Introduction
Nitric oxide (NO) is a signaling molecule that plays
a crucial role in the kidney and participates in a number of
physiological processes, including ion transportation and blood
pressure regulation (1). It has
been previously reported that NO inhibits transepithelial Na+
reabsorption and antidiuretic hormone-stimulated osmotic water
permeability (2,3). Therefore, modulation of NO levels in
the kidney is of importance. NO is synthesized from L-arginine by
three isoforms of NO synthases (NOS), namely neuronal (nNOS),
inducible (iNOS) and endothelial. Asymmetric dimethylarginine
(ADMA) is an endogenous analog of L-arginine that can attenuate the
function of NOS and has been shown to be harmful for the kidney
(4). ADMA exhibits a causative role
in the development of kidney injury in terms of renal fibrosis and
is considered to be a predictive risk factor for diabetic
nephropathy (5). ADMA is
metabolized by dimethylarginine dimethylaminohydrolases (DDAHs)
in vivo that can exist as type 1 and 2 isoforms. The kidney
serves a role in eliminating ADMA, such that the kidney cortex
contains the highest concentration of DDAH enzymes among all organs
(6). The two isoforms of DDAH are
expressed in a tissue-specific manner. Specifically, DDAH-2 is
expressed in the macula densa and distal nephron regions of the
kidney (6-8).
NOS and DDAH systems are important factors in
determining NO production. Therefore, their expression regulates NO
levels. Although mechanism underlying the regulation of NOS
expression has been widely defined (9), reports on the regulatory mechanisms
underlying DDAH expression are limited, with the majority of the
previous studies referring to methylation. Hypermethylation of the
DDAH2 promoter was associated with DDAH2 downregulation in human
umbilical vein endothelial cells and the rat corpora cavernosa,
whereas the inhibition of methylation using 5'-aza-2'-deoxycytidine
enhanced DDAH2 expression in undifferentiated cells of trophoblast
cell lineage (10-12).
Acetylation is an important epigenetic modification that has been
reported to regulate NOS expression by various mechanisms (13,14).
To the best of our knowledge, mechanistically, only Tomikawa et
al (12) previously reported
that histone acetylation regulated the expression of DDAH2 in
trophoblast stem cells and was involved in the regulation of cell
differentiation. However, the accurate mechanism has not been fully
elucidated.
NF-κB is a DNA sequence-specific transcription
factor that is subject to acetylation. The NF-κB acetylation status
may affect its DNA binding affinity, transcriptional activity and
protein interactions, thereby indirectly regulating the
transactivation of numerous genes (15-18).
A previous study and other reports demonstrated that NF-κB
acetylation could regulate the expression of NOS genes (19,20).
Therefore, the present study hypothesized that NF-κB acetylation
may also be involved in regulating DDAH2 expression, resulting in
the changes in both NOS and DDAH levels in turn acting as an
important factor in modulating NO production. Trichostatin A (TSA),
a deacetylase inhibitor, was used to induce acetylation in 293
cells to assess the effect of acetylation on DDAH2 expression and
its mechanisms.
Materials and methods
Cell culture and treatment
293 cells, purchased from the Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences, were
cultured in DMEM (Thermo Fisher Scientific, Inc.) supplemented with
15% FBS in a humidified atmosphere containing 5% CO2 at
37˚C. Subsequently, 24 h after seeding, cells were treated with 250
ng/ml TSA (Sigma-Aldrich; Merck KGaA) at 37˚C for 24 h or
transfected with indicated plasmids and collected 24 h after. All
cultures were tested for mycoplasma.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from 293 cells using a
TRIzol® reagent (Thermo Fisher Scientific, Inc.) and
cDNA was synthesized using the Reverse Transcription System (cat.
no. A3500; Promega Corporation) according to the manufacturer's
protocols. qPCR was performed in an Applied Biosystems 7500
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using a total reaction volume of 20 µl containing 1X
SYBR™-Green PCR master mix (Thermo Fisher Scientific, Inc.), 10 ng
cDNA and 100 nmol/l forward and reverse primers. The sequences of
the intron-spanning primers used were as follows: DDAH2 forward,
5'-TCTCTTTCTTCGTCCTGGGT-3' and reverse, 5'-CACAGGTACCAGGGTGACAT-3'
and β-actin forward, 5'-CCGTCTTCCCCTCCATCG-3' and reverse,
5'-GTCCCAGTTGGTGACGATGC-3'. Thermocycling conditions were 95˚C for
3 min followed by 95˚C for 10 sec and 60˚C for 60 sec for 40
cycles. Dissociation curves were generated to ensure that a single
and specific product was amplified. Cycle threshold values (Ct)
were analyzed using the SDS 2.4 software (Thermo Fisher Scientific,
Inc.) and quantification of the relative DDAH2 expression was
performed using the 2-ΔΔCq method (21) with the β-actin transcript serving as
an internal control.
Western blot analysis
Whole cell lysate was prepared from 293 cells using
RIPA buffer (Thermo Fisher Scientific, Inc.). Protein was
quantified using the DC Protein assay (Bio-Rad Laboratories, Inc.)
following the manufacturer's instructions. In total, 25 µg of each
sample was subjected to 10% SDS-PAGE and transferred onto PVDF
membranes (Bio-Rad Laboratories, Inc.). Subsequently, the membranes
were blocked with 5% non-fat dry milk in TBS containing 0.1%
Tween-20 at room temperature for 1 h and incubated with primary
antibodies at 4˚C overnight, followed by incubation with an
horseradish peroxidase-conjugated secondary antibody (cat. no.
sc-2004 or sc-2005; 1:2,500 dilution; Santa Cruz Biotechnology,
Inc.) at room temperature for 2 h. The protein bands were detected
with an enhanced chemiluminescence detection kit (Bio-Rad
Laboratories, Inc.) and quantified with Quantity One 1-D Analysis
Software version 4.6.8 (Bio-Rad Laboratories, Inc.). The primary
antibodies used were as follows: Anti-DDAH2 (cat. no. sc-32859;
1:500 dilution; Santa Cruz Biotechnology, Inc.), anti-p65 (cat. no.
sc-8008; 1:500 dilution; Santa Cruz Biotechnology, Inc.),
anti-β-actin (cat. no. 12262S; 1:2,500 dilution; Cell Signaling
Technology, Inc.), monoclonal antibody against acetylated lysine
(cat. no. 9681S; 1:1,000 dilution, Cell Signaling Technology, Inc.)
and anti-Lamin B1 antibody (cat. no. 33-2000; 1:500 dilution;
Thermo Fisher Scientific, Inc.).
Analysis of DDAH2 promoter and plasmid
construction
A 2 kbp (from -1983 to +17) sequence of the DDAH2
promoter was analyzed using the Prediction of transcription factor
binding sites analysis of Alibaba-2.1 (default values for all
parameters) and Matrix Search for Transcription Factor Binding
Sites analysis with minimal false negatives of Match-1.0 Public
software (http://www.gene-regulation.com). The DDAH2 promoter
fragment containing a potential NF-κB responsive element (located
between-1582 and -1573 positions) was cloned into pMD18-T vector
(Takara Bio, Inc.), digested with SacI and HindIII
restriction enzymes (New England Biolabs, Inc.) and then subcloned
into SacI/HindIII site of the pGL3_Basic vector
(Promega Corporation) to construct the pGL_1983 wild-type (wt)
plasmid. In addition, a plasmid encompassing a mutated NF-κB
binding site (pGL_1983mut) was generated using the Q5 site-directed
mutagenesis kit following the manufacturer's protocol (New England
BioLabs, Inc.) to change two corresponding G and A residues to T
(sequence was changed from GGGCAGGGAA to GGGCAGGTAT,) to abolish
the potential NF-κB element. All constructs were confirmed by
Sanger sequencing, as previously described (22).
Luciferase assay
293 cells were seeded at 10,000 cells/well into
96-well plates, grown to 60-70% confluence and transiently
transfected with the plasmids using the Lipofectamine®
2000 reagent (Thermo Fisher Scientific, Inc.). To normalize
transfection efficiency, a pRL-TK plasmid (Promega Corporation)
containing a cDNA encoding Renilla luciferase was used as an
internal control. A total of 0.5 µg either of the pGL constructs
(pGL_1983wt, pGL_1983mut or pGL3-Basic) and 0.05 µg pRL-TK were
co-transfected into the 293 cells. Following incubation for 6 h,
cells were treated with TSA (250 ng/ml) for 24 h. The luciferase
activity was determined using the Dual-Luciferase®
Reporter assay system following the manufacturer's instructions
(Promega Corporation) using a Lumat LB 9507 luminometer (Berthold
Technologies, GmbH). Relative luciferase activity was expressed as
the ratio of firefly activity to that of Renilla.
Immunoprecipitation
Nuclear extracts were prepared from 293 cells using
the Nuclear Extract Kit (cat. no. 40010; Active Motif Inc.)
following the manufacturer's protocol and the concentration was
quantified with DC Protein Assay kits (cat. no. 5000112; Bio-Rad
Laboratories, Inc.) following the manufacturer's instructions. In
total, 10 µl antibody against p65 (cat. no. sc-8008; Santa Cruz
Biotechnology, Inc.) was incubated with 400 µg nuclear extract at
4˚C overnight. Subsequently, 20 µl protein A/G-agarose beads (cat.
no. sc-2003; Santa Cruz Biotechnology, Inc.) were added and
incubated with the lysates at 4˚C for 2 h to collect the
immunoprecipitated proteins before the samples were centrifuged at
1,000 x g for 5 min and extensively washed with buffer containing
50 mM Tris (pH 8.0), 150 mM NaCl, 1 mM EDTA and 0.5% NP-40. The
immunoprecipitated proteins were then mixed with SDS loading
buffer, boiled for 5 min and analyzed by SDS-PAGE and western blot
analysis using a monoclonal antibody against acetylated lysine
(Cell Signaling Technology, Inc.).
Electrophoretic mobility shift assay
(EMSA)
Nuclear extracts were first prepared from 293 cells.
The oligonucleotide probes (synthesized by Sangon Biotech Co.,
Ltd.) used were: i) NF-κB consensus, 5'-AGTTGAGGGGACTTTCCCAGGC-3'
and its complementary fragment; ii) for wild-type (WT) NF-κB,
5'-GGGCAGGGAATCCTGGAG-3' and its complementary fragment; and iii)
for mutant (MUT) NF-κB, 5'-GGGCAGGTATTCCTGGAG-3' (mutations
underlined) and its complementary fragment. The probes were labeled
using the Biotin 3' End Labeling Kit (cat. no. 20160, Thermo Fisher
Scientific, Inc.) following the details from the manufacturer. EMSA
was performed using the Lightshift™ Chemiluminescent EMSA kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Next, 10 µg nuclear extract and 25 pmol probe were used
in binding reaction. For competition assays, nuclear extract was
pre-incubated with a 100-fold excess of the unlabeled WT or MUT
competitors with the same sequence as indicated above at 4˚C for 30
min. For the supershift reaction, 1 µg anti-p65 antibody (cat. no.
sc-8008; Santa Cruz Biotechnology, Inc.) or normal IgG (cat. no.
sc-3888; Santa Cruz Biotechnology, Inc.) was pre-incubated with 10
µg nuclear extracts at 4˚C for 30 min. The protein-DNA complexes
were separated by electrophoresis on a 6% non-denaturing acrylamide
gel in 0.5X TBE before transferal onto positively charged nylon
membranes and visualized using streptavidin-horseradish peroxidase
(Thermo Fisher Scientific, Inc.), followed by chemiluminescent
detection (Thermo Fisher Scientific, Inc.) on ChemiDoc Imaging
Systems (Bio-Rad Laboratories, Inc.).
Chromatin immunoprecipitation
(ChIP)
Cultured 293 cells were treated with 1% formaldehyde
and incubated at 37˚C for 20 min. Cells were then harvested,
resuspended in lysis buffer (1% SDS, 10 mmol/l EDTA, 50 mmol/l
Tris-HCl, pH 8.1), incubated at 4˚C for 10 min and sonicated at 20
kHz frequency output for 10 min on ice with cycles of 15 sec on/15
sec off to generate DNA fragments 100-300 bp in length along with
bound proteins. In total, 33% of the lysate was used as the DNA
input control. The remaining lysate was diluted 10-fold, where 50%
of the sample was incubated with 20 ng/µl specific p65 antibody and
the remaining half was incubated with normal rabbit IgG at 4˚C
overnight. Immunoprecipitated complexes were then collected by
incubating with 20 µl protein A/G-agarose beads at 4˚C for 1 h and
centrifuged at 1,000 x g for 5 min at 4˚C. The beads were
extensively washed with low salt immune complex wash buffer (0.1%
SDS, 1% Triton X-100, 2 mmol/l EDTA, 20 mmol/l Tris-Cl, pH 8.1 and
150 mmol/l NaCl), high salt immune complex wash buffer (the same
components apart from the use of 500 mmol/l NaCl) and Tris-EDTA, pH
8.0 successively, and incubated at room temperature for 20 min.
Precipitates were eluted with elution buffer (0.1% SDS and 0.1
mol/l NaHCO3) and cross-linking of protein-DNA complexes
was reversed following incubation at 65˚C for 5 h. DNA was then
extracted using phenol/chloroform, as previously described
(23). qPCR was performed using a
total reaction volume of 20 µl containing 1X SYBR™ Green PCR master
mix (Thermo Fisher Scientific, Inc.) with the following pairs of
primers: i) Forward, 5'-TGGTGGTTTCCSCATTGCTA-3' and reverse,
5'-ACTCATCCCACCCCACAATA-3' to detect the binding capacity of p65 to
the potential NF-κB element; ii) detection of the DDAH2 promoter
region without any potential NF-κB element was used as a negative
control with the following pair of primers: Forward,
5'-GGGTGGGTCAGTGATCTTGA-3' and reverse, 5'-TAGACCTCAGAACAGCGCAA-3';
and iii) a previously identified NF-κB element within the nNOS
promoter (19) was used as a
positive control, using the following primers: Forward,
5'-GCAAGACGATCTGAAAAGCA-3' and reverse, 5'-CTGGCTCTGGGTGATTTGAT-3'.
Thermocycling conditions were 95˚C for 3 min followed by 95˚C for
10 sec and 61˚C for 60 sec for 40 cycles. The enrichment of
immunoprecipitated DNA was calculated relative to the input DNA
using the 2-ΔΔCq method (24).
Statistical analysis
All experiments were performed at least three times.
Results are expressed as the mean ± standard deviation from three
independent experiments. SPSS 22.0 (IBM Corp.) was used for
statistical analyses. P<0.05 was considered to indicate a
statistically significant difference. Differences between DDAH2
mRNA and protein expression levels were analyzed using one-way
ANOVA with Tukey's multiple comparison post hoc test. Luciferase
activity and ChIP results were analyzed using two-way ANOVA with
Sidak correction and Tukey's multiple comparison post hoc test. The
levels of NF-κB acetylation between two groups were compared using
an unpaired two-tailed Student's t-test.
Results
TSA upregulates DDAH2 expression
TSA is a deacetylase inhibitor that can induce the
acetylation of both histones and non-histone proteins (25). Therefore, 293 cells were treated
with TSA to evaluate the effect of acetylation on DDAH2 expression.
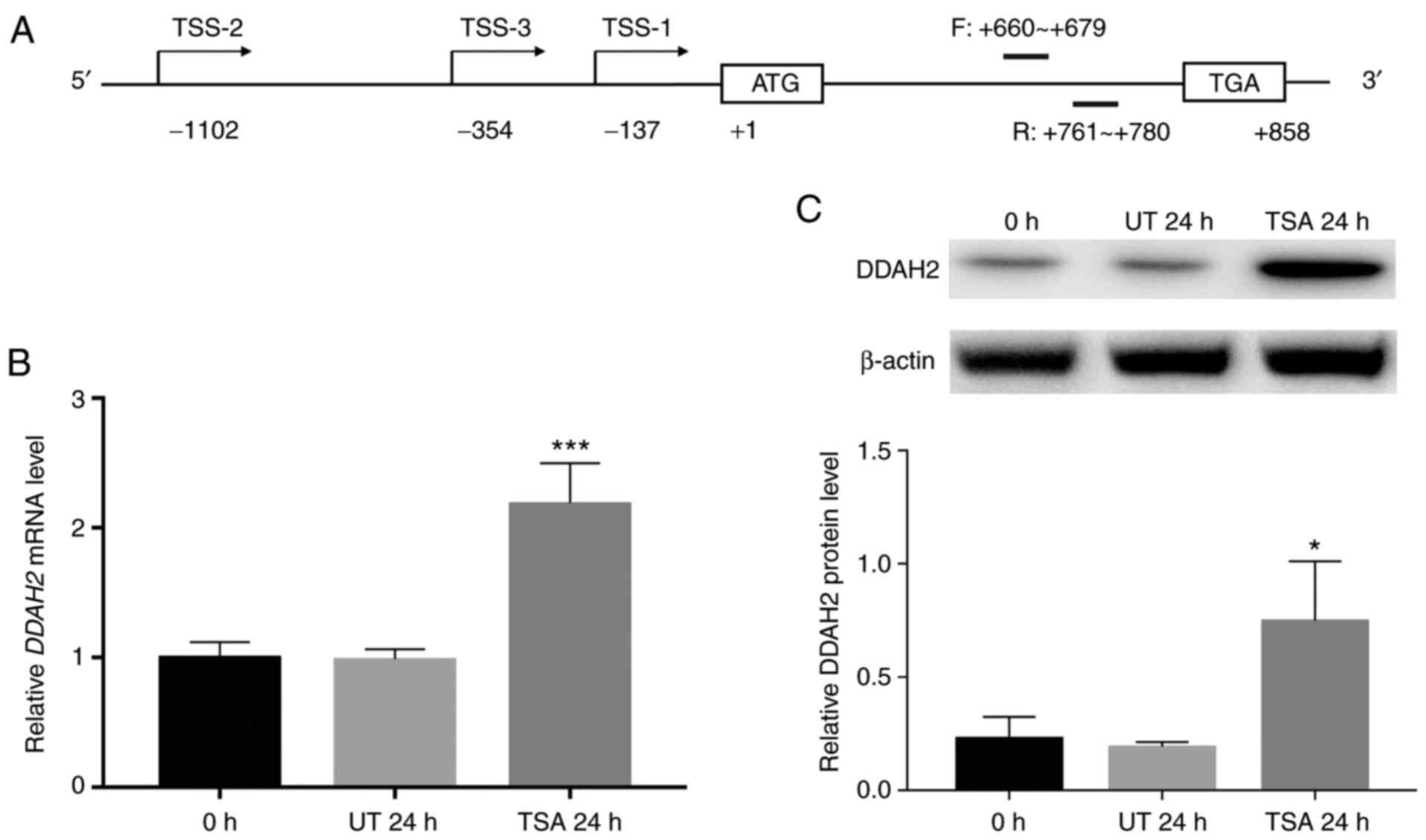
A total of three transcripts of the human DDAH2 gene were
identified, each with different transcription start sites (Fig. 1A). However, all transcripts share
the same 858-bp open reading frame encoding a 285-amino acid
protein. Therefore, qPCR assays were performed to evaluate the
effect of TSA on total DDAH2 mRNA using a pair of intron-spanning
primers targeting the coding sequence (Fig. 1A). Compared with that in the
untreated control cells, DDAH2 mRNA was significantly increased, by
2.2-fold that of untreated control following treatment with TSA for
24 h (Fig. 1B).
Western blotting results showed that in TSA-treated
cells, DDAH2 protein levels were also significantly upregulated, by
3.2-fold that of untreated control (Fig. 1C).
DDAH2 promoter activity is upregulated
by TSA through an NF-κB responsive element
NF-κB is involved in several cellular functions,
including the initiation and propagation of inflammatory and immune
responses, which have also been reported to serve critical roles in
the kidney (26,27). In addition, it was previously shown
that acetylation regulates nNOS gene expression via NF-κB (19). Therefore, the present study aimed to
investigate whether the TSA-induced DDAH2 expression was also
NF-κB-dependent, upstream of the strong effects on NO synthesis.
Therefore, a 2-kbp DDAH2 sequence upstream of the start codon (from
-1983 to +17) was analyzed using bioinformatics tools. The analysis
predicted a potential NF-κB binding site located at -1582 to -1573
(Fig. S1; produced using Alibaba-2.1). This site lies upstream of
the transcription start sites in all three transcripts, indicating
its importance in regulating DDAH2 transcription activation.
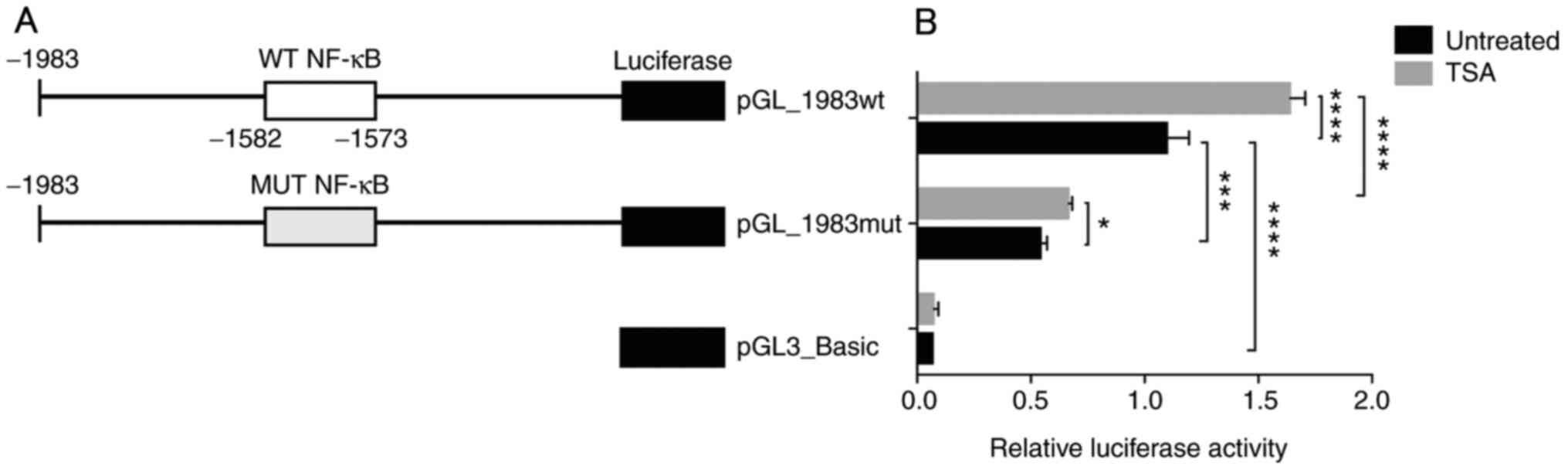
To verify the role of this potential NF-κB element,
luciferase reporter vectors of the DDAH2 promoter were constructed,
containing either WT NF-κB or MUT NF-κB element. The MUT NF-κB
element was produced by changing the two corresponding G and A
residues to T (GGGCAGGTAT). These vectors were subsequently named
pGL_1983wt and pGL_1983mut (Fig.
2A). Alibaba-2.1 analysis revealed that the potential NF-κB
element was abolished, showing no NF-κB binding after the
substitution (Fig. S2). Furthermore, as shown in Fig. 2B, luciferase assay results
demonstrated that under basal conditions, pGL_1983wt exhibited
increased transcriptional activity compared with that by
pGL3_Basic. However, mutation in the NF-κB site (pGL_1983mut)
markedly reduced this activity compared with pGL_1983wt in
untreated cells. Treatment with TSA significantly increased the
activity of pGL_1983wt compared with that of untreated control, by
1.6-fold. Also, after TSA treatment, luciferase activity from the
pGL_1983mut plasmid was significantly lower compared with that
exhibited by pGL_1983wt. However, TSA treatment exhibited a much
less obvious although significant effect (1.2-fold untreated
control) on the activity of the pGL1983_mut plasmid. These results
suggested that the potential NF-κB element could serve a role in
the transactivation of the DDAH2 promoter, such that the
TSA-mediated induction of DDAH2 expression was at least partially
promoted by this element.
NF-κB binding affinity to DDAH2
promoter is enhanced by TSA-induced NF-κB acetylation
It has been previously reported that NF-κB
acetylation is associated with several renal diseases. Enhanced
NF-κB acetylation was found in diabetic nephropathy (28,29)
and cisplatin-induced p65 acetylation resulted in renal proximal
tubule cell injury (30).
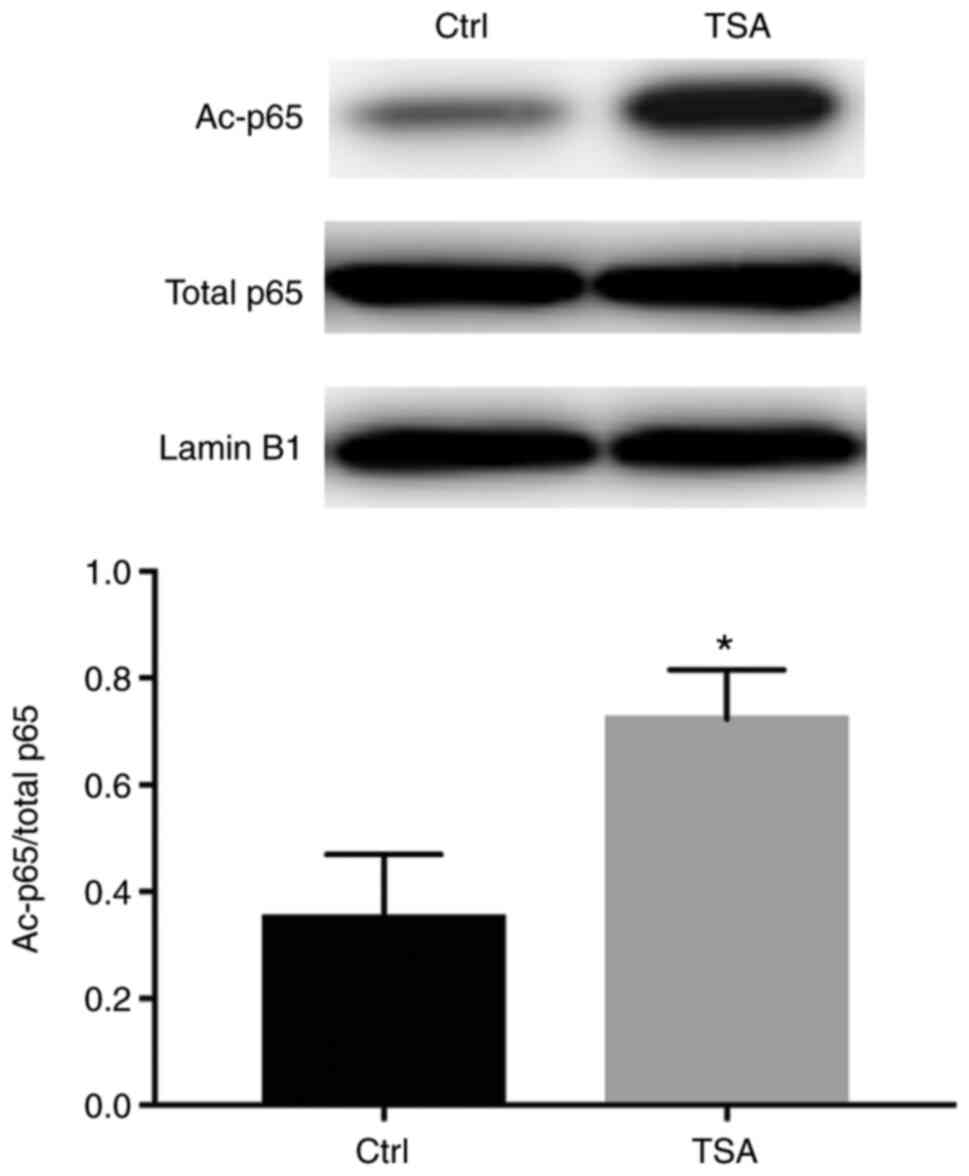
Therefore, the acetylation of NF-κB was assessed in 293 cell
lysates. Nuclear extracts were immunoprecipitated using a
p65-specific antibody, where acetylated protein was detected by
western blotting using a monoclonal antibody specific for
acetylated lysine. The results showed that under basal conditions,
p65 was acetylated, which was significantly increased following TSA
treatment, without any notable effects on total p65 protein levels
(Fig. 3).
The acetylation status of the transcription factors
can influence their DNA binding affinity (19). To reveal the mechanism underlying
the effect of TSA on DDAH2 upregulation in its NF-κB element (from
-1582 to -1573), the binding affinity of NF-κB to the DDAH2
response element was assessed in vitro using EMSA. WT probe
(lane 3) formed a complex with the nuclear extracts isolated from
293 cells, in a similar manner to that of the consensus probe (lane
2; Fig. 4A). Following
site-directed mutagenesis on the NF-κB element by changing the two
corresponding G and A residues to T (MUT probe;
5'-GGGCAGGTATTCCTGGAG-3'), binding ability with nuclear extracts
from 293 cells disappeared (lane 6). The WT probe-nuclear extract
complex was found to be displaced by unlabeled WT competitors (lane
4) but not with unlabeled mutant competitors (lane 5). In addition,
the binding ability of NF-κB to its responsive element (WT probe)
was blocked by a p65-specific antibody, indicating when the nuclear
extract was pre-incubated with the antibody, p65 bound to its
specific antibody, which blocked its binding to the probe. Whereas,
when a normal IgG was used (Fig.
4B), no evident blocking effect was seen, confirming the
specificity of NF-κB binding. Notable, binding was markedly
enhanced in TSA-treated cells (Fig.
4C).
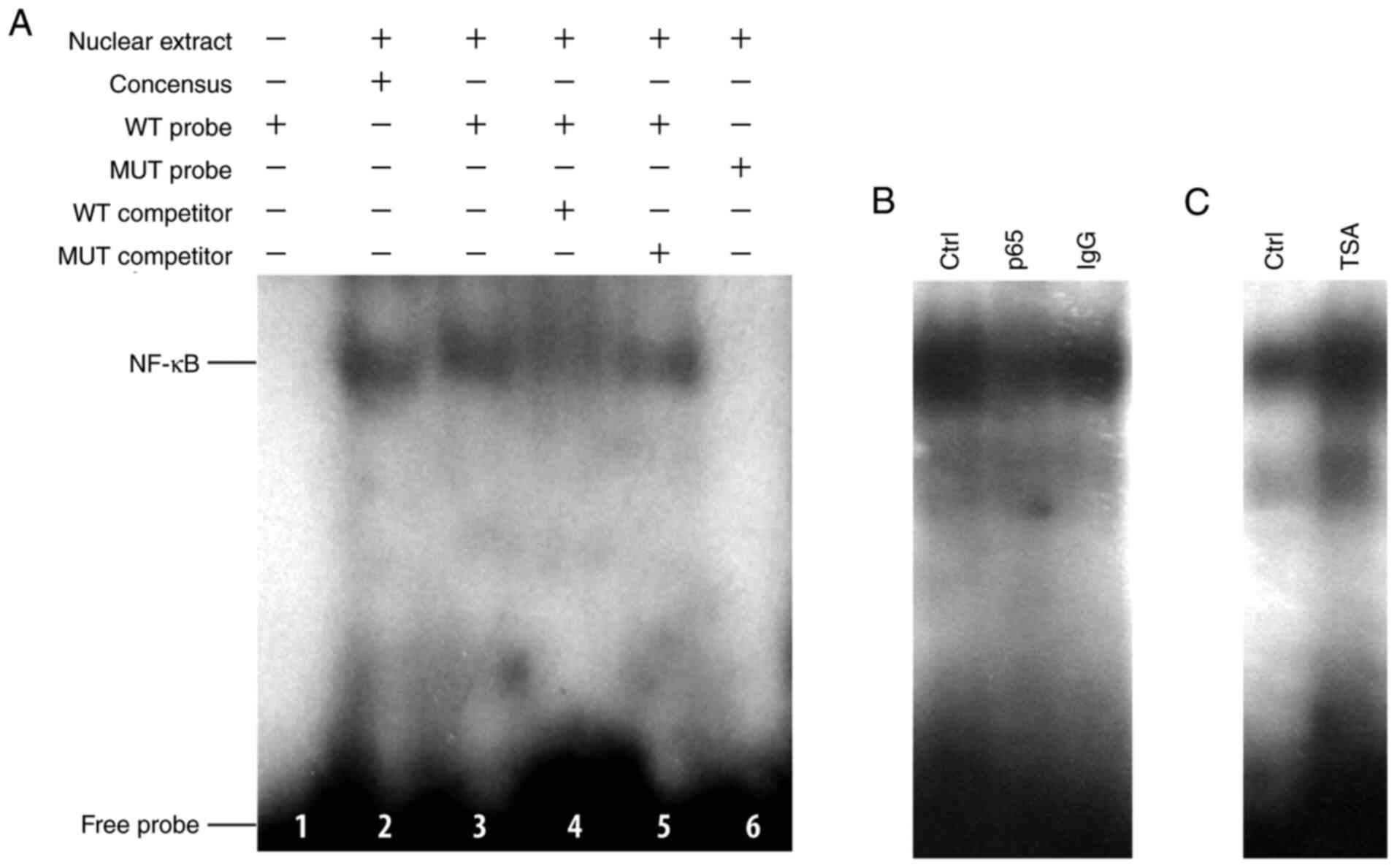 | Figure 4TSA increases the binding of NF-κB to
its responsive element within DDAH2 promoter in vitro by
electrophoresis mobility shift assay. (A) Identification of NF-κB
binding to the DDAH2 promoter. Lane 1, WT probe without nuclear
extract; lane 2, binding of the consensus probe to the nuclear
extract; lanes 3 and 6, binding of WT and MUT probes to the nuclear
extract, respectively; lanes 4 and 5, effect of WT or MUT unlabeled
competitor on probe binding. (B) Blocking of NF-κB binding to WT
probe using the p65 antibody. (C) Effect of TSA treatment on the
binding of NF-κB. DDAH2, dimethylarginine dimethylaminohydrolase 2;
TSA, trichostatin A; WT, wild-type; MUT, mutant; ctrl, control. |
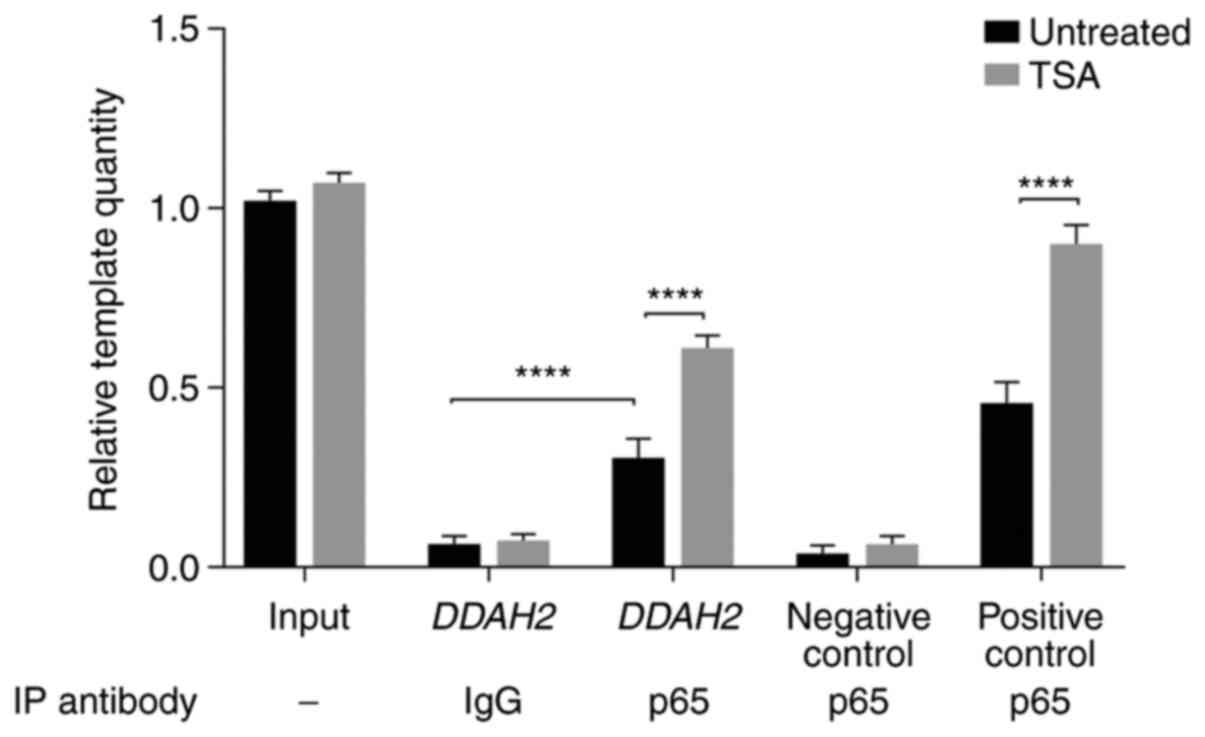
Furthermore, ChIP assay was subsequently performed
to measure NF-κB binding affinity to the DDAH2 promoter. Chromatin
from untreated and TSA-treated cells was immunoprecipitated using
the p65 antibody. A DDAH2 promoter fragment containing the NF-κB
element was amplified by qPCR, whilst DDAH2 promoter fragments
without any potential NF-κB elements and a nNOS gene promoter
region with an NF-κB element (19)
served as negative and positive controls, respectively. The results
showed that p65 specifically bound to the DDAH2 promoter
encompassing the WT NF-κB element, whilst immunoprecipitation using
normal IgG did not show evident binding (Fig. 5). This was demonstrated by the
significantly increased number of DDAH2 promoter templates in p65
antibody-incubated samples compared with those in IgG-incubated
samples prior to TSA treatment (Fig.
5). In addition, TSA significantly augmented p65 binding to the
NF-κB responsive element within the DDAH2 promoter (Fig. 5). These findings indicated that
NF-κB could specifically bind to the NF-κB responsive element
located at the -1582 to -1573 region of the DDAH2 promoter, such
that p65 acetylation significantly enhanced this binding affinity
to this element.
Discussion
The present study demonstrated that DDAH2 mRNA and
protein expression was upregulated in renal cells following NF-κB
acetylation by enhancing its binding to an NF-κB element upstream
of all three DDAH2 transcripts, resulting in increased promoter
activity.
Reversible protein acetylation has been implicated
in the transcriptional regulation of numerous genes. Histone
acetylation was first shown to affect gene expression by modifying
the degree of chromatin condensation (31,32). A
growing number of non-histone proteins, the majority of which are
involved in transcriptional regulation, were rapidly revealed to
undergo acetylation, including p53, Tat and NF-κB (15,33-35).
Histone acetyltransferases (HATs) and histone deacetylases (HDACs)
are involved in regulating the protein acetylation status (36). The balance between these two classes
of enzymes is crucial in maintaining appropriate protein
acetylation homeostasis. It has been reported that TSA specifically
inhibits HDAC activity and recruits HATs in gene transcription
processes, leading to the acetylation of histones and certain
transcription factors (16,25). Therefore, TSA has entered clinical
trials for treating certain diseases, such as such as cancer and
nerve degradation after brain injury (25,37).
Therefore, these aforementioned previous findings support the
present findings that TSA-induced acetylation increased both DDAH2
mRNA and protein expression levels in 293 cells, which was in
accordance with the reported effects of TSA on DDAH2 expression in
trophoblasts (12).
NF-κB is an ubiquitously-expressed transcription
factor that is involved in numerous processes in the kidney,
including inflammation, endothelial function and cellular
transformation (38). Therefore,
the NF-κB signaling pathway has become a potential target for
treatment of certain diseases. For example, NF-κB silencing in
endothelial cells inhibited a signaling cascade leading to reduced
hypertension-induced renal damage (39). It was previously shown that the
acetyltransferase p300 complexes with NF-κB as a coactivator to
acetylate NF-κB as a HAT, resulting in increased acetylation of
NF-κB p65 and p50 subunits, in turn activating the nNOS exon 1f
promoter (13,19). Deng and Wu (20) previously reported that p300 could
acetylate the p50 subunit of NF-κB, thereby increasing NF-κB
binding affinity and NF-κB-mediated transactivation, which is
essential for iNOS transcription. These findings indicate the
importance of NF-κB acetylation in NO modulation. Therefore, to
elucidate the mechanism underlying the acetylation-regulated DDAH2
expression in renal cells, the present study focused on the role of
NF-κB in this process. DDAH2 is transcribed into three transcripts
due to three different transcription start sites. A number of
studies have shown that certain polymorphisms within the DDAH2 core
promoter can affect its basal transcription (40,41).
However, the structure of the DDAH2 promoter has not been fully
elucidated. As a result, the 5'-region upstream sequences of all
three of the transcripts were analyzed, where the analysis revealed
a number of potential responsive elements of certain transcription
factors, including CCAAT-enhancer-binding protein, GATA-binding
factor 1, olfactory neuronal transcription factor 1, activating
protein 2, specificity protein 1, interferon consensus
sequence-binding protein, NF-κB, retinoid X receptor, ETS Like-1
protein, Yin Yang 1, nuclear factor-1, helix-loop-helix
transcription factors E47, myoblast determination protein 1, serum
response factor and cytoplasmic polyadenylation elements binding
protein (data not shown). Among them, a potential NF-κB site was
located at the -1582 to -1573 region. EMSA and ChIP assays
demonstrated that in 293 cells, p65 could specifically bind to the
NF-κB response element site. In particular, mutations in the
binding site blocked the binding of p65, markedly decreasing the
promoter activity, suggesting a role of the NF-κB responsive
element in regulating DDAH2 expression.
In addition, the present study showed that TSA
treatment evidently induced the acetylation of NF-κB p65 subunit.
It has been previously suggested that increased acetylation of
transcription factors may affect their subcellular distribution,
stability, DNA binding affinity and transcriptional activity
(42). Therefore, the effect of
acetylation on the DNA binding ability of NF-κB and DDAH2
transactivation was investigated in the present study. EMSA and
ChIP assays showed that p65 acetylation increased its binding
ability to the DDAH2 promoter. Additionally, luciferase assays
demonstrated that the promoter activity was increased by
acetylation. However, luciferase activity was attenuated following
mutations in the NF-κB responsive element, supporting the role of
NF-κB acetylation in DDAH2 transactivation through its enhanced
binding capacity. The results of the present study were in
accordance with findings from a previous study showing that the
cAMP response element-binding protein/p300-dependent acetylation of
p65 at lys310 was associated with NF-κB transcriptional activation
in activated B cells (43). The
possibility that the TSA-induced histone acetylation could also
affect NF-κB binding should not be completely excluded. However,
this effect was found to be at least partially mediated by its
induction through NF-κB acetylation. Furthermore, a minor increase
in the activity of the DDAH2 promoter harboring mutations in the
NF-κB binding sites was observed in TSA-treated cells compared with
that in the untreated control cells. This observation could result
from the effect of other acetylated transcription factors, which
could also bind to the DDAH2 promoter. Interestingly, following TSA
treatment, changes in the activity of the DDAH2 promoter (1.6-fold
to control), mRNA expression (2.2-fold to control) and protein
levels (3.2-fold to control) were not completely parallel. This
effect could be a result of post-transcriptional and
post-translational regulation of DDAH2, such as the mRNA and
protein degradation rates and translational efficiency. Although
these regulatory mechanisms have not been previously reported in
DDAH2, DDAH1 has been shown to be subject to post-transcriptional
regulation through its 3' untranslated region (44).
NO deficiency in the kidney is commonly observed in
renal diseases, including diabetic and hypertensive nephropathy,
obstructive nephropathy and glomerulosclerosis (35,45).
In addition, NO deficiency has been proposed to be the main
mechanism of systemic hypertension (45,46).
NO insufficiency may be caused by the decreased abundance and
activity of NOS and increase in the levels of the endogenous NOS
inhibitor ADMA (47,48). As aforementioned, NF-κB acetylation
is involved in NOS regulation (19,20).
Taken together, the aforementioned findings indicated that NF-κB
acetylation could modulate both NOS and DDAH systems, supporting
its role in NO synthesis and providing the molecular basis for
treating NO-related diseases by modulating NF-κB acetylation. The
possibility of treating certain renal disorders by changing the
NF-κB acetylation status has been previously discussed. Increased
acetylation of p65 was observed in diabetic nephropathy and
cisplatin-induced nephrotoxicity during chemotherapy in patients
with cancer. Furthermore, overexpression of restored Sirtuin-1
deacetylase ameliorated the increased acetylation of p65, which
attenuated renal cell damage and kidney injury (29,30).
However, Staab et al (49)
reported that treatment with exogenous ADMA enhanced cigarette
smoke-mediated NF-κB binding activity, indicating that the
DDAH/ADMA axis could in turn affect NF-κB signaling. Therefore,
their reciprocal effects need further investigation prior their
application as a therapeutic strategy for certain diseases.
A limitation of the current study was that although
the immortalized 293 cell line derived from human embryonic kidney
has been extensively used as models of human renal cells in in
vitro studies, some evidence in the literature shows that 293
cells display genotypic and phenotypic characteristics that differ
substantially from primary kidney cells (50,51).
Therefore, the current results need to be further confirmed on
renal cell lines, such as HK-2 and podocytes as well.
Overall, the present study demonstrated that NF-κB
acetylation could upregulate its binding affinity to the DDAH2
promoter, thus resulting in the augmentation of its transcriptional
activity and DDAH2 expression in renal cells. These findings
provided a possible mechanism underlying the regulation of NO
production in renal cells and a potential target for treating
certain NO-related renal disorders.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science
Foundation of Liaoning Province (grant no. 2014021061) and National
Natural Science Foundation of China (grant no. 30900807).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and LS performed all the experiments and analyzed
the data. YL designed the study, supervised performance,
interpreted the data and wrote and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zou AP and Cowley AW Jr: Role of nitric
oxide in the control of renal function and salt sensitivity. Curr
Hypertens Rep. 1:178–186. 1999.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Monzon CM and Garvin JL: Nitric oxide
decreases the permselectivity of the paracellular pathway in thick
ascending limbs. Hypertension. 65:1245–1250. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Garcia NH, Stoos BA, Carretero OA and
Garvin JL: Mechanism of the nitric oxide-induced blockade of
collecting duct water permeability. Hypertension. 27:679–683.
1996.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tain YL and Hsu CN: Toxic
dimethylarginines: Asymmetric dimethylarginine (ADMA) and symmetric
dimethylarginine (SDMA). Toxins (Basel). 9(92)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jayachandran I, Sundararajan S,
Paramasivam P, Venkatesan B, Subramanian SC, Balasubramanyam M,
Mohan V and Manickam N: Association of circulatory asymmetric
dimethylarginine (ADMA) with diabetic nephropathy in Asian Indians
and its causative role in renal cell injury. Clinical Biochem.
50:835–842. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Palm F, Onozato ML, Luo Z and Wilcox CS:
Dimethylarginine dimethylaminohydrolase (DDAH): Expression,
regulation, and function in the cardiovascular and renal systems.
Am J Physiol Heart Circ Physiol. 293:H3227–H3245. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tojo A, Welch WJ, Bremer V, Kimoto M,
Kimura K, Omata M, Ogawa T, Vallance P and Wilcox CS:
Colocalization of demethylating enzymes and NOS and functional
effects of methylarginines in rat kidney. Kidney Int. 52:1593–1601.
1997.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nijveldt RJ, Teerlink T, Siroen MP, van
Lambalgen AA, Rauwerda JA and van Leeuwen PA: The liver is an
important organ in the metabolism of asymmetrical dimethylarginine
(ADMA). Clin Nutr. 22:17–22. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Förstermann U and Sessa WC: Nitric oxide
synthases: Regulation and function. Eur Heart J. 33:829–37.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang Z, Zhu LL, Jiang HS, Chen H, Chen Y
and Dai YT: Demethylation treatment restores erectile function in a
rat model of hyperhomocysteinemia. Asian J Androl. 18:763–768.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang JG, Liu JX, Li ZH, Wang LZ, Jiang YD
and Wang SR: Dysfunction of endothelial NO system originated from
homocysteine-induced aberrant methylation pattern in promoter
region of DDAH2 gene. Chin Med J. 120:2132–7. 2007.PubMed/NCBI
|
|
12
|
Tomikawa J, Fukatsu K, Tanaka S and Shiota
K: DNA methylation-dependent epigenetic regulation of
dimethylarginine dimethylaminohydrolase 2 gene in trophoblast cell
lineage. J Biol Chem. 281:12163–12169. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li Y, Li C, Sun L, Chu G, Li J, Chen F, Li
G and Zhao Y: Role of p300 in regulating neuronal nitric oxide
synthase gene expression through nuclear factor-κB-mediated way in
neuronal cells. Neuroscience. 248:681–689. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen J, Zhang J, Shaik NF, Yi B, Wei X,
Yang XF, Naik UP, Summer R, Yan G, Xu X and Sun J: The histone
deacetylase inhibitor tubacin mitigates endothelial dysfunction by
up-regulating the expression of endothelial nitric oxide synthase.
J Biol Chem. 294:19565–19576. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen L, Fischle W, Verdin E and Greene WC:
Duration of nuclear NF-kappaB action regulated by reversible
acetylation. Science. 293:1653–1657. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Huang W, Zhao S, Ammanamanchi S, Brattain
M, Venkatasubbarao K and Freeman JW: Trichostatin A induces
transforming growth factor beta type II receptor promoter activity
and acetylation of Sp1 by recruitment of PCAF/p300 to a Sp1.NF-Y
complex. J Biol Chem. 280:10047–10054. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Izumi H, Ohta R, Nagatani G, Ise T,
Nakayama Y, Nomoto M and Kohno K: p300/CBP-associated factor
(P/CAF) interacts with nuclear respiratory factor-1 to regulate the
UDP-N-acetyl-alpha-d-galactosamine: Polypeptide
N-acetylgalactosaminyltransferase-3 gene. Biochem J. 373:713–722.
2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee JS, Galvin KM, See RH, Eckner R,
Livingston D, Moran E and Shi Y: Relief of YY1 transcriptional
repression by adenovirus E1A is mediated by E1A-associated protein
p300. Genes Dev. 9:1188–1198. 1995.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Y, Zhao Y, Li G, Wang J, Li T, Li W and
Lu J: Regulation of neuronal nitric oxide synthase exon 1f gene
expression by nuclear factor-kappaB acetylation in human
neuroblastoma cells. J Neurochem. 101:1194–1204. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Deng WG and Wu KK: Regulation of inducible
nitric oxide synthase expression by p300 and p50 acetylation. J
Immunol. 171:6581–6588. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Korneluk RG, Quan F and Gravel RA: Rapid
and reliable dideoxy sequencing of double-stranded DNA. Gene.
40:317–323. 1985.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sengüven B, Baris E, Oygur T and Berktas
M: Comparison of methods for the extraction of DNA from
formalin-fixed, paraffin-embedded archival tissues. Int J Med Sci.
11:494–499. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Haring M, Offermann S, Danker T, Horst I,
Peterhansel C and Stam M: Chromatin immunoprecipitation:
Optimization, quantitative analysis and data normalization. Plant
Methods. 3(11)2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yoshida M, Kijima M, Akita M and Beppu T:
Potent and specific inhibition of mammalian histone deacetylase
both in vivo and in vitro by trichostatin A. J Biol Chem.
265:17174–17179. 1990.PubMed/NCBI
|
|
26
|
Guijarro C and Egido J: Transcription
factor-kappa B (NF-kappa B) and renal disease. Kidney Int.
59:415–424. 2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Queisser N and Schupp N: Aldosterone,
oxidative stress, and NF-κB activation in hypertension-related
cardiovascular and renal diseases. Free Radic Biol Med. 53:314–327.
2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shang G, Gao P, Zhao Z, Chen Q, Jiang T,
Zhang N and Li H: 3,5-Diiodo-l-thyronine ameliorates diabetic
nephropathy in streptozotocin-induced diabetic rats. Biochim
Biophys Acta. 1832:674–684. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu R, Zhong Y, Li X, Chen H, Jim B, Zhou
MM, Chuang PY and He JC: Role of transcription factor acetylation
in diabetic kidney disease. Diabetes. 63:2440–2453. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jung YJ, Lee JE, Lee AS, Kang KP, Lee S,
Park SK, Lee SY, Han MK, Kim DH and Kim W: SIRT1 overexpression
decreases cisplatin-induced acetylation of NF-κB p65 subunit and
cytotoxicity in renal proximal tubule cells. Biochem Biophys Res
Commun. 419:206–210. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Turner BM, Birley AJ and Lavender J:
Histone H4 isoforms acetylated at specific lysine residues define
individual chromosomes and chromatin domains in Drosophila polytene
nuclei. Cell. 69:375–384. 1992.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Turner BM and Fellows G: Specific
antibodies reveal ordered and cell-cycle-related use of histone-H4
acetylation sites in mammalian cells. Eur J Biochem. 179:131–139.
1989.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Verdin E and Ott M: 50 years of protein
acetylation: From gene regulation to epigenetics, metabolism and
beyond. Nat Rev Mol Cell Biol. 16:258–264. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Gu W and Roeder RG: Activation of p53
sequence-specific DNA binding by acetylation of the p53 C-terminal
domain. Cell. 90:595–606. 1997.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kiernan RE, Vanhulle C, Schiltz L, Adam E,
Xiao H, Maudoux F, Calomme C, Burny A, Nakatani Y, Jeang KT, et al:
HIV-1 tat transcriptional activity is regulated by acetylation.
EMBO J. 18:6106–6118. 1999.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang XJ and Seto E: HATs and HDACs: From
structure, function and regulation to novel strategies for therapy
and prevention. Oncogene. 26:5310–5318. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Huynh NC, Everts V and Ampornaramveth RS:
Histone deacetylases and their roles in mineralized tissue
regeneration. Bone Rep. 7:33–40. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang H and Sun SC: NF-κB in inflammation
and renal diseases. Cell Biosci. 5(63)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Henke N, Schmidt-Ullrich R, Dechend R,
Park JK, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft FC, et
al: Vascular endothelial cell-specific NF-kappaB suppression
attenuates hypertension-induced renal damage. Circ Res.
101:268–276. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jones LC, Tran CT, Leiper JM, Hingorani AD
and Vallance P: Common genetic variation in a basal promoter
element alters DDAH2 expression in endothelial cells. Biochem
Biophys Res Commun. 310:836–843. 2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Andreozzi F, Presta I, Mannino GC,
Scarpelli D, Di Silvestre S, Di Pietro N, Succurro E, Sciacqua A,
Pandolfi A, Consoli A, et al: A functional variant of the
dimethylarginine dimethylaminohydrolase-2 gene is associated with
insulin sensitivity. PLoS One. 7(e36224)2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Park JM, Jo SH, Kim MY, Kim TH and Ahn YH:
Role of transcription factor acetylation in the regulation of
metabolic homeostasis. Protein Cell. 6:804–813. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ma Z, Chalkley RJ and Vosseller K:
Hyper-O-GlcNAcylation activates nuclear factor
κ-light-chain-enhancer of activated B cells (NF-κB) signaling
through interplay with phosphorylation and acetylation. J Biol
Chem. 292:9150–9163. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Balasubramanian V, Mehta G, Jones H,
Sharma V, Davies NA, Jalan R and Mookerjee RP: Post-transcriptional
regulation of hepatic DDAH1 with TNF blockade leads to improved
eNOS function and reduced portal pressure in cirrhotic rats. Sci
Rep. 7(17900)2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hsu CN and Tain YL: Regulation of nitric
oxide production in the developmental programming of hypertension
and kidney disease. Int J Mol Sci. 20(681)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ahmad A, Dempsey SK, Daneva Z, Azam M, Li
N, Li PL and Ritter JK: Role of Nitric Oxide in the Cardiovascular
and Renal Systems. Int J Mo Sci. 19(2605)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lin HH, Lee TS, Lin SJ, Yeh YC, Lu TM and
Hsu CP: DDAH-2 alleviates contrast medium iopromide-induced acute
kidney injury through nitric oxide synthase. Clin Sci (Lond).
133:2361–2378. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wagner L, Riggleman A, Erdely A, Couser W
and Baylis C: Reduced nitric oxide synthase activity in rats with
chronic renal disease due to glomerulonephritis. Kidney Int.
62:532–536. 2002.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Staab EB, Weigel J, Xiao F, Madayiputhiya
N, Wyatt TA and Wells SM: Asymmetric dimethyl-arginine metabolism
in a murine model of cigarette smoke-mediated lung inflammation. J
Immunotoxicol. 12:273–282. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lin YC, Boone M, Meuris L, Lemmens I, Van
Roy N, Soete A, Reumers J, Moisse M, Plaisance S, Drmanac R, et al:
Genome dynamics of the human embryonic kidney 293 lineage in
response to cell biology manipulations. Nat Commun.
5(4767)2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Stepanenko AA and Dmitrenko VV: HEK293 in
cell biology and cancer research: Phenotype, karyotype,
tumorigenicity, and stress-induced genome-phenotype evolution.
Gene. 569:182–190. 2015.PubMed/NCBI View Article : Google Scholar
|