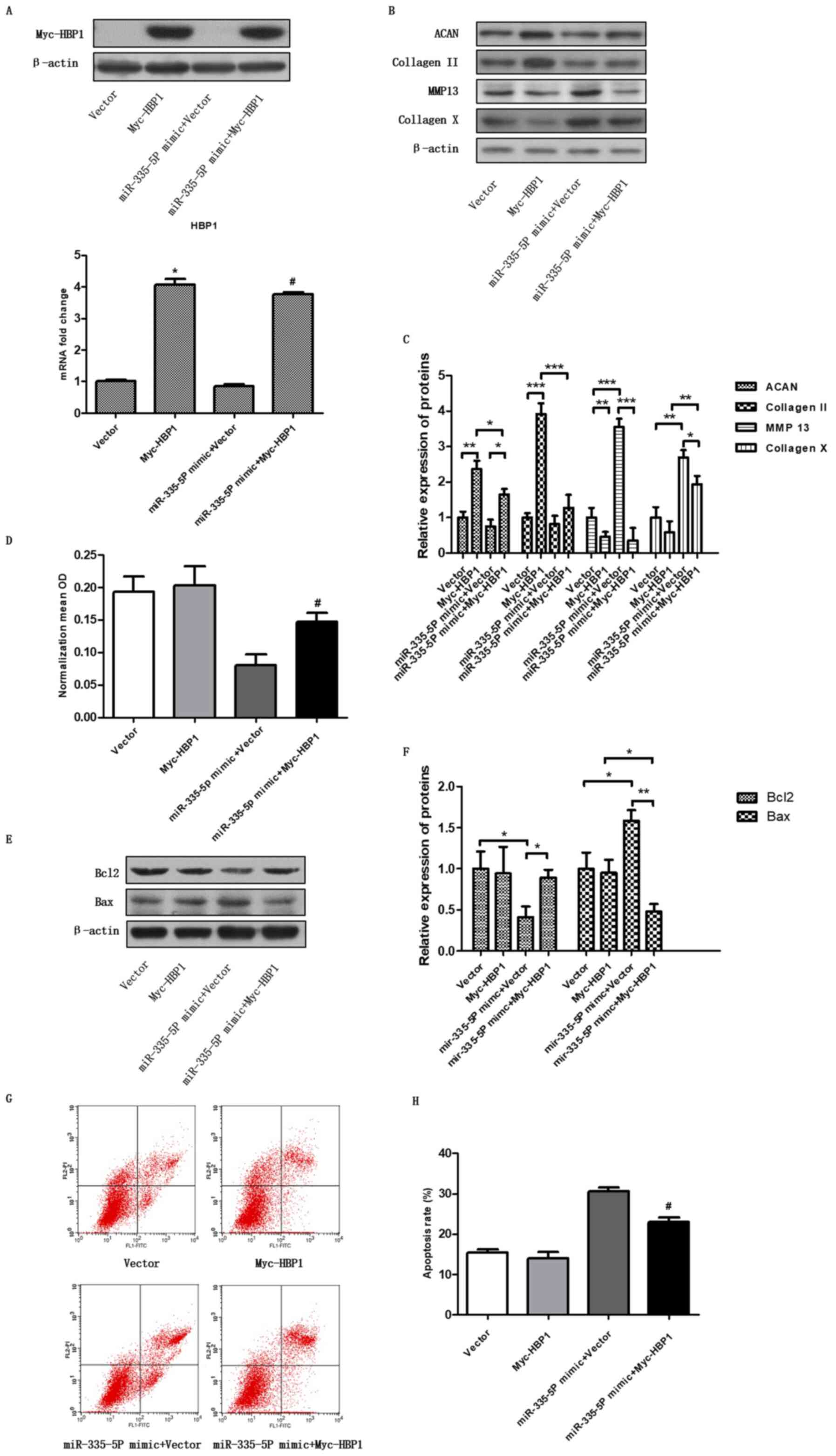 | Figure 4Cartilage-specific genes and
chondrocyte apoptosis are regulated by miR-335-5P via targeting
HBP1. (A) Overexpression of HBP1 was analysed by western blotting
and reverse transcription-quantitative PCR after the chondrocytes
were transfected with the vector, pcDNA3.1-Myc-HBP1, miR-335-5P
mimic alone or miR-335-5P mimic + pcDNA3.1-Myc-HBP1.
*P<0.05 compared with the vector group;
#P<0.05 compared with the miR-335-5P mimic + vector
group. (B and C) Chondrocytes were transfected and the expression
levels of ACAN, collagen II, MMP13 and collagen X were assessed by
western blotting. *P<0.05, **P<0.01,
***P<0.001 as indicated. (D) Cell Counting Kit-8 was
used to determine cell viability, and the data are shown as the
mean ± SEM of three independent experiments. (E and F) Western blot
analysis was used to study the apoptosis-associated protein
expression of Bax and Bcl2. *P<0.05,
**P<0.01as indicated. (G and H) Chondrocyte apoptosis
was analysed by flow cytometry. Cell apoptosis data are shown as
the mean ± SEM of three independent experiments.
*P<0.05 compared with the vector group;
#P<0.05 compared with the miR-335-5P mimic + vector
group. ACAN, aggrecan; miR, microRNA; MMP13, matrix
metalloproteinase 13; HBP1, HMG-box transcription factor 1; OD,
optical density. |
Discussion
In previous studies, the expression of miR-335-5P
was significantly increased in osteoarthritic chondrocytes compared
with normal cartilage (7,13). However, to the best of our
knowledge, the biological function of miR-335-5P in OA has not been
reported, and the molecular mechanism is not yet clear. This study
aimed to investigate the effects of miR-335-5P on chondrocyte
apoptosis and its underlying molecular mechanisms in OA.
In the present study, a miR-335-5P mimic and
inhibitor were transfected into chondrocytes. Notably, chondrocyte
apoptosis was observed after transfection of miR-335-5P mimic.
Although no relationship between chondrocyte apoptosis and the
occurrence and development of OA has previously been demonstrated
(3), the evidence provided here
indicated that chondrocyte apoptosis may occur during the whole
process of OA. Thus, inhibiting chondrocyte apoptosis may serve as
a target for the treatment of OA. Moreover, miR-335-5P could
inhibit the expression of extracellular matrix (ECM)
synthesis-related genes, such as ACAN and collagen II, and promote
the mRNA and protein levels of ECM degradation-related enzymes,
such as MMP13 and collagen X. As inflammation usually accompanies
OA, the NF-κB signalling pathway and inflammatory factors in cells
were also measured. These results showed that the NF-κB signalling
pathway was activated, and that the levels of IL-1α and IL-6 were
significantly increased in cells that were transfected with
miR-335-5p mimics compared with the control group. It is well-known
that inflammation of chondrocytes regulates the expression of
cartilage-specific genes, such as IL-1, which may suppress
expression of cartilage-specific types II and X collagens and
increase types I and III collagens in human chondrocytes. Hence, it
was hypothesized that the molecular mechanisms underlying the
effect of miR-335-5p on aberrant expression of cartilage-specific
genes may be related to inflammation. In summary, these results
indicated that miR-335-5P may have a negative regulatory role in
the pathogenesis of OA. In a study of osteogenic differentiation
induced by OA and normal human bone marrow-derived MSCs, it was
demonstrated that miR-335-5P was associated with the OA process and
had potential to aid in the development of OA therapies (7).
In addition, mechanistic studies have revealed that
miR-335-5P may target a group of negative regulators, including
dishevelled-associated activator of morphogenesis 1 and Rho kinase
1 of the SRY-box-transcription factor 9 (Sox9), and knocking out
Sox9 caused chondrocyte defects (14). The level of miR-335-5P can affect
the expression of its target dickkopf-related protein 1 (DKK1) and
DKK1 can further act on the Wnt signalling pathway (15). In addition, it has been reported
that the Wnt signalling pathway may play an important role in
chondrocyte proliferation and differentiation, and the Wnt pathway
is closely related to the occurrence and development of OA
(16). It is believed that the
expression of miR-335-5P may have a potential influence on the
biology of chondrocytes and thus miR-335-5P may play a role in the
development of OA. This study revealed HBP1 as a newly predicted
target gene of miRNA-335-5P. Through online bioinformatics analysis
and the dual luciferase assay, the 3'-UTR of HBP1 was verified to
bind to miR-335-5P by Specific binding site. These results
suggested that HBP1 is a potential new target of miR-335-5P in
vitro.
The HBP1 gene encodes HMG-box transcription factor
1, which is involved in Wnt signalling inhibition and cell
senescence (17,18). Most reports on this gene have
focused on the activities of transcription factors in various types
of human cancer but less so in OA (19,20).
However, it is known that the Wnt pathway and cellular senescence
play a role in the aetiology of OA (15,16).
It was previously reported that the decreased expression of this
gene was associated with OA susceptibility (21). Thus, the function of HBP1 in the
activation of the Wnt pathway and the attenuation of senescence may
be risk factors for the development of OA.
Another possible reason for the association between
OA and HBP1 is that HBP1 plays a role in the regulation of
superoxide production, which is a cause of OA aetiology (22). Grishko et al (23) demonstrated that oxidative stress in
OA resulted in decreased cell viability, decreased mitochondrial
DNA repair ability after reactive oxygen species (ROS) stimulation
and an increased phenotype of apoptosis in OA pathogenesis,
indicating that the decreased involvement of mitochondrial DNA
damage and repair ability play an important role in the development
of OA. Neutrophils are often found in the joints and synovial
fluids when their surface is attacked by immune complexes and
complement components, and large quantities of free radicals can be
released (24). Under normal
conditions, there is a balance between the oxidation system and the
antioxidant system, and the presence of ROS can prevent the
invasion of pathogens (25).
However, under pathological conditions, the increased production of
ROS inhibits the proliferation of chondrocytes, which results in
their death and inhibits the synthesis of cartilage matrix
proteoglycans and collagen (26-28).
Additionally, the oxidation of cartilage collagen causes collagen
cleavage, which changes the performance of collagen fibres, making
them prone to fatigue damage, accelerating degradation of the
cartilage matrix, reducing the elasticity and strength of
cartilage, damaging chondrocytes and leading to the occurrence of
OA (28). Although this study has
unveiled the influence of miR-335-5P on chondrocytes in
vitro, a lack of the effects of miR-335-3p on an animal model
of OA in vivo is one limitation of the study. Hence, the
effects of miR-335-3p on the animal model of OA remain to be
explored.
In conclusion, the present study has revealed that
miR-335-5P may act as a regulator of OA by inducing chondrocyte
apoptosis, and HBP1 was identified as a novel target of miR-335-5P.
Furthermore, HBP1 was involved in the occurrence of OA via
miR-335-5P. Therefore, miR-335-5P may be a promising therapeutic
target, providing a new repair pathway for the clinical treatment
of OA.
Supplementary Material
Human primary articular chondrocytes
cells were identified by toluidine blue staining and type II
collagen immunohistochemistry staining. (A) Toluidine blue staining
(x100). (B) Type II collagen immunohistochemistry staining
(x100).
miR-335-5P regulates NF-κB pathway and
inflammatory factors in human chondrocytes in vitro. The
chondrocytes were transfected with the miR-335-5P mimic NC,
miR-335-5P mimic, miR-335-5P inhibitor NC, or miR-335-5P inhibitor.
After 48 h, the levels of p65 and IκB were analysed by Western blot
(A-B) and the levels of IL-1 and IL-6 were analysed by qRT-PCR (C).
*P<0.05, **P<0.01,
***P<0.001 compared to the miR-335-5P mimic NC group;
#P<0.05, ###P<0.001 compared with the
miR-335-5P inhibitor NC group.
Acknowledgements
The authors would like to thank Dr Yunfei Pu (School
of Life Sciences, Xiamen University) for critically reading this
manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SC conceived the study. XL, YL and SC designed the
study. XL and YL performed the experiments and wrote the
manuscript. HC, YP and RL performed the statistical analysis. XL
and YL drafted the manuscript. SC reviewed the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The sample was obtained with written informed
consent from the donor. The study was approved by the Ethics
Committee of Fuzhou Second Hospital Affiliated to Xiamen
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ko JY, Lee MS, Lian WS, Weng WT, Sun YC,
Chen YS and Wang FS: MicroRNA-29a counteracts synovitis in knee
osteoarthritis pathogenesis by targeting VEGF. Sci Rep.
7(3584)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Huskisson EC: Modern management of
mild-to-moderate joint pain due to osteoarthritis: A holistic
approach. J Int Med Res. 38:1175–1212. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Goggs R, Carter SD, Schulzetanzil G,
Shakibaei M and Mobasheri A: Apoptosis and the loss of chondrocyte
survival signals contribute to articular cartilage degradation in
osteoarthritis. Vet J. 166:140–158. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Papanagnou P, Stivarou T and Tsironi M:
The role of miRNAs in common inflammatory arthropathies:
Osteoarthritis and gouty arthritis. Biomolecules.
6(44)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Crowe N, Swingler TE, Le LT, Barter MJ,
Wheeler G, Pais H, Donell ST, Young DA, Dalmay T and Clark IM:
Detecting new microRNAs in human osteoarthritic chondrocytes
identifies miR-3085 as a human, chondrocyte-selective, microRNA.
Osteoarthritis Cartilage. 24:534–543. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tornero-Esteban P, Rodríguez-Rodríguez L,
Abásolo L, Tomé M, López-Romero P, Herranz E, González MA, Marco F,
Moro E, Fernández-Gutiérrez B and Lamas JR: Signature of microRNA
expression during osteogenic differentiation of bone marrow MSCs
reveals a putative role of miR-335-5P in osteoarthritis. BMC
Musculoskelet Disord. 16:182–190. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mirzamohammadi F, Papaioannou G and
Kobayashi T: microRNAs in cartilage development, homeostasis, and
disease. Curr Osteoporos Rep. 12:410–419. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Steck E, Boeuf S, Gabler J, Werth N,
Schnatzer P, Diederichs S and Richter W: Regulation of H19 and its
encoded microRNA-675 in osteoarthritis and under anabolic and
catabolic in vitro conditions. J Mol Med (Berl). 90:1185–1195.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Negrini M and Calin GA: Breast cancer
metastasis: A microRNA story. Breast Cancer Res. 10:203–206.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Tomé M, López-Romero P, Albo C, Sepúlveda
JC, Fernández-Gutiérrez B, Dopazo A, Bernad A and González MA:
miR-335 orchestrates cell proliferation, migration and
differentiation in human mesenchymal stem cells. Cell Death Differ.
18:985–995. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kopańska M, Szala D, Czech J, Gabło N,
Gargasz K, Trzeciak M, Zawlik I and Snela S: miRNA expression in
the cartilage of patients with osteoarthritis. J Orthop Surg Res.
12(51)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lin X, Wu L, Zhang Z, Yang R, Guan Q, Hou
X and Wu Q: miR-335-5P promotes chondrogenesis in mouse mesenchymal
stem cells and is regulated through two positive feedback loops. J
Bone Miner Res. 29:1575–1585. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang J, Tu Q, Bonewald LF, He X, Stein G,
Lian J and Chen J: Effects of miR-335-5P in modulating osteogenic
differentiation by specifically downregulating Wnt antagonist DKK1.
J Bone Miner Res. 26:1953–1963. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Yates KE, Shortkroff S and Reish RG: Wnt
influence on chondrocyte differentiation and cartilage function.
DNA Cell Biol. 24:446–457. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sampson EM, Haque ZK, Ku MC, Tevosian SG,
Albanese C, Pestell RG, Paulson KE and Yee AS: Negative regulation
of the Wnt-beta-catenin pathway by the transcriptional repressor
HBP1. EMBO J. 20:4500–4511. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Paulson KE, Riegerchrist K, Mcdevitt MA,
Kuperwasser C, Kim J, Unanue VE, Zhang X, Hu M, Ruthazer R, Berasi
SP, et al: Alterations of the HBP1 transcriptional repressor are
associated with invasive breast cancer. Cancer Res. 67:6136–6145.
2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yee AS, Paulson EK, Mcdevitt MA,
Rieger-Christ K, Summerhayes I, Berasi SP, Kim J, Huang CY and
Zhang X: The HBP1 transcriptional repressor and the p38 MAP kinase:
Unlikely partners in G1 regulation and tumor suppression. Gene.
336:1–13. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen YC, Zhang XW, Niu XH, Xin DQ, Zhao
WP, Na YQ and Mao ZB: Macrophage migration inhibitory factor is a
direct target of HBP1-mediated transcriptional repression that is
overexpressed in prostate cancer. Oncogene. 29:3067–3078.
2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Raine EV, Wreglesworth N, Dodd AW, Reynard
LN and Loughlin J: Gene expression analysis reveals HBP1 as a key
target for the osteoarthritis susceptibility locus that maps to
chromosome 7q22. Ann Rheum Dis. 71:2020–2027. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Berasi SP, Xiu M, Yee AS and Paulson KE:
HBP1 repression of the p47phox gene: Cell cycle regulation via the
NADPH oxidase. Mol Cell Biol. 24:3011–3024. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Grishko VI, Ho R, Wilson GL and Pearsall
AW IV: Diminished mitochondrial DNA integrity and repair capacity
in OA chondrocytes. Osteoarthritis Cartilage. 17:107–113.
2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Stanczyk J, Kowalski ML, Grzegorczyk J,
Szkudlinska B, Jarzebska M, Marciniak M and Synder M: RANTES and
chemotactic activity in synovial fluids from patients with
rheumatoid arthritis and osteoarthritis. Mediators Inflamm.
2005:343–348. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Aikawa C, Nozawa T, Maruyama F, Tsumoto K,
Hamada S and Nakagawa I: Reactive oxygen species induced by
Streptococcus pyogenes invasion trigger apoptotic cell death in
infected epithelial cells. Cell Microbiol. 12:814–830.
2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Huang Z, Li J, Du S, Chen G, Qi Y, Huang
L, Xiao L and Tong P: Effects of UCP4 on the proliferation and
apoptosis of chondrocytes: Its possible involvement and regulation
in osteoarthritis. PLoS One. 11(e0150684)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yu SM and Kim SJ: Production of reactive
oxygen species by withaferin A causes loss of type collagen
expression and COX-2 expression through the PI3K/Akt, p38, and JNK
pathways in rabbit articular chondrocytes. Exp Cell Res.
319:2822–2834. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lepetsos P and Papavassiliou AG:
ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys
Acta. 1862:576–591. 2016.PubMed/NCBI View Article : Google Scholar
|