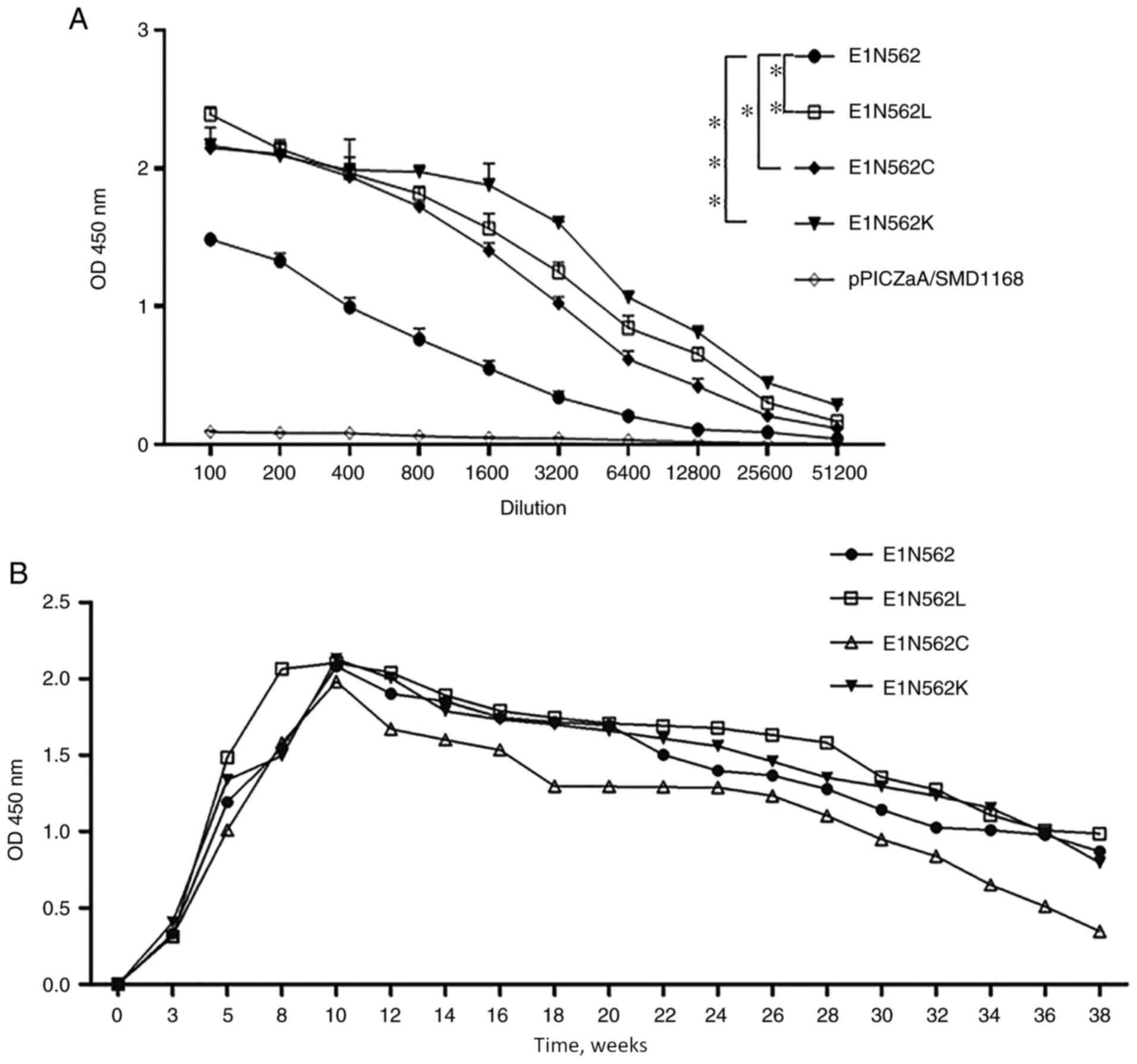
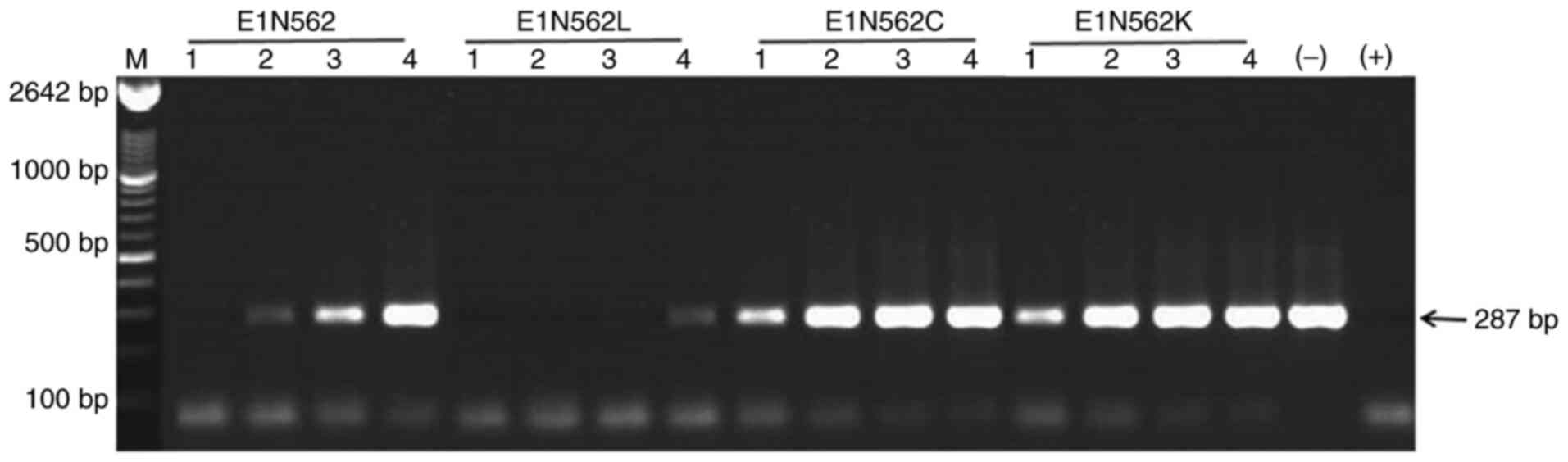
|
1
|
Yamada H, Takahashi K, Lim O, Svay S,
Chuon C, Hok S, Do SH, Fujimoto M, Akita T, Goto N, et al:
Hepatitis E Virus in Cambodia: Prevalence among the general
population and complete genome sequence of genotype 4. PLoS One.
10(e0136903)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pérez-Gracia MT, Suay-García B and
Mateos-Lindemann ML: Hepatitis E and pregnancy: Current state. Rev
Med Virol. 27(e1929)2017.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Abravanel F, Lhomme S, Fougère M, Saune K,
Alvarez M, Péron JM, Delobel P and Izopet J: HEV infection in
French HIV-infected patients. J Infect. 74:310–313. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Debes JD, Pas SD, Groothuismink ZMA, van
der Ende ME, de Man RA and Boonstra A: A mutation in the
progesterone receptor predisposes to HEV infection in HIV-positive
patients. Liver Int. 38:792–796. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kasem A, Izopet J, Pavio N, Aggarwal R,
Labrique A, Wedemeyer H and Dalton HR: Epidemiology of hepatitis E
virus infection. Epidemiol Mikrobiol Imunol. 68:176–182.
2019.PubMed/NCBI
|
|
6
|
Tam AW, Smith MM, Guerra ME, Huang CC,
Bradley DW, Fry KE and Reyes GR: Hepatitis E virus (HEV): Molecular
cloning and sequencing of the full-length viral genome. Virology.
185:120–131. 1991.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li S, Zhang J and Xia N: Lessons from
hepatitis E vaccine design. Curr Opin Virol. 11:130–136.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chandra V, Kalia M, Hajela K and Jameel S:
The ORF3 protein of hepatitis E virus delays degradation of
activated groWTh factor receptors by interacting with CIN85 and
blocking formation of the Cbl-CIN85 complex. J Virol. 84:3857–3867.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Takahashi M, Yamada K, Hoshino Y,
Takahashi H, Ichiyama K, Tanaka T and Okamoto H: Monoclonal
antibodies raised against the ORF3 protein of hepatitis E virus
(HEV) can capture HEV particles in culture supernatant and serum
but not those in feces. Arch Virol. 153:1703–1773. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guu TS, Liu Z, Ye Q, Mata DA, Li K, Yin C,
Zhang J and Tao YJ: Structure of the hepatitis E virus-like
particle suggests mechanisms for virus assembly and receptor
binding. Proc Natl Acad Sci USA. 106:12992–12997. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yamashita T, Mori Y, Miyazaki N, Cheng RH,
Yoshimura M, Unno H, Shima R, Moriishi K, Tsukihara T, Li TC, et
al: Biological and immunological characteristics of hepatitis E
virus-like particles based on the crystal structure. Proc Natl Acad
Sci USA. 106:12986–12991. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yin X, Ying D, Lhomme S, Tang Z, Walker
CM, Xia N, Zheng Z and Feng Z: Origin, antigenicity, and function
of a secreted form of ORF2 in hepatitis E virus infection. Proc
Natl Acad Sci USA. 115:4773–4778. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zafrullah M, Ozdener MH, Kumar R, Panda SK
and Jameel S: Mutational analysis of glycosylation, membrane
translocation, and cell surface expression of the hepatitis E virus
ORF2 protein. J Virol. 73:4074–4082. 1999.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mori Y and Matsuura Y: Structure of
hepatitis E viral particle. Virus Res. 161:59–64. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Graff J, Zhou YH, Torian U, Nguyen H, St
Claire M, Yu C, Purcell RH and Emerson SU: Mutations within
potential glycosylation sites in the capsid protein of hepatitis E
virus prevent the formation of infectious virus particles. J Virol.
82:1185–1194. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shiota T, Li TC, Yoshizaki S, Kato T,
Wakita T and Ishii K: The hepatitis E virus capsid C-terminal
region is essential for the viral life cycle: Implication for viral
genome encapsidation and particle stabilization. J Virol.
87:6031–6036. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Biasini M, Bienert S, Waterhouse A, Arnold
K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M,
Bordoli L and Schwede T: SWISS-MODEL: Modelling protein tertiary
and quaternary structure using evolutionary information. Nucleic
Acids Res. 42:W252–W258. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Arnold K, Bordoli L, Kopp J and Schwede T:
The SWISS-MODEL workspace: A web-based environment for protein
structure homology modelling. Bioinformatics. 22:195–201.
2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang C, Vasmatzis G, Cornette JL and
DeLisi C: Determination of atomic desolvation energies from the
structures of crystallized proteins. J Mol Biol. 267:707–726.
1997.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen D and Meng J: Expression,
purification and immunogenicity of a novel hepatitis E virus-like
particle. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 22:339–342.
2006.PubMed/NCBI(In Chinese).
|
|
21
|
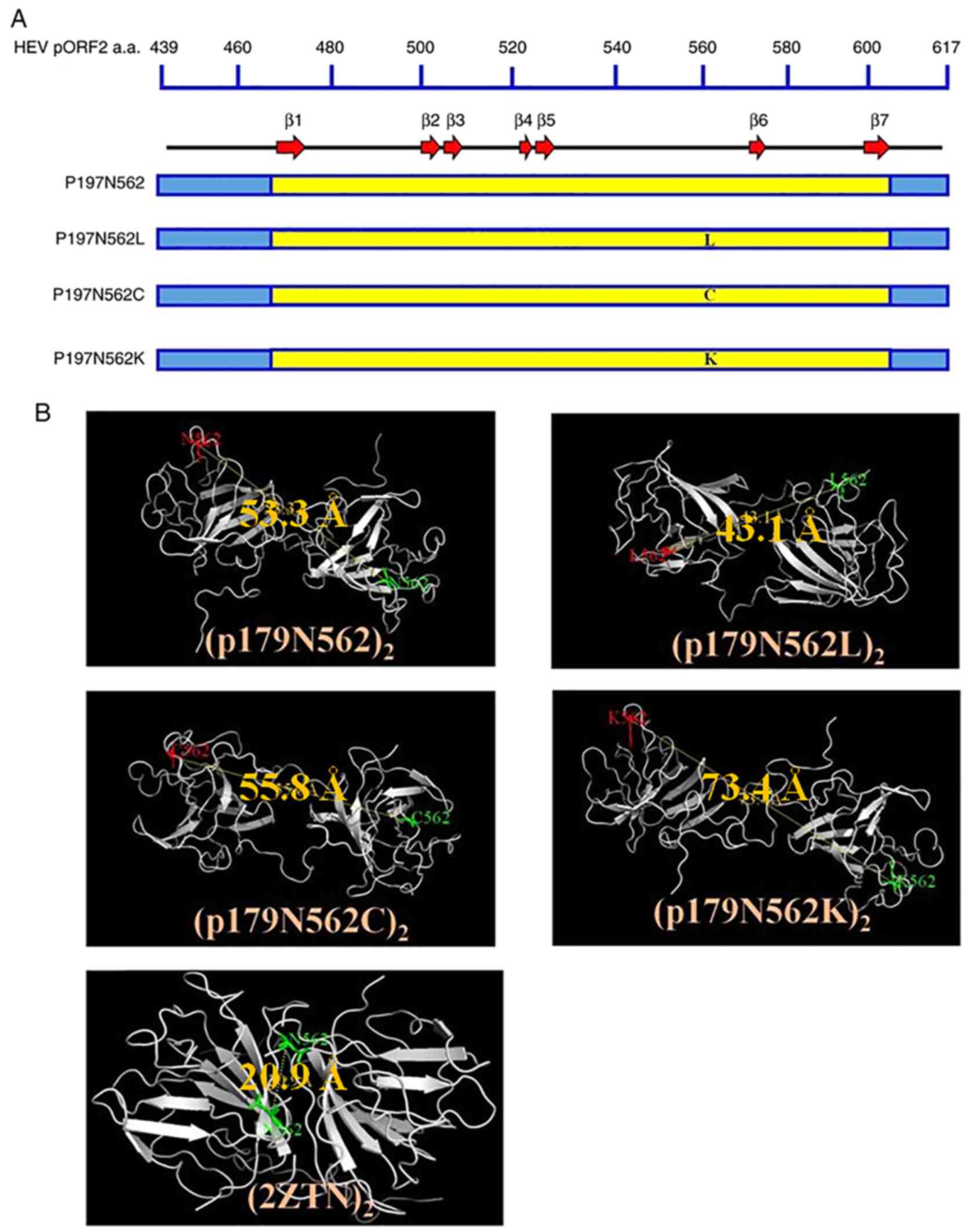
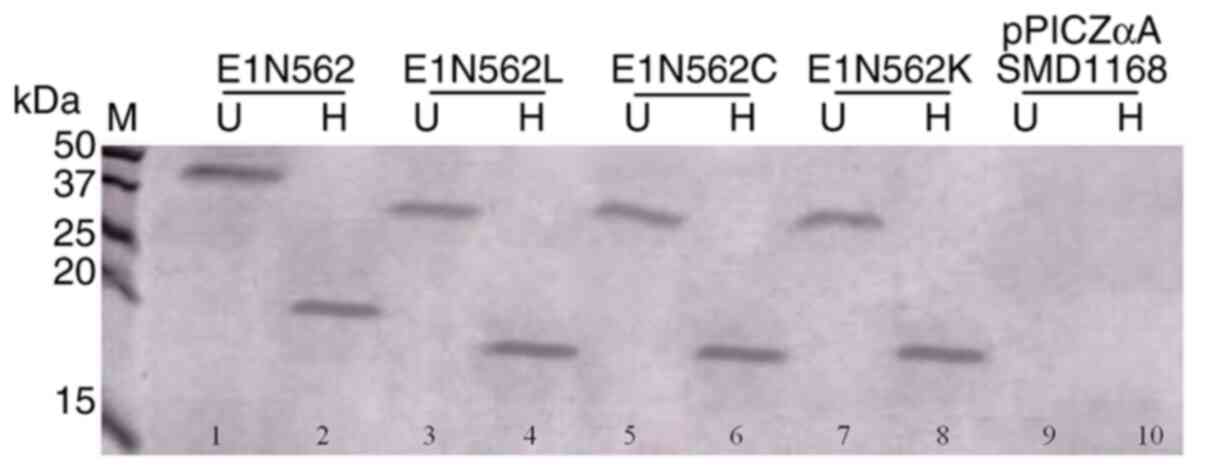
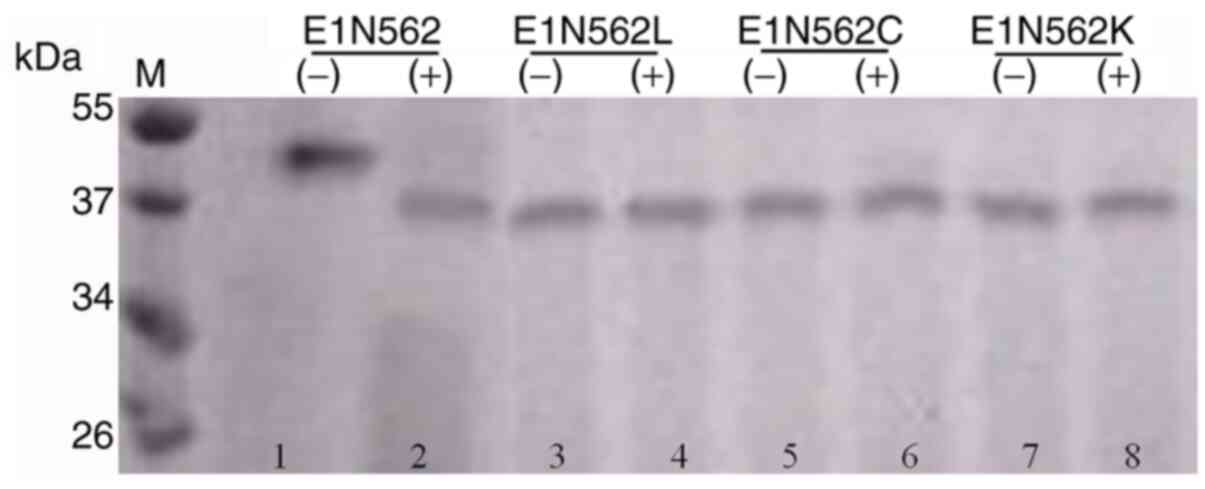
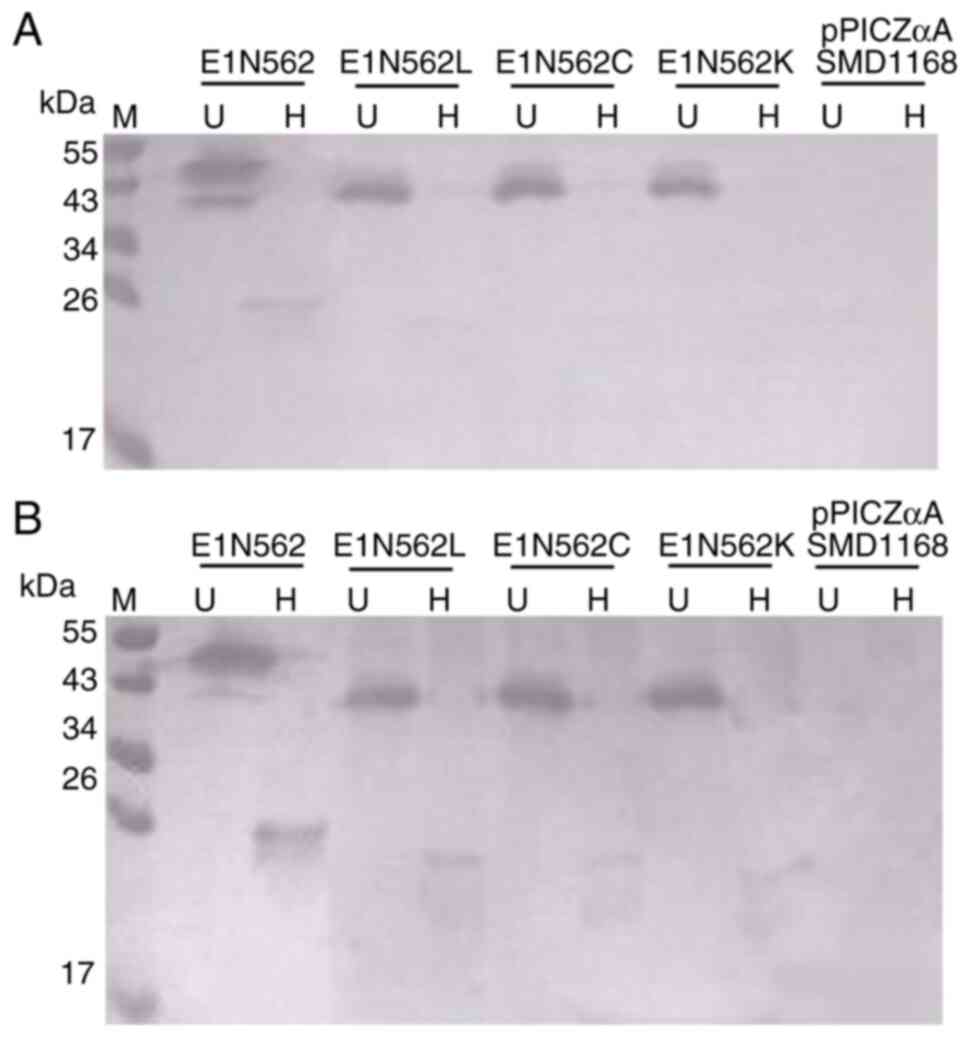
Xu M, Behloul N, Wen J, Zhang J and Meng
J: Role of asparagine at position 562 in dimerization and
immunogenicity of the hepatitis E virus capsid protein. Infect
Genet Evol. 37:99–107. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ito K, Qin Y, Guarnieri M, Garcia T, Kwei
K, Mizokami M, Zhang J, Li J, Wands JR and Tong S: Impairment of
hepatitis B virus virion secretion by single-amino-acid
substitutions in the small envelope protein and rescue by a novel
glycosylation site. J Virol. 84:12850–12861. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hawkins P, Prescott MJ, Carbone L,
Dennison N, Johnson C, Makowska IJ, Marquardt N, Readman G, Weary
DM and Golledge HD: A Good Death? Report of the second Newcastle
meeting on laboratory animal euthanasia. Animals (Basel).
6(50)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang H, Dai X, Shan X and Meng J:
Characterization of antigenic epitopes of the ORF2 protein from
hepatitis E virus genotype 4. Virus Res. 142:140–143.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Meng J, Dubreuil P and Pillot J: A new
PCR-based seroneutralization assay in cell culture for diagnosis of
hepatitis E. J Clin Microbiol. 35:1373–1377. 1997.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Emerson SU, Clemente-Casares P, Moiduddin
N, Arankalle VA, Torian U and Purcell RH: Putative neutralization
epitopes and broad cross-genotype neutralization of Hepatitis E
virus confirmed by a quantitative cell-culture assay. J Gen Virol.
87:697–704. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fiedler K and Simons K: The role of
N-glycans in the secretory pathway. Cell. 81:309–312.
1995.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhou YH, Purcell RH and Emerson SU: An
ELISA for putative neutralizing antibodies to hepatitis E virus
detects antibodies to genotypes 1, 2, 3, and 4. Vaccine.
22:2578–2585. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Meng J, Dai X, Chang JC, Lopareva E,
Pillot J, Fields HA and Khudyakov YE: Identification and
characterization of the neutralization epitope(s) of the hepatitis
E virus. Virology. 288:203–211. 2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Purcell RH, Nguyen H, Shapiro M, Engle RE,
Govindarajan S, Blackwelder WC, Wong DC, Prieels JP and Emerson SU:
Pre-clinical immunogenicity and efficacy trial of a recombinant
hepatitis E vaccine. Vaccine. 21:2607–2615. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li SW, Zhao Q, Wu T, Chen S, Zhang J and
Xia NS: The development of a recombinant hepatitis E vaccine HEV
239. Hum Vaccin Immunother. 11:908–914. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhu FC, Zhang J, Zhang XF, Zhou C, Wang
ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP, et al: Efficacy
and safety of a recombinant hepatitis E vaccine in healthy adults:
A large-scale, randomised, double-blind placebo-controlled, phase 3
trial. Lancet. 376:895–902. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gurramkonda C, Adnan A, Gäbel T, Lünsdorf
H, Ross A, Nemani SK, Swaminathan S, Khanna N and Rinas U: Simple
high-cell density fed-batch technique for high-level recombinant
protein production with Pichia pastoris: Application to
intracellular production of hepatitis B surface antigen. Microb
Cell Fact. 8(13)2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lorenzo FR, Tanaka T, Takahashi H,
Ichiyama K, Hoshino Y, Yamada K, Inoue J, Takahashi M and Okamoto
H: Mutational events during the primary propagation and consecutive
passages of hepatitis E virus strain JE03-1760F in cell culture.
Virus Res. 137:86–96. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Okamoto H: Hepatitis E virus cell culture
models. Virus Res. 161:65–77. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yamamoto H, Suzuki J, Matsuda A, Ishida T,
Ami Y, Suzaki Y, Adachi I, Wakita T, Takeda N and Li TC: Hepatitis
E virus outbreak in monkey facility, Japan. Emerg Infect Dis.
18:2032–2034. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Takahashi K, Kitajima N, Abe N and Mishiro
S: Complete or near-complete nucleotide sequences of hepatitis E
virus genome recovered from a wild boar, a deer, and four patients
who ate the deer. Virology. 330:501–505. 2004.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li S, Tang X, Seetharaman J, Yang C, Gu Y,
Zhang J, Du H, Shih JW, Hew CL, Sivaraman J and Xia N: Dimerization
of hepatitis E virus capsid protein E2s domain is essential for
virus-host interaction. PLoS Pathog. 5(e1000537)2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jameel S, Zafrullah M, Ozdener MH and
Panda SK: Expression in animal cells and characterization of the
hepatitis E virus structural proteins. J Virol. 70:207–216.
1996.PubMed/NCBI View Article : Google Scholar
|