Introduction
In defence against infections, the immune system
regulates the functions of a multitude of cells and molecules to
fight invading pathogens. Physical and psychological stress, sleep
deprivation and malnutrition are likely to cause suboptimal immune
system function, thereby creating an opportunity for infectious
agents to invade the body (1-4).
The effects of intense and prolonged exercise has been documented
to adversely affect the immune system (5), resulting in immunosuppression lasting
for 3-72 h (6), or occasionally 1-2
weeks for athletes in competition (7). The concept of an ‘open window’, where
an individual may be more susceptible to infectious agents
(8), is often used to explain the
reason behind upper respiratory tract infections or symptoms (URTI
or URTS) following a taxing bout of exercise (9-11).
Accordingly, the use of exercise as a model for immune stress has
been employed in previous studies (12,13).
The incidence of URTS has been reported to be higher among athletes
subjected to high-intensity and endurance exercise, such as
marathon and ultramarathon running, particularly in the days
leading up to and after the competition (9,14,15).
Marathon running has been demonstrated to transiently suppress
various aspects of immunity (16),
and an increase in the levels of certain immune markers after a
marathon race have been linked to increased susceptibility to
infections (17). In particular,
the URTI incidence was reported to be 2.5- to ~6-fold higher among
marathon runners in previous studies (18,19).
Therefore, marathon running has been employed by different research
groups as a stressor to the immune system in order to investigate
the impact of dietary supplements on the incidence and severity of
URTS (20-22).
The aim of the present study was to investigate the
potential health benefits of a dietary supplement, IQP-AS-119, in
reducing the incidence and severity of URTS in marathon runners,
providing an indication of the supplement's efficacy in aiding the
body's immune system under conditions of stress, as modelled by the
marathon run.
IQP-AS-119 is a dietary supplement containing a
proprietary blend of ingredients, including a patent-pending
standardized garlic extract, with green chireta (Andrographis
paniculata), plant superoxide dismutase extract, vitamin B
complex, cholecalciferol and zinc. Allicin, which is an
organosulphur compound found in garlic, has been indicated to
reduce the incidence of common cold symptoms in a previous 12 week
study (23). Green chireta has also
been demonstrated to be beneficial in improving URTI symptom scores
(sore throat, sleep disturbances, cough, headache and earache) in
randomized controlled trials in patients with uncomplicated URTI
(24,25).
Plant superoxide dismutase extracts have been
demonstrated to alleviate stress (26,27).
Daily intake of concentrates containing superoxide dismutase has
also been revealed to reduce physical and mental fatigue along with
stress in healthy individuals compared with those receiving placebo
treatment (26).
Cholecalciferol at 300 IU has been indicated to
reduce the risk of reported acute URTI by 50% compared with those
receiving placebo treatment (28).
A review on cholecalciferol supplementation suggested a daily
intake of 1,000 IU per adult as a prophylaxis for respiratory tract
illnesses (29).
Zinc acetate administered in the form of lozenges at
the onset of a common cold has been indicated to reduce the
severity and duration of cold symptoms (30,31).
Thiamine supplementation has also been indicated to be beneficial
in reducing fatigue associated with exercise (32,33).
Folic acid supplementation, as demonstrated in a 12-week study,
resulted in an increase in the serum levels of proteins involved in
the regulation and activation of immune function (34). Based on the reports of beneficial
effects of these ingredients, it was hypothesized that IQP-AS-119
may be used to support immune function and improve energy levels
when the body is under stress, helping individuals maintain optimal
health.
Materials and methods
Ethics and consent
The present trial was performed in accordance with
the principles of the Declaration of Helsinki (35) and the EU recommendations for Good
Clinical Practice (EMA/CHMP/ICH/135/95), E6(36), and was approved by the Ethics
Committee of the Charité-Universitätsmedizin Berlin. All
participants provided written informed consent prior to their
participation in the trial. This trial was registered at Clinicaltrials.gov as NCT02873910 (registered 22
August 2016; retrospectively registered, https://clinicaltrials.gov/ct2/show/NCT02873910).
Design
The current study was a single-centre, double-blind,
randomized, placebo-controlled pilot trial involving participants
in the Berlin Marathon 2016. The study began ~70 days before the
marathon and lasted until ~25 days after the marathon. The
treatment period started 3 weeks prior to and continued for 2 weeks
after the Berlin Marathon on 25 September 2016. The study involved
2 visits to the study centre at the beginning and the end of the
study, with 2 reminder phone calls between the visits.
Participants
Participation in the study was open to registered
runners of the 2016 Berlin marathon. The aim was to recruit 80
participants to be randomized into this exploratory pilot study. No
formal hypothesis testing had been prespecified. The main
eligibility criteria for participation included Caucasians of
either sex, aged 18-69 years, who were residing in either Berlin or
Brandenburg. The participants should have successfully completed at
least 1 marathon in the last 2 years, with a completion time of
3-5.5 h. The participants were required to be non-smokers, or to
have ceased smoking for at least 12 months prior to the screening
visit. They should also have regular sleeping patterns without
suspected sleeping disorders for the 3 months prior to the
screening visit. Participants with a history of severe
cardiovascular disease or collapse during a running event, or
during training, were excluded. Participants with any abnormalities
observed in the screening exercise electrocardiogram that pointed
to an increased cardiovascular risk, systolic blood pressure ≥140
mmHg and/or diastolic blood pressure ≥90 mmHg, were considered
ineligible for the pilot study. Runners with URTS, injuries and/or
were unable to complete all questionnaires were excluded from the
trial. Finally, a total of 80 healthy asymptomatic adults that fit
the inclusion criteria participated in the pilot trial.
Participants were recruited between July and August 2016.
Treatment groups
Numbered days with a minus sign were used to
indicate the time period before the marathon, whereas numbered days
without a minus sign were used to indicate the trial period after
the marathon. Between day -70 and day -26, the participants were
randomly allocated to either the IQP-AS-119 group (V group) or
placebo group (P group). The investigational product (IP) was
either the IQP-AS-119 in tablet form, or a placebo tablet identical
to the IQP-AS-119, which contained microcrystalline cellulose
instead of the active ingredients. Participants in the V group took
one tablet of IQP-AS-119 and participants in the P group took one
placebo tablet daily from day -21 to day 14.
During the study, the participants refrained from
taking nutritional supplements, or other ‘immune support’ products,
systemic analgesics (other than paracetamol up to 2,000 mg/day or
ibuprofen up to 800 mg/day) and antibiotics. Additionally, subjects
were asked to refrain from strenuous exercise (other than
completing training runs).
Assessments
The benefits of the IP were assessed by a number of
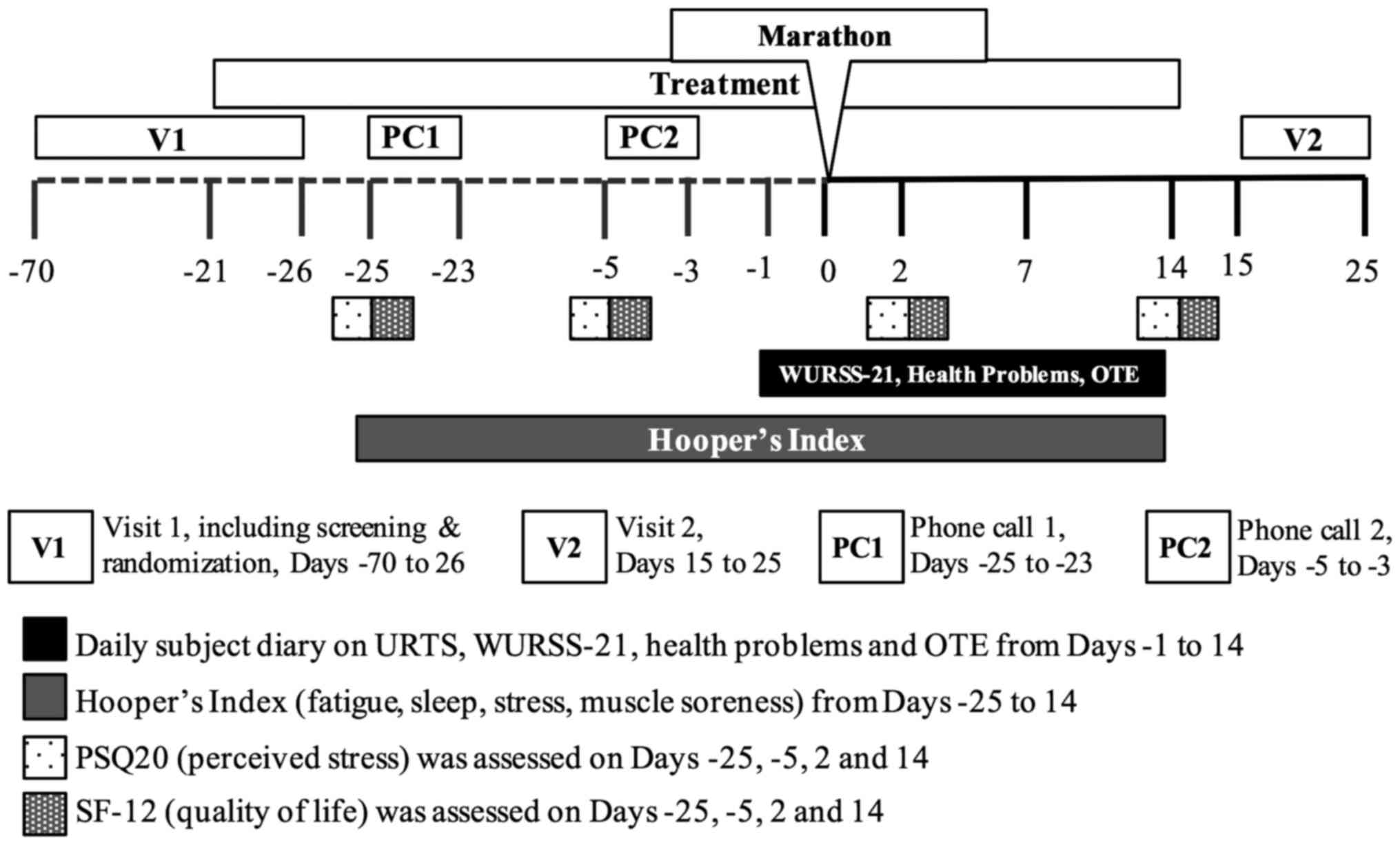
questionnaires distributed during the study. An overview of the
schedule of events for the trial is presented in Fig. 1.
Participants were asked ‘Do you experience any
health problems today?’ and ‘Do you think you have a cold/URTS?’ in
the participant diary from Day -1 to Day 14 (for example, at 14
days after the marathon). On any day that the participant answered
‘yes’ to having health problems, they were asked to report any
ailments they were suffering from, such as gastrointestinal
symptoms, muscle/joint/bone problems, injuries, allergies or other
infections.
Any day of answering ‘yes’ to having a cold/URTS was
considered as a URTS day. Furthermore, in the daily participant
diary from Day -1 to Day 14, the Wisconsin Upper Respiratory
Symptom Survey (WURSS-21) was also completed. The WURSS-21 is a
validated illness-specific instrument used to assess the negative
impact of acute URTI that is presumed to be viral (for example, the
common cold) on the participant's quality of life (QoL) (37). Item 1 in the WURSS-21 asked the
participants ‘How sick do you feel today?’, items 2-11 assessed the
participants' severity of URTS, while items 12-20 assessed the
extent to which the participants' symptoms interfered with daily
activities. Each item was to be rated by on a scale of 0 (no
symptom/not affected), to 7 (severe). Item 21 in the WURSS-21 asked
the participants to assess whether their symptoms were better or
worse compared with the day before. For each URTS day, the severity
was evaluated using the WURSS-21.
At the end of the first week (day 7) and second week
(day 14) after the marathon, the participants were asked to
indicate whether their health had improved, remained the same, or
worsened since the marathon (day 0) using the overall treatment
effect (OTE) assessment (38).
Participants were asked ‘How has your health changed since your
participation in the marathon?’ and they rated their response on a
15-point global rating scale from -7 (a great deal worse), through
0 (no change) to +7 (a great deal better) (38). The change of the participants'
scores was calculated by taking the difference between the scores
for day 14 minus day 7.
On each day of the trial, from day -25 through to
day 25, the participants assessed their well-being by rating their
fatigue, stress, delayed onset of muscle soreness, and sleep
quality/disorders on a scale of 1 to 7 for each of the 4 items in
the Hooper's Index (39). The
Hooper's Index is the summation of the 4 ratings.
Additionally, the participants filled out the
Perceived Stress Questionnaire (PSQ20) (40) and the Short Form 12 Health Survey
Questionnaire (SF-12) (41) on days
-25, -5, 2 and 14 of the trial. The PSQ20 had 20 items in 4 scales:
Worries, tension, joy and demands. For each item, the participants
were asked to evaluate the frequency of occurrence of each item
during the preceding week using values from 1 (almost never) to 4
(usually). The total score of the items for worries, tension, joy
and demands was calculated from the participants' ratings and
transformed according to the instructions provided by the
questionnaire's developers (40),
resulting in transformed scores ranging from 0 to 100. The total
PSQ20 score was used to estimate the participants' stress levels.
The SF-12 assessed the participants' general well-being during the
preceding week, with evaluation of 12 items clustered into 2 scores
for the physical and mental composites according to the
questionnaire's instructions (41).
The changes to the composite scores were calculated by taking the
mean composite scores on day 14 minus the mean composite scores on
either day -5 or day 2.
At the end of the study, both the participants and
investigators independently evaluated the benefit of the IP by
means of a global scale (‘very good’, ‘good’, ‘moderate’ and
‘poor’).
The assessment of tolerability and safety included
physical examination, recording of vital signs at each visit, and
analysis of full blood count parameters, liver and renal function
parameters (alanine transaminase, aspartate aminotransferase, γ-GT,
alkaline phosphatase, bilirubin, creatinine, urea and uric acid),
carbohydrate and lipid metabolism parameters at the beginning and
at the end of the study.
Statistical analysis
The results are presented as the means ± standard
deviation. Non-parametric tests were used for the analyses of the
differences between groups, namely the Mann-Whitney U-test for
independent groups and the χ2 test for the comparison of
percentages. The Kaplan-Meier method was used to compare
differences between groups for cumulative URTS days and Hooper's
indices. Both the full analysis set (FAS) and valid case analysis
set (VCAS) were analysed in order to detect any differences between
them (42). In cases where there
was a major non-compliance of the protocol, the subject data was
excluded from the FAS and only included in the VCAS analyses. All
statistical analyses were performed using SPSS, version 22.0 (IBM
Corp.). All tests were performed with a significance level (type I
error) of 5.0% (two-tailed test).
Results
Participants
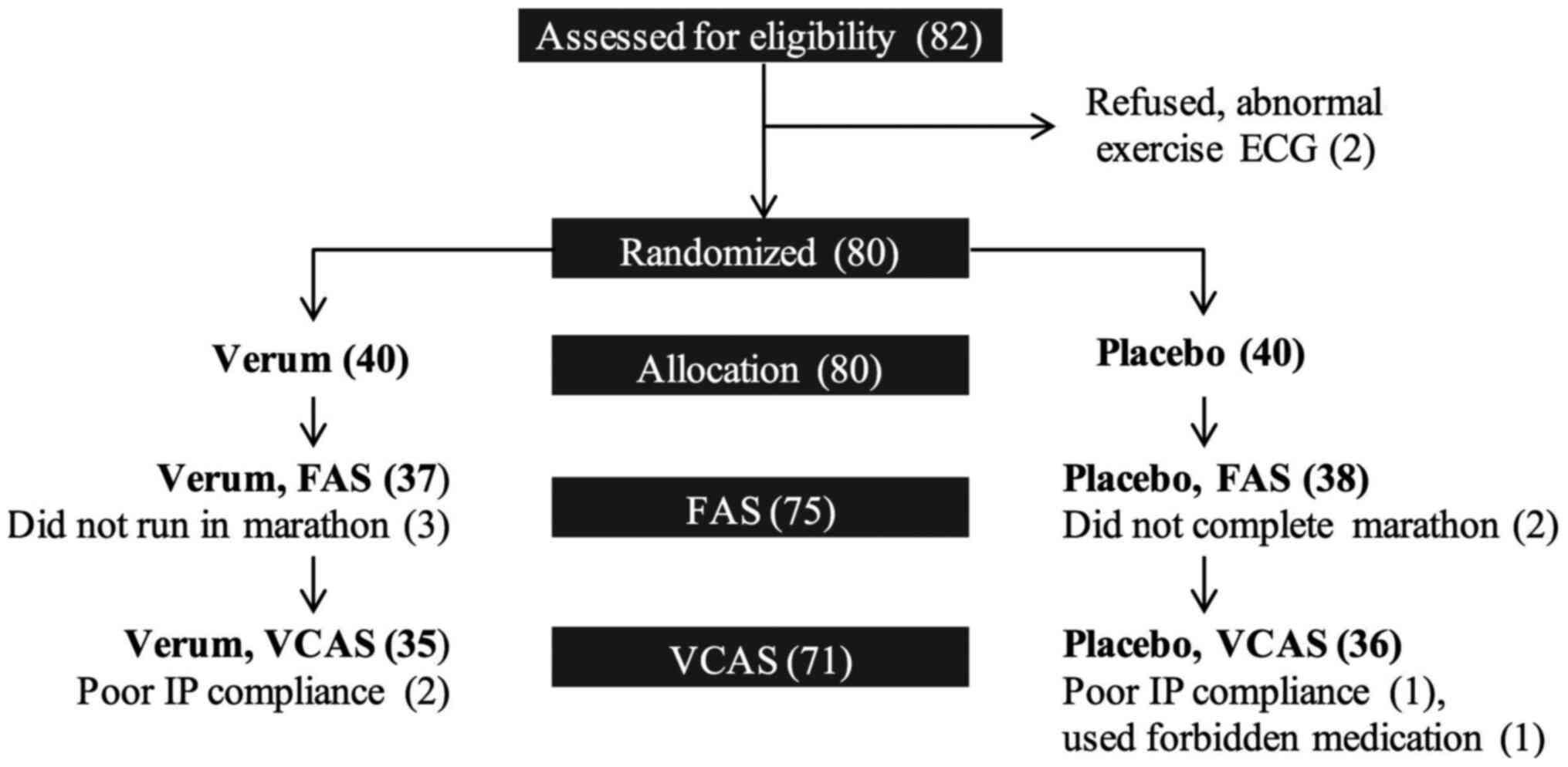
A total of 82 individuals were assessed for
eligibility, and 80 finally participated in the present pilot
study. The participants who completed the marathon were 43 men and
32 women, aged 22-64 years (mean age, 42.4±10.2 years). Three
participants from the V group did not run in the marathon and 2
participants in the P group did not complete the marathon, and they
were excluded from the full FAS. By the end of the study, 2
participants from each group either did not comply with the intake
of IP or had used excluded medication, and they were excluded from
the VCAS. The participant disposition flow chart is presented in
Fig. 2.
Participants from the V and P groups did not differ
significantly in terms of mean age, height, body weight and body
mass index (Table I).
 | Table IParticipant characteristics. |
Table I
Participant characteristics.
|
Characteristics | V group (n=37) | P group (n=38) |
|---|
| Sex |
|
Men | 21 (56.8%) | 22 (57.9%) |
|
Women | 16 (43.2%) | 16 (42.1%) |
| Age (years) | 43.3±10.2 | 40.4±10.3 |
| Height (cm) | 175.4±11.1 | 173.6±8.7 |
| Body weight
(kg) | 72.1±11.5 | 68.8±10.3 |
| BMI
(kg/m2) | 23.29±1.94 | 22.72±2.04 |
URTS
The incidence of URTS reported by participants in
the V group was lower compared with that in the P group (Table II). In the FAS population, the
percentage of participants who had at least 1 day of URTS between
day 1 and day 14 following the marathon was lower by 23.1% in the V
group compared with the P group (P=0.05). Additionally,
participants in the P group reported 48.3% more days with URTS
between day 1 and day 14 post-marathon (P=0.052). In the VCAS
population, the percentage of reported URTS and the number of days
of URTS differed significantly between the two groups (P<0.05),
with the V group reporting more favourable results (Table II). The P group had 2.11±3.25 days
with URTS, twice more when compared with the V group with 1.00±2.38
days (P=0.038).
 | Table IIParticipants with at least 1 day of
URTS reported between Day 1 and Day 14 post-marathon. |
Table II
Participants with at least 1 day of
URTS reported between Day 1 and Day 14 post-marathon.
| | FAS Population | VCAS
Population |
|---|
| Factor | V group (n=37) | P group (n=38) | P-value | V group (n=35) | P group (n=36) | P-value |
|---|
| Proportion of
participants | | | | | | |
|
No URTS | 78.4% | 55.3% | 0.050 | 80.0% | 55.6% | 0.042 |
|
≥1 day
URTS | 21.6% | 44.7% | | 20.0% | 44.4% | |
| Number of days with
URTS/cold1 | 1.05±2.37 | 2.03±3.18 | 0.052 | 1.00±2.38 | 2.11±3.25 | 0.038 |
WURSS-21
Changes to the score for URTS, listed as items 2-11
in the WURSS-21, were evaluated by calculating the mean scores on
the day of assessment minus the score on day -1. Days with milder
URTS exhibited lower scores and the negative values, representing
an improvement of URTS on the day of assessment compared with day
-1. URTS that were assessed by the questionnaire revealed that
participants who had taken IQP-AS-119 reported significantly milder
URTS on days 9, 12, 13 and 14 when compared to participants in the
P group (P<0.05) (Table III).
Changes from baseline (day -1) to day 1 through day 8 were not
statistically significant (data not shown).
 | Table IIIChanges in WURSS-21, mean score
(items 2-11) from baseline (Day -1). |
Table III
Changes in WURSS-21, mean score
(items 2-11) from baseline (Day -1).
| Day | V group (n=37) | P group (n=38) | P-value |
|---|
| Day 0 (marathon
day) | -0.15±0.42 | -0.04±0.15 | 0.400 |
| Day 9 | -0.15±0.63 | 0.14±0.91 | 0.026 |
| Day 10 | -0.14±0.68 | 0.10±0.87 | 0.158 |
| Day 11 | -0.20±0.59 | 0.12±0.83 | 0.051 |
| Day 12 | -0.19±0.61 | 0.11±0.88 | 0.031 |
| Day 13 | -0.16±0.70 | 0.07±0.82 | 0.019 |
| Day 14 | -0.17±0.67 | 0.09±0.88 | 0.028 |
Hooper's index
The summation of the four items (sleep, muscle
soreness, stress, fatigue) was used to yield the Hooper's Index
from the responses of 72 participants on day 7 and 74 participants
on day 14, out of the 75 participants in the FAS. The mean Hooper's
Index at the end of the first and second weeks after the marathon
exhibited no statistically significant differences between the two
groups (Table IV).
 | Table IVSummation of mean scores for Hooper's
Index in the FAS population. |
Table IV
Summation of mean scores for Hooper's
Index in the FAS population.
| Day post
marathon | V group | P group | P-value |
|---|
| Day 7 | 9.64a±3.55 | 8.61a±2.91 | 0.402 |
| Day 14 | 9.57b±3.54 | 9.19b±3.40 | 0.675 |
PSQ20
The scales that had higher values indicated greater
feelings of ‘joy’, ‘worries’, ‘tensions’ and ‘demands’. Changes to
the scores for the individual scales and the total score were
calculated by taking the mean scores on day 14 minus the mean
scores on either day -5 or day 2, so a lower score on day 14 would
result in negative numbers. Increased scores of ‘joy’, and
decreased scores of ‘worries’, ‘tension’, ‘demand’ and ‘total
score’ indicated improvement to participants' QoL. The reduction of
scores for the scales ‘tension’ and ‘demands’ were not
significantly different between the V and P groups. In the
assessment of ‘joy’ in the VCAS population, improvement in ratings
was reported in the V group, while deterioration was reported in
the P group from day 2 to day 14, and the difference was
statistically significant (P=0.021). The similar assessment in the
FAS population did not reveal statistically significant differences
(data not shown). The reduction in the scale for ‘worries’ was
statistically increased in the V group compared with the P group
from day -5 to day 14 and from day 2 to day 14 (P=0.021 and
P=0.012, respectively) in the VCAS population (Table V). The analysis in the FAS
population for the scores of ‘worries’ revealed similar
statistically significant findings (P<0.05) (data not shown). As
regards to the total PSQ20 score, the V group reported an
improvement from day 2 to day 14 compared with the P group
(P=0.035) in the VCAS population.
 | Table VChanges to PSQ20 for joy, worries,
tension and demands; and total score in the VCAS population. |
Table V
Changes to PSQ20 for joy, worries,
tension and demands; and total score in the VCAS population.
| Changes to
PSQ20 | V group | P group | P-value |
|---|
| Joy |
|
Day -5 to
14 | 3.92a±13.84 | -0.95b±13.25 | 0.160 |
|
Day 2 to
14 | 1.52±9.71 | -2.96±9.35 | 0.021 |
| Worries |
|
Day -5 to
14 | -4.51a±10.73 | 1.14b±9.63 | 0.021 |
|
Day 2 to
14 | -2.29±9.56 | 4.07±9.47 | 0.012 |
| Tension |
|
Day -5 to
14 | -2.35b±11.59 | 2.75c±15.05 | 0.204 |
|
Day 2 to
14 | -2.67±11.11 | 0.76±13.11 | 0.419 |
| Demands |
|
Day -5 to
14 | -3.43d±18.79 | -0.76b±15.32 | 0.718 |
|
Day 2 to
14 | -4.51±17.42 | 2.04±14.59 | 0.170 |
| Total Score |
|
Day -5 to
14 | -3.59d±11.34 | 0.93a±9.94 | 0.110 |
|
Day 2 to
14 | -2.75±9.51 | 2.33±8.22 | 0.035 |
SF-12, OTE and assessment of benefit
and tolerability
The items in the SF-12 questionnaire were analysed
by the physical composite (items 1, 2, 3, 4, 5 and 8), and mental
composite scores (items 6, 7, 9, 10, 11 and 12). The changes to the
composite scores were calculated by taking the mean composite
scores on day 14 minus the mean composite scores on either day -5
or day 2. Neither group had significant differences regarding
changes to the physical composite score. For the mental composite,
the V group exhibited a significant improvement from day -5 to day
14 compared with the P group (P=0.038; Table VI).
 | Table VIChanges to physical and mental
composite scores in the SF-12 in the FAS population. |
Table VI
Changes to physical and mental
composite scores in the SF-12 in the FAS population.
| A, PCS |
|---|
| Changes to
SF-12 | V group (n=35) | P group (n=35) | P-value |
|---|
| Day -5 to 14 | -0.60±8.96 | -1.53±5.68 | 0.449 |
| Day 2 to 14 | 0.38±8.58 | 1.36±9.19 | 0.440 |
| B, MCS | | | |
| Changes to
SF-12 | V group (n=35) | P group (n=35) | P-value |
| Day -5 to 14 | 2.13±7.11 | -0.33±4.51 | 0.038 |
| Day 2 to 14 | -0.39±6.04 | -1.47±3.55 | 0.271 |
For each week after the marathon, the OTE scores did
not differ significantly between groups (all P>0.05, Table VII). A negative value in the score
for day 7 indicated rating of worsening health since the marathon.
The change of the participants' scores between the 2 days of
assessment was calculated by taking the difference between the
scores for day 14 minus day 7.
 | Table VIIMean OTE scores for first and second
week after the marathon in the FAS population. |
Table VII
Mean OTE scores for first and second
week after the marathon in the FAS population.
| Day | V group (n=36) | P group (n=38) | P-value |
|---|
| D7 | 0.33±2.19 | -0.11±2.13 | 0.536 |
| D14 | 0.86±2.27 | 0.34±2.88 | 0.343 |
| D14-D7 | 0.53±1.86 | 0.45±2.39 | 0.705 |
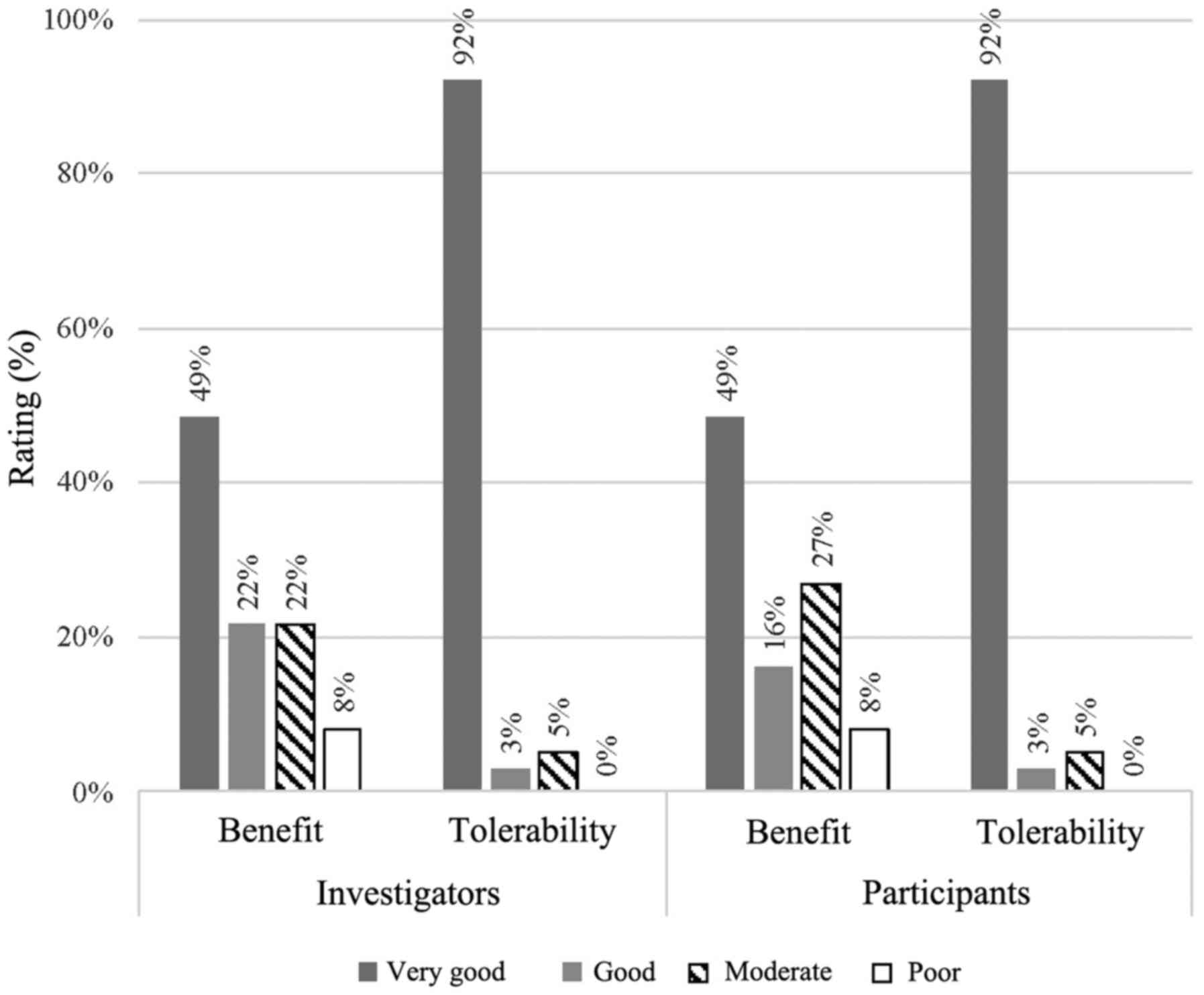
In the global assessment of benefit, IQP-AS-119 was
considered as ‘very good’ or ‘good’ for 71 and 65% of the
participants, as rated by the investigators and participants,
respectively (Fig. 3). The
respective ratings for the placebo were 45% (rating by
investigators) and 42% (rating by participants) (data not shown).
The ratings did not differ significantly between investigators and
participants (P>0.05).
In the assessment of adverse events, physical
examination, recording of vital signs and other laboratory
parameters, there were no clinically relevant differences between
the V and P groups. The tolerance of IQP-AS-119 was rated as ‘very
good’ or ‘good’ for 95% of participants by both investigators and
subjects (Fig. 3). All participants
agreed with the investigators on the tolerability ratings
(P=1.000).
Discussion
The results clearly demonstrated that runners using
IQP-AS-119 had a lower incidence of URTS in the 2 weeks following
the marathon. URTS were reported by 21.6% of the participants
taking IQP-AS-119 compared with 44.7% of those taking the placebo
in the FAS population (Table II).
This was similar in the VCAS population, where the number of
participants in the V group that exhibited URTS following the
marathon was 45% of that in the P group. Additionally, the number
of days with URTS reported by participants taking IQP-AS-119 was
52.6% shorter of that experienced by the subjects in the P group.
Previous dietary supplements had reported similar reduction of URTS
severity (ranging from 33 to 49.5% as compared to placebo) and
shorter duration of days with cold (ranging from 29.4 to 42.3% when
compared with the placebo group) (15,22,30).
In the assessment of the URTS severity per WURSS-21
(Table III), relative differences
were reported in favour of IQP-AS-119 for the URTS score (items
2-11) on post-marathon days 9, 12, 13 and 14. These results suggest
that a larger study population may be required to demonstrate
significant differences in WURSS-21, particularly in the specific
setting of post-exercise URTS, which are usually of a lesser
magnitude compared with those in the context of the classical URTI.
The current study used a non-invasive method for the assessment of
URTI and the WURSS-21 was indicated to be appropriate and accurate.
A statistically significant association was identified between the
questionnaire and the laboratory-assessed biomarkers of induced
rhinovirus infection in a previous study involving 399 participants
(43). Moreover, the WURSS-21 is a
validated (37) assessment for URTI
and had been used in numerous clinical trials for assessing the
treatment of the common cold (44-47).
The overall Hooper's Index assessed on the first and
second week after the marathon did not reveal significant
differences between the groups (Table
IV). The results of the assessment of individual PSQ20 scales
was indicated to be in favour of IQP-AS-119, specifically the ‘joy’
scale (days 2 to 14; P=0.021) and ‘worries’ scale (days 2 to 14;
P=0.012) when compared with placebo (Table V). In addition, the mental composite
score of the SF-12 demonstrated the positive effect of IQP-AS-119
over placebo (Days -5 to 14; P=0.038; Table VI).
Collectively, the WURSS-21, total PSQ20 score and
the mental composite score of the SF-12 indicated that improvements
in well-being were noticeable at 2 weeks after the marathon by
runners who were receiving IQP-AS-119. These observations were in
line with previously observed effects of natural health products in
marathon runners, such as improvement of Profile of Mood States for
confusion, fatigue, tension, anger and vigour (22).
Milder URTS experienced by participants who consumed
IQP-AS-119 on days 9, 12, 13 and 14, together with reduced number
of days with URTS, were consistent with other reported results of
studies on garlic (23) and green
chireta (25,48).
Previous studies by Carillon et al (26) and Milesi et al (27) in healthy subjects demonstrated the
ability of plant superoxide dismutase extract to significantly
reduce stress and fatigue over placebo treatment. The PSQ20's
subscores for ‘joy’ and ‘worries’ were consistent with the findings
of Carillon et al and Milesi et al, where the V group
reported significantly better ‘joy’ between day 2 and day 14, and
lower ‘worries’ from day -5 to day 14 and from day 2 to day 14,
compared with the placebo group.
To the best of our knowledge, the present study was
the first to demonstrate that supplementation with IQP-AS-119 in
immunosuppressed post-marathon runners was beneficial in reducing
the incidence of URTS. A study by Cox et al (49) demonstrated that illness-prone
athletes exhibited higher expression of the pro-inflammatory
cytokine IL-6. These athletes reported at least 4 occurrences of
URTS annually. This population is likely to benefit from the
consumption of IQP-AS-119 to modulate immune response (50).
The aim of the present pilot study was to provide
initial data on the beneficial effects of the IQP-AS-119 on immune
health and its main limitation was the small sample size. The
participants, who were marathon runners, tended to be in a good
physical state and this may not accurately represent the general
population. However, marathon running was used as a model of immune
stress to induce URTS, and the results of the present study may be
applicable to non-athletes who are susceptible to URTS. Future
research should be conducted in the wider population focusing on
the benefits of IQP-AS-119 in alleviating URTS. Of note, it was
hypothesized that the participants maintained a regular diet, but
this was not assessed. The multifactorial nature of immune
regulation was indicated to be affected by stress, nutrition and
sleep patterns, which would require further consideration in future
studies (51). Subject-reported
outcomes, such as those used in the present study, are often
employed in trials evaluating supplements for the common cold
(20,22,24,27).
The inclusion of immune markers and inflammatory markers could
provide objective insights for future similar trials (20,21,52,53).
Furthermore, a recent review had also called for multi-omics
studies for a more comprehensive understanding of the biological
pathways underlying immune regulation (44).
In conclusion, IQP-AS-119 was investigated in terms
of its health benefits in reducing the incidence and severity of
URTS in marathon runners, providing encouraging evidence supporting
the beneficial role of IQP-AS-119 in the immune response. In
addition, given the good tolerability profile of IQP-AS-119 over a
period of 5 weeks, it may be considered as an attractive
nutritional option for prevention or reduction of URTS in
susceptible individuals.
Acknowledgements
The authors would like to thank Dr Norman Bitterlich
(Medizin & Service GmbH), Dr Yvette Röske (analyze and realize
GmbH) and Mr. Constantin Erlenbeck (analyze and realize GmbH) for
their assistance in this study.
Funding
InQpharm Group provided funding for the following:
Design and conduct of the study, collection and analysis of data
and writing of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
RU, UB and UH were responsible for data collection
and management. UP, PWC and PDC contributed to study conception and
design. GB managed the data analysis. LC interpreted the data and
wrote the manuscript. GB, PWC and PDC critically revised and
contributed to the final content of the manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Prior to start of the current study, ethical
approval was gained from ‘Ethikkomission der Charité’ of the
Charité-Universitätsmedizin Berlin, Germany. All participants gave
their informed consent prior to their participation in the trial.
This trial was registered at clinicaltrials.gov as NCT02873910.
Patient consent for publication
Not applicable.
Competing interests
CPW, PDC and LC are employees of InQpharm Group. The
other authors declare that they have no competing interests.
References
|
1
|
Bhaskaram P: Micronutrient malnutrition,
infection, and immunity: An overview. Nutr Rev. 60:S40–S45.
2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Segerstrom SC and Miller GE: Psychological
stress and the human immune system: A meta-analytic study of 30
years of inquiry. Psychol Bull. 130:601–630. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Diment BC, Fortes MB, Edwards JP, Hanstock
HG, Ward MD, Dunstall HM, Friedmann PS and Walsh NP: Exercise
intensity and duration effects on in vivo immunity. Med Sci Sport
Exerc. 47:1390–1398. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Besedovsky L, Lange T and Haack M: The
sleep-immune crosstalk in health and disease. Physiol Rev.
99:1325–1380. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Milne KJ: The immune system and its
response to acute and chronic exercise. University of Windsor,
2018.
|
|
6
|
Kakanis M, Peake J, Hooper S, Gray B and
Marshall-Gradisnik S: The open window of susceptibility to
infection after acute exercise in healthy young male elite
athletes. In: 2010 Asics Conference of Science and Medicine in
Sport,. 13:ppe84–e85. 2010.PubMed/NCBI
|
|
7
|
Boffi El and Amari E: Upper respiratory
tract infections and sports. Rev Med Suisse. 6:1499–1503.
2010.PubMed/NCBI(In French).
|
|
8
|
Nieman DC: Physical activity, fitness and
infection. In: Pysical Activity, Fitness, and Health. Human
Kinetics Publishers, Champaign, IL, pp796-813, 1994.
|
|
9
|
Peters EM and Bateman ED: Ultramarathon
running and upper respiratory tract infections. An epidemiological
survey. South African Med J. 64:582–584. 1983.PubMed/NCBI
|
|
10
|
Kuipers H and Keizer HA: Overtraining in
elite athletes: Review and directions for the future. Sport Med.
6:79–92. 1988.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Meeusen R, Duclos M, Foster C, Fry A,
Gleeson M, Nieman D, Raglin J, Rietjens G, Steinacker J, Urhausen
A, et al: Prevention, diagnosis, and treatment of the overtraining
syndrome: Joint consensus statement of the European College of
Sport Science and the American College of Sports Medicine. Med Sci
Sports Exerc. 45:186–205. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hoffman-Goetz L and Pedersen BK: Exercise
and the immune system: A model of the stress response? Immunol
Today. 15:382–387. 1994.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pedersen BK and Hoffman-Goetz L: Exercise
and the immune system: Regulation, integration, and adaptation.
Physiol Rev. 80:1055–1081. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nieman DC: Exercise, upper respiratory
tract infection, and the immune system. Med Sci Sport Exerc.
26:128–139. 1994.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Peters E, Joseph L, Peters E, Goetzsche J
and Noakes T: Vitamin C as effective as combinations of
anti-oxidant nutrients in reducing symptoms of upper respiratory
tract infection in ultramarathon runners. J Sports Med. 3:23–27.
1996.
|
|
16
|
Rehm KE, Elci OU, Hahn K and Marshall GD:
The impact of self-reported psychological stress levels on changes
to peripheral blood immune biomarkers in recreational marathon
runners during training and recovery. Neuroimmunomodulation.
20:164–176. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gonçalves CA, Dantas PM, dos Santos IK,
Dantas M, da Silva DC, Cabral BG, Guerra RO and Júnior GB: Effect
of acute and chronic aerobic exercise on immunological markers: A
systematic review. Front Physiol. 10(1602)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nieman DC, Johanssen LM, Lee JW and
Arabatzis K: Infectious episodes in runers before and after the Los
Angeles Marathon. J Sports Med Phys Fitness. 30:316–328.
1990.PubMed/NCBI
|
|
19
|
Robson-Ansley P, Howatson G, Tallent J,
Mitcheson K, Walshe I, Toms C, Du Toit G, Smith M and Ansley L:
Prevalence of allergy and upper respiratory tract symptoms in
runners of the London Marathon. Med Sci Sports Exerc. 44:999–1004.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
McFarlin BK, Carpenter KC, Davidson T and
McFarlin MA: Baker's yeast beta glucan supplementation increases
salivary IgA and decreases cold/flu symptomatic days after intense
exercise. J Diet Suppl. 10:171–183. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Scherr J, Nieman DC, Schuster T, Braun S,
Wolfarth B and Halle M: Non-alcoholic beer reduces inflammation and
the incidence of upper respiratory tract infections after a
marathon. Med Sci Sports Exerc. 44:18–26. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Talbott S and Talbott J: Effect of BETA 1,
3/1, 6 GLUCAN on upper respiratory tract infection symptoms and
mood state in marathon athletes. J Sport Sci Med. 8:509–515.
2009.PubMed/NCBI
|
|
23
|
Josling P: Preventing the common cold with
a garlic supplement: A double-blind, placebo controlled survey. Adv
Ther. 18:189–193. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Saxena RC, Singh R, Kumar P, Yadav SC,
Negi MP, Saxena VS, Joshua AJ, Vijayabalaji V, Goudar KS,
Venkateshwarlu K and Amit A: A randomized double blind placebo
controlled clinical evaluation of extract of Andrographis
paniculata (KalmCold) in patients with uncomplicated upper
respiratory tract infection. Phytomedicine. 17:178–185.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Melchior J, Palm S and Wikman G:
Controlled clinical study of standardized Andrographis paniculata
extract in common cold-a pilot trial. Phytomedicine. 3:315–318.
1997.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Carillon J, Notin C, Schmitt K, Simoneau G
and Lacan D: Dietary supplementation with a superoxide
dismutase-melon concentrate reduces stress, physical and mental
fatigue in healthy people: A randomised, double-blind,
placebo-controlled trial. Nutrients. 6:2348–2359. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Milesi MA, Lacan D, Brosse H, Desor D and
Notin C: Effect of an oral supplementation with a proprietary melon
juice concentrate (Extramel) on stress and fatigue in healthy
people: A pilot, double-blind, placebo-controlled clinical trial.
Nutr J. 8(40)2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Camargo CA, Ganmaa D, Frazier AL,
Kirchberg FF, Stuart JJ, Kleinman K, Sumberzul N and Rich-Edwards
JW: Randomized trial of vitamin D supplementation and risk of acute
respiratory infection in Mongolia. Pediatrics. 130:e561–e567.
2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zittermann A, Pilz S, Hoffmann H and März
W: Vitamin D and airway infections: A European perspective. Eur J
Med Res. 21(14)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Prasad AS, Beck FW, Bao B, Snell D and
Fitzgerald JT: Duration and severity of symptoms and levels of
plasma Interleukin-1 receptor antagonist, soluble Tumor Necrosis
Factor Receptor, and adhesion molecules in patients with common
coldtreated with zinc acetate. J Infect Dis. 197:795–802.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Prasad AS, Fitzgerald JT, Bao B, Beck FW
and Chandrasekar PH: Duration of symptoms and plasma cytokine
levels in patients with the common cold treated with zinc acetate.
A randomized, double-blind, placebo-controlled trial. Ann Intern
Med. 133:245–252. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Choi SK, Baek SH and Choi SW: The effects
of endurance training and thiamine supplementation on anti-fatigue
during exercise. J Exerc Nutr Biochem. 17:189–198. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Suzuki M and Itokawa Y: Effects of
thiamine supplementation on exercise-induced fatigue. Metab Brain
Dis. 11:95–106. 1996.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Duthie SJ, Horgan G, De Roos B, Rucklidge
G, Reid M, Duncan G, Pirie L, Basten GP and Powers HJ: Blood folate
status and expression of proteins involved in immune function,
inflammation, and coagulation: Biochemical and proteomic changes in
the plasma of humans in response to long-term synthetic folic acid
supplementation. J Proteome Res. 9:1941–1950. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
World Medical Association: Declaration of
Helsinki. Ethical principles for medical research involving human
subjects. urihttps://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/simplehttps://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/,
2008.
|
|
36
|
ICH: Guideline for Good Clinical Practice
E6 (R2), 2016.
|
|
37
|
Barrett B, Brown RL, Mundt MP, Thomas GR,
Barlow SK, Highstrom AD and Bahrainian M: Validation of a short
form Wisconsin upper respiratory symptom survey (WURSS-21). Health
Qual Life Outcomes. 7(76)2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jaeschke R, Singer J and Guyatt GH:
Measurement of health status. Ascertaining the minimal clinically
important difference. Control Clin Trials. 10:407–415.
1989.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hooper SL and Mackinnon LT: Monitoring
overtraining in athletes. Recommendations. Sport Med. 20:321–327.
1995.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Fliege H, Rose M, Arck P, Walter OB,
Kocalevent RD, Weber C and Klapp BF: The Perceived Stress
Questionnaire (PSQ) reconsidered: Validation and reference values
from different clinical and healthy adult samples. Psychosom Med.
67:78–88. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ware J jr, Kosinski M and Keller SD: A
12-Item Short Health Survey: Construction of scales and preliminary
tests of reliability and validity. Med Care. 34:220–233.
1996.PubMed/NCBI View Article : Google Scholar
|
|
42
|
International Conference on Harmonization:
ICH Topic E 9: Statistical principles for clinical trials,
1998.
|
|
43
|
Barrett B, Brown R, Voland R, Maberry R
and Turner R: Relations among questionnaire and laboratory measures
of rhinovirus infection. Eur Respir J. 28:358–363. 2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Nieman DC and Wentz LM: The compelling
link between physical activity and the body's defense system. J
Sport Heal Sci. 8:201–217. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Smyth AR, Cifelli PM, Ortori CA, Righetti
K, Lewis S, Erskine P, Holland ED, Givskov M, Williams P, Cámara M,
et al: Garlic as an inhibitor of Pseudomonas aeruginosa
quorum sensing in cystic fibrosis-a pilot randomized controlled
trial. Pediatr Pulmonol. 45:356–362. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Barrett B, Brown R, Rakel D, Rabago D,
Marchand L, Scheder J, Mundt M, Thomas G and Barlow S: Placebo
Effects and the Common Cold : A randomized controlled trial. Ann
Fam Med. 9:312–322. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Obasi CN, Brown R, Ewers T, Barlow S,
Gassman M, Zgierska A, Coe CL and Barrett B: Advantage of
meditation over exercise in reducing cold and flu illness is
related to improved function and quality of life. Influenza Other
Respi Viruses. 7:938–944. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Spasov AA, Ostrovskij OV, Chernikov MV and
Wikman G: Comparative controlled study of Andrographis paniculata
fixed combination, Kan Jang and an Echinacea preparation as
adjuvant, in the treatment of uncomplicated respiratory disease in
children. Phyther Res. 18:47–53. 2004.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Cox AJ, Pyne DB, Saunders PU, Callister R
and Gleeson M: Cytokine responses to treadmill running in healthy
and illness-prone athletes. Med Sci Sports Exerc. 39:1918–1926.
2007.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lawson LD and Gardner CD:
Compositionstability, and bioavailability of garlicproductsbeing
used in a clinical trial. J Agric Food Chem. 53:6254–6261.
2005.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Simpson R, Campbell J, Gleeson M, Krüger
K, Nieman DC, Pyne DB, Turner JE and Walsh NP: Can exercise affect
immune function to increase susceptibility to infection? Exerc
Immunol Rev. 26:8–22. 2020.PubMed/NCBI
|
|
52
|
Su QS, Tian Y, Zhang JG and Zhang H:
Effects of allicin supplementation on plasma markers of
exercise-induced muscle damage, IL-6 and antioxidant capacity. Eur
J Appl Physiol. 103:275–283. 2008.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Niemelä M, Kangastupa P, Niemelä O, Bloigu
R and Juvonen T: Acute changes in inflammatory biomarker levels in
recreational runners participating in a marathon or half-marathon.
Sport Med Open. 2(21)2016.PubMed/NCBI View Article : Google Scholar
|