Introduction
Heatstroke is characterised by a core body
temperature >40˚C and central nervous system dysfunction
resulting in delirium, convulsions or coma (1). Heatstroke is a life-threatening
condition that is often accompanied by organ injury and a poor
prognosis including sequela or even mortality (2). Acute kidney injury (AKI) is a common
complication of heatstroke. Increased pathogenesis of
heatstroke-associated AKI is likely due to decreased perfusion
caused by dehydration and subsequent hypovolemia, direct thermal
injury, rhabdomyolysis-associated myoglobinuria and systemic
inflammatory response syndrome (3).
In addition, the release of inflammatory factors and direct heat
damage can induce apoptosis in cells, as observed in a baboon
animal model (4) of heatstroke
(5).
Currently, the pathologic process responsible for
tissue and cell damage induced by heatstroke is not clearly
understood. In addition, no effective clinical methods have been
developed for early diagnosis, and affordable treatment options are
also lacking, resulting in elevated rates of mortality in patients
with heatstroke.
Heat is one of the most influential external factors
that affects cellular function and structure (6). Rapidly increasing ambient temperatures
can lead to extensive cell degeneration and necrosis in tissues,
and when core body temperatures exceed 41.6-42°C for
>45 min, cells undergo apoptosis (7). These extreme temperatures can induce
destruction of cell structures and necrosis within minutes
(8). Sakaguchi et al
(9) demonstrated that exposure of a
rat model of heat shock to temperatures of 41.5°C for 2
h induced apoptosis in healthy tissue cells. Therefore, cellular
apoptosis may be one of the mechanisms responsible for the
development of renal injury induced by heatstroke. Therefore, it is
important to identify compounds that are capable of reversing or
inhibiting these apoptotic pathways, thereby limiting AKI in
patients with heatstroke.
Curcumin is a phenol that is extracted from
turmeric. Studies have demonstrated that curcumin exhibits a wide
range of biological functions, including inhibition of cell
proliferation, antioxidant properties, anti-apoptotic effects and
scavenging of oxygen free radicals (10-12).
In addition, multiple studies have demonstrated a protective effect
of curcumin on AKI induced by a number of different factors
including toxic drugs (13-15).
It can therefore be hypothesised that curcumin
exhibits a protective effect in renal injury caused by heat stress.
In the current study, the previously described dry-heat environment
protocol was used to establish a rat model of heatstroke, determine
the effect of curcumin pre-treatment on renal pathological changes
and explore the possible underlying mechanism governing this
interaction.
Materials and methods
Animals
A total of 50 male Sprague–Dawley (SD) rats (65-70
days old; 190-220 g) were purchased from the Experimental Animal
Center of Xinjiang Medical University. Animals were housed in cages
in groups (five rats per cage) at 20±2°C and 40-50%
humidity with a 12 h light/dark cycle. The rats were fed a standard
pellet diet and provided with water ad libitum. The present
study was approved by the ethical committee of the General Hospital
of Xinjiang Military Region of the PLA. Animal care and experiments
were conducted according to the National Science Council
guidelines.
Establishment of a rat model of
heatstroke, curcumin pre-treatment and collection of blood, urine
and kidney tissue samples
The 50 SD rats were randomly divided into five
groups (n=10): Normal temperature control (NT control), dry-heat
control (DH control), and curcumin treatment groups including 50,
100 and 200 mg/kg groups, the curcumin concentrations were based on
the previous relevant research (16,17).
The control rats were pretreated with 0.9% saline by gavage while
experimental rats were administered curcumin orally. All rats were
pretreated once a day for seven consecutive days. Curcumin was
dissolved in 0.5% sodium carboxymethyl cellulose (CMCNa) solution
prior to administration.
The dry-heat heatstroke rat model was replicated
after 7 days of pre-treatment from our previous study (18). The NT control group rats were
incubated at room temperature (20±2°C) with a humidity
of 40-50%. The remaining rats were incubated in the dry-heat
environment (The Simulated Climate Cabin for Special Environment of
Northwest of China, Urumqi, China) at a temperature of
41°C±0.5°C and 10±1% humidity. The rat core
body temperature was monitored (using a thermometer to measure
rectal body temperature) every 30 min. The rats were removed from
the experimental cabin following 150 min of incubation and were
anesthetized by intraperitoneal injection of 3% sodium
pentobarbital 0.2 ml/100 g (30 mg/kgb). After the animals were
anesthetized, blood was collected from the inferior vena cava for
analysis of blood indicators, and urine was collected by puncturing
the bladder to assess the renal injury index. Renal tissue was
stored at -80°C for subsequent analysis. After the
specimen was extracted, the rats were euthanized using cervical
dislocation.
Biochemical analysis
Serum was separated via centrifugation at 1,006.2 x
g for 10 min at 4˚C and stored at -20°C for analyses of
creatinine and blood urea nitrogen (BUN) levels using a fully
automatic biochemical analyser (Mindray BS-180; Shenzhen Mindray
Bio-Medical Electronics Co., Ltd.).
Measurement of kidney injury
molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin
(NGAL) levels in urine
The expression of KIM-1 (cat. no. MKM100) and NGAL
(cat. no. MLCN20) in urine were quantified using commercial ELISA
kits (R&D Systems, Inc.) according to the manufacturer's
instructions.
Western blot analysis
The kidney samples were ground with liquid nitrogen
followed by lysis with a Cell Lysis Buffer (cat. no. ab152163;
Abcam) for 2 h on ice. The lysates were centrifuged at 4,024.8 x g
for 20 min at 4˚C, and supernatants were collected and stored at
-80°C in Eppendorf tubes. Samples were mixed with 2X
loading buffer (Abcam) and boiled for 8 min before they were
subjected to electrophoresis. Protein samples (120 ng) were
quantified using a BCA protein assay kit (Pierce™ BCA
Protein Assay kit; Thermo Fisher Scientific, Inc.). Electrophoresis
was then performed at 60 V (5% stacking gel) and then at 100 V (10%
separating gel) for 1.5 h, and electrotransferred for 20-30 min
according to different molecular weights (sample amount, 0.5 µg).
The PVDF membrane was blocked using 5% non-fat milk powder for 2 h
at room temperature, followed by incubation with the following
primary antibodies: Cytochrome c, cat. no. 4272; JNK, cat.
no. 9252; caspase-9, cat. no. 9504 and caspase-3, cat. no. 9662;
all from Cell Signaling Technology, Inc.) at 4˚C overnight.
Secondary antibodies (goat anti-rabbit IgG H&L, cat. no.
ab6721; rabbit anti-mouse IgG H&L, cat. no. ab6728; both from
Abcam) were then added, and the membrane was incubated at room
temperature for 1 h. The target band was detected by
chemiluminescence (ChemDoc-IT® 510 Imager, Ultra-Violet
Products Ltd.), and the protein was semi-quantified using
Visionworks LS (version 8.1.2; Ultra-Violet Products Ltd.)
following analysis of the grey intensity.
TUNEL staining for detection of
apoptotic cells
Apoptotic cells (fixed in 10% methanol at 4˚Cfor 24
h) in the kidney sections were detected using a TUNEL assay kit
(In Situ Cell Death Detection kit; Roche Applied Science)
according to the manufacturer's instructions. For each study group,
the apoptosis index was calculated using a 10x field of view.
Apoptotic cells were observed via an optical microscope
(magnification, x400) and imaged, Each group was captured in 10
fields. Apoptotic cells were manually identified by their specific
morphological characteristics (presence of apoptotic bodies,
chromatin condensation, marginalisation and membrane lysis). The
apoptosis index was calculated as follows: Apoptosis cells/total
cells within a high-power field.
Changes in morphology observed under
an electron microscope
Kidney specimens were cut into 2-mm sized fragments
and fixed in 2.5% glutaraldehyde at room temperature in 0.1 M
phosphate buffer overnight. The tissues were washed thrice in 0.1 M
phosphate buffer and fixed with 1% osmium tetroxide in phosphate
buffer for 1 h at 4°C. The fixed tissues were then
washed thrice in 0.1 M phosphate buffer. The specimens were
dehydrated using a graded series of ethanol (50, 70, 80, 90, 95 and
100%) for 15-20 min at each step and transferred to absolute
acetone for 20 min. The specimens were then placed in a 1:1 mixture
of absolute acetone and a Spurr resin mixture for 1 h at room
temperature and then transferred to a 1:3 mixture of the same
solution overnight. The next day, specimens were placed in capsules
containing embedding medium and heated at 70°C for ~9 h.
The sections were then sequentially stained with uranyl acetate and
alkaline lead citrate at 25˚C for 15 min each and observed under a
transmission electron microscope (JEM-1230; JEOL, Ltd.).
Statistical analysis
In the current study, repeated measurement data are
presented as the mean ± SD (n=10 in each group). One-way ANOVA was
used for comparison between groups, and Bonfferoni was used as a
post-hoc test. All statistical analysis was performed by SPSS
software (version 21.0; IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
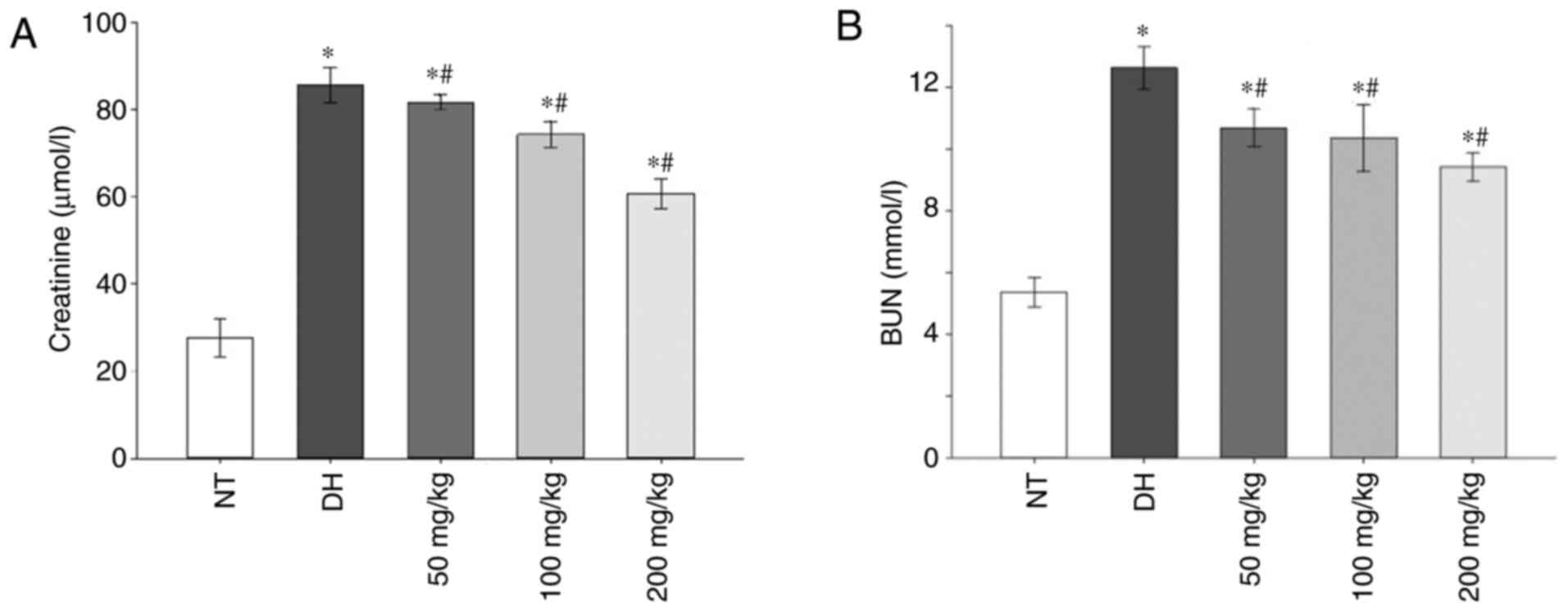
Compared with the NT control group, the
concentration of creatinine and BUN in serum samples was
significantly increased in the DH and all curcumin treatment groups
(P<0.05). However, pre-treatment with increasing concentrations
of curcumin resulted in a significant decrease in BUN and
creatinine levels compared with the DH control (P<0.05; Fig. 1A and B).
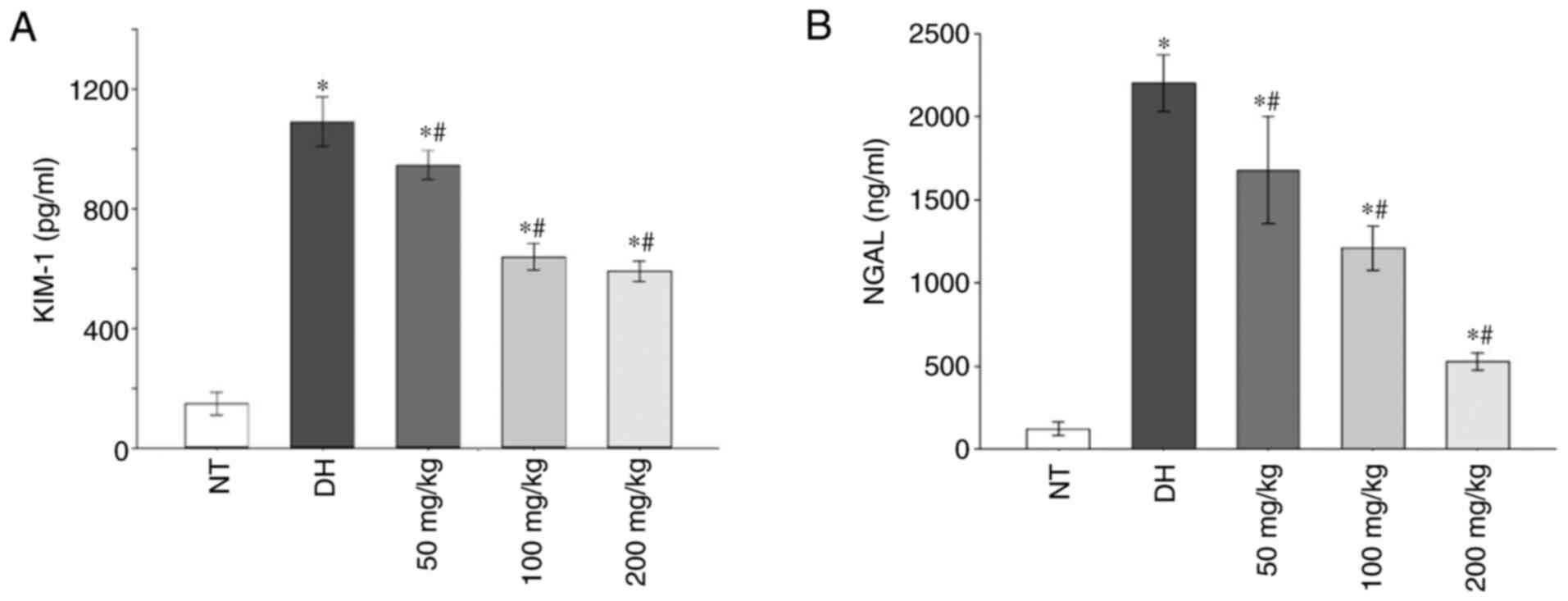
Additionally, in the DH and all curcumin treatment
groups, the levels of early renal injury markers, namely KIM-1 and
NGAL, increased significantly compared with the NT control group.
At all different curcumin concentrations, the expression of KIM-1
and NGAL were significantly decreased compared with the DH control
group (P<0.05; Fig. 2A and
B).
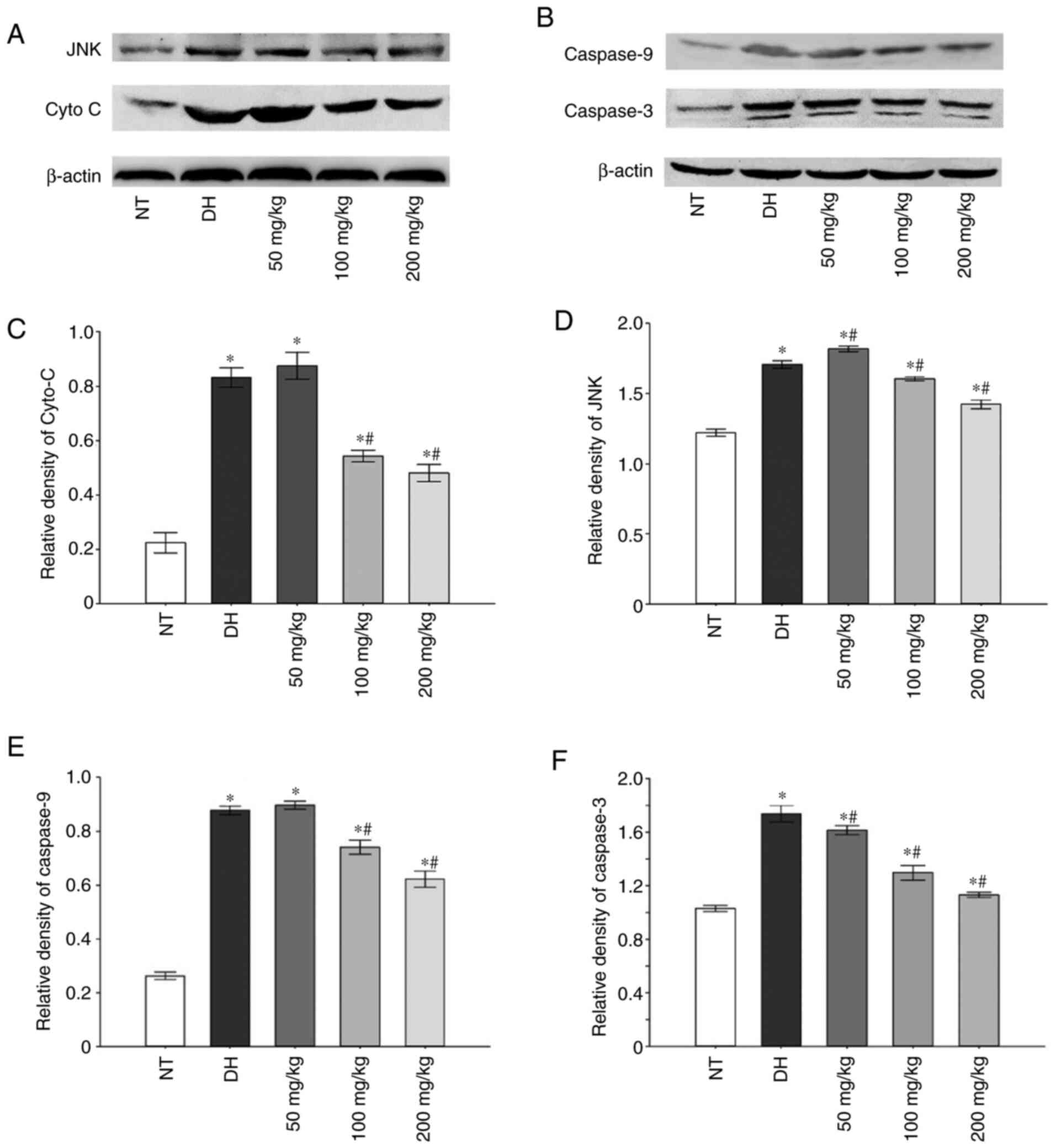
Following incubation for 150 min in a dry-heat
environment, in the DH and all curcumin treatment groups, the
expression of cytochrome c (Cyt c), JNK, caspase-3
and caspase-9 was revealed to be increased compared with the NT
control group. However, 100 and 200 mg/kg curcumin pre-treatment
group caused the expression of these apoptosis-related proteins to
significantly decrease (Fig.
3).
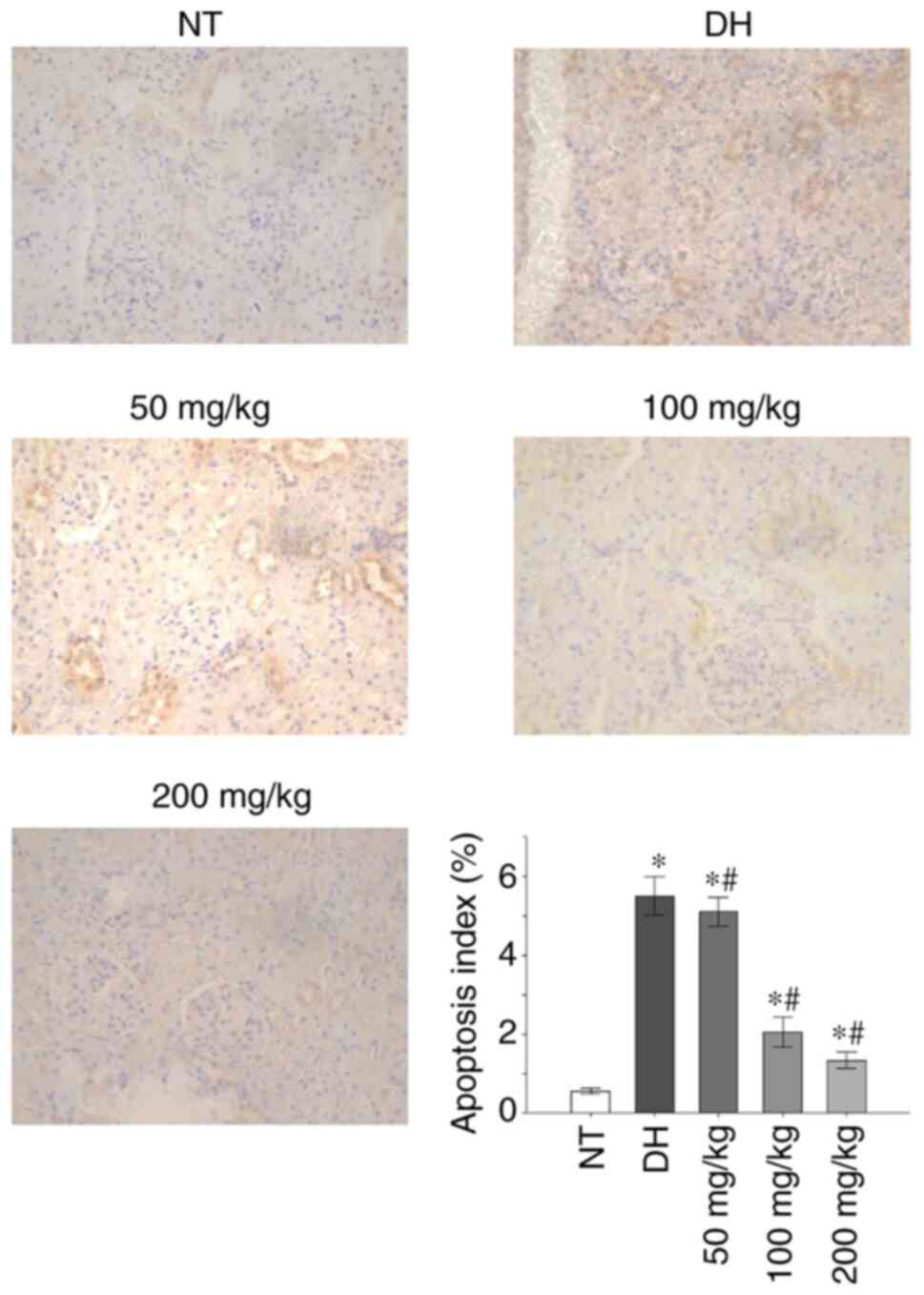
Paraffin sections of renal tissues were stained
using TUNEL. The renal tissues obtained from the NT control group
rats did not appear to exhibit significant apoptosis (apoptosis
index, 0.55+0.071%), whereas those from the DH and all curcumin
treatment groups indicated significantly higher levels of apoptosis
in the renal tubular cells compared with the NT group (apoptosis
index, 5.5+0.48%; P<0.05). The level of apoptosis observed in
the renal tissue of rats treated with 50 mg/kg curcumin (apoptosis
index, 5.1+0.37%) was significantly lower compared with the DH
control group (P<0.05). Similarly, the apoptosis index
determined for the renal tissue in rats treated with either 100
mg/kg (2.05+0.37%) or 200 mg/kg (1.33+0.20%) of curcumin was also
significantly decreased compared with the DH control group
(P<0.05; Fig. 4).
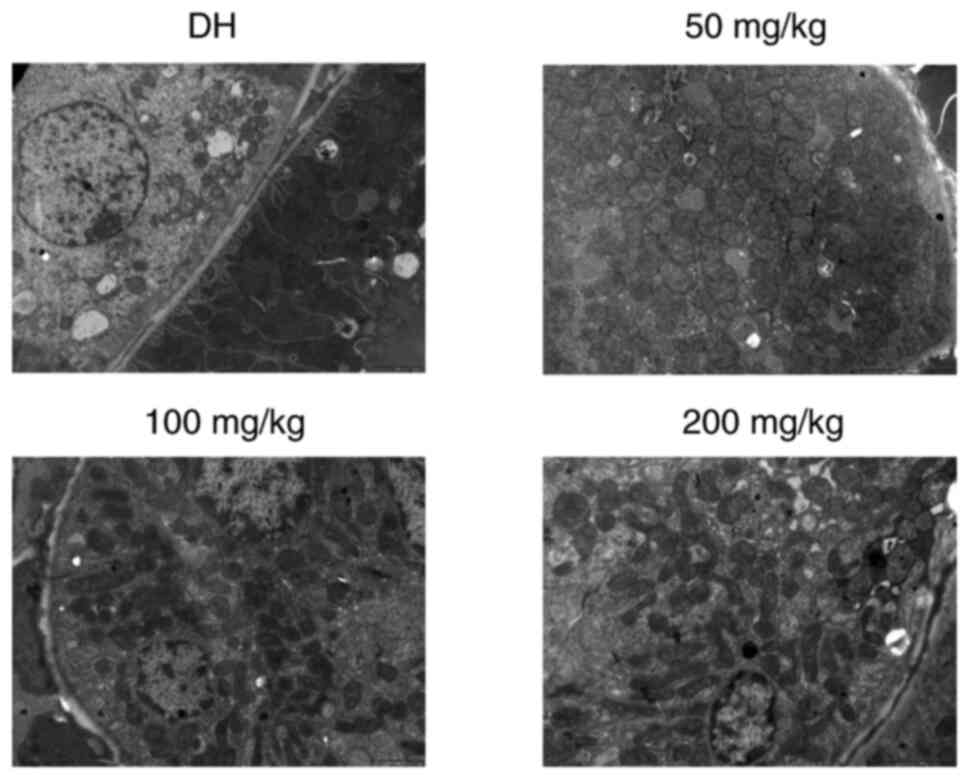
Electron microscopy revealed severe mitochondrial
damage within the DH control group, which was characterised by
mitochondrial swelling and vacuolisation, as well as disappearance
of the mitochondrial cristae (Fig.
5). Alternatively, compared with the DH control group, samples
from rats treated with 50 mg/kg of curcumin demonstrated markedly
reduced mitochondrial damage and mitochondrial swelling. In the
animals treated with 100 and 200 mg/kg of curcumin, the
mitochondrial swelling was even further reduced, and mitochondria
vacuolisation was nearly invisible (Fig. 5).
Discussion
Increasing evidence has suggested that apoptosis may
serve an essential role in the pathological process of heatstroke
(19,20). In recent years, researchers have
indicated that heatstroke regulates apoptosis by regulating the
expression of specific caspases (21). Hsu et al (22) demonstrated that in a heat stress
model of corneal cells, cell death was directly related to the
heat-induced expression of caspase-8 and caspase-9, as well as the
activation of specific mitochondrial pathways. In addition,
Milleron and Bratton (23)
demonstrated that inhibition of caspase activity could
significantly reduce heat-induced apoptosis. Further research has
demonstrated that the direct influence of heat on cells, including
cellular damage, production of oxygen metabolism products and
production of proteinases and various cytokines that are released
during the heat shock process, can activate or inhibit specific
signal transduction pathways (24,25),
thereby mediating the survival or death of cells.
Apoptosis is characterised by a series of
morphological changes, including membrane blebbing, cell shrinkage,
chromatin condensation and DNA fragmentation, followed by rapid
engulfment of the dead cell by neighbouring cells, without rupture
of the cell membrane (26).
Mitochondria regulate apoptosis through the activation of a variety
of death stimuli; therefore, mitochondrial dysfunction serves a
prominent function in apoptosis (27). Mitochondrial dysfunction leads to
release of Cyt c from the mitochondrial membrane space into
the cytoplasm where it serves a vital role in mediating apoptosis
(28,29). Released Cyt c binds Apoptotic
Peptidase Activating Factor 1 and forms an activation complex with
caspase-9, which then serves to activate caspase-3 to induce cell
apoptosis and resulting in the activation of downstream cascade
reactions.
However, apoptosis is regulated by many upstream
signalling pathways. MAPKs are considered to be some of the most
important signalling molecules in the transmission of apoptotic
signals to the mitochondria (30).
Specific MAPK family members, namely JNK and p38, have been
determined to be upstream stimulators of classical apoptotic
pathways (31). These molecules
have been indicated to reduce the expression and activity of the
anti-apoptotic Bcl-2 family members by interfering with cellular
localisation and dimer formation (32). Alternatively, MAPKs have also been
revealed to promote the expression and activity of pro-apoptotic
proteins and induce apoptosis via the mitochondrial pathway
(33,34).
In the present study, the identification of
apoptosis based on the pathological changes in the renal tissue is
facile. Following incubation in a dry-heat environment for 150 min,
expression of apoptosis-related proteins increased significantly.
Therefore, apoptosis may serve an essential role in kidney injury
in a dry-heat environment.
To study the benefits of curcumin pre-treatment,
rats were pre-treated with different concentrations of curcumin
prior to incubation in a dry-heat environment for 150 min and
subsequent changes were observed in the renal tissue, as well as
changes in the expression of JNK, Cyt c, caspase-3 and
caspase-9. The results of electron microscopy revealed that the
changes in the renal tissues of the DH control group were
noticeable, with mitochondrial injury including swelling and
vacuolisation being observed. Increased expression of
apoptosis-related proteins, specifically, JNK, Cyt c,
caspase-3 and caspase-9 were also observed following incubation in
the dry-heat environment. Therefore, mitochondria may serve a
significant role in regulating apoptosis within kidney tissues
exposed to a dry-heat environment, and inhibiting pathways
associated with mitochondrial apoptosis may prove to be beneficial
in preventing renal injury following extended exposure to a
dry-heat environment. Curcumin pre-treatment (100 and 200 mg/kg)
resulted in decreased expression of JNK, Cyt c, caspase-3,
and caspase-9. Electron microscopy revealed that the rats treated
with 50 mg/kg of curcumin demonstrated reduced mitochondrial damage
and mitochondrial swelling compared with the DH control. This
effect was even more pronounced in tissues from animals treated
with 100 mg/kg or 200 mg/kg of curcumin, resulting in near
elimination of mitochondrial vacuolisation. These findings indicate
a dose-dependent protective effect of curcumin on mitochondria.
In conclusion, the results of the current study
demonstrated that pre-treatment with curcumin prevents
heatstroke-induced AKI in rats. Curcumin (100 and 200 mg/kg) groups
markedly reduced the expression of specific markers of AKI and
apoptosis-related proteins. By reducing the degree of cellular
damage through inhibition of the mitochondrial apoptotic pathway,
curcumin prevents renal tissue injury induced by heatstroke.
Therefore, curcumin may be a potential prophylactic treatment that
may prevent the adverse, severe effects of AKI in heatstroke by
reducing the degree of cellular damage through inhibition of the
mitochondrial apoptotic pathway.
Although some beneficial results have been obtained
from the current study, the study has some limitations. A
horizontal comparative study was performed. The experiment would
have been improved if a vertical comparison at different time
points was performed, or relevant factors and mechanism were
diversified. Therefore, the correlation between dose and time
should be examined in future experiments in order to identify an
optimal curcumin concentration and treatment period.
Acknowledgements
The authors thank Professor Zhao Rong and Professor
Xu Qin (Key Laboratory of the Special Environmental Medicine of
Xinjiang, General Hospital of Xinjiang Military Region of the PLA)
for providing extensive assistance in the experiments presented in
this manuscript.
Funding
The current study was funded by The Clinical
Medicine Major Projects of New and High Technology Research of the
PLA (grant no. 2010gxjs016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YHZ and CFS analyzed and interpreted the data and
wrote the manuscript. YK assisted in study design. AQ, WJX and WHS
analyzed the data and revised the manuscript. JWL designed the
study and drafted the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethical
committee of the General Hospital of Xinjiang Military Region of
the PLA. Animal care and experiments were conducted according to
the National Science Council guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bouchama A and Knochel JP: Heat stroke. N
Engl J Med. 346:1978–1988. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Al Mahri S and Bouchama A: Heatstroke.
Handb Clin Neurol. 157:531–545. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Heled Y, Fleischmann C and Epstein Y:
Cytokines and their role in hyperthermia and heatstroke. J Basic
Clin Physiol Pharmacol. 24:85–96. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bouchama A, Roberts G, Al Mohanna F,
El-Sayed R, Lach B, Chollet-Martin S, Ollivier V, Al Baradei R,
Loualich A, Nakeeb S, et al: Inflammatory, hemostatic, and clinical
changes in a baboon experimental model for heatstroke. J Appl
Physiol (1985). 98:697–705. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Roberts GT, Ghebeh H, Chishti MA,
Al-Mohanna F, El-Sayed R, Al-Mohanna F and Bouchama A:
Microvascular injury, thrombosis, inflammation, and apoptosis in
the pathogenesis of heatstroke: A study in baboon model.
Arterioscler Thromb Vasc Biol. 28:1130–1136. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Khogali M: Heat-related illnesses. Middle
East J Anaesthesiol. 12:531–572. 1994.PubMed/NCBI
|
|
7
|
Gong L, Zhang Q, Pan X, Chen S, Yang L,
Liu B, Yang W, Yu L, Xiao ZX, Feng XH, et al: p53 protects cells
from death at the heatstroke threshold temperature. Cell Rep.
29:3693–3707.e5. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lim CL and Mackinnon LT: The roles of
exercise-induced immune system disturbances in the pathology of
heat stroke: The dual pathway model of heat stroke. Sports Med.
36:39–64. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sakaguchi Y, Stephens CL, Makino M, Kaneko
T, Strebel FR, Danhauser LL, Jenkins GN and Bull JM: Apoptosis in
tumors and normal tissues induced by whole body hyperthermia in
rats. Cancer Res. 55:5459–5464. 1995.PubMed/NCBI
|
|
10
|
Unlu A, Nayir E, Dogukan Kalenderoglu M,
Kirca O and Ozdogan M: Curcumin Turmeric) and cancer. J BUON.
21:1050–1060. 2016.PubMed/NCBI
|
|
11
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: Problems and promises.
Mol Pharm. 4:807–818. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mirzaei H, Shakeri A, Rashidi B, Jalili A,
Banikazemi Z and Sahebkar A: Phytosomal curcumin: A review of
pharmacokinetic, experimental and clinical studies. Biomed
Pharmacother. 85:102–112. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
He L, Peng X, Zhu J, Liu G, Chen X, Tang
C, Liu H, Liu F and Peng Y: Protective effects of curcumin on acute
gentamicin-induced nephrotoxicity in rats. Can J Physiol Pharmacol.
93:275–282. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Abdel-Moneim AM, El-Toweissy MY, Ali AM,
Awad Allah AA, Darwish HS and Sadek IS: Curcumin ameliorates lead
(Pb(2+))-induced hemato-biochemical alterations and renal oxidative
damage in a rat model. Biol Trace Elem Res. 168:206–220.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hismiogullari AA, Hismiogullari SE, Karaca
O, Sunay FB, Paksoy S, Can M, Kus I, Seyrek K and Yavuz O: The
protective effect of curcumin administration on carbon
tetrachloride (CCl4)-induced nephrotoxicity in rats. Pharmacol Rep.
67:410–416. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jiang J, Liu J, Li J, Tao L, Wang Z, Yang
L, Shi W and Ma N: Effect of curcumin on expressions of CD11b and
CD19 in peripheral blood of heat stroke rats in a simulation
dry-heat environment. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue.
31:221–224. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
17
|
Cao W, Cao JJ, Liu JW, Li JJ, Shen CF,
Song LY, Ma N, Shi WH and Xu Q: Effects of curcumin pretreatment on
lung injury and HMGB-1 and ICAM-1 mRNA in heat stroke rats in
desert dry heat environment. Prog Mod Biomed. 18:652–656. 2018.
|
|
18
|
ou Zhou R, Liu JW, Zhang D and Zhang Q:
Heatstroke model for desert dry-heat environment and observed organ
damage. Am J Emerg Med. 32:573–579. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhu YH and Pei ZM: GSK2193874 treatment at
heatstroke onset reduced cell apoptosis in heatstroke mice. Cell
Mol Biol (Noisy-le-grand). 64:36–42. 2018.PubMed/NCBI
|
|
20
|
Geng Y, Ma Q, Liu YN, Peng N, Yuan FF, Li
XG, Li M, Wu YS, Li BL, Song WB, et al: Heatstroke induces liver
injury via IL-1β and HMGB1-induced pyroptosis. J Hepatol.
63:622–633. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ji J, Hong X, Su L and Liu Z: Proteomic
identification of hippocalcin and its protective role in
heatstroke-induced hypothalamic injury in mice. J Cell Physiol.
234:3775–3789. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hsu YL, Yu HS, Lin HC, Wu KY, Yang RC and
Kuo PL: Heat shock induces apoptosis through reactive oxygen
species involving mitochondrial and death receptor pathways in
corneal cells. Exp Eye Res. 93:405–412. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Milleron RS and Bratton SB: Heat shock
induces apoptosis independently of any known initiator
caspase-activating complex. J Biol Chem. 281:16991–17000.
2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
North S and Hainaut P: P53 and cell cycle
control: A finger in every pie. Pathol Biol (Paris). 48:255–270.
2000.PubMed/NCBI
|
|
25
|
Ye F, Deng PY, Li D, Luo D, Li NS, Deng S,
Deng HW and Li YJ: Involvement of endothelial cell-derived CGRP in
heat stress-induced protection of endothelial function. Vasc
Pharmacol. 46:238–246. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257.
1972.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jeong SY and Seol DW: The role of
mitochondria in apoptosis. BMB Rep. 41:11–22. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Santra S, Kaittanis C and Perez JM:
Cytochrome C encapsulating theranostic nanoparticles: A novel
bifunctional system for targeted delivery of therapeutic
membrane-impermeable proteins to tumors and imaging of cancer
therapy. Mol Pharm. 7:1209–1222. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yamada Y and Harashima H: Mitochondrial
drug delivery systems for macromolecule and their therapeutic
application to mitochondrial diseases. Adv Drug Deliv Rev.
60:1439–1462. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang Y, Xia C, Lun Z, Lv Y, Chen W and Li
T: Crosstalk between p38 MAPK and caspase-9 regulates
mitochondria-mediated apoptosis induced by
tetra-α-(4-carboxyphenoxy) phthalocyanine zinc photodynamic therapy
in LoVo cells. Oncol Rep. 39:61–70. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen YJ, Liu WH, Kao PH, Wang JJ and Chang
LS: Involvement of p38 MAPK- and JNK-modulated expression of Bcl-2
and Bax in Naja nigricollis CMS-9-induced apoptosis of human
leukemia K562 cells. Toxicon. 55:1306–1316. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Deng YT, Huang HC and Lin JK: Rotenone
induces apoptosis in MCF07 human breast cancer cell-mediated ROS
through JNK and p38 signaling. Mol Carcinog. 49:141–151.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Kang YH and Lee SJ: The role of p38 MAPK
and JNK in Arsenic trioxide-induced mitochondrial cell death in
human cervical cancer cells. J Cell Physiol. 217:23–33.
2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Su JC, Lin KL, Chien CM, Lu CM, Chen YL,
Chang LS and Lin SR: Novel indoloquinoline derivative, IQDMA,
induces G(2)/M phase arrest and apoptosis in A549 cells through
JNK/p38 MAPK signaling activation. Life Sci. 85:505–516.
2009.PubMed/NCBI View Article : Google Scholar
|