Introduction
A pathological scar is a fibroproliferative disorder
that is characterized by the excessive repair by tissue repair
cells, mainly fibroblasts, through the excessive synthesis and
secretion of extracellular matrix during skin wound healing
(1). Not only do pathological scars
seriously affect the physical appearance, but they are also usually
accompanied with infection, itching, pain and ulceration (2,3). In
addition, they can cause serious dysfunction or disfigurement,
which obviously affects the quality of life of the patient
(2,3). Despite the existence of different
clinical treatments for pathological scars, such as surgical
resection, laser treatment, cortisol injection therapy, and
compression therapy, no treatment method is known to achieve a
satisfactory therapeutic effect (4,5).
Mesenchymal stem cells, derived from the mesoderm at
the embryonic stage, are adult stem cells with self-renewal and
multi-directional differentiation potential. During wound healing,
mesenchymal stem cells have been shown to regulate macrophages and
T-cell function (6,7), neutralize oxidizing substances
(8), secrete anti-fibrotic factors
(9), strengthen the function of
dermal fibroblasts (10), promote
vascularization and stability of blood vessels, and induce the
differentiation of dermal layer cells, which can help in healing of
the tissue (11). In addition,
previous studies have shown that mesenchymal stem cells, bone
marrow mesenchymal stem cells (12,13),
umbilical cord mesenchymal stem cells (14) and chorionic mesenchymal stem cells
(15) can promote wound healing and
treat various types of fibrotic diseases.
Adipose-derived stem cells (ADSCs), which have been
isolated from human adipose tissue suspensions, have multipotential
differentiation capacity (16,17).
In addition to possessing the characteristics of general stem
cells, ADSCs have the ability of self-renewal and multiplication,
and can also differentiate into many specific functional cell lines
(16,17). Compared with other mesenchymal stem
cells, ADSCs have a wide range of sources, only lead to minor
damage in the donor site, have a good tissue compatibility, are
easy to culture in vitro, have weak immunogenicity and
relatively uncontroversial ethically (16,17).
It has been shown that ADSCs can help repair tissue and organ
damage (18,19), as well as promote wound healing
through their paracrine effects in diabetic and nude mice (20,21).
However, the molecular mechanisms by which ADSCs promote wound
healing remain to be elucidated.
The present study demonstrated that co-culture with
ADSCs inhibited the proliferation, migration, and protein
expression of extracellular matrix, and also inhibited the
transforming growth factor β1 (TGF-β1)/mothers against
decapentaplegic homolog (Smad) pathway in hypertrophic scar
fibroblasts and keloid fibroblasts.
Materials and methods
Tissue specimens and patients
Adipose tissue, used to extract adipose-derived
mesenchymal stem cells, was derived from 5 healthy subjects (2
males and 3 females; 25-42 years old) undergoing local liposuction
from October 2018 to May 2019 at Plastic Surgery Hospital, Chinese
Academy of Medical Sciences and Peking Union Medical College
(Beijing, China). Hypertrophic scar tissues were obtained from 9
patients with hypertrophic scars, keloid tissues were obtained from
14 keloid patients, and 5 normal skin tissues were obtained from
post-reconstruction cat ear malformation and cosmetic outpatient
surgeries. Patients with the following criteria were excluded from
the study: i) Less than 6 months with the condition; ii) infection
in the lesion; iii) radiation therapy or steroid injection; iv)
pathological scar disease combined with other hereditary diseases,
body fluid transmission diseases (such as HIV and HBV), malignant
tumors and skin diseases; and, v) age >55 years or <16
years.
All the participants in the present study signed
informed consents, and the study was approved by the Ethics
Committee of Plastic Surgery Hospital, Chinese Academy of Medical
Sciences and Peking Union Medical College.
Isolation, culture and identification
of ADSCs
The isolation, culture and identification (Fig. S1) of ADSCs was performed according
to the protocol of Gao et al (22). Briefly, the adipose tissue was
obtained under aseptic conditions and cut into 1-2 mm slices, then
incubated with an equal volume of 0.1% type I collagen (cat. no.
17018029; Thermo Fisher Scientific, Inc.) at 37˚C for 45 min. The
precipitate was centrifuged (1,200 x g at room temperature) for 15
min and the cell pellet was resuspended in a low-glucose DMEM
medium (cat. no. 21885108; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 1% penicillin-streptomycin (cat. no. 15140163;
Thermo Fisher Scientific, Inc.), 10% fetal bovine serum (cat. no.
10437028; Thermo Fisher Scientific, Inc.), and 640 µg/ml glutamine
(cat. no. G3126; Sigma-Aldrich; Merck KGaA). The cells were
incubated at 37˚C with 5% CO2, and the medium was
changed after 4 days of culture. Following that, the medium was
changed every two days. Cells from passages 3-6 were used in the
experiments.
Isolation, culture and identification
of fibroblasts
Fibroblasts, including normal skin fibroblasts,
hypertrophic scar fibroblasts and keloid fibroblasts, were
isolated, cultured and identified as previously described (23,24).
Briefly, normal skin tissue, hypertrophic scar tissue or keloid
tissue was incubated in a PBS buffer solution containing 1%
streptomycin for 15 min, then cut into 0.5x0.5 cm sections, which
were incubated in a cell culture dish containing low-glucose DMEM
medium (cat. no. 21885108; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 1% penicillin-streptomycin (cat. no. 15140163;
Thermo Fisher Scientific, Inc.), 10% fetal bovine serum (cat. no.
10437028; Thermo Fisher Scientific, Inc.), and 640 µg/ml glutamine
(cat. no. G3126; Sigma-Aldrich; Merck KGaA). The incubation was
continued until a dense monolayer (80% confluency) of cells formed
around the tissue pieces. Cells from passages 3-6 were used in the
experiments.
Transwell chamber co-culture
system
A Transwell chamber (cat. no. 140652; Thermo Fisher
Scientific, Inc.) was used to culture ADSCs with fibroblasts. The
co-culture system was performed as follows: 1.5x103
ADSCs were added per well in the upper chamber of a 12-well plate
Transwell chamber, with 0.5 ml of culture medium, and
3x103 ADSCs per well were inoculated into the lower
chamber with 1.5 ml culture medium. For the single culture system
only 3x103 ADSCs were inoculated per well into the lower
chamber, with or without adding SB431542 (cat. no. s4317;
Sigma-Aldrich; Merck KGaA) into the culture medium.
Cell proliferation assay
The viability of fibroblasts was evaluated using the
MTT Cell Proliferation and Cytotoxicity Assay kit and the BrdU Cell
Proliferation Assay kit (cat. no. C0075S; Beyotime Institute of
Biotechnology). Briefly, after 4 h of incubation with MTT (10 µl,
10 mg/ml), the supernatant was removed and 100 µl DMSO was added.
After 30 min, the optical density (OD) was measured using a plate
reader (ELx808; BioTek Instruments, Inc.).
Cell scratch test
A scratch was created perpendicular to the back of
the horizontal line using a vertically positioned (non-tilted) 200
µl pipette tip. The scratched cells were removed by washing the
cells 3 times with PBS. The cells were then cultured at 37˚C and 5%
CO2 in a serum-free DMEM medium, and images captured
after 4 days using a light microscope (magnification, x200; CKX41;
Olympus Corporation).
Western blot analysis
Collagen-I, collagen-III, fibronectin (FN), α-smooth
muscle actin (α-SMA), TGF-β1, Smad2, Smad3, phosphorylated (p-)
Smad2, p-Smad3 and Smad7 proteins were detected by western blotting
as previously described (25).
Total protein was extracted from cells using a cell total protein
extraction kit (cat. no. P1250), and the protein concentration was
measured using a BCA kit (cat. no. P1511; both from Applygen
Technologies, Inc.). Subsequently, 40 µg total protein/sample were
analyzed via 8-10% SDS-PAGE, and the proteins were transferred onto
a PVDF membrane (cat. no. 3010040001; Merck & Co., Inc.). The
antibodies (Abcam) were diluted according to the manufacturer's
guidelines. The membranes were blocked for 1 h at room temperature
using a blocking solution containing 5% skimmed milk in TBS-0.05%
Tween-20. The membranes were then incubated with the primary
antibodies: Collagen-I (1:1,000; cat. no. ab34710), collagen-III
(1:1,000; cat. no. ab184993), FN (1:2,000; cat. no. ab2413), α-SMA
(1:1,500; cat. no. ab5694), TGF-β1 (1:1,000; cat. no. ab215715),
Smad2 (1:200; cat. no. ab40855), Smad3 (1:1,000; cat. no. ab40854),
p-Smad2 (1:500; cat. no. ab188334), p-Smad3 (1:1,000; cat. no.
ab52903) and Smad7 (1:1,000; cat. no. ab216428), diluted in the
blocking solution, for 2 h at room temperature, then incubated with
the secondary antibodies: Goat anti-rabbit IgG H&L
HRP-conjugated (1:2,000; cat. no. ab6721) and goat Anti-Mouse IgG
H&L HRP-conjugated (1:3,000; cat. no. ab6789), diluted in
blocking solution, for 1 h at room temperature. Finally, the signal
was visualized with ECL solution (cat. no. K22020; Abbkine
Scientific Co., Ltd.). ImageJ software (v3.0; National Institutes
of Health) was used to analyze the protein bands, and β-actin was
for normalization.
Statistical analysis
SPSS 20.0 (SPSS, Inc.) was used for statistical
analysis of the data. Student's t-test was used to compare between
two groups, while multi-group comparisons were performed using
one-way ANOVA followed with Duncan's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
ADSCs inhibit the proliferation of
HSFs and KFs
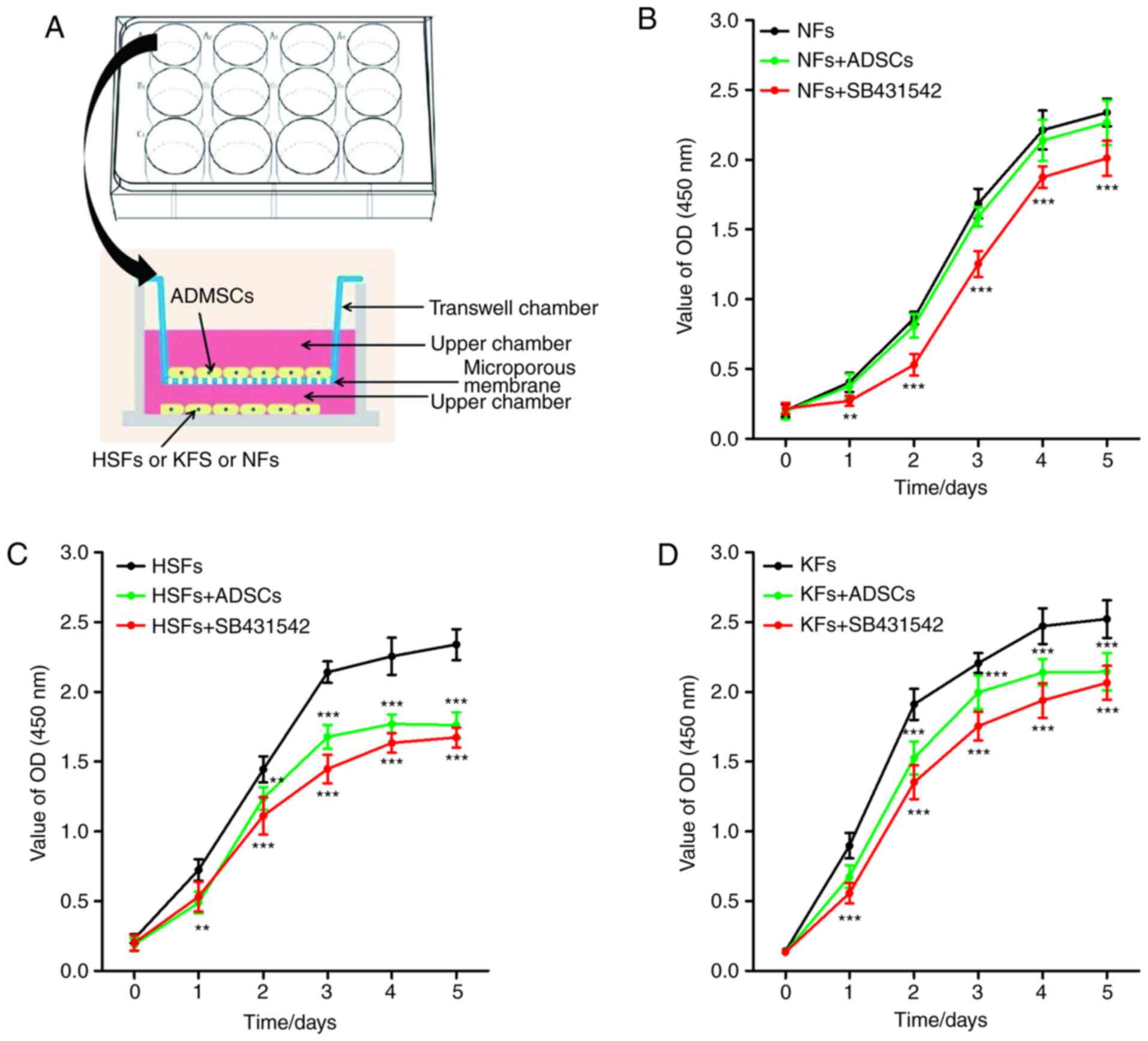
Transwell chambers were used to establish a
co-culture system of ADSCs and NFs, HSFs or KFs (Fig. 1A). No significant difference in the
proliferation of NFs was observed between NFs single culture system
and ADSCs + NFs co-culture system. However, SB431542 inhibited the
proliferation of NFs in the NFs single culture system (Fig. 1B). As shown in Fig. 1C and D, the proliferation of HSFs and KFs was
higher in the single culture system, compared with that in the
co-culture system with ADSCs. As with the NFs, SB431542
significantly inhibited the proliferation of HSFs and KFs in the
single culture system.
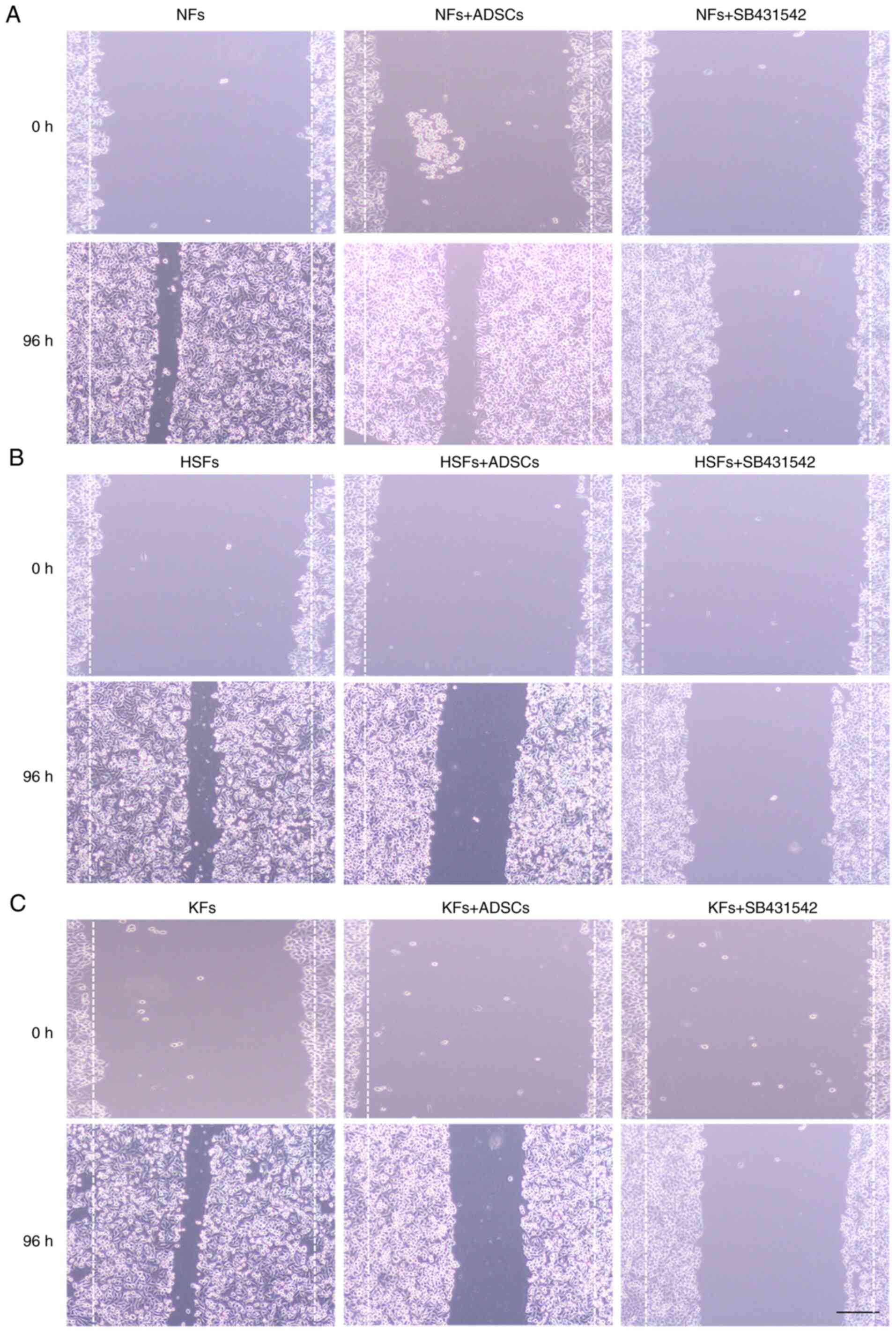
ADSCs inhibit the migration of HSFs
and KFs
While co-culture with ADSCs did not affect the
migration of NFs, SB431542 significantly inhibited the cell
migration of NFs in NFs single culture system (Fig. 2A). In addition, the migration
distance of HSFs and KFs were significantly higher in the single
culture system, compared with their migration distance following
co-culture with ADSCs (Fig. 2B and
C). SB431542 significantly
inhibited the migration of HSFs and KFs in the single culture
system.
ADSCs reduce the expression of
extracellular matrix proteins in HSFs and KFs
The expression of extracellular matrix proteins,
such as collagen-I, collagen-III and FN, and the extracellular
matrix-related proteins, such as α-SMA, in NFs, HSFs and KFs, were
measured in the single culture systems and in the co-cultured cells
(Fig. 1A). There was no significant
difference in the expression of collagen-I, collagen-III, FN and
α-SMA protein in NFs cultured alone and in those co-cultured with
ADSCs. However, the expression of collagen-I, collagen-III, FN and
α-SMA proteins was significantly decreased in NFs treated with
SB431542. Of importance, the expression of collagen-I,
collagen-III, FN and α-SMA proteins in HSFs and KFs from the single
culture system were significantly lower than those from the
co-culture system with ADSCs (Fig.
3A-C). On the other hand, SB431542 reduced the protein
expression of collagen-I, collagen-III, FN and α-SMA in HSFs and
KFs from the single culture system (Fig. 3A-C). In addition, the concentration
of hydroxyproline (HYP) in HSFs and KFs from the single culture
system was significantly lower compared with that from the
co-culture system with ADSCs, and SB431542 reduced the
concentration of HYP from the single culture system (Fig. 3D).
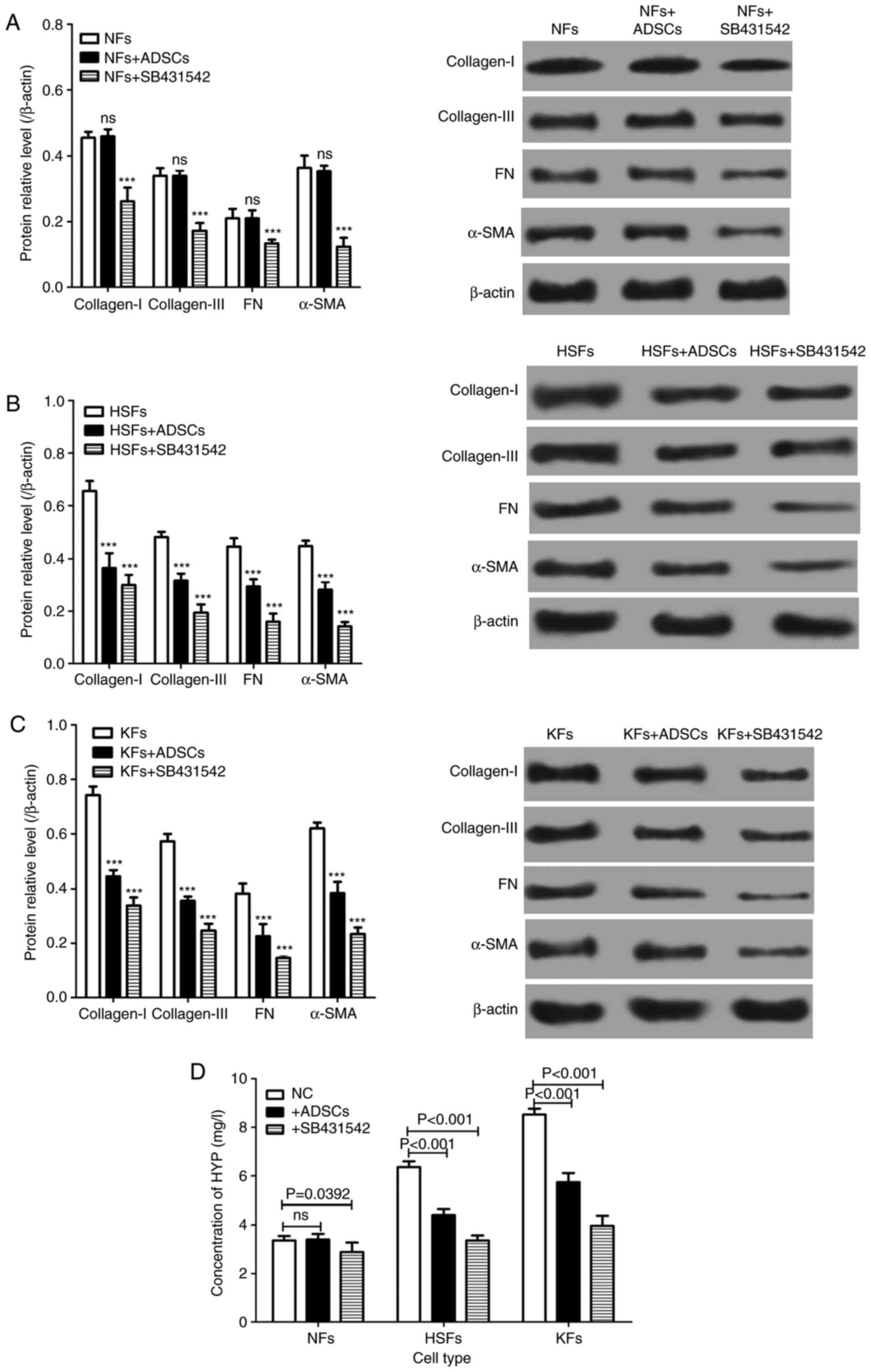 | Figure 3Effects of ADSCs on the synthesis of
extracellular matrix in NFs, HSFs and KFs. Western blotting was
used to detect the expression of collagen-I, collagen-III, FN and
α-SMA protein in (A) NFs (***P<0.01 vs. NFs group),
(B) HSFs (***P<0.01 vs. HSFs group) and (C) KFs
(***P<0.01 vs. KFs group). (D) The concentration of
HYP in the culture medium of NFs, HSFs and KFs with different
culture environments. HYP; hydroxyproline; ADSCs, adipose-derived
mesenchymal stem cells; NFs, normal skin fibroblasts; HSFs,
hypertrophic scar fibroblasts; KFs, keloid fibroblasts; ns, not
significant.. |
ADSCs inhibit TGF-β1/Smad pathway in
HSFs and KFs
The TGF-β1/Smad pathway is a signaling pathway that
is closely associated with cell proliferation and migration, and to
extracellular matrix synthesis in fibroblasts. As shown in Fig. 4, the protein expression of TGF-β1,
p-Smad2/Smad2, p-Smad3/Smad3 and Smad7 in NFs from the single
culture system were similar to that from the co-culture systems.
However, the selective inhibitor of the TGF-β1/Smad signaling
pathway, SB431542, significantly decreased the protein expression
of TGF-β1, p-Smad2/Smad2, p-Smad3/Smad3 and Smad7 in NFs. In
addition, the protein expression of TGF-β1, p-Smad2/Smad2,
p-Smad3/Smad3 and Smad7 in HSFs and KFs from the single culture
systems were significantly higher than those from the co-culture
systems with ADSCs (Fig. 4B and
C). Furthermore, SB431542 also
suppressed TGF-β1, p-Smad2, p-Smad3 and Smad7 expression in HSFs
and KFs in the single culture systems (Fig. 4B and C).
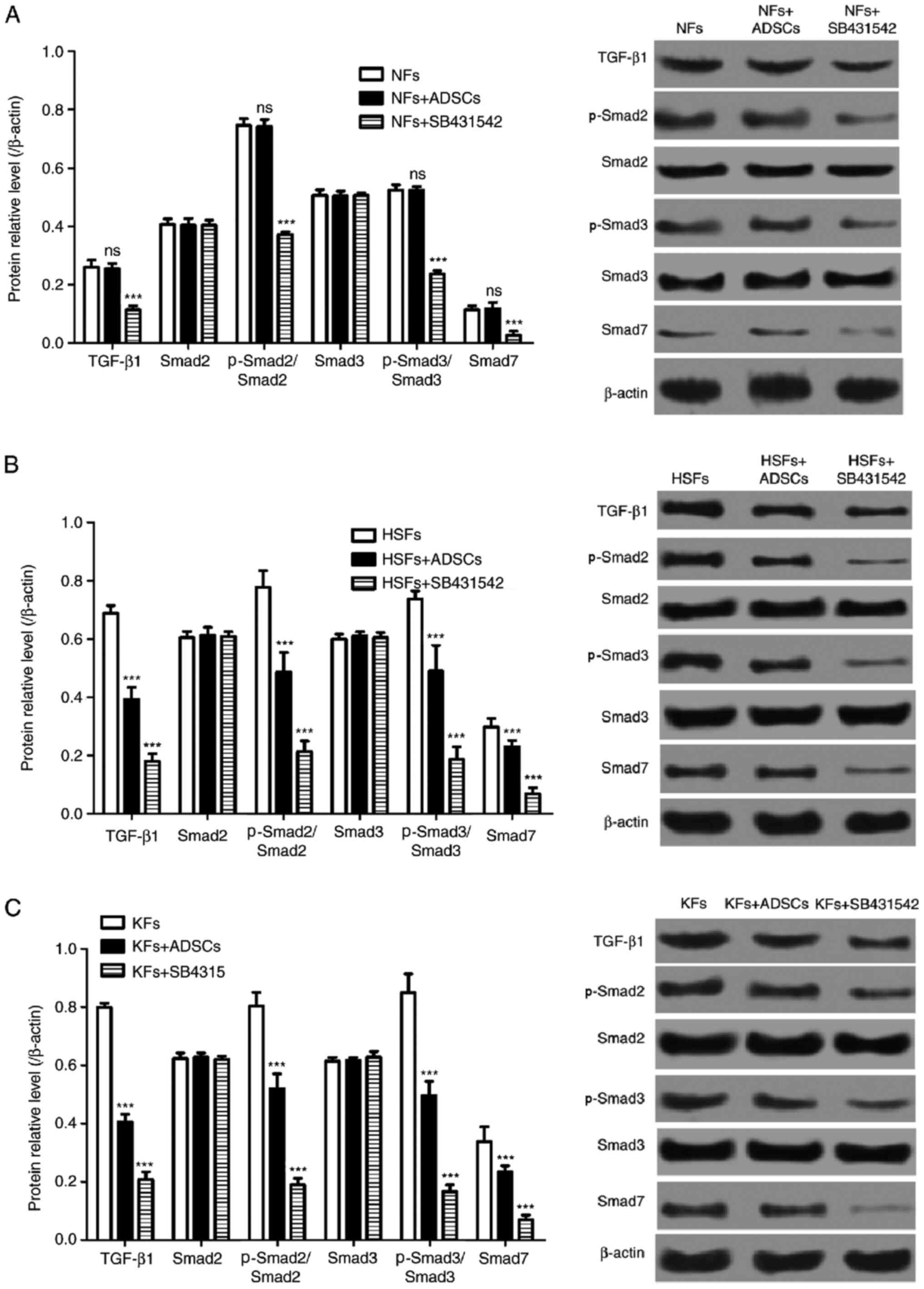 | Figure 4Effects of ADSCs on TGF-β1/Smad
pathway in NFs, HSFs and KFs. A-C, Western blot was used to detect
the expression of TGF-β1, Smad2, Smad3, p-Smad 2, p-Smad 3 and Smad
7 protein in (A) NFs (***P<0.01 vs. NFs group), (B)
HSFs (***P<0.01 vs. HSFs group) and (C) KFs
(***P<0.01 vs. KFs group). ADSCs, adipose-derived
mesenchymal stem cells; TGF-β1, transforming growth factor β1;
Smad, mothers against decapentaplegic homolog; NFs, normal skin
fibroblasts; HSFs, hypertrophic scar fibroblasts; KFs, keloid
fibroblasts; p-, phosphorylated.; ns, not significant. |
Discussion
The complex causes and mechanisms have led to a
number of hypotheses to explain pathological scars formation, such
as the immunoinflammatory over-the-sun holiday hypothesis (i.e.
excessive inflammation results in extracellular matrix deposition
and tissue fibrosis), the cytokine regulatory disorder hypothesis,
the cell matrix line disorder hypothesis and the epigenetic
hypothesis (26,27). However, no single hypothesis can
fully explain the mechanism of pathological scars formation.
Despite that, various hypotheses can hold several views on the
causes of pathological scar formation; excessive fibroblast
proliferation and deposition of extracellular matrix are considered
the most significant pathological changes during the development of
pathological scars (26,27). Therefore, inhibition of fibroblasts
proliferation and the suppression of extracellular matrix synthesis
by fibroblasts could be potential targets for the prevention and
treatment of pathological scars (28).
Previous studies have shown that the transplantation
of mesenchymal stem cells into the large area of wounds can
accelerate wound healing, improve healing quality and reduce scar
formation (29,30). This suggests that mesenchymal stem
cells can inhibit scar formation, which provides an approach for
the treatment of wounds and pathological scars (29,30).
Previous studies have shown that mesenchymal stem cells can inhibit
scar hyperplasia through myofibroblasts regulation (31,32),
immune response regulation (33),
ROS/RNS homeostasis (34) and
angiogenesis induction (35). The
present study demonstrated that ADSCs inhibited cell proliferation
and migration, as well as the protein expression of collagen-I,
collagen-III, FN and α-SMA in hypertrophic scar fibroblasts and
keloid fibroblasts. Evidently, the present study only investigated
the effect of ADSCs on proliferation, migration and the synthesis
of extracellular matrix in HSFs and KFs in vitro. The
current study was limited to outside the body to circumvent the
complex environment inside the body, and its conclusion needs to be
confirmed in vivo. With the advancements in cell therapy and
stem cells understanding, ADSCs are regarded as model seed cells
for cell therapy due to their ability to secrete a large number of
active factors (36,37) that can act through paracrine
mechanisms to exert multiple effects, such as the induction of
wound healing (19), angiogenesis
(22), the inhibition of scar
formation following myocardial infarction (38) and multi-directional differentiation
(39). Yoshihiko et al
(40) demonstrated that
adipose-derived stem/stromal cells can inhibit the formation of
vocal cord scars through the regulation of the biological behavior
of vocal fold fibroblasts and through the regulation of vocal folds
inflammation. Yun et al (41) demonstrated that human ADSCs can
stimulate scar remodeling in a pig wound model by decreasing the
activity of mast cells, inhibiting the effects of TGF-β on
fibroblasts and decreasing the expression of MMP molecules. In
vitro, human ADSCs were shown to inhibit TGF-β1-induced
differentiation of human dermal fibroblasts and keloid scar-derived
fibroblasts in a paracrine manner (42).
The mode of action of ADSCs in the regulation of
scar fibroblasts can occur either through direct contact, or
through indirect non-contact mechanisms (16,17).
The present study established an indirect co-culture system of
ADSCs and fibroblasts, including hypertrophic scar fibroblasts and
keloid fibroblasts, using a Transwell chamber wherein ADSCs were
not in direct contact with fibroblasts. However, in animal
experiments, ADSCs are in direct contact with scar fibroblasts.
While a study has indicated that local injection of adipose stem
cells can promote healing and reduce the risk of scar formation
during healing of the injury site (43), ADSCs-conditioned medium was alone
able to alter the biological behavior of target cells (44,45).
Therefore, the interaction between the two cell types could be
achieved through the influence of receptors, in addition to their
direct interaction.
The present study observed that co-culture with
ADSCs inhibited the protein expression of TGF-β1, p-Smad2/Smad2,
p-Smad3/Smad3 and Smad7 in HSFs and KFs. The TGF-β family is highly
conserved and its members are widely expressed during embryonic and
tissue development, where they have been shown to exhibit different
biological functions in a cell-dependent and condition-dependent
manner (46). TGF-β1 is a
representative cytokine of the TGF-β family that plays an important
role in the regulation of the biological behavior of different cell
types at different stages of development (46). TGF-β1 exists in complex regulatory
networks with different cell signaling pathway molecules that can
regulate the expression of each other (46). In the process of wound healing,
moderate secretion of TGF-β1 can promote the proliferation and
migration of fibroblasts, and can also accelerate the healing of
wounds (47,48). Jung et al (49) demonstrated that ADSCs can
downregulate the expression of type-1 collagen and hyaluronic acid
at the mRNA level via paracrine TGF-β1 activity. Overexpression of
TGF-β1 has been reported to promote the secretion of extracellular
matrix, which leads to scars formation (50,51).
In summary, the present study demonstrated that
ADSCs can affect the biological behavior of HSFs and KFs in
vitro, specifically proliferation, migration and extracellular
matrix synthesis, by regulating the TGF-β1/Smad pathway.
Supplementary Material
Identification of ADSCs by flow
cytometry. (A) Statistics of different surface antigen-positive
cells by detecting cell-surface antigen of (B) CD29, (C) CD44, (D)
CD105, (E) CD45, (F) CD34 and (G) CD31. Red traces indicates
negative control for antibody, and blue traces indicates positive
staining. ADSCs, adipose-derived mesenchymal stem cells; CD,
cluster of differentiation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LT conceived and designed the current study and
contributed to writing the manuscript. FX and JX performed the
experiments. JL, CZ, LY, XM and MZ analyzed and interpreted the
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All participants in the present study signed
informed consents, and the study was approved by the Ethics
Committee of Plastic Surgery Hospital, Chinese Academy of Medical
Sciences and Peking Union Medical College (Beijing, China).
Patient consent for publication
All the participants in the present study signed
informed consents.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fearmonti RM, Bond JE, Erdmann D, Levin
LS, Pizzo SV and Levinson H: The modified patient and observer scar
assessment scale: A novel approach to defining pathologic and
nonpathologic scarring. Plast Reconstr Surg. 129:242–247.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bock O, Schmid-Ott G, Malewski P and
Mrowietz U: Quality of life of patients with keloid and
hypertrophic scarring. Arch Dermatol Res. 297:433–438.
2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rohrer TE and Gold M: Introduction to
special issue on hypertrophic scars and keloids. Dermatol Surg. 43
(Suppl 1):S1–S2. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Seifert O and Mrowietz U: Keloid scarring:
Bench and bedside. Arch Dermatol Res. 301:259–272. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gauglitz GG, Korting HC, Pavicic T,
Ruzicka T and Jeschke MG: Hypertrophic Scarring and Keloids:
Pathomechanisms and current and emerging treatment strategies. Mol
Med. 17:113–125. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nakajima H, Uchida K, Guerrero AR,
Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT and
Johnson WE: Transplantation of mesenchymal stem cells promotes an
alternative pathway of macrophage activation and functional
recovery after spinal cord injury. J Neurotrauma. 29:1614–1625.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schurgers E, Kelchtermans H, Mitera T,
Geboes L and Matthys P: Discrepancy between the in vitro and in
vivo effects of murine mesenchymal stem cells on T-cell
proliferation and collagen-induced arthritis. Arthritis Res Ther.
12(R31)2010.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Kim WS, Park BS, Kim HK, Park JS, Kim KJ,
Choi JS, Chung SJ, Kim DD and Sung JH: Evidence supporting
antioxidant action of adipose-derived stem cells: Protection of
human dermal fibroblasts from oxidative stress. J Dermatol Sci.
49:133–142. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hiwatashi N, Bing R, Kraja I and Branski
RC: Mesenchymal stem cells have antifibrotic effects on
transforming growth factor-β1-stimulated vocal fold fibroblasts.
Laryngoscope. 127:E35–E41. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Smith AN, Willis E, Chan VT, Muffley LA,
Isik FF, Gibran NS and Hocking AM: Mesenchymal stem cells induce
dermal fibroblast responses to injury. Exp Cell Res. 316:48–54.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Harvestine JN, Orbay H, Chen JY, Sahar DE
and Leach JK: Cell-secreted extracellular matrix, independent of
cell source, promotes the osteogenic differentiation of human
stromal vascular fraction. J Mater Chem B. 6:4104–4115.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Antoniou KM, Papadaki HA, Soufla G,
Kastrinaki MC, Damianaki A, Koutala H, Spandidos DA and Siafakas
NM: Investigation of bone marrow mesenchymal stem cells (BM MSCs)
involvement in idiopathic pulmonary fibrosis (IPF). Respir Med.
104:1535–1542. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang D, Sun S, Wang Z, Zhu P, Yang Z and
Zhang B: Stromal cell-derived factor-1 receptor
CXCR4-overexpressing bone marrow mesenchymal stem cells accelerate
wound healing by migrating into skin injury areas. Cell Reprogram.
15:206–215. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yuben M, Daniel A, Ursula M, Samuel CS,
Jorge T, Sivakami I, Richard B and Alan T: Human umbilical cord
mesenchymal stem cells reduce fibrosis of bleomycin-induced lung
injury. Am J Pathol. 175:303–313. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee MJ, Jung J, Na KH, Moon JS, Lee HJ,
Kim JH, Kim GI, Kwon SW, Hwang SG and Kim GJ: Anti-fibrotic effect
of chorionic plate-derived mesenchymal stem cells isolated from
human placenta in a rat model of CCl(4)-injured liver: Potential
application to the treatment of hepatic diseases. J Cell Biochem.
111:1453–1463. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bunnell BA: Adipose-derived stem cells.
Methods Mol Biol. 5:59–67. 2008.
|
|
17
|
Ma T, Sun J, Zhao Z, Lei W, Chen Y, Wang
X, Yang J and Shen Z: A brief review: Adipose-derived stem cells
and their therapeutic potential in cardiovascular diseases. Stem
Cell Res Ther. 8(124)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang D, Wang W, Li L, Peng Y, Chen P,
Huang H, Guo Y, Xia X, Wang Y and Wang H: The relative contribution
of paracine effect versus direct differentiation on adipose-derived
stem cell transplantation mediated cardiac repair. PLoS One.
8(e59020)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kim WS, Park BS, Sung JH, Yang JM, Park
SB, Kwak SJ and Park JS: Wound healing effect of adipose-derived
stem cells: A critical role of secretory factors on human dermal
fibroblasts. J Dermatol Sci. 48:15–24. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee SH, Lee JH and Cho KH: Effects of
human adipose-derived stem cells on cutaneous wound healing in nude
mice. Ann Dermatol. 23:150–155. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Maharlooei MK, Bagheri M, Solhjou Z,
Jahromi BM, Akrami M, Rohani L, Monabati A, Noorafshan A and Omrani
GR: Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes
skin wound healing in diabetic rats. Diabetes Res Clin Pract.
93:228–234. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gao W, Qiao X, Ma S and Cui L:
Adipose-derived stem cells accelerate neovascularization in
ischaemic diabetic skin flap via expression of hypoxia-inducible
factor-1α. J Cell Mol Med. 15:2575–2585. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bock O, Yu H, Zitron S, Bayat A, Ferguson
MWJ and Mrowietz U: Studies of transforming growth factors beta 1-3
and their receptors I and II in fibroblast of keloids and
hypertrophic scars. Acta Derm Venereol. 85:216–220. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wong VW, You F, Januszyk M, Gurtner GC and
Kuang AA: Transcriptional profiling of rapamycin-treated
fibroblasts from hypertrophic and keloid scars. Ann Plast Surg.
72:711–719. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tao J, Zhang J, Ling Y, Mccall CE and Liu
TF: Mitochondrial sirtuin 4 resolves immune tolerance in monocytes
by rebalancing glycolysis and glucose oxidation homeostasis. Front
Immunol. 9(419)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
O'Leary R, Wood EJ and Guillou PJ:
Pathological scarring: Strategic interventions. Eur J Surg.
168:523–534. 2002.PubMed/NCBI
|
|
27
|
Sarrazy V, Billet F, Micallef L, Coulomb B
and Desmoulière A: Mechanisms of pathological scarring: Role of
myofibroblasts and current developments. Wound Repair Regen. 19
(Suppl 1):S10–S15. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wagner JA: Therapy of pathological scars.
J Dtsch Dermatol Ges. 11:1139–1157. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu YL, Liu WH, Sun J, Hou TJ, Liu YM, Liu
HR, Luo YH, Zhao NN, Tang Y and Deng FM: Mesenchymal stem
cell-mediated suppression of hypertrophic scarring is p53 dependent
in a rabbit ear model. Stem Cell Res Ther. 5(136)2014.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Zhang Q, Liu LN, Yong Q, Deng JC and Cao
WG: Intralesional injection of adipose-derived stem cells reduces
hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther.
6(145)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jia SS, Li WY, Liu X and Li LY:
Transforming growth factor-β1 induces differentiation of bone
marrow-derived mesenchymal stem cells into myofibroblasts via
production of reactive oxygen species. Beijing Da Xue Xue Bao Yi
Xue Ban. 47:737–742. 2015.PubMed/NCBI(In Chinese).
|
|
32
|
Lichtman MK, Otero-Vinas M and Falanga V:
Transforming growth factors β (TGF-β) isoforms in wound healing and
fibrosis. Wound Repair Regen. 24:215–222. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Stagg J: Immune regulation by mesenchymal
stem cells: Two sides to the coin. Tissue Antigens. 69:1–9.
2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Park JE, Seo YK, Yoon HH, Kim CW, Park JK
and Jeon S: Electromagnetic fields induce neural differentiation of
human bone marrow derived mesenchymal stem cells via ROS mediated
EGFR activation. Neurochem Int. 62:418–424. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Manieri NA, Mack MR, Himmelrich MD,
Worthley DL, Hanson EM, Lars E, Wang TC and Stappenbeck TS:
Mucosally transplanted mesenchymal stem cells stimulate intestinal
healing by promoting angiogenesis. J Clin Invest. 125:3606–3618.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zuk P, Zhu M, Mizuno H, Huang J, Futrell
J, Katz A, Benhaim P, Lorenz H and Hedrick M: Multilineage cells
from human adipose tissue: Implications for cell-based therapies.
Tissue Eng. 7:211–228. 2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bayesgenis A, Soler-Botija C, Farré J,
Sepúlveda P, Raya A, Roura S, Prat-Vidal C, Gálvez-Montón C,
Montero JA and Büscher D: Human progenitor cells derived from
cardiac adipose tissue ameliorate myocardial infarction in rodents.
J Mol Cell Cardiol. 49:771–780. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cao Y, Sun Z, Liao L, Meng Y, Han Q and
Zhao RC: Human adipose tissue-derived stem cells differentiate into
endothelial cells in vitro and improve postnatal neovascularization
in vivo. Biochem Biophys Res Commun. 332:370–379. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yoshihiko K, Kobler JB, Herrera VLM and
Zeitels SM: Perspectives on adipose-derived stem/stromal cells as
potential treatment for scarred vocal folds: Opportunity and
challenges. Curr Stem Cell Res Ther. 5:175–181. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yun IS, Jeon YR, Lee WJ, Lee JW, Rah DK,
Tark KC and Lew DH: Effect of human adipose derived stem cells on
scar formation and remodeling in a pig model: A pilot study.
Dermatol Surg. 38:1678–1688. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Spiekman M, Przybyt E, Plantinga JA, Gibbs
S, van der Lei B and Harmsen MC: Adipose tissue-derived stromal
cells inhibit TGF-β1-induced differentiation of human dermal
fibroblasts and keloid scar-derived fibroblasts in a paracrine
fashion. Plast Reconstr Surg. 134:699–712. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zonari A, Martins TM, Paula AC, Boeloni
JN, Novikoff S, Marques AP, Correlo VM, Reis RL and Goes AM:
Polyhydroxybutyrate-co-hydroxyvalerate structures loaded with
adipose stem cells promote skin healing with reduced scarring. Acta
Biomater. 17:170–181. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang Y, Dong W, Wang J, Cai J and Wang Z:
Human omental adipose-derived mesenchymal stem cell-conditioned
medium alters the proteomic profile of epithelial ovarian cancer
cell lines in vitro. Onco Targets Ther. 10:1655–1663.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ivanova-Todorova E, Bochev I, Dimitrov R,
Belemezova K, Mourdjeva M, Kyurkchiev S, Kinov P, Altankova I and
Kyurkchiev D: Conditioned medium from adipose tissue-derived
mesenchymal stem cells induces CD4+FOXP3+
cells and increases IL-10 secretion. J Biomed Biotechnol.
2012(295167)2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Pardali E, Sanchez-Duffhues G,
Gomez-Puerto MC and Dijke PT: TGF-β-induced endothelial-mesenchymal
transition in fibrotic diseases. Int J Mol Sci.
18(2157)2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yichiang H, Chen MJ, Yu YM, Shunyao K and
Chang CC: Suppression of TGF-β1/SMAD pathway and extracellular
matrix production in primary keloid fibroblasts by curcuminoids:
Its potential therapeutic use in the chemoprevention of keloid.
Arch Dermatol Res. 302:717–724. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Emami A, Halim AS, Salahshifar I, Yussof
SJ, Khoo TL and Kannan TP: Association of TGFβ1 and SMAD4 variants
in the etiology of keloid scar in the Malay population. Arch
Dermatol Res. 304:541–547. 2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Jung H, Kim HH, Lee DH, Hwang YS, Yang HC
and Park JC: Transforming growth factor-beta 1 in adipose derived
stem cells conditioned medium is a dominant paracrine mediator
determines hyaluronic acid and collagen expression profile.
Cytotechnology. 63:57–66. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Shah M, Foreman DM and Ferguson MW:
Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition
of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci.
108:985–1002. 1995.PubMed/NCBI
|
|
51
|
Pohlers D, Brenmoehl J, Löffler I, Müller
CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW and Wolf G:
TGF-β and fibrosis in different organs-molecular pathway imprints.
Biochim Biophys Acta. 1792:746–756. 2009.PubMed/NCBI View Article : Google Scholar
|