Introduction
Venous thromboembolism (VTE), including deep venous
thrombosis (DVT) and pulmonary embolism (PE), is the third most
common cardiovascular disease following myocardial infarction and
stroke, with ~10 million new cases diagnosed worldwide each year
(1). VTE is associated with a
relatively high mortality rate and in a 25-year (1966-1990)
inception cohort (n=2218) of Olmsted County, Minnesota, 6% of
patients with DVT and 10% of patients with PE died within 30 days
after disease onset (2). The
long-term morbidity and mortality related to VTE mainly results
from complications such as post-thrombotic syndrome, recurrent
venous thromboembolism and chronic thromboembolic pulmonary
hypertension (3). Due to its
nonspecific clinical manifestations, the misdiagnosis and omission
rate of VTE is relatively high (1).
It is important to examine the dynamic changes of thrombus and the
underlying molecular mechanisms of VTE in order to provide patients
with a diagnosis and treatment strategies.
Previous studies have revealed that risk factors
such as hypercoagulability, stasis, vascular wall damage or
dysfunction may increase the risk of VTE (1). Thrombomodulin (TM), which is a
specific molecular marker for vascular endothelial cell damage, is
a transmembrane glycoprotein that exists on the surface of vascular
endothelial cells and serves as a cofactor for thrombin-mediated
activation of protein C (PC), which is a major anticoagulant that
downregulates thrombin formation and decreases thrombus formation
(4,5). Elevated TM levels can be detected in
different types of body fluid in a variety of diseases associated
with endothelial cell injuries (4).
In a previous study, plasma TM levels were reported to be increased
in patients with VTE and were positively correlated with Caprini
score (6). Furthermore, familial TM
deficiency has been linked to the most common inherited thrombotic
disorders (7). TM has also been
reported to be associated with the formation of DVT; however, few
reports have examined the dynamic changes of TM during the
evolution of DVT.
The increased expression of TM commonly occurs
during inflammation and tumor development, both of which are
closely associated with the NF-κB signaling pathway (8,9).
Increased TM expression has been observed in bladder cancer,
pancreatic cancer, prostate cancer and sepsis, and has been
demonstrated to be closely associated with the activation of NF-κB
(10-14).
Previous studies have suggested that the occurrence of DVT is
closely associated with inflammation (15), and is regulated via the
Sirtuin-1/NF-κB signaling pathway (16). However, to the best of our
knowledge, whether TM is also regulated by NF-κB during the
evolution of DVT has yet to be determined.
The current study aimed to examine the expression of
TM during the evolution of DVT and to explore its potential
association with the NF-κB pathway using pyrrolidine
dithiocarbamate (PDTC), which is an inhibitor of NF-κB (17,18).
The current study also aimed to identify novel diagnosis and
treatment strategies that could be used in patients with DVT.
Materials and methods
Patients
A total of 48 consecutive patients diagnosed with
DVT at the Affiliated Hospital of Nantong University (Nantong,
Jiangsu, China) from April 2019 to December 2019 were enrolled in
the present study. The levels of TM of DVT patients were analyzed
in the 3 age groups, which were defined as the Y group (<64
years; n=26), M group (65-74 years; n=11) and the O group (>75
years; n=10). Patients with any acute or chronic infection or
cancer-related diseases were excluded. Additionally, a total of 23
age and sex matched healthy individuals were enrolled at Affiliated
Hospital of Nantong University (Nantong, Jiangsu, China) from May
2019 to September 2019 as controls in the current study. Venous
blood (2 ml) was drawn from the patients with DVT and healthy
controls, and collected in sterile tubes. The present study was
approved by the Ethics Committee of Affiliated Hospital of Nantong
University, and all participants were required to provide their
written informed consent before enrollment. Clinical
characteristics of patients in these two groups are summarized in
Table I.
 | Table IBaseline characteristics of patients
in the DVT and control group. |
Table I
Baseline characteristics of patients
in the DVT and control group.
| Characteristic | Control (N=23) | DVT (N=48) | P-value |
|---|
| Age, years | 52.77±13.93 | 59.68±18.16 | 0.119 |
| Sex, male | 11 | 21 | 0.802 |
| Body mass index,
kg/m2 | 24.33±3.48 | 24.50±3.29 | 0.849 |
| Diabetes | 0 | 10 | 0.02 |
| Hypertension | 0 | 15 | 0.002 |
| Smoking | 0 | 0 | - |
| Malignant
disease | 0 | 0 | - |
| Recent surgery or
immobilization | 0 | 0 | - |
| VTE history | 0 | 0 | - |
Experimental animals and grouping
Sexually mature male Sprague-Dawley rats (weight,
300±20 g; n=76) that were 10-12 weeks of age, were purchased from
the Laboratory Animal Center of Nantong University. The rats were
kept under a 12 h light/dark cycle, a temperature of
23±3˚C and a humidity of 55-60%, and had free access to
chow and water. All protocols were approved by the institutional
review board for animal experiments at Nantong University. A total
of 9 different time points of the DVT model group (1, 4, 6, 12 and
24 h, and at 3, 7, 14 and 21 days) and a control group were used to
investigate the expression of TM and NF-κB during the evolution of
DVT (n=6x10). In order to further investigate the underlying NF-κB
pathway, another 16 rats were divided randomly into 4 groups: i)
DVT model group (0 mg/kg PDTC); ii) DVT rats treated with 50 mg/kg
PDTC; iii) DVT rats treated with 100 mg/kg PDTC; iv) DVT rats
treated with 150 mg/kg PDTC, n=4x4).
DVT animal model
Inferior vena cava (IVC) stenosis procedures were
performed as described previously under aseptic conditions to
establish the DVT model (16,19).
Specifically, 90% ligation of IVC and complete ligation of side
branches were performed in order to recapitulate human DVT
(20) (Fig. S1). The control animals only
received dissection of IVC and side branches without vein ligation.
Anesthesia was induced in an isoflurane chamber (3-4%) and
maintained with a face mask using 1-2% isoflurane. The rats were
allowed to recover post-surgery and housed under a 12 h light/dark
cycle, a temperature of 23±3˚C and a humidity of 55-60%, and had
free access to chow and water during recovery. Finally, the rats
were sacrificed at h 1, 4, 6 and 12 and day 1, 3, 7, 14 and 21 post
operation, and the control animals were sacrificed at a median time
point of day 1 post surgery. A total of 2 ml venous blood was
collected via the apex cordis into EDTA-coated capillary tubes
prior to the rats being sacrificed. All rats were sacrificed by
cervical dislocation under deep anesthesia with 2% isoflurane.
Post-sacrifice, the thrombosed IVC was carefully dissected on ice,
while the weight (G) and length (cm) of thrombi harvested from the
IVC were measured with an electronic balance (Shanghai Mettler
Toledo Co., Ltd.) and vernier caliper (Deli Co., Ltd.). Thrombi and
endothelial tissues were soaked in paraformaldehyde and used for
hematoxylin and eosin (H&E) staining and immunofluorescence
staining. The remaining tissues were stored at -80˚C and
subsequently used for western blot analysis.
Digital subtraction angiography
(DSA)
Venography was performed on day 1 after receiving
IVC-ligations or sham operations. A fluoroscopy unit (Shimadzu type
C-VISION PLUS; Philips Medical Systems, Inc.) was used to estimate
the thrombus mass within the IVC after ligation. Inhalant
isoflurane induced in an isoflurane chamber (3-4%) and maintained
with a face mask using 1-2% isoflurane was used for the induction
and maintenance of anesthesia on day 1 post operation. Rats were
placed on supine position under the X-ray source, and 2 ml of
contrast medium (Iohexol; OMNIPAQUE 350; GE Healthcare) was
injected through the tail vein at a rate of 0.3 ml/sec to visualize
the adequate filling of the IVC (21). The venographic images were acquired
at 6 frames/sec. The length and width of clots were measured using
interventional workspot (Allura 3D-RA Release 5; Philips Medical
Healthcare, Inc.).
ELISA
Venous blood collected from humans and rats was
centrifugated at 1,789 x g at 4˚C for 10 min and the
plasma was collected and stored at -80˚C until subsequent use.
Plasma levels of TM (Human TM ELISA kit, cat. no. AE90697Hu; Rat TM
ELISA kit; cat. no. AE90697Ra) and NF-κB (Rat nuclear factor Kappa
B ELISA kit; cat. no. AE91992Ra) was quantified using an ELISA kit
(Shanghai United Biotech Co., Ltd.) according to manufacturer's
protocol. All samples were repeated in triplicate.
Histopathological analysis
The thrombotic and endothelial tissues of control
rats and rats sacrificed at each of the nine time-points were fixed
in 4% paraformaldehyde overnight at room temperature and processed
as 4-6 µm thick paraffin sections, then stained with H&E (20
min in hematoxylin solution and 60 sec in eosin solution at room
temperature) following standard procedures. Light microscopy
(magnification, x4) was used to observe typical pathological
changes in rat inferior vena cava thrombus at different time
points.
Immunofluorescence
The thrombotic and endothelial tissues on day 1 post
surgery were fixed in 4% paraformaldehyde overnight at room
temperature and sliced as 4-6 µm thick paraffin sections.
Immunofluorescence staining was performed on paraffin embedded
tissue sections to detect the distribution of TM and NF-κB (p65).
The slices were blocked with 5% bovine Serum Albumin
(Sigma-Aldrich; Merck KGaA) for 2 h at room temperature and
incubated independently with different primary antibodies at 4˚C
overnight. Anti-TM antibodies (rabbit polyclonal; 1:600;
Mybiosource, Inc; cat. no. MBS9606019) and anti-p65 (rabbit
polyclonal; 1:1,000; ProteinTech Group, Inc; cat. no. 10745-1-AP)
were used as primary antibodies. CD34 was used as a marker to
define the distribution of vascular endothelial cell (CD34 mouse
monoclonal; 1:400; NOVUS Biologicals, LLC; cat. no. SA00003-1).
Western blot analysis
Protein was extracted from thrombus and venous
endothelium tissues of control rats and DVT rats at nine
time-points by dissolving in a lysis buffer using a Tissue or Cell
Total Protein Extraction kit (Nanjing KeyGen Biotech Co., Ltd.)
following manufacturer's instructions. The concentration of protein
was quantified using a Drop One spectrophotometer (Thermo Fisher
Scientific, Inc.). 50 µg total protein per gel lane was then
electrophoretically separated on 10% polyacrylamide gels and
transferred onto nitrocellulose membranes. After blocking with 5%
non-fat milk for 1.5 h at room temprature, blots were incubated
with anti-TM (1:1,000; rabbit anti-rat TM; Abcam; cat. no.
ab187075.), anti-p65 (1:1,000; rabbit anti-rat p65, Cell Signaling
Technology, Inc; cat. no. 8242S.) overnight at 4˚C. Membranes were
then were rinsed six times with 1x tris buffered saline with 0.1%
Tween (TBST), before incubation with a secondary antibody
(1:10,000; goat anti-rabbit IgG; Absin Bioscience, Inc; cat. no.
abs20040ss.) for 2 h at room temperature. After rinsing six times
for 10 min each time, images were acquired using a Tanon 2500 Gel
Imaging System (Yph-Bio Co., Ltd.) and processed using ImageJ
software (v.1.8.0; National Institutes of Health). β-actin was used
as the loading control.
Administration of PDTC
PDTC (Selleck Chemicals) was dissolved in sterilized
water and injected at a concentration of 25 mg/ml, similar to a
previously reported protocol (17).
We defined the four groups as follows: C, DVT model group (0 mg/kg
PDTC); LI, DVT rats treated with 50 mg/kg PDTC; MI, DVT rats
treated with 100 ml/kg PDTC; HI, DVT rats treated with 150 mg/kg
PDTC. A total of 4 different concentrations 100 ml/kg of PDTC were
injected into 100 ml/kg the PDTC groups and DVT model group
respectively at 6 h, day 1, 2 and 3 post operation via the tail
vein at a rate of 0.05-0.1 ml/sec. Blood samples were taken 6 h
following each administration via retro-orbital sinus as previously
reported to detect the expression of TM and NF-κB in plasma
(22,23).
Statistical analysis
Statistical analysis was performed using the
statistical software package SPSS (Version 16.0; SPSS, Inc.), and
the graphs were generated using GraphPad Prism 6.0 (GraphPad
Software, Inc.). One-way ANOVA followed by the Bonferroni post-hoc
test was used to compare differences of age and body mass index
between the DVT group and the control group. Differences in
categorical variables between groups were tested using the
χ2 or Fisher’s exact test. One-way ANOVA followed by the
Bonferroni post hoc test was also used to compare differences
between multiple groups. Two-way ANOVA followed by the Bonferroni
post hoc test was only used to compare differences between
different time points in the PDTC groups and DVT model group.
Receiver operating characteristic (ROC) curves and the area under
the ROC curve (AUC) were used to compare the sensitivity and
specificity of the plasma levels of TM for the diagnosis of DVT.
All data were expressed as mean ± SEM, and P<0.05 was considered
to indicate a statistically significant difference.
Results
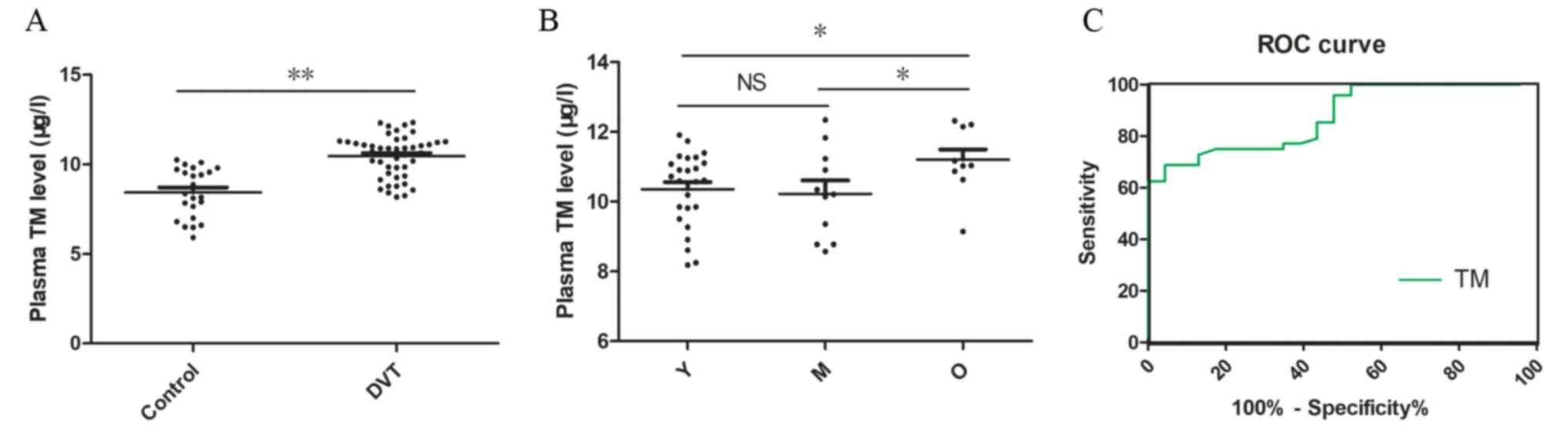
The level of TM is upregulated in
patients with DVT
The levels of TM in plasma collected from 48
patients with DVT and 23 healthy controls was measured using ELISA.
Clinical characteristics of patients in these two groups are
summarized in Table I. Compared
with the control group (8.43±0.29 µg/l), plasma TM levels were
significantly increased in patients with DVT (10.46±0.17 µg/l;
P<0.01; Fig. 1A). The results
also indicated that the levels of TM in the O group (11.20±0.94
µg/l) were significantly higher compared with the Y (10.36±1.05
µg/l; P<0.05) and M group (10.23±1.27) µg/l; P<0.05; Fig. 1B). ROC curve analysis for TM was
performed and the AUC value ranged from 0.795 to 0.953 for TM in
identifying patients with DVT from healthy controls (Fig. 1C). The results suggested that TM
level could differentiate patients with DVT from non-thrombotic
controls with an AUC of 0.874, and the best cut off value for
predicting DVT was 10.12 µg/l.
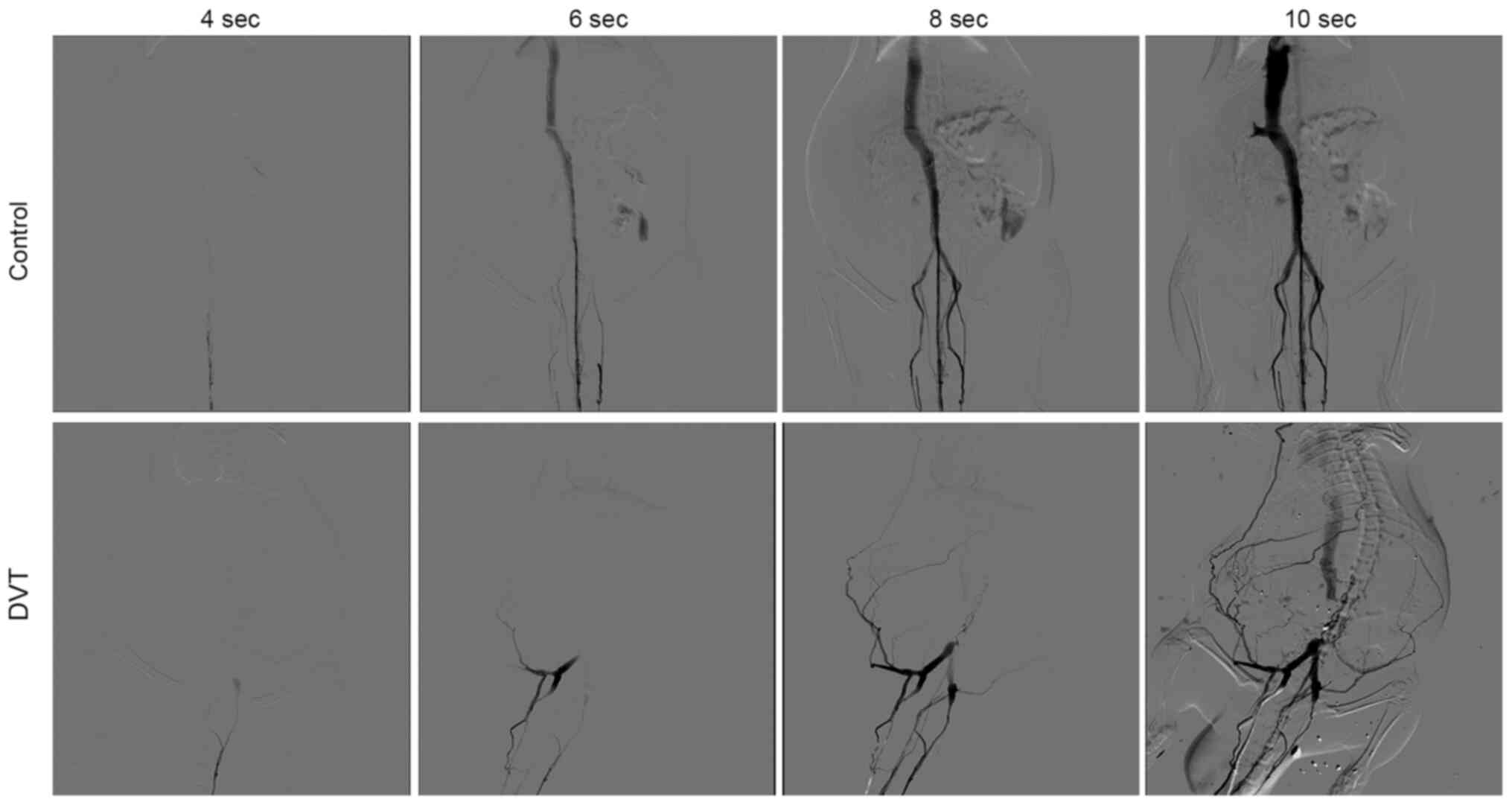
Visualizing venous thrombosis by
DSA
DSA was performed to ensure the successful
establishment of thrombosis in the IVC. Anesthetized rats were
monitored by continuous camera recording. Fig. 2 presents the images at different
time points after the injection of contrast agent. In the control
group who did not receive ligation, the IVC was unobstructed and no
filling defect and occlusion was observed. In the ligation groups,
a partial intraluminal filling defect of the IVC was visualized
under the ligation point, with the formation of side branches. The
images indicate that only a small amount of contrast agent
successfully passed through the occlusion site and reached vessels
above the renal vein through side branches. The results
demonstrated that the DVT model was successfully established via
stenosis of IVC.
Thrombi size progression over
time
Using a vernier caliper and electronic balance, the
length and weight of thrombi formed at h 1, 4, 6 and 12 and day 1,
3, 7, 14 and 21 were measured after the model was established. A
visible thrombus was observed as early as 1 h after ligation.
Thrombus was found only in 4 out of 6 rats at 1 h and 21 days after
modeling. Thrombus weight and length were relatively low in the
early stages of modeling (1, 4, 6, 12 h) and no significant
difference was observed among the four subgroups. The thrombi size
was stabilized at a higher level at 24 h, day 3 and 7 after
modeling and began to decrease afterwards. However, there was no
significant difference between the results of 14 and 21 day
(P>0.05). In general, the results suggested that the thrombi
size and weight exhibited three distinct periods (1-12, 24 h-day 7
and 14-21), as presented in Fig.
3.
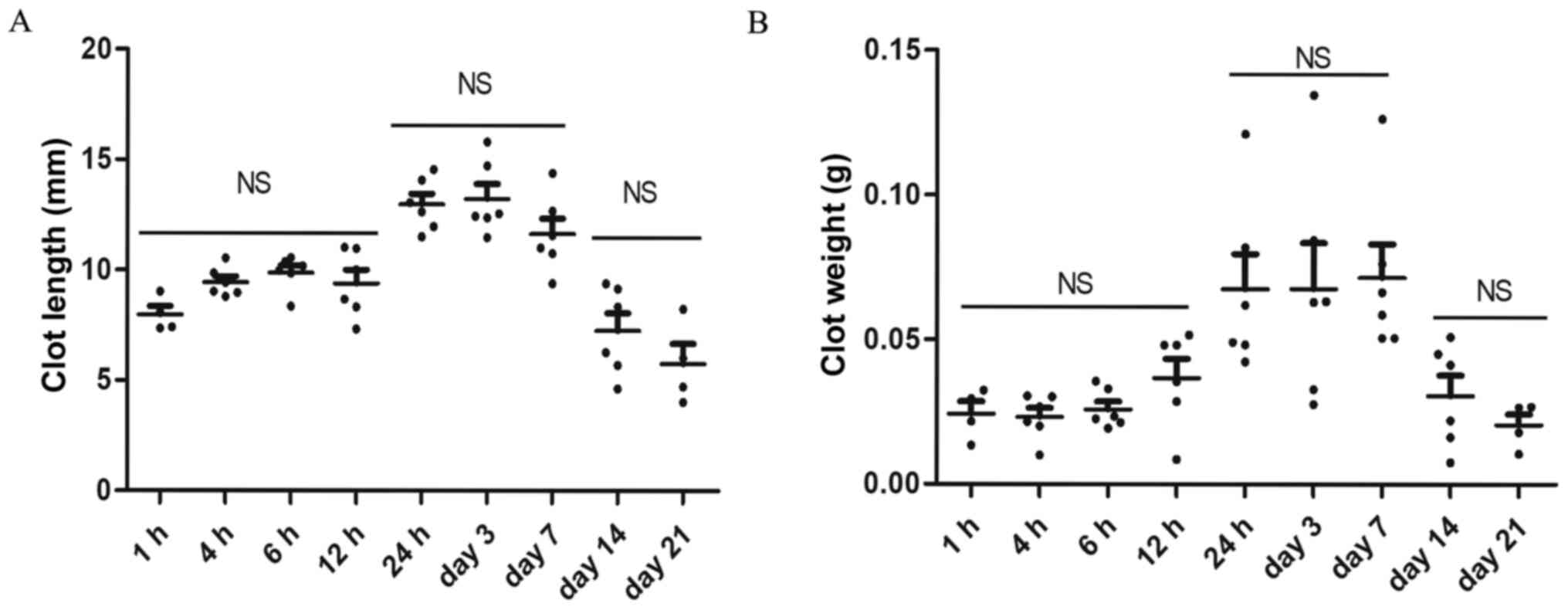 | Figure 3Measurement of thrombus size at
different time points. (A) Thrombus length at h 1, 4, 6 and 12 and
day 1, 3, 7, 14 and 21 post ligation after modeling. (B) Thrombus
weight at h 1, 4, 6 and 12 and day 1, 3, 7, 14 and 21 post
ligation. Data are presented as mean ± SEM. n=6, thrombus was found
only in 4 out of 6 rats at 1 h and 21 days after modeling. NS, no
statistical significance between groups. |
Histopathological results
The examination of the H&E-stained thrombi and
endothelium sections revealed that no thrombosis and disruption of
endothelial cell layer was observed in the control group.
Meanwhile, visible thrombus composed of fibrin red blood cells
which infiltrated with an abundance of inflammatory cells was
identified in the experiment group. The mechanization process
(obvious fibrosis hyperplasia was observed around the thrombus)
started as early as the first day after modeling, and the
completely mechanization was observed at about 2 weeks. Organized
thrombus was firmly adhered to the blood vessel walls and massive
fissures were presented due to the dried thrombus (recanalization).
A period of 21 days after modeling, only a small amount of red
blood cells and fibrin remained in the inferior vena cava. The
representable pathological changes of the thrombus as well as vein
wall at various time points were presented in Fig. S2.
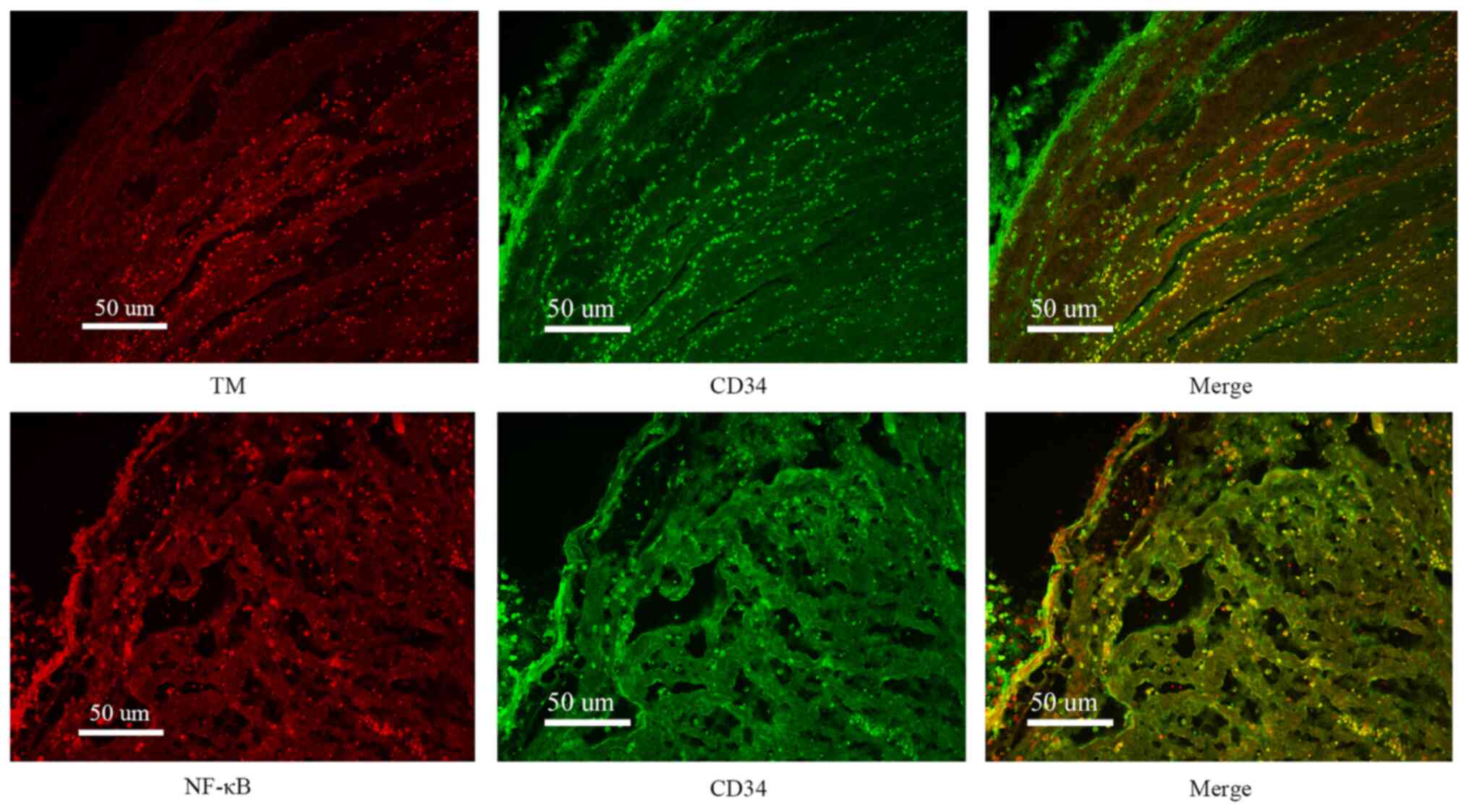
Immunofluorescence results
In order to study the distribution of TM and NF-κB
(p65), immunofluorescence examination was performed. It was
indicated that TM and NF-κB (p65) co-localized within cells
expressing CD34. Additional examination further confirmed the
co-localization of TM and NF-κB (p65) in endothelial cells
(Fig. 4).
TM and NF-κB expression over time
In order to elucidate the role and regulatory
mechanism of TM during the evolution of DVT, the levels of TM and
NF-κB were monitored and compared with the control group in plasma
and thrombi and endothelium tissues at the h 1, 4, 6 and 12 and day
1, 3, 7, 14 and 21 post ligation. The results revealed that DVT
induced the expression of TM and NF-κB in plasma over time
(Fig. 5A and B). The plasma TM levels from 1-12 h post
operation (1 h, 18.70±0.08 µg/l; 4 h, 18.94±0.15 µg/l; 6 h,
19.00±0.09 µg/l; 12 h, 19.03±0.13 µg/l, P<0.01) were increased
compared with the control group (16.26±0.08 µg/l). The plasma TM
levels then plateaued during 1 to 7 days after modeling (24 h,
21.88±0.06 µg/l; day 3, 22.26±0.09 µg/l; day 7, 21.75±0.06 µg/l)
with a significant difference compared with the control group
(P<0.01). Plasma TM levels then subsequently increased on days
14 to 21 following ligation (day 14, 24.04±0.08 µg/l; day 21,
23.97±0.14 µg/l), which was significant when compared with the
control group (P<0.01).
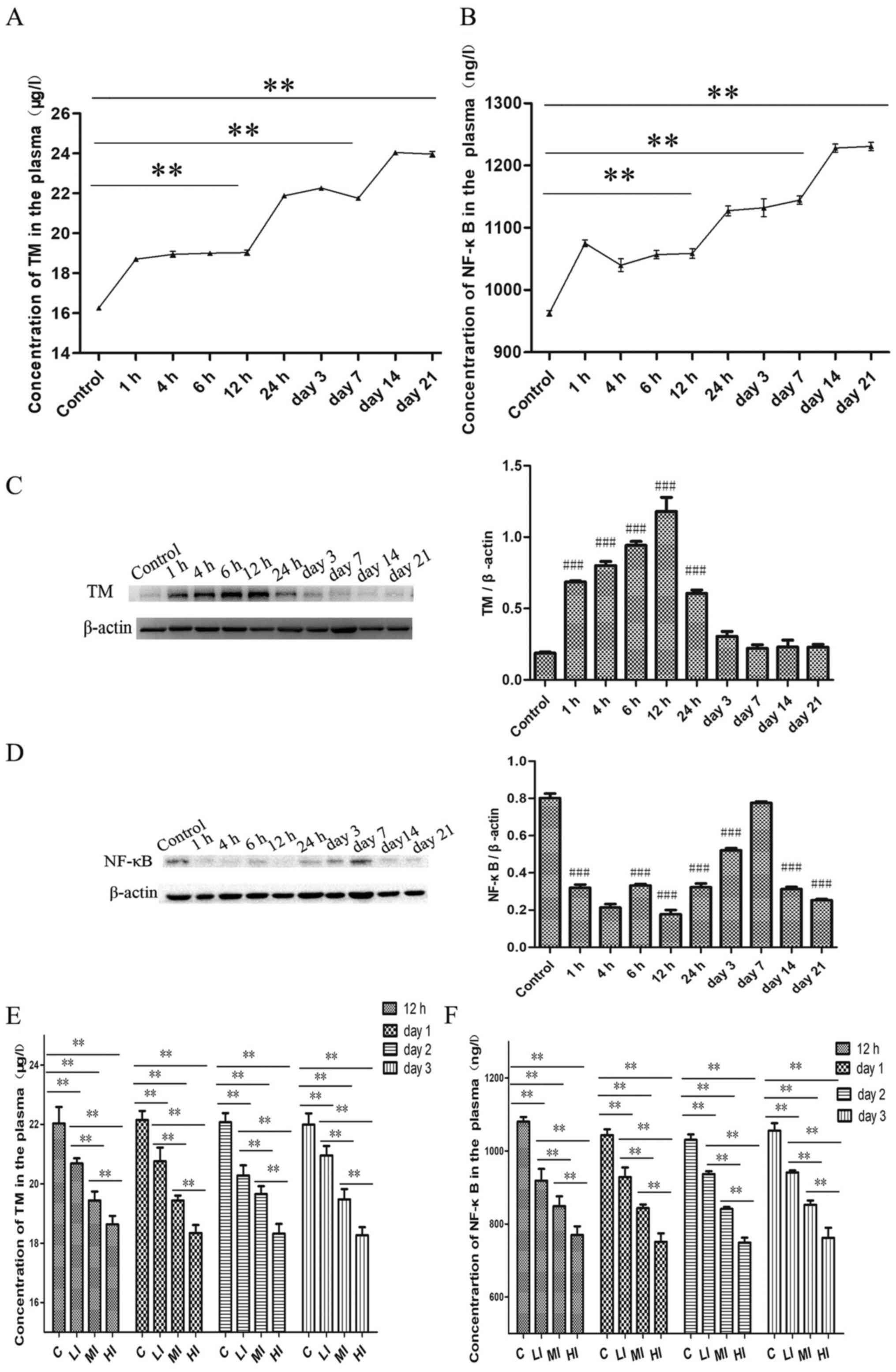 | Figure 5TM and NF-κB expression over time.
(A) The concentration of TM in the plasma during the evolution of
DVT. DVT group vs. the control group: *P<0.05,
**P<0.01, ***P<0.001. (B) The plasma
level of NF-κB at different time points after modeling. DVT group
vs. the control group. (C) The protein expression changes of TM and
(D) NF-κB in endothelium tissues over time. #P<0.05,
##P<0.01 and ###P<0.001 vs. the control
group. (E) The concentration of TM and (F) NF-κB in the plasma
after the administration of PDTC. C, DVT model group (0 mg/kg
PDTC); LI, DVT rats treated with 50 mg/kg PDTC; MI, DVT rats
treated with 100 mg/kg PDTC; HI, DVT rats treated with 150 mg/kg
PDTC. LI, MI, HI vs. the control group; *P<0.05,
**P<0.01 and ***P<0.001 as indicated.
TM, Thrombomodulin; DVT, deep vein thrombosis; PDTC, pyrrolidine
dithiocarbamate. |
Plasma NF-κB levels exhibited similar trend to
plasma TM levels (control, 962.96±4.31 ng/l; 1 h, 1075.22±5.23
ng/l; 4 h, 1039.99±10.25 ng/l; 6 h, 1056.73±6.66 ng/l; 12 h,
1058.89±7.64 ng/l; 24 h, 1127.41±7.90 ng/l; 3 day, 1132.12±14.42
ng/l; 7 day, 1144.41±6.73 ng/l; 14 day, 1228.10±6.52 ng/l; 21 day,
1230.68±6.69 ng/l). The results suggested that thrombi size, TM and
NF-κB expression exhibited three distinct periods (1-12, 24 h-day 7
and 14-21) of markedly different results between periods. Compared
with the control group, TM in the vascular endothelium of the DVT
group was upregulated up until day 1 post surgery while NF-κB (p65)
was downregulated except for day 7, where no significant difference
was demonstrated (Fig. 5C and
D). The concentrations of TM and
NF-κB in the thrombus were undetectable (data not shown).
Subsequently, PDTC was used as an inhibitor of NF-κB
and injected into the DVT model rats via the vein tail. The effect
of inhibiting NF-κB activation was confirmed by ELISA and the
result revealed that compared with DVT rats that received saline,
the plasma levels of TM and NF-κB were downregulated in rats
received PDTC treatment in a dose-dependent manner (C vs. LI; C vs.
MI; C vs. HI; LI vs. MI; P<0.01; LI vs. HI; MI vs. HI; all,
P<0.01), as presented in Fig. 5E
and F.
Discussion
In the present study, the level of TM as a marker of
vascular endothelial dysfunction during DVT evolution and its
possible association with the NF-κB signaling pathway. A DVT animal
model was established using the ‘stenosis’ method and the levels of
TM at 9 different time points were measured during the evolution of
DVT. The data demonstrated that plasma TM levels and thrombus size
were increased along with the activation of NF-κB signaling
pathway.
DVT formation has been previously thought to be
associated with blood stagnancy, hypercoagulability and venous
endothelial dysfunction (24).
However, there is increasing evidence suggesting that inflammation
also serves an important role in the pathogenesis of DVT (15,25,26).
TM is a glycoprotein and serves as a cofactor for
thrombin-mediated activation of protein C (PC), which is a major
anticoagulant that downregulates thrombin formation and decreases
thrombus formation (4,10). In addition to its anticoagulant
activity, studies have revealed that TM exhibits potent
anti-inflammatory capabilities via a variety of molecular
mechanisms (4,10).
TM exists not only as a cellular membrane bound form
but also as a soluble form within plasma (5). It has been previously indicated that
increased levels of soluble TM are associated with vascular damage,
infection and sepsis (5). In the
current study, it was revealed that the levels of soluble TM were
increased significantly in patients with DVT compared with healthy
controls with an AUC of 0.874, providing a promising diagnostic
method for DVT, as previously reported (6). Consistent with a previous study, the
results of the current study also indicated that circulating TM was
associated with age (27). Due to
the limited sample size in the current study, further
investigations should enroll additional patients to assess the
levels of TM among different age groups. A previous report revealed
that levels of soluble TM were higher in controls compared with
patients with DVT (28); however,
another studies results were consistent to the current study in
which the VTE group exhibited significantly higher levels of
soluble TM, and positively correlated with Caprini risk
stratification (6,29). The discrepancy among the studies may
be due to the different study population and sample size. The
population enrolled in the study by Bombeli et al (28) were Swiss (n=229), whereas patients
were Chinese in the current study (n=71). Moreover, there were
differences in the inclusion and exclusion criteria. The present
study included patients with first-onset acute DVT, while Bombeli
et al included patients with a history of one or more venous
thromboembolic events. Different populations and sample sizes may
have affected the results. It has also been previously suggested
that TM exerts anti-inflammatory and anti-fiber deposition effects
(4,10). As DVT is an inflammatory disease,
the anti-inflammatory effects may also significantly increase by
upregulating the expression of TM to exert its anti-inflammatory
and anti-fiber deposition effects (15). Based on the effects of TM, the
levels of TM may increase in patients with DVT.
Many patients with DVT may be asymptomatic, so their
optimal diagnosis period may be missed (30). Despite of its low specificity, the
level of plasma TM may be used as a potent dynamic monitoring
biomarker, reflecting the evolution of DVT. The current study aimed
to investigate the dynamic changes of TM during the evolution of
DVT. In further studies, the expression of TM in other inflammatory
diseases, such as sepsis, inflammatory bowel disease, ulcerative
colitis and pneumonia will be further explored to investigate the
specificity of TM. In the current study, plasma levels and
endothelial expression of TM at different time points were measured
during DVT evolution. At the thrombus formation period (1-12 h
after modeling), plasma levels and endothelial expression of TM
were increased. It was speculated that the increase of TM level
resulted from the initiation of the inflammatory response and
endothelial cell damage. Although the expression of TM in the
endothelium decreased after 24 h, the expression of TM in the
plasma remain increased, indicating that soluble TM in the plasma
may be derived from other sources at this period. In line with the
results gained in the present study, Sane et al (29) reported that the plasma TM levels of
patients with PE were lower in the acute phase compared with the
stable phase (seven months later). However, the exact mechanism
behind this phenomenon needed to be further evaluated in future
studies.
Recombinant human soluble TM (ART-123) has been
reported to be a highly effective antithrombotic agent in the
prevention of VTE post total hip replacement surgeries (31). Previous studies have reported that
increased TM levels indicated worse prognosis of pregnancy-induced
hypertension and pre-eclampsia (32). Meanwhile, recombinant-TM improved
not only the fetal-placental blood flow but also oxygenation in the
placenta and fetal brain, making it a possible candidate treatment
strategy for pre-eclampsia complications (4). Therefore, it was speculated that the
vascular endothelial cell injury and inflammation promoted
prothrombotic changes and induced the observed increase in TM
secretion. Therefore, TM may not only be a marker for DVT but also
be a potential treatment target.
It has been previously indicated that NF-κB
transcription factor p50 is essential for the pathogenesis of DVT,
and suggested that specific inhibitors of p50, such as Andro, may
be therapeutically valuable for preventing and treating venous
thrombosis (33). In order to
identify the mechanism of how TM contributes to the pathogenesis of
DVT, NF-κB in plasma and endothelium was investigated In the
current study, although the expression of NF-κB in the endothelium
was decreased in the DVT groups, plasma NF-κB levels were
increased. The increase of NF-κB levels in plasma was consistent
with the results of a previous study (34). Further studies are required to
evaluate the mechanism behind the downregulation of NF-κB in the
endothelium. The current results revealed that the increased
secretion of TM during the evolution of DVT was associated with the
NF-κB pathway, confirming its significant role in DVT.
Furthermore, the current study attempted to verify
the regulatory effect of NF-κB on TM via the induction of PDTC,
which is a specific NF-κB inhibitor that inhibits the activation
and nuclear translocation of NF-κB through inhibiting the
phosphorylation of I κB (17,18).
The results of present study revealed that inhibition of NF-κB
expression by PDTC reduced plasma TM concentration in a
dose-dependent manner. Similarly, Deng et al (35) demonstrated in a lipopolysaccharide
(LPS)-induced vascular endothelial injury model in human umbilical
vein endothelial cells that Puerarin could prevent vascular
endothelial injury by suppressing the activation of NF-κB, and
identified that this mechanism was associated with the
downregulation of inflammatory factors and coagulation-related
factors including TM.
There were some limitations of the current study.
Firstly, it should be noted that rat models were used and these may
not perfectly recapitulate all human features of DVT. Especially
when considering the fact that the animal model was built on
healthy animals with no underlying inflammatory or pro-coagulant
conditions. Secondly, the changes of TM and NF-κB were not measured
in the in endothelium following the administration of PDTC.
Thirdly, this was a single-center study with a cohort of a limited
number of patients that may not be necessarily be representable to
the general population.
In conclusion, the results of the current study
suggested that plasma TM levels were increased significantly in
patients with DVT. Furthermore, the results of the animal
experiment revealed that the increased secretion of TM during the
evolution of DVT was associated with the NF-κB pathway and changed
along with thrombus size, indicating that the level of plasma TM
may be a potent biomarker for the evolution of DVT.
Supplementary Material
Rat DVT model modeling process diagram
(stenosis). Skin preparation and disinfection of the abdominal
surgery area. (B) Making a 2 cm incision in the middle of the
abdomen. (C) Full exposure and separating the inferior vena cava
branches below the left renal vein to the level of the iliac vein.
(D) Ligation of the visible branches one by one using a 5.0 silk
suture. (E) Placing a 5.0 silk suture tightly around the IVC
together with a 4.0 silk suture. (F) Ligation of the IVC. (G)
Removing the 4.0 silk suture. (H) Suturing the abdominal muscle,
skin and skin disinfection. DVT, deep vein thrombosis; IVC,
Inferior vena cava.
Typical pathological changes in rat
inferior vena cava thrombus at different time points.
Magnification, x100.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Medical
Innovation Team of Jiangsu Province (grant no. Suweikejiao
2017).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC, BS and XY, YZ contributed to the conception and
design of the project. SL and DL collected the clinical samples. XC
and BS performed all the experiments. XC drafted the manuscript
with critical review by BS, XY and YZ. YZ supervised the findings
of this work. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The protocol of the present study was approved by
the Ethics Committee of Affiliated Hospital of Nantong University
and informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Di Nisio M, van Es N and Büller HR: Deep
vein thrombosis and pulmonary embolism. Lancet. 388:3060–3073.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Thomas M, Hollingsworth A and Mofidi R:
Endovascular management of acute lower limb deep vein thrombosis: A
systematic review and meta-analysis. Ann Vasc Surg. 58:363–370.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Galanaud JP, Monreal M and Kahn SR:
Epidemiology of the post-thrombotic syndrome. Thromb Res.
164:100–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shin M, Hino H, Tamura M, Ishizuka B,
Tanaka M, Suzuki N and Tateda T: Thrombomodulin improves maternal
and fetal conditions in an experimental pre-eclampsia rat model. J
Obstet Gynaecol Res. 40:1226–1234. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Anastasiou G, Gialeraki A, Merkouri E,
Politou M and Travlou A: Thrombomodulin as a regulator of the
anticoagulant pathway: Implication in the development of
thrombosis. Blood Coagul Fibrinolysis. 23:1–10. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fu Y, Liu Y, Chen S, Jin Y and Jiang H:
The combination of caprini risk assessment scale and thrombotic
biomarkers to evaluate the risk of venous thromboembolism in
critically ill patients. Medicine (Baltimore).
97(e13232)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Salvagno GL, Pavan C and Lippi G: Rare
thrombophilic conditions. Ann Transl Med. 6(342)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer.
12(86)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang Q, Lenardo MJ and Baltimore D: 30
Years of NF-κB: A blossoming of relevance to human pathobiology.
Cell. 168:37–57. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shirai Y, Uwagawa T, Shiba H, Shimada Y,
Horiuchi T, Saito N, Furukawa K, Ohashi T and Yanaga K: Recombinant
thrombomodulin suppresses tumor growth of pancreatic cancer by
blocking thrombin-induced PAR1 and NF-κB activation. Surgery.
161:1675–1682. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ma J and Bai J: Protective effects of
heparin on endothelial cells in sepsis. Int J Clin Exp Med.
8:5547–5552. 2015.PubMed/NCBI
|
|
12
|
Wu CT, Chang YH, Lin P, Chen WC and Chen
MF: Thrombomodulin expression regulates tumorigenesis in bladder
cancer. BMC Cancer. 14(375)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Menschikowski M, Hagelgans A, Tiebel O,
Vogel M, Eisenhofer G and Siegert G: Regulation of thrombomodulin
expression in prostate cancer cells. Cancer Lett. 322:177–184.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Song D, Ye X, Xu H and Liu SF: Activation
of endothelial intrinsic NF-{kappa}B pathway impairs protein C
anticoagulation mechanism and promotes coagulation in endotoxemic
mice. Blood. 114:2521–2529. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liang W, Wei F, Yang C, Xie F, Shuai XX,
Wang M and Yu M: GDF-15 is associated with thrombus burden in
patients with deep venous thrombosis. Thromb Res. 187:148–153.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yao X, Chen W, Liu J, Liu H, Zhan JY, Guan
S, Lu Z, Tang P, Li P and Lin B: Deep vein thrombosis is modulated
by inflammation regulated via sirtuin 1/NF-κB signalling pathway in
a rat model. Thromb Haemost. 119:421–430. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Qin JD, Cao ZH, Li XF, Kang XL, Xue Y, Li
YL, Zhang D, Liu XY and Xue YZ: Effect of ammonium pyrrolidine
dithiocarbamate (PDTC) on NF-κB activation and CYP2E1 content of
rats with immunological liver injury. Pharm Biol. 52:1460–1466.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yin J, Wu M, Duan J, Liu G, Cui Z, Zheng
J, Chen S, Ren W, Deng J, Tan X, et al: Pyrrolidine dithiocarbamate
inhibits NF-KappaB activation and upregulates the expression of
Gpx1, Gpx4, occludin, and ZO-1 in DSS-induced colitis. Appl Biochem
Biotechnol. 177:1716–1728. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Z, Hu L, Chen W, Zhou C, Gui G and
Lin B: Danhong huayu koufuye prevents deep vein thrombosis through
anti-inflammation in rats. J Surg Res. 201:340–347. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Diaz JA, Obi AT, Myers DD Jr, Wrobleski
SK, Henke PK, Mackman N and Wakefield TW: Critical review of mouse
models of venous thrombosis. Arterioscler Thromb Vasc Biol.
32:556–562. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vukojevic J, Siroglavic M, Kasnik K, Kralj
T, Stancic D, Kokot A, Kolaric D, Drmic D, Sever AZ, Barisic I, et
al: Rat inferior caval vein (ICV) ligature and particular new
insights with the stable gastric pentadecapeptide BPC 157. Vascul
Pharmacol. 106:54–66. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sharma A, Fish BL, Moulder JE, Medhora M,
Baker JE, Mader M and Cohen EP: Safety and blood sample volume and
quality of a refined retro-orbital bleeding technique in rats using
a lateral approach. Lab Anim (NY). 43:63–66. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Goicoechea M, Cía F, San José C, Asensio
A, Emparanza JI, Gil AG, López de Cerain A, Aldazabal P, Azpitarte
M, Otaegui D and López de Munain A: Minimizing creatine kinase
variability in rats for neuromuscular research purposes. Lab Anim.
42:19–25. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bagot CN and Arya R: Virchow and his
triad: A question of attribution. Br J Haematol. 143:180–190.
2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Iba T and Levy JH: Inflammation and
thrombosis: Roles of neutrophils, platelets and endothelial cells
and their interactions in thrombus formation during sepsis. J
Thromb Haemost. 16:231–241. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vazquez-Garza E, Jerjes-Sanchez C,
Navarrete A, Joya-Harrison J and Rodriguez D: Venous
thromboembolism: Thrombosis, inflammation, and immunothrombosis for
clinicians. J Thromb Thrombolysis. 44:377–385. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ochi A, Adachi T, Inokuchi K, Ogawa K,
Nakamura Y, Chiba Y, Kawasaki S, Onishi Y, Onuma Y, Munetsugu Y, et
al: Effects of aging on the coagulation fibrinolytic system in
outpatients of the cardiovascular department. Circ J. 80:2133–2140.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bombeli T, Jutzi M, De Conno E, Seifert B
and Fehr J: In patients with deep-vein thrombosis elevated levels
of factor VIII correlate only with von Willebrand factor but not
other endothelial cell-derived coagulation and fibrinolysis
proteins. Blood Coagul Fibrinolysis. 13:577–581. 2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sane M, Granér M, Laukkanen JA, Harjola VP
and Mustonen P: Plasma levels of haemostatic factors in patients
with pulmonary embolism on admission and seven months later. Int J
Lab Hem. 40:66–71. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kalayci A, Gibson CM, Chi G, Yee MK,
Korjian S, Datta S, Nafee T, Gurin M, Haroian N, Qamar I, et al:
Asymptomatic deep vein thrombosis is associated with an increased
risk of death: Insights from the APEX trial. Thromb Haemost.
118:2046–2052. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kearon C, Comp P, Douketis J, Royds R,
Yamada K and Gent M: Dose-response study of recombinant human
soluble thrombomodulin (ART-123) in the prevention of venous
thromboembolism after total hip replacement. J Thromb Haemost.
3:962–968. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ayala-Ramírez P, Buitrago T, Poveda A,
Rodríguez JL, Olaya-C M and García-Robles R: Increased tissue
factor and thrombomodulin expression and histopathological changes
in placentas of pregnancies with preeclampsia. J Neonatal Perinatal
Med. 9:31–39. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li YD, Ye BQ, Zheng SX, Wang JT, Wang JG,
Chen M, Liu JG, Pei XH, Wang LJ, Lin ZX, et al: NF-kappaB
transcription factor p50 critically regulates tissue factor in deep
vein thrombosis. J Biol Chem. 284:4473–4483. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li G, Zhou R, Zhao X, Liu R and Ye C:
Correlation between the expression of IL-18 and deep venous
thrombosis. Int J Mol Med. 42(2972)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Deng HF, Wang S, Li L, Zhou Q, Guo WB,
Wang XL, Liu MD, Liu K and Xiao XZ: Puerarin prevents vascular
endothelial injury through suppression of NF-ΚB activation in
LPS-challenged human umbilical vein endothelial cells. Biomed
Pharmacother. 104:261–267. 2018.PubMed/NCBI View Article : Google Scholar
|